Abstract
Background
Medication adherence may be a proxy for healthy behaviors and other factors that affect outcomes. Prior studies of the association between placebo adherence and health outcomes have been limited primarily to men enrolled in clinical trials and cardiovascular disease outcomes. We examined associations between adherence to placebo and the risk of fracture, coronary heart disease, cancer, and all-cause mortality in the two Women’s Health Initiative (WHI) hormone therapy randomized trials.
Methods
Postmenopausal women randomized to placebo with adherence measured at least once were eligible for analysis. Time-varying adherence was assessed by dispensing history and pill counts. Outcome adjudication was based on physician review of medical records. Cox proportional hazards models evaluated the relation between high adherence (≥80%) to placebo and various outcomes, referent to low adherence (<80%).
Results
A total of 13,444 postmenopausal women were under observation for 106,066 person-years. High placebo adherence was inversely associated with most outcomes including hip fracture (HR 0.50, 95% CI 0.33–0.78), myocardial infarction (HR 0.69, 95% CI 0.50–0.95), cancer death (HR 0.60, 95% CI 0.43–0.82) and all cause mortality (HR 0.64, 95% CI 0.51–0.80) after adjustment for potential confounders. Women with low adherence to placebo were 20% more likely to have low adherence to statins and osteoporosis medications.
Conclusions
In the WHI clinical trials, high adherence to placebo was associated with favorable clinical outcomes and mortality. Until the healthy behaviors and/or other factors for which high adherence is a proxy can be better elucidated, caution is warranted when interpreting the magnitude of benefit of medication adherence.
Keywords: fracture, adherence, placebo, compliance, mortality, myocardial infarction, malignancy, osteoporosis
Introduction
Medication adherence is an important topic within the medical community, with numerous studies demonstrating suboptimal adherence with a wide variety of drugs, including oral osteoporosis medications and therapies for hyperlipidemia and hypertension (1–7). Between 25 and 50% of new users of these medications discontinue within one year of initiation. The potential importance of this finding is underscored by observational studies demonstrating significant differences in the risk for fracture, cardiovascular events, and mortality comparing adherent and non-adherent persons (8–12).
However, a largely unexplored potential source of confounding related to medication adherence is the possibility that other healthy behaviors are also present in persons adherent to medications. This might lead to beneficial effects independent of the medication effect, which has been called a “healthy adherer” effect. In fact, randomized, placebo-controlled trials (RCTs) evaluating drug therapy, mainly after myocardial infarction (MI) in men, have demonstrated lower mortality risk in patients with higher placebo adherence (13–15) lending credence to the possibility that good adherence itself is associated with a lower risk of adverse outcomes. The healthy adherer effect is hypothesized to be a surrogate for other healthy behaviors (16, 17), which may or may not be able to be measured and controlled for in analyses of observational data or trials. It is not clear if the healthy adherer effect extends to clinical outcomes other than MI, to healthy populations, or to women.
To examine these issues, we used data from the placebo arms of the two hormone therapy (HT) randomized, placebo-controlled trials of the Women’s Health Initiative (WHI), which enrolled relatively healthy postmenopausal women. We studied the relationship between placebo adherence and the risk for fracture, coronary heart disease (CHD), malignancy, cause-specific mortality and all-cause mortality. We also examined the extent to which the healthy adherer effect carried over to other medication taking behavior for hyperlipidemia and osteoporosis. We selected these conditions because they are chronic and asymptomatic; thus, good adherence is more likely due to a healthy adherer effect and less likely to be attributable to symptom-relieving behavior.
Methods
Cohort and Patient Eligibility Criteria
All women participating in the two hormone therapy (HT) trials of the WHI who were randomized to placebo were eligible for analysis. More complete descriptions of the HT trials (18–20), patient recruitment and study implementation have been previously published (21). A three month wash-out period was required for women presenting with current hormone therapy use. The WHI trial evaluating estrogen plus progestin randomized 16,608 postmenopausal women with no prior hysterectomy to active hormone therapy or placebo. The separate WHI trial evaluating estrogen alone randomized 10,739 postmenopausal women with prior hysterectomy to active hormone therapy or placebo. Differences in the characteristics of participants in these two trials have been previously described (19). Women were not eligible for this analysis if they experienced an outcome of interest or withdrew from the HT study before the first measurement of adherence.
