Abstract
Acute erythroleukemia (AML-M6) is an uncommon subtype of acute myeloid leukemia (AML); it is considered to have a poor prognosis. From January 1st, 1980 to May 21st, 2008, 108 patients with newly diagnosed AML-M6 were seen at the University of Texas – M.D. Anderson Cancer Center (UT-MDACC). Half (50%) had a history of myelodysplatic syndrome (MDS), compared to 41% in our control group (patients with other AML subtypes) (p=0.05). Poor risk cytogenetics was more common in patients with AML-M6 (69% versus 46%, p<0.001). Complete remission rates were 63% for patients with AML-M6, comparing to 58% for the control group (p = 0.285). Median disease free survival (DFS) for patients with AML-M6 was 31 weeks, versus 49 weeks for the control group (p = 0.004). Median overall survival (OS) of patients with AML-M6 was 33 weeks, compared to 42 weeks for the control group (p = 0.13). On multivariate analysis for DFS and OS, AML-M6 was not an independent risk factor. Acute erythroleukemia is commonly associated with a previous diagnosis of MDS and poor risk karyotype. The diagnosis of AML-M6 does not impart by itself a worse prognosis, and treatment decisions on this disease should be guided by well know AML prognostic factors.
Keywords: Acute erythroleukemia, adults, clinical features, prognosis
Introduction
Acute erythroleukemia was first described in 1912 by Copelli1, but it was Giovanni Antonio Di Guglielmo who coined the term ‘eritroleucemia’, describing an abnormal proliferation of erythroid cells, myeloblasts and megakaryocytes2,3. In 1923 Di Guglielmo described a case of pure erythroleukemia characterized by pure proliferation of erythroid immature elements, akin to acute leukemia4. Finally, in 1953 Dameshek proposed the term ‘Di Guglielmo's Syndrome’, which describes three phases of alterations in the bone marrow: an erythremic phase, an erythromyeloblastic phase and a myeloblastic phase, similar to acute myeloid leukemia5.
The French-American-British (FAB) classification in 1976 established the first set of criteria for the diagnosis of acute erythroleukemia, which was called Acute Myeloid Leukemia-M6 (AML-M6)6. A revision of the FAB classification defined AML-M6 as the presence of more than 50% erythroid cells with more than 30% blasts of non-erythroid bone marrow nucleated cells7. The WHO classification recognizes the FAB criteria plus the presence of pure erythroleukemia cases, where there is an isolated proliferation of immature erythroid elements8.
Acute erythroleukemia has been considered to be a subtype of acute myeloid leukemia with a worse prognosis9-13. It is very uncommon in children14,15, and it corresponds to 3-5% of adult acute myeloid leukemia cases12. Familial and congenital forms have been described16,17. It is often associated with an antecedent diagnosis of myelodysplastic syndrome (MDS) and the presence of poor chromosome abnormalities9,18-20. Due to its rarity, the clinical experience with AML-M6 is limited. It is also not clear if the pathological diagnosis of AML-M6 is associated with a worse prognosis or if the worse prognosis reported with this leukemia is due to its frequent association with poor-risk karyotype and other poor-risk features.
We sought to describe the clinical experience at the UT-MDACC with patients diagnosed with AML-M6. The objective of this study is to describe the clinical and biological characteristics of these patients and to compare them with non-M6 AML patients. We also sought to evaluate the impact of the diagnosis of AML-M6 in complete remission rates and survival independent of other risk factors.
Patients and Methods
Patients
Adult patients with newly diagnosed AML-M6 seen at UT-MDACC between January 1st, 1980 and May 21st, 2008 were compared with patients with other AML subtypes, except those with acute promyelocytic leukemia (APL). Charts were manually reviewed. Patients were treated on studies conducted at UT-MDACC. Studies were approved by the Institutional Review Board and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent prior to study entry
A diagnosis of AML-M6a (mixed erythroid/myeloid) was considered if more than 50% of the total nucleate cells were of erythroid lineage and more than 20% of non-erythroid cells were blasts8. We defined pure erythroid leukemia (AML-M6b) as patients with an increased number of blasts (>30%) demonstrating aberrant erythroblast-like morphology, positivity by immunophenotyping for glycophorin A and/or coarse block positivity for periodic acid-Schiff (PAS) stain (in the absence of other lineage specific markers, such as myeloperoxidase (MPO), terminal deoxynucleotyidyl transferase (TdT) and CD41/61)21-25.
Laboratory Data
Bone marrow reports were reviewed for information about dysplasia in one or more hematopoietic lineages. Cytogenetic data was stratified into three subgroups (modified from previous reports26-28): Poor-risk (-5, -7, 11q abnormalities, +8, miscellaneous abnormalities and complex karyotype – defined as more than 3 cytogenetic abnormalities), good-risk (t(8;21) or Inv(16), t(16;16)) and intermediate-risk (diploid or -Y).
Treatment
Patients received different treatment regimens according to the period of diagnosis and prevailing studies. The treatment regimens were divided into: Group 1 – Regimens with Ara-C and Anthracyclines. Group 2 – Regimens with Ara-C and Fludarabine, without anthracyclines. Group 3 – Regimens with Ara-C plus Topotecan. Group 4 – Other Ara-C containing regimens. Group 5 – Miscellaneous intensive chemotherapy regimens.
Criteria for Response, Definition of Disease Free Survival and Overall Survival
Complete remission (CR) was defined by the presence of less than 5% blasts in the bone marrow (BM) with more than 1×109/L neutrophils and more than 100×109/L platelets in the peripheral blood29,30. A relapse was defined by more than 5% blasts in a bone marrow aspirate unrelated to bone marrow recovery or by the presence of extramedullary disease. Disease-free survival (DFS) was calculated from the time of CR until relapse, death in CR or last follow-up. Event free survival (EFS) was calculated from the beginning of treatment until an event or last follow-up. An event was defined as relapse, death during induction or death in CR. Overall survival (OS) was calculated from the time of diagnosis until death or last follow-up.
Statistical analysis
Categorical and continuous variables were compared by the Chi-Square/Fischer exact test and Mann-Whitney U Test, respectively31. The survival curves were estimated using Kaplan Meier plots and compared by the log rank test32. Clinical and biological characteristics were analyzed for their association with survival using multivariate Cox proportional hazards models33. All computations were done in SAS (Cary, NC) and Statistica, version 6.0 (Tulsa, OK).
