Background
15.4 million women worldwide are infected with HIV.[1] The majority are of child-bearing age and not in immediate need of antiretroviral therapy (ART). Provision of safe and effective contraception prevents maternal mortality, reduces unsafe abortions, and allows control of child-bearing.[2, 3] The World Health Organization also promotes effective contraception as a major component of preventing perinatal HIV infection.[4] Two of the most common methods of modern contraception in sub-Saharan Africa are oral contraceptive pills (OCPs) and injectable progestins, such as Depo-Medroxyprogesterone Acetate (DMPA). Use of DMPA appears to be rising in many countries owing to its discreteness and its convenient 3-monthly dosing schedule.[5]
We recently reported a randomized trial that examined the contraceptive efficacy and safety of the intrauterine contraceptive device (IUD) among HIV-infected women in Lusaka, Zambia.[6] That study found the IUD to be safe and effective, but subsequent analysis indicated women allocated to the hormonal contraception control arm had faster progression of their HIV disease (based on death and decline in CD4+ count). This finding corroborated by other research in both macaques [7] and humans [8] suggests a link between progesterone exposure and both HIV acquisition and faster disease progression. We therefore hypothesized that progesterone might be primarily responsible for study’s observation and sought to examine the question in greater detail.
Methods
We have described details of this randomized trial and its methods previously.[6] Briefly, post-partum HIV-infected women desiring contraception were randomized to either the copper-T IUD or hormonal contraception. Women allocated to the hormonal arm were allowed to choose between DMPA and OCPs. If breastfeeding, women choosing OCPs were placed on progesterone-only pills until their baby was six months old (according to Zambian national guidelines). Contraceptives were dispensed every three months. Study visits occurred at enrollment, one month post-enrollment, and then every six months until the last randomized woman had been followed for 24 months. At each study visit, women were asked whether they were still using their randomized method of contraception, and if not, which method they were currently using (if any). Patient report was our only measure of adherence; we did not perform pill counts. Patients who missed scheduled study visits were followed-up at home by community volunteers. Although study assignment was not masked, these volunteers were not informed of the patients’ contraceptive methods, in an effort to avoid differential follow-up by study arm.
CD4+ cell counts were obtained every 12 months until August 2003, when ART became available in the public sector through the MTCT Plus Initiative. [9] Thereafter, we obtained CD4+ cell counts every six months. Women were allowed to switch from their originally-allocated contraceptive method and remain in the study.
Statistical analysis
We defined HIV disease progression as either death or becoming eligible for ART (decline in CD4+ cell count below 200 cells/mm3 or commencement of ART for any reason). Our primary analysis employs this composite outcome, but we also examined each factor separately (e.g. CD4+ cell decline). Data were analyzed using SAS® version 9.1.4 (SAS Institute, Cary, NC, USA). Continuous covariates were compared across the three contraceptive exposures using unpaired, two-tailed t tests to evaluate means, and the non-parametric Wilcoxon rank-sum test was used to evaluate differences in medians. Dichotomous and categorical variables were compared using the Pearson chi-square test statistic. We used the Kaplan-Meier method and hazard rate ratios (HRs) from Cox proportional hazard modeling to compare rates of clinical disease progression among the three contraceptive exposures. Multivariate models controlled for maternal baseline CD4+ count. Women were censored at time of pregnancy, death, CD4+ cell count falling below 200 cells/mm3, or starting ART. Of the 599 enrolled women, four were excluded from this analysis because they were already on ART at study enrollment. Women with initial CD4+ counts below 200 cells/mm3 were excluded from the analysis of the clinical outcome of CD4+ count falling below 200 cells/mm3 (N =538). The composite analysis included all subjects since those who had a CD4+ count less than 200 were still “eligible” to have the death outcome.
We categorized contraceptive exposure in the primary analysis by the method a woman was dispensed at her first study visit. (Whether their exposure was IUD or hormonal was random, but women allocated to the hormonal arm were allowed to choose between OCPs and DMPA.) We refer to this as the intent-to-treat analysis. Since women were allowed to switch among methods at any time during the trial, including from IUD to hormonal or vice versa, we also performed an actual-use analysis in which the contraceptive method was treated as a time-varying exposure in extended Cox proportional hazards regression. In this analysis, women who used more than one type of contraception contributed person-time to more than one exposure group. The study received continuing ethical review from the relevant authorities at the University of Zambia and at the University of Alabama at Birmingham. All participants provided written informed consent.
