Abstract
Objective
Determine whether reconstitution of Vγ2Vδ2 T cells in patients with HIV is due to new cell synthesis with recovery of the T-cell receptor repertoire or proliferative expansion of residual cells from the time of treatment initiation.
Design
Perform a cross-sectional analysis of the T-cell receptor complexity of Vγ2 chain in patients treated for HIV, natural virus suppressors who control viremia to undetectable levels, patients with chronic low-level viremia in the absence of therapy, and uninfected controls. Apply quantitative methods for repertoire analysis to assess the degree of Vδ2 repertoire loss or reconstitution.
Methods
T-cell receptor Vγ2 chain DNA clones (up to 300 per patient sample) were sequenced and aligned to enumerate the antigen-reactive subset with Vγ2-Jγ1.2 rearrangements. Predominant shared (public) sequences in each patient were compared to a reference library of public sequences from uninfected controls to assess the extent of similarity. Repertoire comparisons were quantified through bioinformatics testing.
Results
Patients with prolonged virus suppression due to antiretroviral therapy reconstituted the Vγ2 T-cell repertoire to near-normal levels. Natural virus suppressors were similar to the treatment group. Severe defects in the Vγ2 T-cell receptor repertoire were observed in patients with chronic viremia despite the absence of overt disease.
Conclusion
Prolonged HIV suppression with antiretroviral therapy leads to reconstitution of the Vγ2Vδ2 T-cell subset deleted in HIV disease. Direct evidence for repair of the T-cell receptor repertoire supports a view that treatment-associated immune reconstitution is due to new cell synthesis and not to expansion of residual cell populations.
Keywords: antiretroviral therapy, HIV, immune reconstitution, repertoire, T-cell receptor, thymic function, Vgamma9, Vgamma2
Introduction
HIV infection causes specific depletion of CD4-negative Vγ2Vδ2 T cells (a subset of γδ T cells) in patients with HIV disease [1,2]. Vγ2Vδ2 T-cell depletion is remarkably consistent among individuals with HIV disease and may occur when Envelope glycoprotein binds to elevated levels of CCR5, which activates caspase and causes cell death; CCR5 levels are very high in these cells [3]. Prolonged antiretroviral therapy (ART) appears to reconstitute Vγ2Vδ2 T cells [4]. Untreated natural virus suppressors (viral loads <400 RNA copies/ml of blood in the absence of ART [5]) also called elite controllers, had Vγ2Vδ2 T-cell counts similar to uninfected controls, but the Vγ2 repertoire was damaged [6], and higher cell counts reflected expansion of the residual population [7]. Untreated HIV-positive patients with persistent low-level viremia had low Vγ2Vδ2 T cells [8] and significant damage to the T-cell receptor (TCR) repertoire [2].
Here, we compared the Vγ2 repertoires (also designated Vγ9 in an alternate nomenclature) among three groups of HIV patients to determine the mechanism for Vγ2Vδ2 T-cell reconstitution after long-term virus suppression. We focus on public Vγ2 chains found in many unrelated individuals due to the lack of major histocompatibility complex (MHC) restriction for γδ T-cell antigen responses [9]; at least eight public clonotypes were found in more than 50% of all healthy volunteers [10]. Comparing the presence or absence of these public Vγ2 chains among HIV-positive groups defines the extent of depletion or reconstitution of the TCR repertoire.