All study pills, hormones or identical appearing placebo, were dispensed at the WHI clinical centers using a computerized dispensing system, blinding both participants and clinical staff to randomization allocation. For breast cancer safety, all participants were required to have annual clinical breast exams (performed at the clinical centers) and annual screening mammography.
Characterization of Adherence
For this analysis, and consistent with recommendations from the International Society of Pharmacoeconomics and Outcomes Research (ISPOR), adherence was used as a general term and defined by the extent to which a patient’s behavior coincides with the prescribed treatment regimen (22). It can be quantified as persistence, which captures the length of time a patient continues with therapy, or as compliance, typically quantified as a medication possession ratio (MPR), or as the proportion of days covered (PDC). The MPR and the PDC are equivalent if the MPR is capped at a maximum of 100%.
For this analysis, we quantified adherence as the PDC, calculated as the number of days for which the study medication was dispensed (based on dispensing history) minus the number of days of untaken pills (based on remaining study pills returned) divided by the number of days between visits. While enrolled in the study, women had an annual clinic visit and a semiannual contact by phone, mail, or in-person clinic visit. At each clinic visit, women were asked to return all their study medication bottles. To assess PDC, pill counts in the returned medication bottles were evaluated. From 1993–1996, a pill counter was used; subsequently, pill weighing was used to estimate remaining pill number. This process was not observed by or discussed with participants. However, a PDC below 80% initiated future staff efforts to increase adherence for both the placebo and intervention arms, and study personnel remained blinded to treatment arm. The mean interval between study visits at which adherence was assessed was approximately 6 months; 95% of intervals over which adherence was assessed were one year or less.
Although selection of an adherence threshold is arbitrary, we initially categorized PDC as <= 50%, >50 and < 80%, and >= 80%, following prior conventions (8, 23). Because some outcome models were unstable, with <10 events in at least one of the lower two categories of adherence, PDC was subsequently collapsed to two categories of < 80% vs. >= 80%. The main exposure variable of interest was cumulative (i.e. average) PDC since the beginning of observation. Since there were multiple adherence assessments available per participant, PDC varied over time in the analysis. As part of a sensitivity analysis, we examined outcomes in relation to adherence only for the most recent time interval; as results were minimally different compared to the main analysis, these data are not presented.
Outcome Assessment
Outcomes of interest were hip, clinical vertebral, and distal forearm (i.e. wrist) fracture, CHD (myocardial infarction or CHD death), invasive breast cancer, colorectal cancer, all invasive cancer, cancer death and all-cause mortality. Case definitions for these outcomes were as defined in the WHI protocol. Self reports of clinical outcomes were verified by medical record and pathology report review by trained physician adjudicators. Outcomes were then centrally reviewed by physicians based upon medical record review (24–26), with blinding to randomization allocation.
Statistical Analysis
Descriptive statistics compared demographics, comorbidities, medication use, and other risk factors across adherence categories. Proportional hazards models modeled the time-varying relationship between adherence to placebo and outcomes of interest (27). Adherence prior to fracture has been shown to differ from adherence after the fracture in a previous report (8), so adherence was measured for all participants prior to events. Observation time began at the time of the first measurement of adherence and continued until a participant died or became lost to follow-up. In cases were a participant did not bring her pills in for a particular visit, the adherence value from the previous visit was carried forward until her next known adherence value. Age and BMI were modeled continuously; all other covariates were modeled categorically (categories shown as in table 1). Covariates measured at baseline were selected based upon a-priori interest and their inclusion in previous WHI reports examining the same outcomes; covariates used in the analysis are reported in the footnote to Table 2.
Table 1.