Results
We randomized 599 HIV-infected women between June 12, 2002 and October 2, 2003. Of those remaining, 303 were randomized to hormonal contraception and 296 to the IUD. Four women entered the study on ART and are excluded from this analysis (1 in the hormonal arm and 3 in the IUD arm). One hundred and ninety (63%) of the 302 women randomized to the hormonal arm chose DMPA, and 112 (37%) chose OCPs. Women starting OCPs, DMPA, or IUD did not differ substantially by any clinical or socio-demographic factor that we measured at baseline [Table 1].
Table I.
Baseline characteristics of participants and contraceptive method started postpartum
| IUD (n=293) | DMPA (n=190) | P value** | OCPs (n=112) | P value** | |
|---|---|---|---|---|---|
| Age, mean (±sd*) | 26.3 (+/− 4.9) | 25.9 (+/− 5.0) | 0.48 | 25.9 (+/− 4.8) | 0.47 |
| Married, # (%) | 261 (89.1) | 169 (88.9) | 0.96 | 102 (91.1) | 0.56 |
| Total number of pregnancies, median (IQR**) | 2.0 (1.0,3.0) | 2.0 (1.0,3.0) | 0.77 † | 2.0 (1.0,3.0) | 0.11 † |
| Total number of living children, median (IQR) | 2.0 (1.0,3.0) | 2.0 (1.0,3.0) | 0.61 † | 2.0 (1.0,3.0) | 0.54 † |
| Report at least 1 child death, # (%) | 83 (28.3) | 52 (27.4) | 0.82 | 29 (25.9) | 0.62 |
| Years of schooling, mean (±sd) | 7.2 (+/− 3.0) | 7.5 (+/− 2.9) | 0.31 | 7.1 (+/− 3.4) | 0.57 |
| Any prior contraceptive use, n (%) | 178 (60.8) | 125 (65.8) | 0.26 | 60 (53.6) | 0.18 |
| Enrollment CD4+ count, mean (±sd) | 517.6 (+/− 265.1) | 497.4 (+/− 256.3) | 0.41 | 488.6 (+/−239.9) | 0.31 |
| CD4+ count < 200 cells/μL, # (%) | 28 (9.6) | 17 (8.9) | 0.81 | 12 (10.7) | 0.73 |
| Enrollment Hgb, g/dL (sd) | 11.7 (+/− 1.9) | 11.6 (+/− 2.2) | 0.64 | 11.7 (+/− 1.4) | 0.91 |
| BMI at enrollment, mean, kg/m2 (sd) | 24.2 (+/− 3.9) | 23.9 (+/− 4.3) | 0.47 | 24.4 (+/− 3.6) | 0.62 |
| Time in study-months (median, Ist and 3rd quarter) | 27.6 (21.1,32.9) | 26.2 (13.6,32.9) | 0.05 † | 25.7 (14.4,32.1) | <0.00† |
| Breast feeding at enrollment | 269 (93.7) | 186 (98.4) | 0.01 | 107 (96.4) | 0.30 |
Use of Fisher’s Exact Test due to small expected cell sizes
P-derived from Kruskal-Wallis Test
Compared to the IUD arm
Overall, 208 of the 595 women either died (n=27; 5%), became pregnant (n=21; 4%), withdrew (n=73; 12%) or were lost to follow-up (n=90; 15%) prior to the end of the study period of 24 months. Of these women censored before 24 months, 81 (38.9%) initiated the IUD, 80 (37.9%) initiated DMPA, and 50 (23.7%) initiated OCPs. Among the 293 in the IUD group, 63 (21.5%) were either lost to follow-up or withdrawn, compared to 60/190 (31.6%) women in the DMPA group and 40/112 (35.7%) women in the OCP group. Compared to the IUD arm, women in the DMPA and OCP groups had 1.47 (95%CI, 1.09–1.99; p=0.01) and 1.66 (95%CI, 1.19–2.31; p<0.001) increased hazard of becoming lost to follow-up or withdrawn at 24 months, respectively. We observed no statistically significant differences in the baseline CD4+ counts or changes in CD4+ counts at 12 and 18 months within each exposure category prior to being lost-to-follow-up (data not shown).