Results
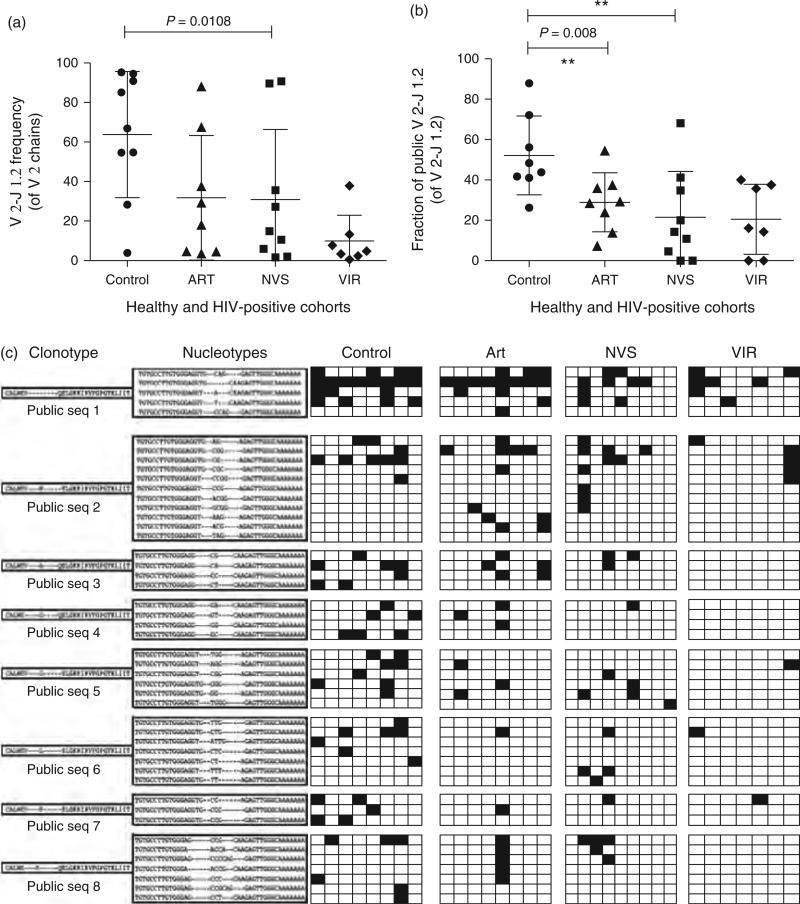
Peripheral blood mononuclear cells from four groups: patients receiving ARTand having CD4 counts above 300 cells/μl (ART), natural virus suppressors who control HIV to undetectable levels in the absence of therapy (NVS), patients with chronic viremia not receiving therapy (VIR), and HIV-negative controls. Specimens were used for cloning cDNA for individual Vγ2 chains, followed by analysis of the T-cell receptor repertoire (Supplemental digital content Table 1 and Methods, http://links.lww.com/QAD/A342). Approximately 250 Vγ2 chain sequences were analyzed for each patient or control specimen. In control donors, more than 60% have the Vγ2-Jγ1.2 rearrangement. All HIV-infected patients had profound depletion of phosphoantigen-reactive Vγ2-Jγ1.2 chains (Fig. 1b). This value was as low as 10% for the VIR group. For the ARTand NVS groups, Vγ2-Jγ1.2 comprised approximately 30% of all Vγ2 chains.
Fig. 1. HIV infection depletes circulating Vγ2-Jγ1.2Vδ2 T cells.
(a) The fraction of Vγ2 chains expressing Jγ1.2 segment is lowest for the VIR group. The fraction of chains expressing Jγ1.2 was calculated and plotted for the four groups (mean + SD). On average, approximately 60% of Jγ2 chains expressed the Jγ1.2 segment in healthy controls, whereas only approximately 10% Jγ2 chains were Jγ1.2 in VIR. For NVS and ART groups this number was approximately 30%. (b) The fraction of Vγ2-Jγ1.2 chains expressing public Jγ1.2 clonotype is lowest for the VIR group. Public clonotypes were identified as aa sequences present in more than one patient in the control group, and these sequences were scored for their presence in HIV-positive groups. For control group, approximately 52% of Vγ2-Jγ1.2 chains were found in two or more donors, whereas approximately 20% of Vγ2-Jγ1.2 chains were public for VIR and NVS groups. ART group had approximately 30% public Vγ2-Jγ1.2 chain. For each panel horizontal lines represent mean values. Statistical comparisons used Kruskal–Wallis test; P values less than 0.05 were considered significant. (c) Clonotype and nucleotype abundance for the most common public Vγ2-Jγ1.2 chains in control, ART, NVS, and VIR groups. The eight most common public clonotypes (PubSeq 1–8) are listed along with all their nucleotypes found in this study. Vertical columns are grouped into control, ART, NVS, and VIR groups as described in the text. Each individual column represents sequencing data for an individual patient. A shaded box indicates that nucleotype was present in the set of TCR-Vγ2 sequences from the individual patient or control. ART, antiretroviral therapy; NVS, natural virus suppressor; TCR, T-cell receptor; VIR, viremic and not receiving therapy.