Baseline Features of the WHI-HT Placebo Participants, by Average Adherence over the HT Trial, N=13,444 women
| Cumulative Adherence at the end of Observation* | |||||||
|---|---|---|---|---|---|---|---|
| < 50% N = 926 |
50 – <80% N = 2153 |
≥ 80% N = 10365 |
P-value | ||||
| N | % | N | % | N | % | ||
| Age, years | <.001 | ||||||
| 50 – 59 | 390 | 42.1 | 808 | 37.5 | 3131 | 30.2 | |
| 60 – 69 | 386 | 41.7 | 858 | 39.9 | 4836 | 46.7 | |
| 70 – 79 | 150 | 16.2 | 487 | 22.6 | 2398 | 23.1 | |
| Ethnicity | <.001 | ||||||
| White | 646 | 69.8 | 1459 | 67.8 | 8716 | 84.1 | |
| African American | 154 | 16.6 | 399 | 18.5 | 850 | 8.2 | |
| Hispanic | 86 | 9.3 | 198 | 9.2 | 448 | 4.3 | |
| Other / Unknown | 40 | 4.3 | 97 | 4.5 | 351 | 3.4 | |
| Education | 0.001 | ||||||
| ≤ High school | 254 | 27.4 | 641 | 29.8 | 2935 | 28.3 | |
| Some college | 403 | 43.5 | 864 | 40.1 | 4105 | 39.6 | |
| College graduate | 257 | 27.8 | 624 | 29.0 | 3262 | 31.5 | |
| Income | <.001 | ||||||
| < $20,000 | 253 | 27.3 | 517 | 24.0 | 2151 | 20.8 | |
| $20,000 – $49,999 | 367 | 39.6 | 934 | 43.4 | 4895 | 47.2 | |
| $50,000 – $74,999 | 134 | 14.5 | 306 | 14.2 | 1650 | 15.9 | |
| ≥ $75,000 | 100 | 10.8 | 254 | 11.8 | 1109 | 10.7 | |
| Married / Living as married | 485 | 52.4 | 1173 | 54.5 | 6200 | 59.8 | <.001 |
| Occupation | <.001 | ||||||
| Managerial / Professional | 259 | 28.0 | 698 | 32.4 | 3258 | 31.4 | |
| Technical / Sales / Admin | 254 | 27.4 | 520 | 24.2 | 2960 | 28.6 | |
| Service / Labor | 197 | 21.3 | 454 | 21.1 | 2061 | 19.9 | |
| Homemaker | 84 | 9.1 | 210 | 9.8 | 980 | 9.5 | |
| BMI (kg/m2), % | 0.084 | ||||||
| < 25 | 219 | 23.7 | 543 | 25.2 | 2802 | 27.0 | |
| 25 – <30 | 345 | 37.3 | 733 | 34.0 | 3638 | 35.1 | |
| ≥ 30 | 356 | 38.4 | 863 | 40.1 | 3864 | 37.3 | |
| Current smoking | 130 | 14.0 | 272 | 12.6 | 996 | 9.6 | <.001 |
| Current alcohol use | 582 | 62.9 | 1395 | 64.8 | 7062 | 68.1 | <.001 |
| Total physical activity ≥ 11 MET-hr/wk | 273 | 29.5 | 635 | 29.5 | 3510 | 33.9 | <.001 |
| Total calcium intake, mg | <.001 | ||||||
| < 600 | 301 | 32.5 | 584 | 27.1 | 2212 | 21.3 | |
| 600 – 1200 | 330 | 35.6 | 823 | 38.2 | 4122 | 39.8 | |
| > 1200 | 248 | 26.8 | 628 | 29.2 | 3677 | 35.5 | |
| Fruit/Vegetable servings | <.001 | ||||||
| < 3 | 405 | 43.7 | 853 | 39.6 | 3865 | 37.3 | |
| 3 – <5 | 252 | 27.2 | 693 | 32.2 | 3433 | 33.1 | |
| ≥ 5 | 222 | 24.0 | 489 | 22.7 | 2713 | 26.2 | |
| Red meat servings | <.001 | ||||||
| < 0.5 | 378 | 40.8 | 800 | 37.23 | 3836 | 37.0 | |
| 0.5 – <1 | 273 | 29.5 | 656 | 30.5 | 3557 | 34.3 | |
| ≥ 1 | 228 | 24.6 | 579 | 26.9 | 2618 | 25.3 | |
| Parental history of fracture | 295 | 31.9 | 706 | 32.8 | 3798 | 36.6 | <.001 |
| Family history of breast cancer | 120 | 13.0 | 323 | 15.0 | 1594 | 15.4 | 0.175 |
| Bilateral oophorectomy | 176 | 19.0 | 360 | 16.7 | 1581 | 15.3 | <.001 |
| Age at menarche, years | 0.339 | ||||||
| ≤ 10 | 74 | 8.0 | 163 | 7.6 | 722 | 7.0 | |
| 11–13 | 621 | 67.1 | 1422 | 66.0 | 7070 | 68.2 | |
| 14–15 | 177 | 19.1 | 452 | 21.0 | 2050 | 19.8 | |
| 16+ | 47 | 5.1 | 104 | 4.8 | 484 | 4.7 | |
| Age at first birth, years | <.001 | ||||||
| No term pregnancy | 100 | 10.8 | 217 | 10.1 | 966 | 9.3 | |