Women randomized to the IUD arm were much more likely to discontinue their method than women in the DMPA or OCP groups. 146 women switched from the IUD over 504 woman-years of follow up (switching rate: 29.0/100 woman-years; 95%CI, 24.6–34.0); 43 women switched from DMPA over 334 woman- years (switching rate: 12.9/100 woman-years; 95%CI, 9.5–17.3); and 43 women switched from OCPs over 163 woman-years (switching rate: 26.4/100 woman-years; 95%CI, 19.6–35.6). Compared to the IUD arm, the hazard rate ratio for switching contraception methods for the women initiated on DMPA was 0.44 (95%CI, 0.31–0.62) and on OCPs was 0.94 (95%CI, 0.68–1.32). The median months of follow-up for women initiating the IUD was 27.6 months, compared to 26.2 months among women initiating DMPA, and 25.7 months among women initiating OCPs. To explore whether change in methods might be driven by disease progression, we evaluated the baseline CD4+ counts and changes in CD4+ counts at 12 and 18 months (delta CD4) prior to switching within strata of initial contraceptive exposure. We found no statistical evidence to suggest an association between more rapid CD4+ cell decline and switching from a particular contraceptive method (data not shown).
Death
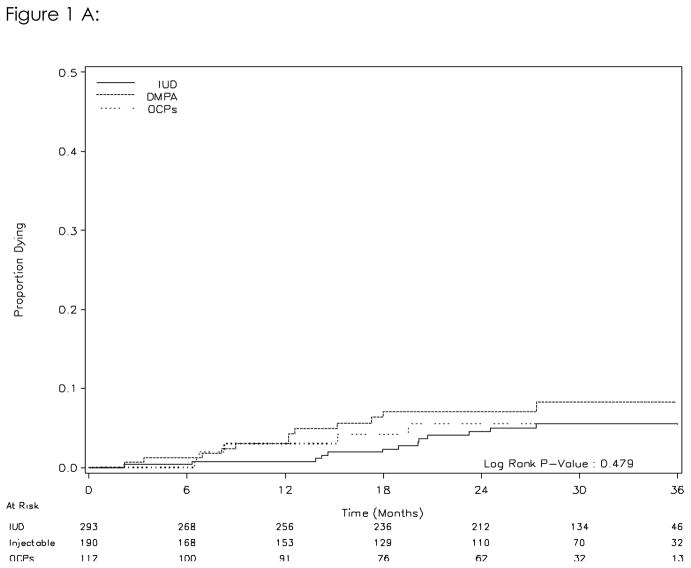
Thirty women died over 1,211 woman-years of follow-up (rate 2.5/100 woman-years). The mortality rate among women who started on the IUD was 2.0/100 woman-years. The mortality rates were slightly higher in women initiating DMPA (3.2/100 woman years) and OCPs (2.4/100 woman years). Compared to the IUD group, the crude hazard ratios for death in the DMPA group and OCP group were 1.39 (95%CI, 0.63–3.06) and 1.06 (95%CI, 0.38–2.97), respectively. [Table II] Kaplan-Meier analysis indicated no significant difference in death between women initiating the different methods (log rank, p=0.48; Figure 1A). In an actual use- analysis that treated current contraceptive method as a time-varying exposure, the hazard ratios for DMPA and OCPs were 1.83 (95%CI, 0.82–4.08) and 1.24 (95%CI, 0.42–3.63; Table II), respectively. Controlling for baseline BMI, education, and breast-feeding did not significantly change the results (data not shown)
Table 2.