The normal Vγ2 repertoire is dominated by public clonotypes [11], each of which is encoded by multiple nucleotypes. We matched Vγ2 clonotype sequences from our HIV-positive groups to a table of public clonotypes from HIV-negative African Americans (Supplemental Digital Content Table 2, http://links.lww.com/QAD/A342) [10]. A significant decrease among public clonotypes was apparent for the ART, NVS, and VIR groups. In healthy controls, 52% of all Vγ2-Jγ1.2 rearrangements were public clonotypes (Fig. 1c). The NVS group had approximately 20%, a result similar to the VIR group. The ART group had approximately 30% public Vγ2 clonotypes, a value lower than controls but higher than other HIV-infected groups. Our earlier studies implied higher levels of Vγ2-Jγ1.2 cells in ART compared to other groups, but did not enumerate public clonotypes [4,12].
Next, we examined the distribution of nucleotypes for several of the most common public clonotypes including (PubSeq1) (Fig. 1c). PubSeq1 was encoded by five different nucleotypes in controls and ART patients, but was less frequent in NVS or VIR groups (Supplemental digital content Table 3, http://links.lww.com/QAD/A342). Controls had an average of 2.4 nucleotypes for PubSeq1; values were lower in all HIV-positive groups. However, we found examples where nucleotype abundance was similar to controls even though clonotype expression was lower (e.g. PubSeq2 for ART and NVS groups). For PubSeq6 and PubSeq7 there were substantial defects in both clonotype and nucleotype expression for all HIV-positive groups. PubSeq8 had low clonotype abundance for all HIV-positive groups, but nucleotype levels were similar to controls for ART and NVS groups.
For the VIR group, five of the seven patients had PubSeq1 and only one patient had more than one nucleotype (Fig. 1c). For PubSeq2, two of the seven VIR and six of the eight controls had this clonotype. Patients in the NVS group were likely to express PubSeq1 (five of nine) or PubSeq2 (four of nine). Most surprising was the group of ART patients where all the eight patients expressed PubSeq1 with an average of 1.9 nucleotypes per person (Fig. 1c). Thus, every control or ART patient had PubSeq1 and had similar nucleotype abundance. When PubSeq1 was present in NVS or VIR groups, it was encoded by nine nucleotypes in five of the NVS patients (average 1.8) and seven nucleotypes in five of the VIR Vγ2 patients (average 1.4). Patient 5 in the ART group showed strong selection for PubSeq1 (three nucleotypes), PubSeq2 (three nucleotypes), and the less frequent PubSeq8. A similar pattern was seen for patient #2 of the NVS group who had three nucleotypes encoding PubSeq1 and six nucleotypes encoding PubSeq2. Surprisingly, nucleotype diversity for ART or NVS patients was sometimes greater than for uninfected controls and we found several nucleotypes that were not present in control groups. Whether these Vγ2 sequences are selected for their response to HIV, another infectious agent, malignancy, or unknown pathology could not be determined.