| <20 | 203 | 21.9 | 406 | 18.9 | 1727 | 16.7 | |
| 20+ | 500 | 54.0 | 1262 | 58.6 | 6688 | 64.5 | |
| Visit to usual care provider in the last year | 772 | 83.4 | 1828 | 84.9 | 9206 | 88.8 | <.001 |
| Any health insurance | 770 | 83.2 | 1862 | 86.5 | 9442 | 91.1 | <.001 |
| Mammogram in past 2 years | 600 | 64.8 | 1404 | 65.2 | 7216 | 69.6 | <.001 |
| Pap smear in past 2 years | 451 | 48.7 | 1008 | 46.8 | 5348 | 51.6 | <.001 |
| Visit to usual care provider in last year | 610 | 65.9 | 1437 | 66.7 | 7373 | 71.1 | <.001 |
| Colonoscopy ever | 314 | 33.9 | 843 | 39.2 | 4195 | 40.5 | <.001 |
| Self-reported general health | <.001 | ||||||
| Excellent | 138 | 14.9 | 299 | 13.9 | 1727 | 16.7 | |
| Very good | 325 | 35.1 | 783 | 36.4 | 4376 | 42.2 | |
| Good | 340 | 36.7 | 772 | 35.9 | 3390 | 32.7 | |
| Fair | 107 | 11.6 | 260 | 12.1 | 756 | 7.3 | |
| Poor | 6 | 0.6 | 19 | 0.9 | 52 | 0.5 | |
| Cardiovascular disease | 53 | 5.7 | 134 | 6.2 | 568 | 5.5 | 0.005 |
| Treated diabetes (pills or shots) | 57 | 6.2 | 141 | 6.5 | 562 | 5.4 | 0.262 |
| Number of CVD risk factors | 0.243 | ||||||
| None | 362 | 39.1 | 829 | 38.5 | 4176 | 40.3 | |
| 1–2 | 525 | 56.7 | 1212 | 56.3 | 5744 | 55.4 | |
| ≥ 3 | 39 | 4.2 | 112 | 5.2 | 445 | 4.3 | |
| G-I Symptoms reported | 0.038 | ||||||
| None | 234 | 25.3 | 506 | 23.5 | 2669 | 25.8 | |
| 1 | 290 | 31.3 | 724 | 33.6 | 3559 | 34.3 | |
| 2 | 270 | 29.2 | 615 | 28.6 | 2881 | 27.8 | |
| 3 | 126 | 13.6 | 298 | 13.8 | 1205 | 11.6 | |
| Vasomotor symptoms (HF/NS) sweats)F/NS) | 447 | 48.3 | 939 | 43.6 | 4022 | 38.8 | <.001 |
| Years since menopause | <.001 | ||||||
| < 10 | 283 | 30.6 | 585 | 27.2 | 2639 | 25.5 | |
| 10 – <20 | 277 | 29.9 | 675 | 31.4 | 3506 | 33.8 | |
| ≥ 20 | 257 | 27.8 | 654 | 30.4 | 3187 | 30.7 | |
| Depression (based upon shortened CES-D) | 142 | 15.3 | 303 | 14.1 | 1015 | 9.8 | <.001 |
| Falls in the last year | 0.146 | ||||||
| None | 576 | 62.2 | 1310 | 60.8 | 6461 | 62.3 | |
| 1 time | 171 | 18.5 | 425 | 19.7 | 1957 | 18.9 | |
| 2 or more times | 101 | 10.9 | 281 | 13.1 | 1288 | 12.4 | |
| History of fracture after 55 | 90 | 9.7 | 253 | 11.8 | 1438 | 13.9 | <.001 |
| Aspirin use (≥80mg day for 30+ days) | 141 | 15.2 | 361 | 16.8 | 2220 | 21.4 | <.001 |
| NSAID use | 276 | 29.8 | 676 | 31.4 | 3730 | 36.0 | <.001 |
| Corticosteroid use | 1 | 0.1 | 5 | 0.2 | 12 | 0.1 | 0.395 |
| Statin use | 48 | 5.2 | 148 | 6.9 | 761 | 7.3 | 0.045 |
| Bisphosphonate use | 6 | 0.6 | 25 | 1.2 | 127 | 0.2 | 0.295 |
| Calcitonin use | 2 | 0.2 | 1 | 0.0 | 13 | 0.1 | 0.423 |
| Beta-blocker use | 47 | 5.1 | 112 | 5.2 | 712 | 6.9 | 0.003 |
| Thiazide use | 36 | 3.9 | 83 | 3.9 | 498 | 4.8 | 0.091 |
| Loop diuretic use | 21 | 2.3 | 59 | 2.7 | 215 | 2.1 | 0.156 |
| PPI use | 15 | 1.6 | 37 | 1.7 | 173 | 1.7 | 0.978 |
| Lifetime HT use | 0.003 | ||||||
| No prior use | 571 | 61.7 | 1399 | 65.0 | 6775 | 65.4 | |
| < 5 years | 190 | 20.5 | 471 | 21.9 | 2196 | 21.2 | |
| ≥ 5 years | 164 | 17.7 | 283 | 13.1 | 1393 | 13.4 | |
| Number of meds taken | <.001 | ||||||
| None | 332 | 35.9 | 702 | 32.6 | 3062 | 29.5 | |
| 1–2 | 325 | 35.1 | 788 | 36.6 | 3836 | 37.0 | |