Results
Clinical and Biological Features
There were 2,992 patients with newly diagnosed AML seen at the UT-MDACC during the specified period, and 108 had the diagnosis of AML-M6, corresponding to 3.6% of all patients with AML. The characteristics of patients with AML-M6 are summarized in Table 1. They were different from with other AML subtypes in a number of characteristics. There was a slight male predominance (65% vs 55%, P= 0.05). They were older (P = 0.009), most often had previously received chemotherapy or radiotherapy for other neoplastic diseases (25% vs 15%, P = 0.003), and had a higher prevalence of antecedent hematologic disorder (AHD)/MDS (50% vs 41%, P = 0.05). They also had, at presentation, lower hemoglobin (Hb) levels (P < 0.001), lower white blood cell (WBC) count (P < 0.001), lower platelet count (P < 0.001), lower circulating blasts (P < 0.001), lower β2-microglobulin level (P=0.004), lower lactate dehydrogenase (LDH) level (P=0.01) and a higher prevalence of poor-risk cytogenetic abnormalities (69% vs 46%, P<0.001), including complex karyotypes (52% vs 23%, P<0.001). BM dysplasia of all three lineages was also more frequent in patients with AML-M6. Seventy-eight percent of evaluable patients with AML-M6 had erythroid lineage dysplasia (vs 31% in non-M6 AML, p < 0.001), 45% had granulocytic lineage dysplasia (vs 33% in non-M6 AML, p = 0.026) and 42% had megakaryocytic lineage dysplasia (vs 19% in non-M6 AML, p < 0.001).
Table 1. Characteristics of AML-M6 vs AML-Other Patients (excluding APL).
| Characteristics | AML-M6 | AML-Other | P |
|---|---|---|---|
| No. Patients | 108 | 2,884 | |
| Male Sex, no. (%) | 70 (65) | 1,600 (55) | 0.05 |
| Median Age, years (range) | 60 (17-85) | 59 (14-89) | 0.009 |
| PS 3-4, no. (%) | 7 (6) | 259 (9) | 0.37 |
| Prior AHD/MDS, no. (%) | 54 (50) | 1,169 (1,169) | 0.05 |
| Prior Chemo or XRT, no. (%) | 27 (25) | 423 (15) | 0.003 |
| Median WBC, ×109/L (range) | 2.4 (0.34-45.1) | 9.7 (0.2-433) | < 0.001 |
| Median Hb, g/dL (range) | 7.7 (2.5-12.6) | 8.3 (2-16.1) | < 0.001 |
| Median Platelets, × 109/L (range) | 38 (3-222) | 49 (1-2,292) | < 0.001 |
| Median PB Blasts, % (range) | 3 (0-78) | 24 (0-99) | < 0.001 |
| Median BM Blasts, % (range) | 22 (2-92) | 53 (0-99) | < 0.001 |
| Median Beta 2 Microglobulin, mg/L (range) | 2.3 (1.2-13.3) | 2.8 (0-31.3) | 0.004 |
| Median LDH, IU/L (range) | 739 (226-25,980) | 916 (66-58,586) | 0.019 |
| Cytogenetics | < 0.001 | ||
| • Poor | 75 (69) | 1,329 (46) | |
| • Intermediate | 31 (29) | 1,129 (39) | |
| • Favorable | 0 (0) | 255 (9) | |
| • Complex | 56 (52) | 654 (23) | < 0.001 |
| BM Dysplasia* | 85 | 1830 | |
| • Granulocytic | 38 (45) | 605 (33) | 0.026 |
| • Erythroid | 66 (78) | 563 (31) | < 0.001 |
| • Megakaryocytic | 36 (42) | 353 (19) | < 0.001 |
| Protected Environment | 66 (61) | 1,713 (59) | 0.71 |
There were 85 evaluable patients in the AML-M6 group and 1,830 patients in the AML-Other group. BM – bone marrow; Chemo – chemotherapy; PB – peripheral blood; PS – performance status; XRT – radiotherapy
Among these 108 patients, 78 (72%) presented with mixed proliferation of myeloid and erythroid blasts (AML-M6a). The median number of erythroblasts was 60% (43%-87%). One case had 43% erythroblasts on the bone marrow aspirate, but sheets of immature erythroblast were seen on biopsy. The median number of myeloid blasts (out of non-erythroid cells) was 45% (range 19-90.3%). One case had 19% blasts seen on aspirate, but the number of immature myeloid cells was estimated to be 30% by the biopsy. Thirty patients (28%) presented with isolated proliferation of erythroid series alone (AML-M6b). The median number of blasts was 69% (31-94.6%). Twelve patients were tested for glycophorin A; it was positive in 11 (92%). The PAS stain was performed in 28 patients and was positive in 27 cases (96%), demonstrating coarse block-positivity, which is characteristic of erythroleukemias. There was a high correlation between PAS and glycophorin A positivity, with both tests being positive in 10 out of 12 samples (83%). Also, one patient was positive for CD71 (transferrin receptor), two patients were positive for CD36 and in one case iron was detected in blast cells. In one case the diagnosis was confirmed by electron microscopy. Iron stains were performed in 57 cases, and the median number of ringed sideroblasts was 3% (range 0-89%).
The two subgroups of AML-M6 (M6a and M6b) had different clinical and biological features, which are summarized in Table 2. Patients with pure erythroid leukemia (M6b) more often had history of prior treatment for other malignancies (p = 0.01). They also had lower platelet counts (p = 0.01), higher β2-microglobulin (p = 0.01), higher LDH (p = 0.004) and higher incidence of poor-risk karyotype abnormalities (p=0.03).
Table 2. Characteristics of AML-M6a versus AML-M6b patients.
| Characteristics | AML-M6a | AML-M6b | P |
|---|---|---|---|
| No. Patients | 78 | 30 | |
| Sex: Male, no. (%) | 53 (68) | 17 (55) | 0.36 |
| Median Age, Years (range) | 57 (17-76) | 61 (18-85) | 0.07 |
| Prior AHD/MDS, no. (%) | 37 (47) | 17 (57) | 0.51 |
| Prior Chemo or XRT, no. (%) | 14 (18) | 13 (43) | 0.01 |
| Median WBC, ×109/L (range) | 2.1 (0.5-45.1) | 3.6 (0.3-21.8) | 0.07 |
| Median Hb, g/dL (range) | 7.7 (2.5-12.6) | 7.7 (5.3-11.8) | 0.33 |
| Median Platelets, × 109/L (range) | 45 (3-222) | 21 (3-119) | 0.01 |
| Median β2 Microglobulin, mg/L (range) | 2.1 (1.2-8.1) | 3.2 (1.7-13.3) | 0.01 |
| Median LDH, IU/L (range) | 677 (226-25,980) | 1087 (399-17,313) | 0.004 |
| Cytogenetics*, no. (%) | |||
| Poor | 50 (65) | 25 (86) | 0.03 |
| Intermediate | 27 (35) | 4 (14) | |
| BM Dysplasia**, no. (%) | 56 (80) | 14 (93) | 0.28 |
| Protected Environment, no. (%) | 60 (77) | 16 (53) | 0.02 |
there were 77 available patients in M6a group and 29 in M6b
there were 71 available patients in M6a group and 14 in M6b
History of AHD/MDS or Previous Therapy
The details of the previous diagnosis of hematological diseases and other neoplasms in patients with AML-M6 are summarized in Table 3. Fifty-four patients (50%) had a previous history of MDS or other AHD. Fifty (46%) had MDS and/or cytopenias, with a median duration of 3.5 months (1-72) prior to the diagnosis of AML-M6. Three patients had a prior diagnosis of myeloproliferative disorder (one patient with polycythemia vera, one with essential thrombocythemia and one with both polycythemia vera and MDS), and one case chronic lymphocytic leukemia as well as MDS. Twenty-seven patients had received previous chemotherapy and/or radiotherapy. The most common non-hematological malignancy was breast cancer in 7 patients (6.5%). Two additional patients had a history of solid tumors but had only received surgical treatment (one had lung cancer and one had prostate cancer).