T-cell receptor-Vγ2 repertoires are similar for antiretroviral therapy and uninfected control groups
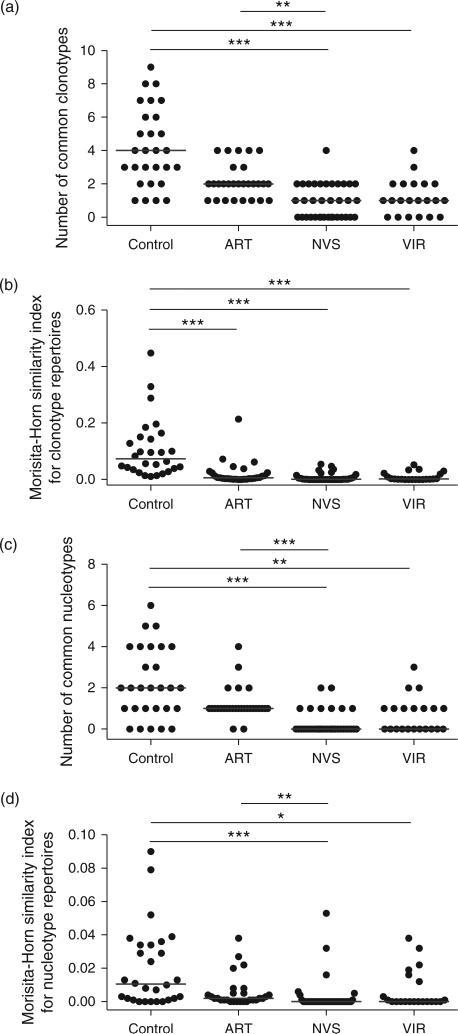
Individual TCR repertoires are complex because of clonal diversity (increasing the number of unique TCR sequences) and selection, which causes individual clones to be present above or below the population averages. In ‘shallow’ sequencing studies like ours, the Vγ2 sequence sample size (about 250 sequences per sample) is below the number required to cover an entire repertoire. Most Vγ2 sequences are present once within each sample set and a few are present at higher, and sometimes much higher frequencies. Specialized statistical methods are required to make group-wise comparisons of repertoire complexity (see online Methods). One approach is to compare, between groups, the level of similarity between pairs of individual repertoires within each group [13,14].
We compared the appearance of common clonotypes between pairs of individual repertoires. Differences in the median number of common clonotypes per group between control and ART patients were not statistically significant (Fig. 2a). There were significantly more common clonotypes between the control and ART groups compared with NVS, or between the control and VIR groups. We calculated the Morisita-Horn similarity index between pairs of clonotype repertoires in each group, which measures the abundance of individual clonotypes (or nucleotypes) and total number of clonotypes (or nucleotypes) in pair-wise comparisons; significant differences were observed between control and all other groups and there was a trend to lower similarity indices for NVS and VIR groups (Fig. 2b). Statistically significant differences in Morisita-Horn similarity indices between control and ART groups reflect differences in the abundance of common clonotypes, showing the ART group is actually more heterogeneous than the control group. Similar results were obtained when comparing the numbers of common nucleotypes (Fig. 2c). The Morisita-Horn similarity analysis for nucleotype repertoires showed significantly greater similarity in the control group compared with either NVS or VIR repertoires and in ART compared with NVS groups (Fig. 2d). Again, control and ART groups were distinguishable from the NVS and VIR groups in terms of nucleotype complexity.
Fig. 2. Similarity analysis between the TCR-Vγ2 repertoires of pairs of individuals within each group.
The number of clonotypes (a) or nucleotypes (c) common between individual repertoires and the Morisita-Horn similarity indices for clonotype (b) and nucleotype (d) repertoires were estimated for a sample size of 98 TCR-Vγ2 sequences per individual repertoire. Horizontal lines represent the median similarity values per group and asterisks show P < 0.0005 (***), (P < 0.005 (**), or P < 0.05 (*). Statistical comparisons were made using a Kruskal–Wallis test and Dunn's multiple comparison post-tests. In terms of common nucleotypes (c) or similarity index for nucleotype repertoire (d), control and ART groups were not different at P < 0.05. ART, antiretroviral therapy; TCR, T-cell receptor.
Discussion
Profound depletion of CD4-negative Vγ2Vδ2 T cells in HIV disease is specific for cells expressing a Vγ2-Jγ1.2Vδ2 TCR [1], the same receptor that recognizes stimulatory phosphoantigens [15–17] and tumor cells [18–20]. Now, nearly 10 years after the introduction of combination therapy, we can assess the long-term effects of virus suppression on reconstitution of the Vγ2Vδ2 TCR repertoire.