| ≥ 3 | 269 | 29.0 | 663 | 30.8 | 3467 | 33.4 | |
CVD = cardiovascular disease; HF = hot flashes; NS = night sweats; HT = hormone therapy; NSAID = non-steroidal anti-inflammatory drugs; CES-D = Right for Epidemiologic Studies Depression Scale
Note: all p-values are from chi-square tests using a significance criterion of 0.05.
adherence was assessed as a fixed covariate for the purposes of descriptively comparing women; all other analyses evaluated adherence as time-varying
Note: column % may not sum exactly to 100% due to rounding or to missing data
Table 2.
Relationship Between Adherence to Placebo and Various Events, High Compared to Low Adherence, WHI Clinical Trial Placebo Arm (N=13,444 Women)
| Outcome | Adherence Category |
Events | Ann % |
Crude Hazard Ratio (95% CI) |
Adjusted* Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| Death (all cause) | <80% | 109 | 0.99 | 1.00 | 1.00 |
| ≥80% | 355 | 0.59 | 0.65 (0.52, 0.80) | 0.64 (0.51, 0.80) | |
| Hip Fracture | <80% | 28 | 0.25 | 1.00 | 1.00 |
| ≥80% | 99 | 0.16 | 0.68 (0.44, 1.03) | 0.50 (0.33, 0.78) | |
| Clinical Vertebral fracture | <80% | 21 | 0.19 | 1.00 | 1.00 |
| ≥80% | 106 | 0.18 | 0.92 (0.58, 1.48) | 0.79 (0.49, 1.27) | |
| Distal forearm/wrist fracture | <80% | 78 | 0.53 | 1.00 | 1.00 |
| ≥80% | 475 | 0.63 | 1.18 (0.93, 1.50) | 1.08 (0.85, 1.38) | |
| Clinical MI | <80% | 50 | 0.46 | 1.00 | 1.00 |
| ≥80% | 191 | 0.32 | 0.73 (0.53, 0.99) | 0.69 (0.50, 0.95) | |
| CHD Death | <80% | 19 | 0.17 | 1.00 | 1.00 |
| ≥80% | 70 | 0.12 | 0.75 (0.44, 1.25) | 0.82 (0.48, 1.40) | |
| Any Cancer | <80% | 138 | 1.32 | 1.00 | 1.00 |
| ≥80% | 726 | 1.24 | 0.94 (0.79, 1.13) | 0.91 (0.76, 1.10) | |
| Invasive breast cancer | <80% | 50 | 0.46 | 1.00 | 1.00 |
| ≥80% | 215 | 0.36 | 0.76 (0.56, 1.04) | 0.73 (0.53, 1.00) | |
| Colorectal cancer | <80% | 14 | 0.13 | 1.00 | 1.00 |
| ≥80% | 105 | 0.17 | 1.36 (0.78, 2.38) | 1.41 (0.80, 2.48) | |
| Cancer death | <80% | 52 | 0.47 | 1.00 | 1.00 |
| ≥80% | 163 | 0.27 | 0.62 (0.45, 0.85) | 0.60 (0.43, 0.82) |
CHD = coronary heath disease; MI = myocardial infarction; Ann % = Annualized %
Adjusted for age, ethnicity, education, smoking, alcohol, fruit/vegetables intake, red meat intake, BMI, physical activity, physical function, any insurance, mammogram, visit to usual care provider in the past year, colonoscopy ever, family history of fracture, family history of breast cancer, self-reported health, history of diabetes, bilateral oophorectomy, age at first birth, age at menarche, depression, aspirin, corticosteroids, fracture medication, beta blockers, thiazides, loop diuretics, PPIs, NSAIDs, lifetime hormone therapy duration, number of medications taken
We then examined the correlation between placebo adherence and adherence to hyperlipidemia and osteoporosis medications in the subset of clinical trial participants taking medications for these conditions (N=883 and n=158, respectively). In the WHI, use of non-study medications was not captured with sufficient detail that allowed for calculation of their PDC. Therefore, adherence with hyperlipidemia and osteoporosis medications was quantified as one-year persistence, which was determined by self-report and medication bottle review at baseline and one year later. We defined high persistence with statins and osteoporosis pharmacotherapy as individuals remaining on therapy at one year.