Hazard of Disease progression by type of contraceptive exposure
| Outcome | Contraceptive method | Intent-to-treat analysis (Crude hazard ratio) | Time-varying analysis* (Adjusted hazard ratio) |
|---|---|---|---|
| Death | IUD | ref | ref |
| OCPs | 1.06 (0.38–2.97) | 1.24 (0.42–3.63) | |
| DMPA | 1.39 (0.63–3.06) | 1.83 (0.82–4.08) | |
| CD4+ count dropping below 200 cells/m3 or Initiating ART | IUD | ref | ref |
| OCPs | 1.54(0.98 – 2.42) | 1.69(1.09 – 2.64) | |
| DMPA | 1.81(1.26 – 2.60) | 1.56(1.08 – 2.26) | |
| Death or CD4+ count dropping below 200 cells/m3 or initiating ART | IUD | ref | ref |
| OCPs | 1.52(1.00 – 2.32) | 1.67(1.10 – 2.51) | |
| DMPA | 1.81(1.30 – 2.53) | 1.62(1.16 – 2.28) |
extended Cox model treating contraceptive exposure as a time-varying covariate, censored at time of pregnancy; models control for baseline CD4+ count.
Figure 1.
Figure 1A. Risk of death by initial contraceptive method (intent-to-treat analysis)
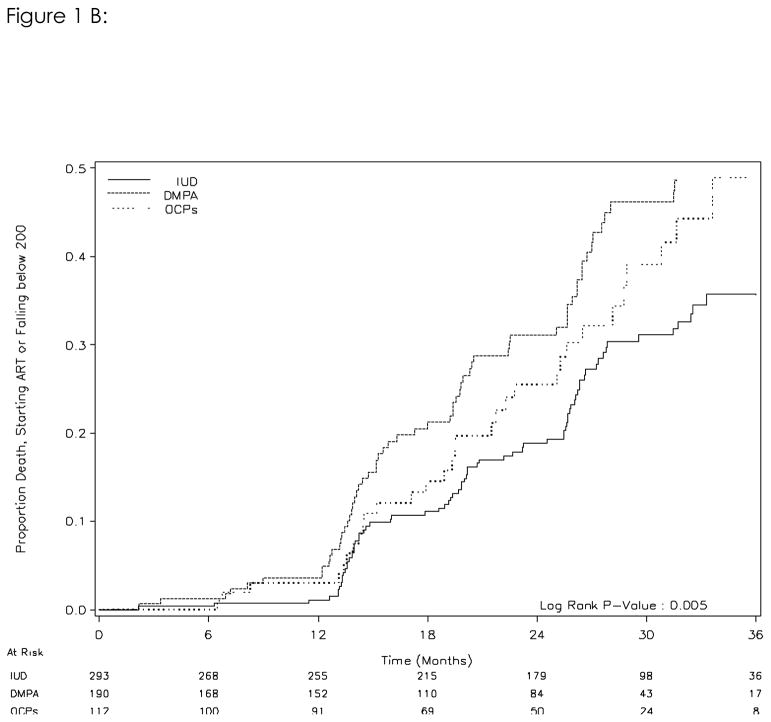
Figure 1B. Risk of HIV disease progression (either CD4 cell count falling below 200 cells/mm3 or initiation of ART) by initial contraceptive method (intent-to-treat analysis)
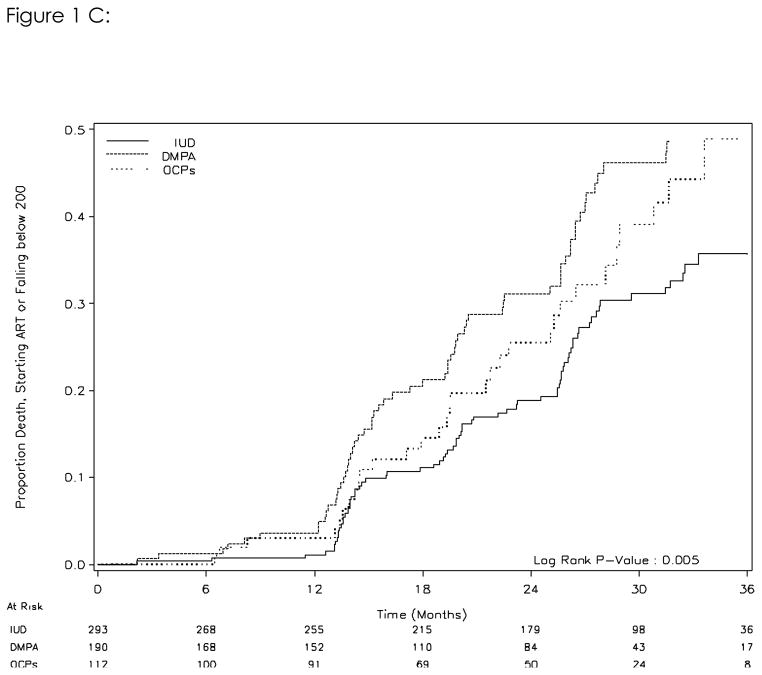
Figure 1C. Risk of death or HIV disease progression (either death or CD4 cell count falling below 200 cells/mm3 or initiation of ART) by initial contraceptive method (intent-to-treat analysis)
CD4+ cell count falling below 200 cells/mm3 or initiating ART
A total of 148 women either had their CD4+ cell count fall below 200 cells/mm3 and/or initiated ART during follow-up. The rates of women meeting these criteria in the IUD arm was 11.2/100 woman-years of follow-up, versus 17.5/100 in the DMPA group and 14.3/100 in the OCP group. In crude analysis using the IUD as the referent group, the hazard ratios for DMPA and OCP were 1.81 (95%CI, 1.26–2.60) and 1.54 (95%CI, 0.98–2.42) respectively. A Kaplan-Meier analysis indicates a difference