Table 3. History of AHD/MDS and Chemotherapy/Radiotherapy in Patients with AML-M6.
| Disease | Number (%) |
|---|---|
| AHD/MDS | |
| MDS | 45 (41.6) |
| Cytopenias | 5 (4.6) |
| Polycythemia Vera | 1 (0.9) |
| Essential Thrombocythemia | 1 (0.9) |
| Polycythemia Vera + MDS | 1 (0.9) |
| Chronic Lymphocytic Leukemia + MDS | 1(0.9) |
| Previous Chemotherapy and/or Radiaton Therapy | |
| Breast Cancer | 7 (6.5) |
| Non-Hodgkin's Lymphoma | 4 (3.7) |
| Multiple Myeloma | 3 (2.7) |
| Hodgkin's Lymphoma | 2 (1.8) |
| Prostate Cancer | 2 (1.8) |
| Head and Neck Cancer | 2 (1.8) |
| Acute Lymphoblastic Leukemia | 2 (1.8) |
| Chronic Lymphocytic Leukemia | 1 (0.9) |
| Lung Cancer | 1 (0.9) |
| Osteosarcoma | 1 (0.9) |
| Ovarian Cancer | 1 (0.9) |
| Allogeneic SCT for MDS | 1 (0.9) |
Cytogenetic Analysis
The details of the cytogenetic analysis in patients with AML-M6 are summarized in table 4. Cytogenetic data was available in 106 patients (98%). In one case there were insufficient metaphases and in the second pre G-banding technique was used, and it was not considered in the final analysis. Overall, cytogenetic abnormalities were detected in 77 patients (71%). The most common abnormality was deletion of chromosome 5 and/or 7, which occurred in 50 patients (46%). The karyotype was diploid in 29% of patients, and trisomy 8 was present in 8.3%. No patient with AML-M6 presented with good-risk cytogenetics. Also, 56 patients (52%) presented with a complex karyotype. Among these, 48 had deletion of chromosome 5 and/or 7. There were 13 cases of chromosome 3 abnormalities (12%), including three monosomies and three translocations – one t(3;3)(q24;29), one t(3;5)(q23;q31) and one t(3;21)(q26;q22). Most of these patients had other associated poor chromosome abnormalities (12 patients, 11%). There were five patients (4.6%) with 19q13 abnormalities, including one translocation t(11;19)(q11;q13.3). All of these patients had other poor chromosome abnormalities with complex karyotypes.
Table 4. Cytogenetic Data of Patients with AML-M6.
| Affected Chromosome | Number (%) |
|---|---|
| Chromosome 3 | |
| Monosomy 3 | 3 (2.7) |
| t(3;3)(q24;q29) | 1 (0.9) |
| t(3;5)(q23;q31) | 1 (0.9) |
| t(3;21)(q26;q22) | 1 (0.9) |
| Chromosome 5 and/or 7 | |
| -5 and/or -7 | 50 (46) |
| Chromosome 8 | |
| Trisomy 8 | 9 (8.3) |
| Chromosome 11 | |
| 11q abnormalities | 1 (0.9) |
| Chromosome 19 | |
| 19q13 abnormalities | 5 (4.6) |
| CBF Translocations | |
| t(8;21) | 0 (0) |
| Inv(16) or t(16;16) | 0 (0) |
| Diploid or –Y | 31 (28.7) |
| Complex Karyotype* | 56 (69) |
| Other | |
| Miscellaneous | 15 (14) |
| Pre-banding techniques | 1 (0.9) |
| Insufficient metaphases | 1 (0.9) |
Included in this group are 48 of the 50 patients with -5/-7
Treatment Response and Survival
Patients were treated using different regimens of intensive induction chemotherapy, according to the time of diagnosis and the prevailing protocols. There was no difference in the proportions of patients with AML-M6 and non-M6 AML in the different treatment groups (data not shown).
Response and survival outcomes for all patients are summarized in table 5 and figure 1. The median follow-up for the entire cohort is 322 weeks. The CR rate for AML-M6 was 63% (versus 58% for non-M6 AML, p = 0.28). Seventeen patients with AML-M6 were refractory (15.7%) and 23 (21.3%) died at induction. The median OS was 33 weeks for AML-M6 versus 43 weeks for non-M6 AML (p=0.13). The median EFS was 16 weeks for AML-M6 versus 20 weeks for non-M6 AML (p=0.33). For those patients who achieved CR, the median DFS was 31 weeks for AML-M6 versus 49 weeks nor non-M6 AML (p=0.004).
Table 5. Response and Survival Data – AML-M6 versus AML-Other.
| Parameter | AML-M6 | AML-Other | P |
|---|---|---|---|
| CR rate, no. (%) | 68 (63) | 1666 (58) | 0.25 |
| Median DFS, weeks (range) | 31 (3-1,158+) | 49 (0-1,281+) | 0.004 |
| Median EFS, weeks (range) | 16 (0-1,170+) | 20 (0-1,285+) | 0.33 |
| Median OS, weeks (range) | 33 (0-1,170+) | 43 (0-1,285+) | 0.13 |
Figure 1.