Focusing on γδ T cells is a new approach for studying treatment effects. Immune reconstitution in HIV disease is usually judged by treatment-associated increases in the CD4+T-cell count [21] which are related to fewer opportunistic infections and improved responses to vaccination [22]. The extent of CD4+T-cell reconstitution has been related to nadir CD4 cell count [23] and the duration of viremia prior to treatment [24].
Proliferative expansion of surviving CD4+T cells is one model for immune reconstitution [25]. This mechanism would increase CD4 cell counts without affecting TCR complexity. Alternately, reconstitution of CD4+T cells by de-novo thymic output would increase both counts and repertoire complexity. Studies on TCR recombination excisional circles (TRECs), a byproduct of TCR rearrangement [26], and phenotypically defined recent thymic emigrants [27] supported thymic output as the driver of immune reconstitution. Using spectratyping methods, the TCR Vβ repertoire complexity was increasing during reconstitution [28] although spectra-type data are not corrected for sample size effects which can be especially important for low CD4+ cell counts, and expansion of individual clones can skew the spectratype and confuse interpretation of data. Sequencing studies are needed to confirm whether reconstitution is associated with increasing TCR repertoire complexity due to new cell synthesis. However, the large size of the TCRVβ repertoire with more than 105 rearranged sequences in healthy adults, and MHC restriction differences reduce the frequency of public clonotypes. These technical issues are obstacles to proving whether or not reconstitution is associated with increased TCR repertoire complexity.
Knowing that the thymus produces γδ, CD4αβ and CD8αβ T cells from the same precursor cell pool [29], reconstitution of the CD4+ TCR repertoire must be matched by similar changes in the γδ TCR repertoire. Our finding that ART reconstitutes Vγ2 repertoire complexity supports the thymic output model for immune reconstitution of both γδ and CD4+ T cells. Although these are cross-sectional studies, our earlier work documented near-extinction of Vγ2-Jγ1.2 sequences in viremic patients with CD4+T-cell counts below 200 cells/μl [2]. All patients in the ART group had nadir CD4+ cell counts below 200 cells/μl two of them with nadir CD4+ below 70 cells/μl. In our experience, it is unlikely that these individuals with such low nadir CD4+ cell counts were harboring substantial Vγ2-Jγ1.2 cells in blood. Surviving γδ T cells sequestered in tissues are unlikely to account for repertoire changes because longitudinal studies on patients initiating combination ART did not detect a rapid rebound in Vγ2Vδ2 T cells [2]. The increased complexity of reconstituted γδ TCR repertoire should be matched by similar changes in the αβ TCR repertoire in CD4+ cells, a result consistent with some recent studies [30].
Even though treatment reconstituted the Vγ2 chain receptor repertoire, functional responses to phosphoantigen were not restored fully [12]. We did find that bisphosphonate (zoledronate) induced Vγ2Vδ2 cell proliferation and antibody-dependent cellular cytotoxicity (ADCC) in HIV-positive patients who had received ART [31]. Bisphosphonate treatment has been developed as an immunotherapy for cancer, by increasing tumor cytolysis or elevating ADCC activity [32,33], and was well tolerated by HIV patients [34]. Knowing that treated HIV patients can recover a functional Vγ2 chain repertoire and their effector function can be stimulated by bisphosphonates, we are encouraged to initiate bisphosphonate therapy studies in patients with HIV disease.
Supplementary Material
Acknowledgements
We thank Dr Mohammad Sajadi and Dr Robert R. Redfield for providing ART, NVS and VIR specimens collected under approved protocols.
This work was supported by PHS grant CA142458 (C.D.P.). V.V. is an Australian Research Council Future Fellow.
Footnotes
Author contributions: S.C. and C.C. designed and conducted the study. V.V. performed bioinformatics analysis of repertoire complexity. C.D.P. conceived the study and wrote the manuscript.
Conflicts of interest
The authors have no conflict of interests related to these studies.