between the three contraception arms in the time to either of the two outcome. (log-rank test p=0.01, figure 1B) Treating contraception as a time-varying exposure and adjusting for initial CD4+ cell count, the hazard ratios for DMPA and OCP for this outcome were 1.56 (95%CI, 1.08–2.26) and 1.69 (95%CI, 1.09–2.64) respectively. Controlling for baseline BMI, education, and breast-feeding did not significantly change the results (data not shown).
Composite outcome of Death, CD4+ count falling <200 cells/mm3, or Initiating ART
One hundred and seventy-five of 595 women met the criteria of the composite outcome. In the IUD group, the rate was 13.1/100 woman-years; in the DMPA group it was 20.9/100 woman-years and in the OCP group 16.9/100 woman years. Compared to the IUD or non-hormonal group, the crude hazard ratios from the intent-to-treat Cox analysis for the composite outcome in the DMPA group and OCP group were 1.81(95%CI, 1.30–2.53) and 1.52(95%CI, 1.00–2.32). The Kaplan-Meier analysis indicates a difference between the three contraceptive groups in the time to any of the three outcomes. (log-rank test p=0.005, Figure 1C) Treating the contraception exposure as a time-varying exposure, the hazard ratios for DMPA and OCPs were 1.62 (95%CI, 1.16–2.28) and 1.67 (95%CI, 1.10–2.51), respectively. Controlling for baseline BMI, education, and breast-feeding did not significantly change the results (data not shown).
Discussion
The contraceptive trial that formed the basis for this report was designed to evaluate the efficacy and safety of the IUD in HIV-infected women. Our study found unexpectedly that women initiating hormonal contraception had more rapid progression of their HIV disease. In this secondary report, we examined the hormonal contraceptive groups separately with HIV disease progression as an endpoint and found that compared to the IUD both OCPs and DMPA were associated with accelerated HIV disease progression.