Application of external adjustment methods to control for the healthy adherer effect in an observational analysis
Assuming women who were high placebo adherers were more likely to adhere to osteoporosis and lipid medications, this suggests that the behaviors and factors associated with healthy adherer effect may be in part independent of the medication itself and thus generalizable across medication classes. For this reason, we considered higher adherence with osteoporosis and lipid medications as a proxy for unmeasured confounders related to the healthy adherer effect. An unmeasured confounder can be controlled for using external adjustment methods (28) by using information obtained from other studies where that confounder was measured to estimate 1) the differential prevalence of the confounder between groups and 2) the association between the confounder and the outcome. We demonstrated how one might use our results (from the placebo group) to adjust for the healthy adherer effect in an observational analysis of adherence to an active medication (e.g. hormone therapy) where there is no placebo group in which to directly measure adherence behavior. In this example, we use the result from the placebo adherence association with hip fractures, and the differential prevalence to osteoporosis and lipid medications between women adherent and non-adherent to placebo, to more fully adjust the effect of adherence to HT on fractures.
Using identical methods to those described above for women randomized to placebo, we studied women in the HT arm of the WHI trial to estimate the relationship between adherence to HT and hip fracture. We compared women highly adherent (>=80%) to those less adherent (<80%) to HT, adjusting for the same confounders as for our placebo-adherence models (Table 2). External adjustment techniques (28), with the confounder of interest being non-adherence behavior, were used to yield a healthy adherer-adjusted HT-hip fracture result. In other words, the ‘missing’ confounder that we controlled using external adjustment represented whatever factor(s) for which placebo adherence served as a proxy. This healthy adherer adjusted result was compared to the previously-reported result for the association between HT and hip fracture (18, 20, 29).
Results
Descriptive characteristics of the 13,444 WHI HT trial participants receiving placebo are in Table 1. Older women, those better educated, and with higher household incomes were more adherent with the placebo regimen. Adherent women were less likely to smoke and consume low amounts of fruits and vegetables and had better self-reported health. Adherent women were more likely to have had pap smears and colonoscopies and to have seen a healthcare provider in the previous year. In general, differences between groups defined by adherence were modest. Some statistically significant differences were of small magnitude and of little clinical significance.