References
- 1.Enders PJ, Yin C, Martini F, Evans PS, Propp N, Poccia F, et al. HIV-mediated gammadelta T cell depletion is specific for Vgamma2+ cells expressing the Jgamma1.2 segment. AIDS Res Human Retrovirus. 2003;19:21–29. doi: 10.1089/08892220360473934. [DOI] [PubMed] [Google Scholar]
- 2.Hebbeler AM, Propp N, Cairo C, Li H, Cummings JS, Jacobson LP, et al. Failure to restore the Vgamma2-Jgamma1.2 repertoire in HIV-infected men receiving highly active antiretroviral therapy (HAART). Clin Immunol. 2008;128:349–357. doi: 10.1016/j.clim.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Pauza CD. HIV envelope-mediated, CCR5/alpha4beta7-dependent killing of CD4-negative gammadelta T cells which are lost during progression to AIDS. Blood. 2011;118:5824–5831. doi: 10.1182/blood-2011-05-356535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bordon J, Evans PS, Propp N, Davis CE, Jr, Redfield RR, Pauza CD. Association between longer duration of HIV-suppressive therapy and partial recovery of the V gamma 2 T cell receptor repertoire. J Infect Dis. 2004;189:1482–1486. doi: 10.1086/382961. [DOI] [PubMed] [Google Scholar]
- 5.Sajadi MM, Heredia A, Le N, Constantine NT, Redfield RR. HIV-1 natural viral suppressors: control of viral replication in the absence of therapy. AIDS. 2007;21:517–519. doi: 10.1097/QAD.0b013e328013d9eb. [DOI] [PubMed] [Google Scholar]
- 6.Riedel DJ, Sajadi MM, Armstrong CL, Cummings JS, Cairo C, Redfield RR, et al. Natural viral suppressors of HIV-1 have a unique capacity to maintain gammadelta T cells. AIDS. 2009;23:1955–1964. doi: 10.1097/QAD.0b013e32832ff1ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pauza CD, Riedel DJ, Gilliam BL, Redfield RR. Targeting gammadelta T cells for immunotherapy of HIV disease. Future Virol. 2011;6:73–84. doi: 10.2217/FVL.10.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boudova S, Li H, Sajadi MM, Redfield RR, Pauza CD. Impact of persistent HIV replication on CD4 negative Vgamma2Vdelta2 T cells. J Infect Dis. 2012;205:1448–1455. doi: 10.1093/infdis/jis217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 10.Cairo C, Armstrong CL, Cummings JS, Deetz CO, Tan M, Lu C, et al. Impact of age, gender, and race on circulating gamma-delta T cells. Human Immunol. 2010;71:968–975. doi: 10.1016/j.humimm.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venturi V, Price DA, Douek DC, Davenport MP. The molecular basis for public T-cell responses? Nature Rev Immunol. 2008;8:231–238. doi: 10.1038/nri2260. [DOI] [PubMed] [Google Scholar]
- 12.Cummings JS, Cairo C, Armstrong C, Davis CE, Pauza CD. Impacts of HIV infection on Vgamma2Vdelta2 T cell pheno-type and function: a mechanism for reduced tumor immunity in AIDS. J Leukocyte Biol. 2008;84:371–379. doi: 10.1189/jlb.1207847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venturi V, Kedzierska K, Turner SJ, Doherty PC, Davenport MP. Methods for comparing the diversity of samples of the T cell receptor repertoire. J Immunol Methods. 2007;321:182–195. doi: 10.1016/j.jim.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Venturi V, Kedzierska K, Tanaka MM, Turner SJ, Doherty PC, Davenport MP. Method for assessing the similarity between subsets of the T cell receptor repertoire. J Immunol Methods. 2008;329:67–80. doi: 10.1016/j.jim.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 16.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- 17.Tanaka Y, Brenner MB, Bloom BR, Morita CT. Recognition of nonpeptide antigens by T cells. J Mol Med. 1996;74:223–231. doi: 10.1007/BF00196576. [DOI] [PubMed] [Google Scholar]