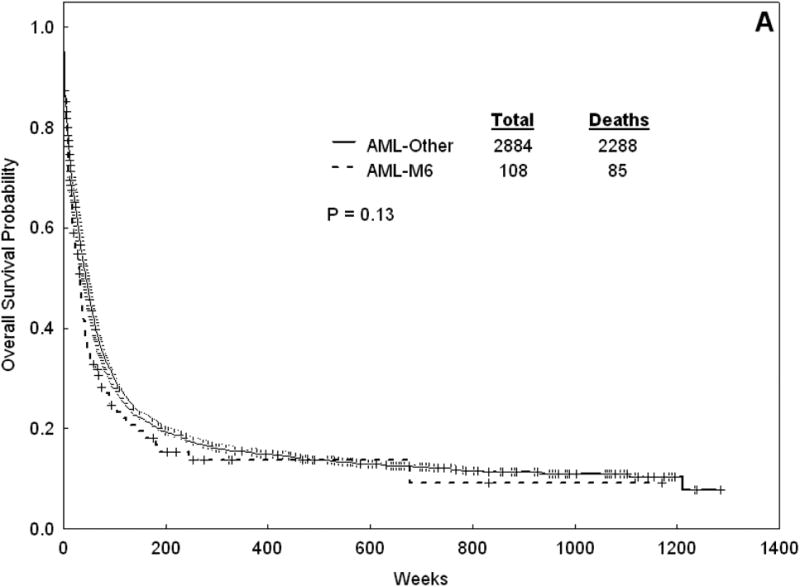
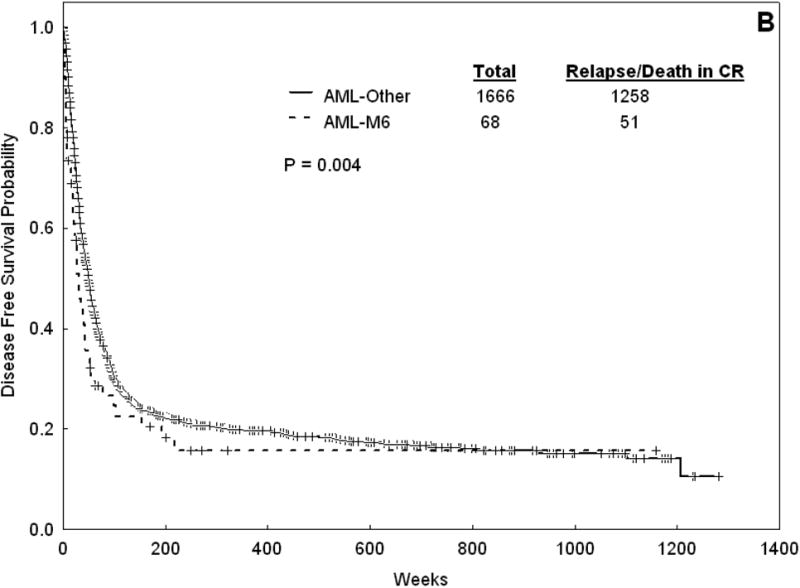
Overall survival (A) and DFS (B) for Patients with AML-M6 versus AML-Other.
Twenty-four patients with AML-M6 received a stem cell transplantation (SCT). Their median age was 50 years (compared to 62 years for those who didn't receive a SCT, P < 0.001). The stem cell source was a matched related donor in 13 patients, a matched unrelated donor in 9 patients, a haploidentical related donor in one patient and a cord blood unit in one patients. Transplants were performed in first remission in 13 patients, in second remission or first relapse in 6 patients and for refractory disease in 5 patients. The median time to SCT was 24 weeks (range 12-80 weeks). Thirteen patients have died after SCT (relapse = 7, infection = 4, GVHD = 2). The median OS after SCT was 37 weeks (range 1-1,130+).
The treatment response and outcome of patients with AML-M6a and AML-M6b are summarized in Table 6. The two groups were treated similarly with a few notable exceptions. There was a lower proportion of patients with M6a receiving treatment group 2 (Fludarabine + Ara-C) than those with M6b (2.5% versus 20%, p =0.005). There was a higher proportion of patients in the AML-M6a who underwent induction therapy in the protected environment (77% vs 53%, p = 0.02). There were no difference in the response rate, with a CR rate of 64% and 60% in AML-M6a and AML-M6b cohorts, respectively (p = 0.82). The median OS for AML-M6a was 39 weeks versus 15 weeks for AML-M6b (p = 0.007). The median EFS was 24 weeks for AML-M6a versus 10 weeks for AML-M6b (p = 0.02). The median DFS for AML-M6a was 35 weeks versus 8 weeks for AML-M6b (p = 0.02) (Figure 2). As more patients with AML-M6a group underwent a SCT (23 patients with M6a were transplanted compared to one with M6b), we repeated the analysis comparing survival outcomes for the cohorts, including only with patients who did not receive a stem cell transplant. We found similar differences in OS, EFS and DFS (data not shown).
Table 6. Response and Survival Data – AML-M6a versus AML-M6b.
| Parameter | AML-M6a | AML-M6b | P |
|---|---|---|---|
| Complete Response, no. (%) | 50 (64) | 18 (60) | 0.82 |
| Median DFS, weeks (range) | 35 (3-822+) | 8 (3-1,158+) | 0.02 |
| Median EFS, weeks (range) | 24 (1-831+) | 10 (0-1,170+) | 0.02 |
| Median OS, weeks (range) | 39 (1-831+) | 15 (0-1,170+) | 0.007 |
Figure 2.
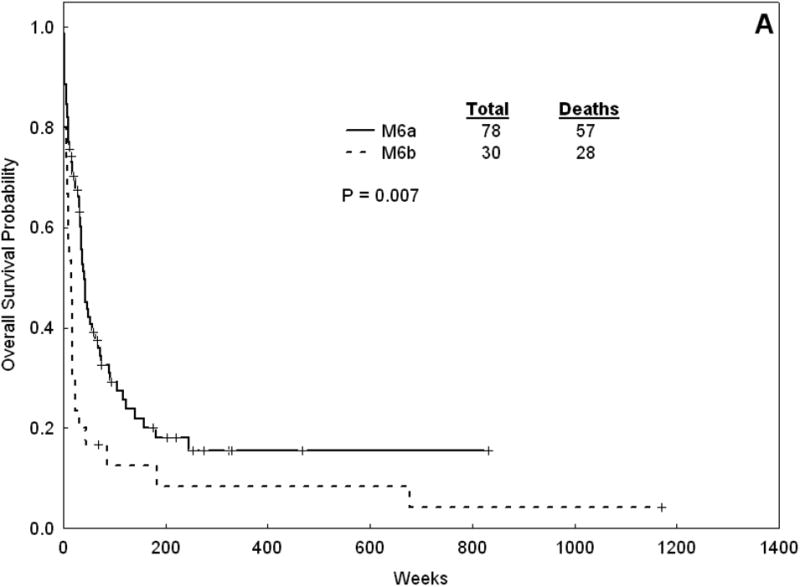
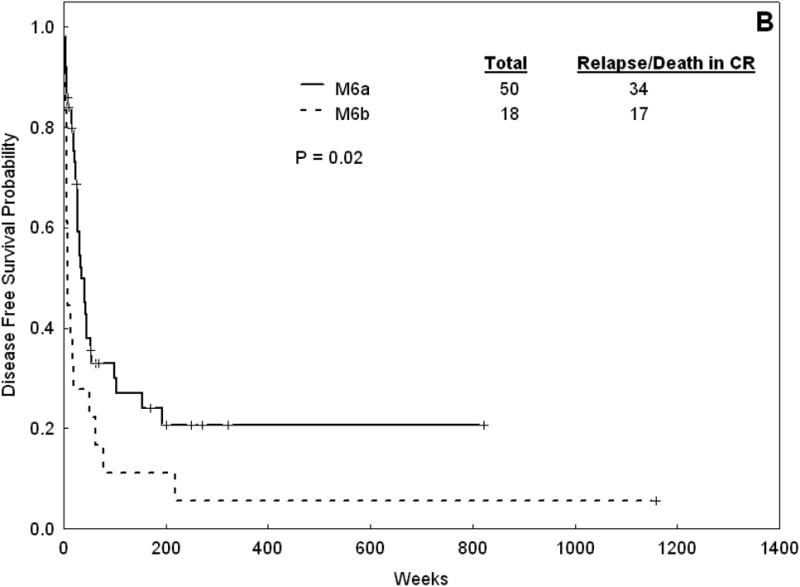
Overall survival (A) and DFS (B) for AML-M6a patients versus AML-M6b.