Animal models suggest that hormones like progesterone may promote simian immunodeficiency disease,[7, 10] as does at least one study in humans. Lavreys and colleagues showed that newly HIV-infected Kenyan sex workers using DMPA at the time of HIV acquisition, had higher viral load set points compared to those without the exposure.[11] (high viral set points have been shown to be predictive of HIV disease progression.) In addition, the Kenya study found that multiple viral genotypes were more commonly detected in women who acquired HIV while using hormonal contraception (either oral contraceptive pills or DMPA) and women with multiple viral genotypes had higher viral loads 4–24 months, lower CD4+ counts, and faster CD4+ count declines over time.[8]
Other studies have not observed an association between hormonal contraception exposure and HIV disease progression. [12, 13] Richardson and colleagues analyzed data on 193 women, some of whom were using hormonal contraception (both DMPA and OCPs) and others who were not. Hormonal contraception was not associated with appreciable changes in CD4+ cell count or viral load, either after short term (<5 months) or long term follow-up (up to 24 months).[13]
A large body of literature suggests that estrogen and progesterone have a broad array of effects on immune function. Estrogen and progesterone receptors are found on many immune cells including T-lymphocytes, B-lymphocytes, monocytes, and neutrophils.[14, 15] Potential effects on the immune system include the following: modulation of cellular activation levels (measured through CD38, CD25, CD69) which can impact both the number of lymphocytes infected with HIV and the rate of clearance of the infected cells[16]; disruption of the cytokine balance between TH1 and TH2 which diminishes the clearance of HIV infected cells[17]; and increased cellular senescence (measured through CD57, ki67, IDO).[18–20]
Based on the basic science literature, we expected to find in our current study that DMPA would hasten HIV disease progression more than OCPs, however, this is not what we observed. In crude analysis, there was a suggestion that DMPA might be worse than OCPs, but in the time-varying analysis, which accounts for switching among methods, as well as adherence to methods, this association all but disappeared. It is important to note, however, that a relationship between hormonal contraception and disease progression was not an a priori hypothesis of our trial.
Among this study’s limitations is our inability to assess the specific contribution of progesterone and estrogen to the outcomes. No women received estrogen monotherapy (this is not a contraceptive method), and of those who received OCPS, there was exposure to combination estrogen/progesterone, progesterone-only, or both formulations All breastfeeding women who chose OCPs were, per Ministry of Health protocol, prescribed progesterone-only pills until their babies were 6 months old. Thereafter, they were switched to combination formulations. Unfortunately, we do not have data available distinguishing the various OCP formulations.
Another limitation of this analysis is the large proportion of women who switched contraceptive methods, withdrew from the study, or were lost to follow-up (n=281, 47.2 % in total). We addressed the switching within our proportional hazards regression by treating contraceptive method as a time-varying exposure. Although the women who were lost to follow-up appeared to have similar prognosis based on the change in their CD4 prior to leaving the study, we cannot rule out informative censoring. Furthermore, women who became pregnant were censored in this study. This is another possible source of bias based on differential censoring among the contraceptive methods. Finally, although the initial method allocation was randomized (IUD vs hormonal contraception), women were allowed to choose their type of hormonal contraception. This is a potential source of confounding for which we may not have controlled completely.
Safe and effective contraception provides many benefits, especially to HIV-infected women. The risk of maternal mortality increases with each subsequent pregnancy, and nowhere is this more evident than in sub-Saharan Africa, where a woman’s lifetime risk of dying in pregnancy can be as high as in one in 22.[21] Our findings raise the possibility that hormonal contraception, relative to the IUD, may hasten HIV disease progression. Although previous animal studies suggest a greater effect of progesterone-only methods (e.g. DMPA), this finding was not confirmed in this secondary analysis. Women using DMPA and OCPs had similarly elevated risk for HIV disease progression when compared to those without hormonal exposure. While concerning, we feel strongly that these results are not definitive, and as such should not influence current prescribing practice. A randomized trial designed specifically to evaluate the potential relationship between HIV disease progression and hormonal contraception is urgently needed.
Acknowledgments
The authors would like to thank the Mark Giganti and Dwight Rouse for their helpful comments on this manuscript, and as always, our hard-working study team and all our participants.
Footnotes
Disclosure of funding: This work was supported by a grant from the Elizabeth Glaser Pediatric AIDS Foundation (PG-51161) with complementary resources from the US Agency for International Development (HRN-A-00-98-00020-00; SA-04-395). Investigators received salary or stipend support from the National Institutes of Health (D43-TW01035, K23-AI01411, K01-TW05708, K01-TW06670, D43-TW010035).