- 18.Hebbeler AM, Cairo C, Cummings JS, Pauza CD. Individual Vgamma2-Jgamma1.2+ T cells respond to both isopentenyl pyrophosphate and Daudi cell stimulation: generating tumor effectors with low molecular weight phosphoantigens. Cancer Immunol Immunother. 2007;56:819–829. doi: 10.1007/s00262-006-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamb LS, Jr, Lopez RD. gammadelta T cells: a new frontier for immunotherapy? Biol Blood Marrow Transplant. 2005;11:161–168. doi: 10.1016/j.bbmt.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Viey E, Laplace C, Escudier B. Peripheral gammadelta T-lymphocytes as an innovative tool in immunotherapy for meta-static renal cell carcinoma. Expert Rev Anticancer Ther. 2005;5:973–986. doi: 10.1586/14737140.5.6.973. [DOI] [PubMed] [Google Scholar]
- 21.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 22.Kroon FP, Rimmelzwaan GF, Roos MT, Osterhaus AD, Hamann D, Miedema F, et al. Restored humoral immune response to influenza vaccination in HIV-infected adults treated with highly active antiretroviral therapy. AIDS. 1998;12:F217–223. doi: 10.1097/00002030-199817000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lange CG, Lederman MM, Medvik K, Asaad R, Wild M, Kalayjian R, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–2023. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann GR, Zaunders JJ, Cunningham P, Kelleher AD, Grey P, Smith D, et al. Rapid restoration of CD4 T cell subsets in subjects receiving antiretroviral therapy during primary HIV-1 infection. AIDS. 2000;14:2643–2651. doi: 10.1097/00002030-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Renaud M, Katlama C, Mallet A, Calvez V, Carcelain G, Tubiana R, et al. Determinants of paradoxical CD4 cell reconstitution after protease inhibitor-containing antiretroviral regimen. AIDS. 1999;13:669–676. doi: 10.1097/00002030-199904160-00007. [DOI] [PubMed] [Google Scholar]
- 26.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Lewin SR, Markowitz M, Lin HH, Skulsky E, Karanicolas R, et al. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med. 1999;190:725–732. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin L, Kou ZC, Rodriguez C, Hou W, Goodenow MM, Sleasman JW. Antiretroviral therapy restores diversity in the T-cell receptor Vbeta repertoire of CD4 T-cell subpopulations among human immunodeficiency virus type 1-infected children and adolescents. Clin Vaccine Immunol. 2009;16:1293–1301. doi: 10.1128/CVI.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dik WA, Pike-Overzet K, Weerkamp F, de Ridder D, de Haas EF, Baert MR, et al. New insights on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. J Exp Med. 2005;201:1715–1723. doi: 10.1084/jem.20042524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012;55:291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poonia B, Pauza CD. Gamma delta T cells from HIV+ donors can be expanded in vitro by zoledronate/interleukin-2 to become cytotoxic effectors for antibody-dependent cellular cytotoxicity. Cytotherapy. 2012;14:173–181. doi: 10.3109/14653249.2011.623693. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M, et al. Zoledronate-activated Vgamma9gammadelta T cell-based immunotherapy is feasible and restores the impairment of gammadelta T cells in patients with solid tumors. Cytotherapy. 2011;13:92–97. doi: 10.3109/14653249.2010.515581. [DOI] [PubMed] [Google Scholar]
- 33.Santini D, Martini F, Fratto ME, Galluzzo S, Vincenzi B, Agrati C, et al. In vivo effects of zoledronic acid on peripheral gammadelta T lymphocytes in early breast cancer patients. Cancer Immunol Immunother. 2009;58:31–38. doi: 10.1007/s00262-008-0521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poccia F, Gioia C, Martini F, Sacchi A, Piacentini P, Tempestilli M, et al. Zoledronic acid and interleukin-2 treatment improves immunocompetence in HIV-infected persons by activating Vgamma9Vdelta2 T cells. AIDS. 2009;23:555–565. doi: 10.1097/QAD.0b013e3283244619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.