Treatment outcome also differed between patients with intermediate and poor-risk karyotype. The median DFS and OS were 51 (range 3-1,158+) and 117 (range 1-1,170+) weeks, respectively, for patients with intermediate risk karyotype. The same parameters were 20 (range 3-218) and 17 (range 0-677) weeks for patients in the poor karyotype subgroup (p < 0.001 for both) (Figure 3).
Figure 3.
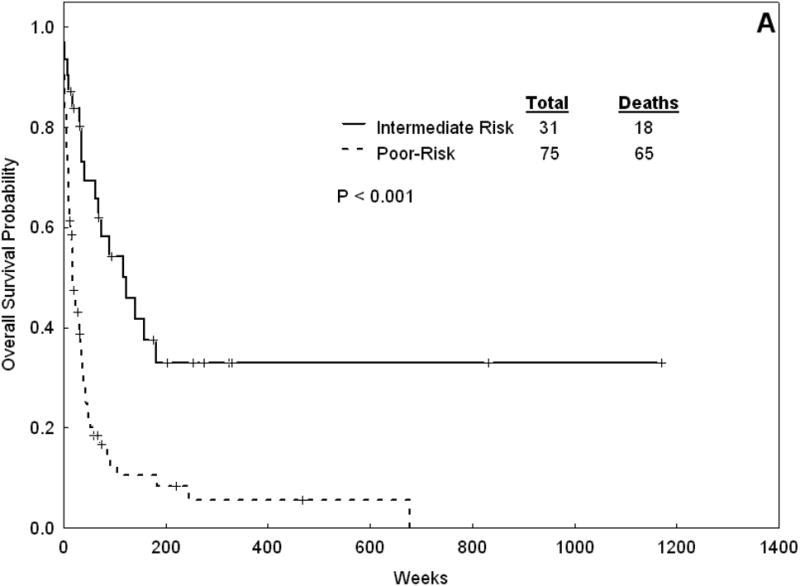
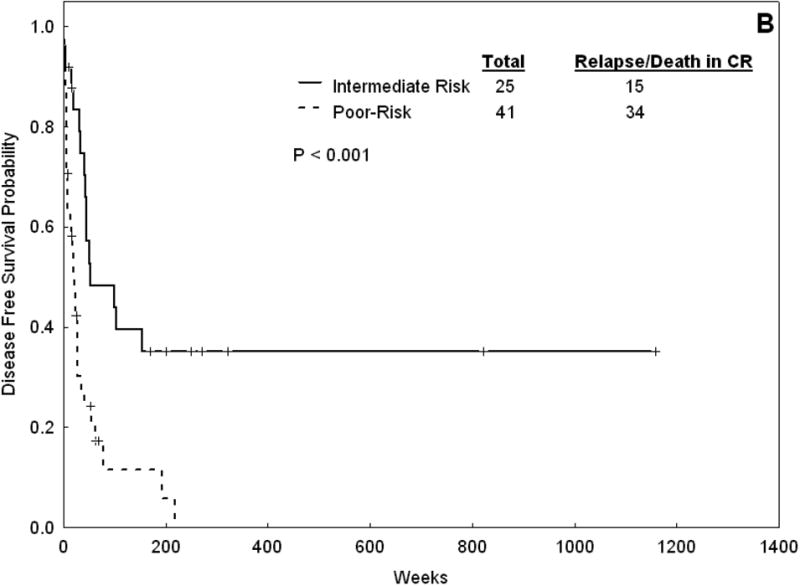
Overall survival (A) and DFS (B) for Patients with AML-M6 with intermediaterisk karyotype versus those with poor-risk karyotype.
Prognostic Factors Associated with Survival
The following factors were associated with a worse disease-free survival by multivariate analysis: age, intermediate and poor-risk cytogenetics (versus good-risk cytogenetics), induction outside the protected environment, performance status greater than two, lower hemoglobin, and the presence of antecedent hematological disease/chemotherapy (Table 7). The diagnosis of AML-M6 was not an independent predictive factor for worse disease free-survival (p=0.39). Similar results were found in the multivariate analysis for overall survival (Table 8). The subtype of AML-M6 (M6a and M6b) was also not an independent prognostic factor for disease-free and overall survival (data not shown).
Table 7. Multivariate analysis for Disease Free Survival.
| Characteristic | Hazard Ratio | P-value |
|---|---|---|
| Age | 1.012 | < 0.0001 |
| Protective Environment | 0.867 | 0.02 |
| Hemoglobin | 0.952 | 0.001 |
| PS 2-4 | 1.34 | 0.02 |
| Cytogenetics | ||
| Intermediate vs Favorable | 1.42 | <0.0001 |
| Poor vs Favorable | 2.34 | <0.0001 |
| AHD/MDS | 1.17 | 0.009 |
| AML-M6 | 1.13 | 0.39 |
Table 8. Multivariate analysis for Overall Survival.
| Characteristic | Hazard Ratio | P-value |
|---|---|---|
| Age | 1.02 | < 0.0001 |
| Protective Environment | 0.70 | < 0.0001 |
| Hemoglobin | 0.95 | 0.0001 |
| PS 2-4 | 2.31 | < 0.0001 |
| Cytogenetics | ||
| Intermediate vs Favorable | 1.45 | <0.0001 |
| Poor vs Favorable | 2.49 | <0.0001 |
| AHD/MDS | 1.21 | <0.0001 |
| Prior Chemotherapy | 1.34 | <0.0001 |
| AML-M6 | 1.13 | 0.39 |
Discussion
Acute myeloid leukemia is a heterogeneous disease34, and its outcome is directed by the presence or absence of several prognostic factors13,35. The most important ones include age, cytogenetics, performance status and response to treatment13,35. Most recently, molecular abnormalities such as mutations of NPM1 and FLT3 genes have been identified that are associated with a worse or better prognosis in patients with diploid AML36. The perception that genetic and molecular abnormalities define unique subtypes of leukemias with important clinical and prognostic features has lead to an overall change of direction in the classification of AML, going from a pure morphological classification to a more genetic and molecular-based one, as seen in the most recent WHO classification. In this context, it is important to assess what is the independent prognostic value of the FAB morphological classification. Some subtypes (M0, M7) have a worse prognosis37-39, while others (M5) do not40.
In this report we analyzed the clinical and laboratory characteristics and survival outcomes of 108 patients with acute erythroleukemia and compared them with other patients with AML diagnosed and treated at the same time. We did not find a worse response to treatment or OS for these patients compared to patients with non-M6 AML. We did, however, note that AML-M6 is associated with a shorter DFS. In the multivariate analysis, the diagnosis of AML-M6 was not an independent prognostic factor, indicating that the shorter DFS of these patients is accounted for by the presence of other covariates, such as poor cytogenetic characteristics (which were more common in AML-M6 patients).