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS), World Health Organization. Report on the global HIV/AIDS epidemic 2007. Geneva: UNAIDS; 2007. [Google Scholar]
- 2.Grimes DA, Benson J, Singh S, Romero M, Ganatra B, Okonofua FE, Shah IH. Unsafe abortions: the preventable pandemic. Lancet. 2006;368:1908–1919. doi: 10.1016/S0140-6736(06)69481-6. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Darroch JE, Vlassoff MJN. (UNFPA) The Allan Guttmacher Institute, editor. Adding it up: The Benefits of Investing in Sexual and Reproductive Health Care. New York: 2003. [Google Scholar]
- 4.UNAIDS. Report on the global HIV/AIDS epidemic: 4th global report. Geneva: 2004. [Google Scholar]
- 5.Seiber EE, Bertrand JT, Sullivan TM. Changes in contraceptive method mix in developing countries. International Family Planning Perspectives. 2007;33:117–123. doi: 10.1363/3311707. [DOI] [PubMed] [Google Scholar]
- 6.Stringer EM, Kaseba C, Levy J, Sinkala M, Goldenberg RL, Chi BH, et al. A randomized trial of the intrauterine contraceptive device (IUD) versus hormonal contraception in HIV-1-infected women. AJOG. 2007;197 (2):144, e1–8. doi: 10.1016/j.ajog.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 8.Baeten JM, Lavreys L, Sagar M, Kreiss JK, Richardson BA, Chohan B, et al. Effect of contraceptive methods on natural history of HIV: studies from the Mombasa cohort. J Acquir Immune Defic Syndr. 2005;38 (Suppl 1):S18–S21. doi: 10.1097/01.qai.0000167030.18278.0e. [DOI] [PubMed] [Google Scholar]
- 9.Rabkin M, El Sadr W. Perspectives and practice in antiretroviral treatment. Geneva: WHO; 2003. Saving mothers, saving families: the MTCT-Plus initiative: case study. [Google Scholar]
- 10.Trunova N, Tsai L, Tung S, Schneider E, Harouse J, Gettie A, et al. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology. 2006;352:169–177. doi: 10.1016/j.virol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Lavreys L, Baeten JM, Chohan V, McClelland RS, Hassan WM, Richardson BA, et al. Higher Set Point Plasma Viral Load and More-Severe Actua HIV Type (HIV-1) Illness Predict Mortality among High-Risk HIV infected African Women. Clin Infect Dis. 2006;42:1333–1339. doi: 10.1086/503258. [DOI] [PubMed] [Google Scholar]
- 12.Cejtin HE, Jacobson L, Springer G, Watts D, Levine A, Greenblatt R, et al. Effect of hormonal contraceptive use on plasma HIV-1 RNA levels among HIV-infected women. AIDS. 2003;17:1702–1704. doi: 10.1097/00002030-200307250-00019. [DOI] [PubMed] [Google Scholar]
- 13.Richardson BA, Otieno PA, Mbori-Ngacha D, Overbaugh J, Farquhar C, John-Stewart GC. Hormonal contraception and HIV-1 disease progression among postpartum Kenyan women. AIDS. 2007;21:749–753. doi: 10.1097/QAD.0b013e328032790f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danel L, Vincent C, Rousset F, Klein B, Bataille R, Flacher M, et al. Estrogen and progesterone receptors in some human myeloma cell lines and murine hybridomas. J Steroid Biochem. 1988;30:363–367. doi: 10.1016/0022-4731(88)90124-0. [DOI] [PubMed] [Google Scholar]
- 15.Pasanen S, Ylikomi T, Palojoki E, Syvala H, Pelto-Huikko M, Tuohimaa P. Progesterone receptor in chicken bursa of Fabricius and thymus: evidence for expression in B-lymphocytes. Mol Cell Endocrinol. 1998;141:119–128. doi: 10.1016/s0303-7207(98)00086-0. [DOI] [PubMed] [Google Scholar]
- 16.Enomoto LM, Kloberdanz KJ, Mack DG, Elizabeth D, Weinberg A. Ex Vivo Effect of Estrogen and Progesterone Compared With Dexamethasone on Cell-Mediated Immunity of HIV-Infected and Uninfected Subjects. J Acquir Immune Defic. 2007;45:137–143. doi: 10.1097/QAI.0b013e3180471bae. [DOI] [PubMed] [Google Scholar]
- 17.Maret A, Coudert JD, Garidou L, Foucras G, Gourdy P, Krust A, et al. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hemaotpoietic cells. European J Immunology. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- 18.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 19.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp Gerontol. 2007;42:432–437. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization, UNICEF, UNFPA, The World Bank. Maternal Mortality in 2005. World Health Organization; Geneval, Switzerland: 2007. [Google Scholar]