The classification of the erythroleukemias has always been fraught with controversy. Since their initial description, this group of patients have been variably considered as having leukemia, myelodysplastic syndrome and myeloproliferative disorders21. Di Guglielmo described two subtypes of erythroleukemias2,4. Di Guglielmo's syndrome is a mixed proliferation of myeloid blasts and erythroid elements. Di Guglielmo's disease is a pure proliferation of immature erythroid cells. The FAB classification in 1976 established the first set of criteria for AML-M6, which required the presence of abnormal erythroid cells accounting for more than 50% of total nucleate cells, as well as more than 30% blasts and promyelocytes6. The FAB recommendations were revised in 1985, and the requirement for diagnosis included abnormal erythroid cells being more than 50% of total nucleate cells and more than 30% of non-erythroid elements being blasts7. Both sets of criteria acknowledged Di Guglielmo's syndrome but excluded Di Guglielmo's disease. Many subsequent reports described patients presenting with a proliferation of immature erythroid cells without the presence of myeloid blasts, and suggested that those patients had a worse prognosis than the ‘mixed’ subtype of AML-M624,25,41-46. The availability of glycophorin-A antibodies and other monoclonal antibodies for erythroid antigens (such as ABH antigens) permitted the recognition that some cases of ‘undifferentiated’ acute leukemia were actually minimally differentiated erythroleukemias25,45,47,48. Others have advocated a sub-classification of AML-M6 based on the percentage of pronormoblasts in the erythroid compartment49,50. The WHO classification in 2001 subdivided AML-M6 into two subgroups: one corresponding to the classic FAB, ‘mixed’ type (only now the lower threshold for myeloid blasts was 20%) and the other corresponding to the pure erythroid leukemia subtype, in which more than 80% of bone marrow cells are immature cells (with an undifferentiated or pronormoblastic appearance) committed exclusively to the erythroid lineage8. Others, however, have described patients with clinical and pathological features similar to the WHO “pure erythroid leukemia” in which the percentage of erythroid blasts did not reach 80%51,52. In our series, we found 30 patients with characteristics suggestive of pure erythroid leukemia, but not all of them fulfilled the WHO criteria. These patients commonly had dysplastic features in the erythroid series and a high rate of PAS-positivity. PAS is not specific for AML-M6, and can be positive in other leukemias including acute lymphoblastic leukemia53. However, the presence of coarse block-like PAS staining in blasts with absence of other markers (such as MPO and TdT) is suggestive of AML-M6. Glycophorin A is a sialoglycoprotein of the red cell surface which contains the antigen for the MN system54. It is a specific red cell marker, but it can be negative in AML-M648. The patients with AML-M6b in our series had a worse outcome than the AML-M6a patients, confirming previous data50. In the multivariate analysis, however, the subtype of AML-M6 did not emerge as an independent risk factor.
Other reports of adult AML-M6 are summarized in Table 811,19,55. Our series confirm some of the findings in previous reports. In particular, the presence of pancytopenia, with low circulating blasts is typical of AML-M6, with very few patients presenting with elevated WBC (only six cases in our series had more than 12×109/L). No recurrent chromosomal abnormalities have been reported. Abnormalities of chromosome 5 and/or 7 with a complex karyotype were the most common cytogenetic aberration detected in our series, as well as in previous reports9,18,20,56. Abnormalities of chromosome 3 were also frequent in our series as well as other reports57. Approximately one-third of our patients had a normal karyotype, and this was associated with a higher DFS and overall survival. No cases of core binding factor (CBF) translocations (t(8;21) and Inv(16)/t(16;16)) were identified in our series. A recent study reported a high rate (10%) of chromosome 19 aberrations, in band 19q13.158. Five patients in our series had abnormalities of chromosome 19 at the same band, confirming this data.
The role of allogeneic SCT in acute erythroleukemia has been the subject of two reports59,60. In a recent report by the European Group of Blood and Marrow Transplantation (EBMT), among 207 patients with AML-M6 in first CR, 103 underwent an autologous and 104 an allogeneic SCT. At 5 years, OS was 34 +/- 5% for patients receiving an autologous SCT, and 57+/- 5% for patients undergoing allogeneic SCT. DFS at 5 years was 26+/- 5% for autologous SCT and 57+/-5% for allogeneic SCT. However, only patients with de novo AML-M6 in first CR were included in this study, and cytogenetics data was available in only 79 patients. Among them, only 12 patients (15%) had poor-risk cytogenetics. In our report, patients who underwent allogeneic stem cell transplantation had a high survival rate after transplant (median 37 weeks). Furthermore, among the 10 patients who had long-term survival (> 3 years), 5 had undergone a stem cell transplant.
The most recent criteria for diagnosis of acute myeloid leukemia re-classify most cases of AML-M6 as other subtypes61. According to the recently published 4th edition of the WHO classification of hematologic and lymphoid tissues neoplasms61, cases of AML with complex karyotype and/or other cytogenetic abnormalities similar to those found in MDS (such as -5 and/or -7 abnormalities) are classified as ‘acute myeloid leukemia, myelodysplasia-related’. Cases of AML who had previously received chemotherapy and/or radiotherapy for other malignancies are classified as ‘therapy-related acute myeloid leukemia’. Approximately 50-60% of patients in our series would not be classified as AML-M6 according to these criteria. Nevertheless, we sought to describe our clinical experience with this subtype of AML following the historical definition in order to better define its prognostic impact.
In conclusion, our results confirm the importance of classic prognostic factors (especially cytogenetics) in the outcome of patients with AML-M6. The pathological diagnosis of AML-M6 does not impart by itself a worse outcome. There is need for more studies to define the specific pathological features of the two subgroups of this disease, in special the pure erythroid leukemia subtype. Treatment decisions for patients with AML-M6 (including the decision to do a stem cell transplant) should be guided by well established risk factors.
Table 9. Selected Reports of AML-M6.
| MDACC | Colita et al.55 | Olopade et al.11 | Atkinson et al.19 | |
|---|---|---|---|---|
| Time Period | 1981-2006 | 1976-1999 | 1969-1991 | 1977-1990 |
| Number | 108 | 54 | 26 | 15 |
| Median Age, years (range) | 60 (17-85) | 59 (19-84) | 66 (6-85) | 65 (16-85) |
| Male, no. (%) | 70 (65%) | 40 (74%) | 16 (62%) | 7 (47%) |
| AHD/MDS, no. (%) | 54 (50%) | NR | NR | 3 (20%) |
| Treatment-related, no. (%) | 27 (25%) | NR | NR | 4 (27%) |
| Median WBC, ×109/L (range) | 2.4 (0.3-45.1) | 3.24 (0.4-367) | 3.7 (0.8-10.6) | 2.9 (1.2-6.2) |
| Median Hb, g/dL (range) | 7.7 (2.5-12.6) | 8.5 (4.7-13.4) | 6.5 (1.0-10.1) | 7.2 (2.4-14.7) |
| Median Platelet count, ×109/L (range) | 38 (3-222) | 47 (6-334) | 48 (12-819) | 48 (3-259) |
| Median Erythroblasts, % (range) | 61 (31-95) | NR | 64 (40-84) | NR |
| Poor-risk Karyotype, no. (%) | 75 (69%) | 18 (33%) | 17 (65%) | 4/6 (66%) |
| CR Rate, % | 63 | 54 | 62 | 30 |
| OS, months (range) | 8.2 (0-292+) | 9 (NR) | 5.7 (0.5-73.5) | 3.2 (1-84+) |
NR – not reported
References
- 1.Copelli M. Di una emopatia sistemizzata rapresentata da una imperplasia eritroblastica (eritronatosis) Pathologica. 1912;4:460. [Google Scholar]
- 2.Di Guglielmo G. Un caso di eritroleucemia Megacariociti in circolo e loro funzione piastrinopoietico. Vol. 13. Folia Medica (Pavia); 1917. Richerche di ematologia I; p. 386. [Google Scholar]
- 3.Bain BJ. Di Guglielmo and his syndromes. Br J Haematol. 2003;120:939–943. doi: 10.1046/j.1365-2141.2003.04181.x. [DOI] [PubMed] [Google Scholar]
- 4.Di Guglielmo G. Eritremie acute. XXIX Congr Italiano Medical International; Roma: 1923. [Google Scholar]
- 5.Dameshek W, Baldini M. The Di Guglielmo syndrome. Blood. 1958;13:192–194. [PubMed] [Google Scholar]
- 6.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 7.Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 8.Brunning RD, Matutes E, Flandrin G, et al. Acute Myeloid Leukemia not otherwise categorised. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, et al., editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. pp. 91–105. [Google Scholar]
- 9.Davey FR, Abraham N, Jr, Brunetto VL, et al. Morphologic characteristics of erythroleukemia (acute myeloid leukemia; FAB-M6): a CALGB study. Am J Hematol. 1995;49:29–38. doi: 10.1002/ajh.2830490106. [DOI] [PubMed] [Google Scholar]
- 10.Peterson BA, Levine EG. Uncommon subtypes of acute nonlymphocytic leukemia: clinical features and management of FAB M5, M6 and M7. Semin Oncol. 1987;14:425–434. [PubMed] [Google Scholar]
- 11.Olopade OI, Thangavelu M, Larson RA, et al. Clinical, morphologic, and cytogenetic characteristics of 26 patients with acute erythroblastic leukemia. Blood. 1992;80:2873–2882. [PubMed] [Google Scholar]
- 12.Bennett JM, Begg CB. Eastern Cooperative Oncology Group study of the cytochemistry of adult acute myeloid leukemia by correlation of subtypes with response and survival. Cancer Res. 1981;41:4833–4837. [PubMed] [Google Scholar]
- 13.Lowenberg B. Prognostic factors in acute myeloid leukaemia. Best Pract Res Clin Haematol. 2001;14:65–75. doi: 10.1053/beha.2000.0116. [DOI] [PubMed] [Google Scholar]
- 14.Barnard DR, Alonzo TA, Gerbing RB, Lange B, Woods WG. Comparison of childhood myelodysplastic syndrome, AML FAB M6 or M7, CCG 2891: report from the Children's Oncology Group. Pediatr Blood Cancer. 2007;49:17–22. doi: 10.1002/pbc.20951. [DOI] [PubMed] [Google Scholar]
- 15.Malkin D, Freedman MH. Childhood erythroleukemia: review of clinical and biological features. Am J Pediatr Hematol Oncol. 1989;11:348–359. [PubMed] [Google Scholar]
- 16.Lazure T, Beauchamp A, Croisille L, Ferlicot S, Feneux D, Fabre M. Congenital anerythremic erythroleukemia presenting as hepatic failure. Arch Pathol Lab Med. 2003;127:1362–1365. doi: 10.5858/2003-127-1362-CAEPAH. [DOI] [PubMed] [Google Scholar]
- 17.Novik Y, Marino P, Makower DF, Wiernik PH. Familial erythroleukemia: a distinct clinical and genetic type of familial leukemias. Leuk Lymphoma. 1998;30:395–401. doi: 10.3109/10428199809057551. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura H. Cytogenetic heterogeneity in erythroleukemia defined as M6 by the French-American-British (FAB) Cooperative Group criteria. Leukemia. 1989;3:305–309. [PubMed] [Google Scholar]
- 19.Atkinson J, Hrisinko MA, Weil SC. Erythroleukemia: a review of 15 cases meeting 1985 FAB criteria and survey of the literature. Blood Rev. 1992;6:204–214. doi: 10.1016/0268-960x(92)90016-j. [DOI] [PubMed] [Google Scholar]
- 20.Lessard M, Struski S, Leymarie V, et al. Cytogenetic study of 75 erythroleukemias. Cancer Genet Cytogenet. 2005;163:113–122. doi: 10.1016/j.cancergencyto.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Mazzella FM, Alvares C, Kowal-Vern A, Schumacher HR. The acute erythroleukemias. Clin Lab Med. 2000;20:119–137. [PubMed] [Google Scholar]
- 22.Schumacher HR. Case 3. In: Schumacher HR, editor. Acute Leukemia: Approach to Diagnosis. New York: Igaku-Shoin; 1990. pp. 127–135. [Google Scholar]
- 23.Schumacher HR. Morphology - Difficult and controversial acute leukemias. In: Schumacher HR, editor. Acute leukemia : difficult, controversial, automated analysis. Baltimore: Williams & Wilkins; 1998. p. 12. [Google Scholar]
- 24.Hasserjian RP, Howard J, Wood A, Henry K, Bain B. Acute erythremic myelosis (true erythroleukaemia): a variant of AML FAB-M6. J Clin Pathol. 2001;54:205–209. doi: 10.1136/jcp.54.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garand R, Duchayne E, Blanchard D, et al. Minimally differentiated erythroleukaemia (AML M6 ‘variant’): a rare subset of AML distinct from AML M6. Groupe Francais d'Hematologie Cellulaire. Br J Haematol. 1995;90:868–875. doi: 10.1111/j.1365-2141.1995.tb05208.x. [DOI] [PubMed] [Google Scholar]
- 26.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 27.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 28.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 29.Cheson BD, Cassileth PA, Head DR, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 30.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 31.Snedecor G, Cochran W. Statistical Methods. 7th. Armes, IA: Iowa State University Press; 1980. [Google Scholar]
- 32.Kaplan EL, Meire P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 33.Cox D. Regression models and life tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 34.Gilliland DG, Jordan CT, Felix CA. The molecular basis of leukemia. Hematology Am Soc Hematol Educ Program. 2004:80–97. doi: 10.1182/asheducation-2004.1.80. [DOI] [PubMed] [Google Scholar]
- 35.Avivi I, Rowe JM. Prognostic factors in acute myeloid leukemia. Curr Opin Hematol. 2005;12:62–67. doi: 10.1097/01.moh.0000148760.15412.df. [DOI] [PubMed] [Google Scholar]
- 36.Gaidzik V, Dohner K. Prognostic implications of gene mutations in acute myeloid leukemia with normal cytogenetics. Semin Oncol. 2008;35:346–355. doi: 10.1053/j.seminoncol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Bene MC, Bernier M, Casasnovas RO, et al. Acute myeloid leukaemia M0: haematological, immunophenotypic and cytogenetic characteristics and their prognostic significance: an analysis in 241 patients. Br J Haematol. 2001;113:737–745. doi: 10.1046/j.1365-2141.2001.02801.x. [DOI] [PubMed] [Google Scholar]
- 38.Oki Y, Kantarjian HM, Zhou X, et al. Adult acute megakaryocytic leukemia: an analysis of 37 patients treated at M.D. Anderson Cancer Center. Blood. 2006;107:880–884. doi: 10.1182/blood-2005-06-2450. [DOI] [PubMed] [Google Scholar]
- 39.Barbaric D, Alonzo TA, Gerbing RB, et al. Minimally differentiated acute myeloid leukemia (FAB AML-M0) is associated with an adverse outcome in children: a report from the Children's Oncology Group, studies CCG-2891 and CCG-2961. Blood. 2007;109:2314–2321. doi: 10.1182/blood-2005-11-025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tallman MS, Kim HT, Paietta E, et al. Acute monocytic leukemia (French-American-British classification M5) does not have a worse prognosis than other subtypes of acute myeloid leukemia: a report from the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:1276–1286. doi: 10.1200/JCO.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 41.Griesser GH, Horny HP. Hematopathological features of acute erythremia (morbus Di Guglielmo). A contribution to the classification and differential diagnosis of erythroid neoplasias. Acta Haematol. 1987;77:193–197. doi: 10.1159/000205994. [DOI] [PubMed] [Google Scholar]
- 42.Kowal-Vern A, Cotelingam J, Schumacher HR. The prognostic significance of proerythroblasts in acute erythroleukemia. Am J Clin Pathol. 1992;98:34–40. doi: 10.1093/ajcp/98.1.34. [DOI] [PubMed] [Google Scholar]
- 43.Mazzella FM, Schumacher HR. Acute erythroleukemia, M6b. Arch Pathol Lab Med. 2000;124:330–331. doi: 10.5858/2000-124-0330-AEM. [DOI] [PubMed] [Google Scholar]
- 44.Mazzella FM, Smith D, Horn P, et al. Prognostic significance of pronormoblasts in erythrocyte predominant myelodysplastic patients. Am J Hematol. 2006;81:484–491. doi: 10.1002/ajh.20563. [DOI] [PubMed] [Google Scholar]
- 45.Shichishima T. Minimally differentiated erythroleukemia: recognition of erythroid precursors and progenitors. Intern Med. 2000;39:761–762. doi: 10.2169/internalmedicine.39.761. [DOI] [PubMed] [Google Scholar]
- 46.Yonezumi M, Miyagishima T, Kudo M, et al. A case of de novo early erythroblastic leukemia supporting a proposal of AML M6 ‘variant’. Leuk Lymphoma. 2000;39:667–668. doi: 10.3109/10428190009113400. [DOI] [PubMed] [Google Scholar]
- 47.Greaves MF, Sieff C, Edwards PA. Monoclonal antiglycophorin as a probe for erythroleukemias. Blood. 1983;61:645–651. [PubMed] [Google Scholar]
- 48.Villeval JL, Cramer P, Lemoine F, et al. Phenotype of early erythroblastic leukemias. Blood. 1986;68:1167–1174. [PubMed] [Google Scholar]
- 49.Kowal-Vern A, Mazzella FM, Cotelingam JD, Shrit MA, Rector JT, Schumacher HR. Diagnosis and characterization of acute erythroleukemia subsets by determining the percentages of myeloblasts and proerythroblasts in 69 cases. Am J Hematol. 2000;65:5–13. doi: 10.1002/1096-8652(200009)65:1<5::aid-ajh2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 50.Mazzella FM, Kowal-Vern A, Shrit MA, et al. Acute erythroleukemia: evaluation of 48 cases with reference to classification, cell proliferation, cytogenetics, and prognosis. Am J Clin Pathol. 1998;110:590–598. doi: 10.1093/ajcp/110.5.590. [DOI] [PubMed] [Google Scholar]
- 51.Domingo-Claros A, Larriba I, Rozman M, et al. Acute erythroid neoplastic proliferations. A biological study based on 62 patients. Haematologica. 2002;87:148–153. [PubMed] [Google Scholar]
- 52.Goldberg SL, Noel P, Klumpp TR, Dewald GW. The erythroid leukemias: a comparative study of erythroleukemia (FAB M6) and Di Guglielmo disease. Am J Clin Oncol. 1998;21:42–47. doi: 10.1097/00000421-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Hayhoe FGJ, Quaglino D. Haematological Cytochemistry. 2nd. Edinburgh: Churchill Livingstone; 1988. [Google Scholar]
- 54.Chasis JA, Mohandas N. Red blood cell glycophorins. Blood. 1992;80:1869–1879. [PubMed] [Google Scholar]
- 55.Colita A, Belhabri A, Chelghoum Y, Charrin C, Fiere D, Thomas X. Prognostic factors and treatment effects on survival in acute myeloid leukemia of M6 subtype: a retrospective study of 54 cases. Ann Oncol. 2001;12:451–455. doi: 10.1023/a:1011133115435. [DOI] [PubMed] [Google Scholar]
- 56.Cuneo A, Van Orshoven A, Michaux JL, et al. Morphologic, immunologic and cytogenetic studies in erythroleukaemia: evidence for multilineage involvement and identification of two distinct cytogenetic-clinicopathological types. Br J Haematol. 1990;75:346–354. doi: 10.1111/j.1365-2141.1990.tb04347.x. [DOI] [PubMed] [Google Scholar]
- 57.Kwong YL. Translocation (3;5)(q21;q34) in erythroleukemia: a molecular and in situ hybridization study. Cancer Genet Cytogenet. 1998;103:15–19. doi: 10.1016/s0165-4608(97)00366-x. [DOI] [PubMed] [Google Scholar]
- 58.Cigudosa JC, Odero MD, Calasanz MJ, et al. De novo erythroleukemia chromosome features include multiple rearrangements, with special involvement of chromosomes 11 and 19. Genes Chromosomes Cancer. 2003;36:406–412. doi: 10.1002/gcc.10180. [DOI] [PubMed] [Google Scholar]
- 59.Fouillard L, Labopin M, Gorin NC, et al. Hematopoietic stem cell transplantation for de novo erythroleukemia: a study of the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2002;100:3135–3140. doi: 10.1182/blood.V100.9.3135. [DOI] [PubMed] [Google Scholar]
- 60.Killick S, Matutes E, Powles RL, et al. Acute erythroid leukemia (M6): outcome of bone marrow transplantation. Leuk Lymphoma. 1999;35:99–107. doi: 10.3109/10428199909145709. [DOI] [PubMed] [Google Scholar]
- 61.Swerdlow SH, Campo E, Harris NL, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th. Lyon: IARC Press; 2008. [Google Scholar]