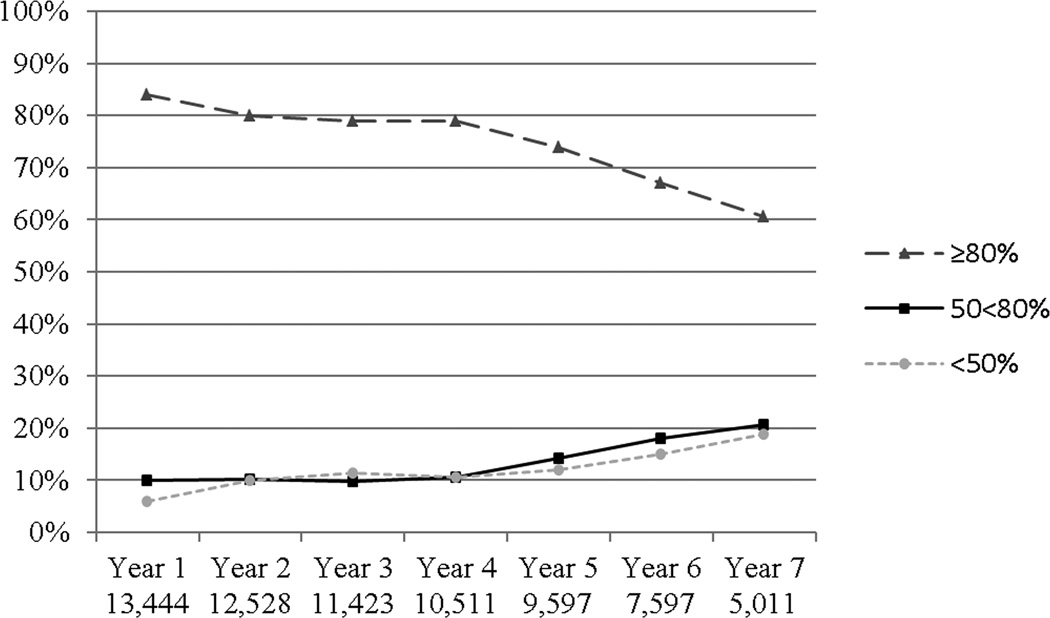
The pattern of adherence over the course of the study is shown in Figure 1. On average, a majority of women were highly adherent to study medication, although adherence decreased over time. Five years into the trial, about 25% of women were < 80% adherent. Women reporting baseline moderate to severe climacteric symptoms had similar adherence to placebo as others (data not shown).
Figure 1.
Proportion of Women with Various Levels of Adherence to Study Medication (Placebo) over the Course of the WHI Randomized Trial
The numbers at the bottom of the figure describes the sample size under observation at that time point
The main results of the study are shown in Table 2. Adherence to placebo was significantly and inversely associated with all-cause mortality, hip fracture, myocardial infarction, invasive breast cancer, and cancer-related death. Non-significant trends suggested a reduced incidence of all other outcomes except for wrist fracture and colon cancer. The crude and adjusted hazard ratios were minimally different for most outcomes. In analyses for which we did have adequate numbers of outcome events to examine 3 categories of adherence (0–50%, >50–<80%, >= 80%), results for intermediate adherence (PDC 50–80%) were in-between those for high PDC and low PDC.
Table 3 shows the association between adherence to placebo and persistence with statins and osteoporosis medications. Women highly adherent to placebo dispensed through the clinical trial had a 20% greater absolute difference in persistence with medications for hyperlipidemia and osteoporosis (prescribed for clinical indications) compared to women not highly adherent to placebo.
Table 3.
Relationship Between Adherence to Placebo and Adherence to Other Medications, WHI HT Clinical Trial Placebo Arm Women
| Cumulative Adherence † to placebo |
Statin Users* (n = 883) | Osteoporosis Drug** Users* (n = 158) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Persistent *** |
Non- Persistent |
Missing | N | Persistent *** |
Non- Persistent |
Missing | |
| ≥ 80% | 708 | 82% | 17% | 2% | 132 | 70% | 29% | 1% |
| < 80% | 175 | 63% | 21% | 15% | 26 | 50% | 35% | 15% |
| p value, Fisher's exact test | <.001 | <.001 | ||||||
Data shown are % in each row
Differences in persistence for both statin use and osteoporosis drug use are statistically different between women adherent vs. non-adherent to placebo by Fisher’s exact test, p < 0.001 for each
A user is defined as someone taking this medication at baseline
Osteoporosis drugs include bisphosphonates, raloxifene, and calcitonin
Persistent is defined by continued use to one year after baseline
Evaluated one year after baseline
We could not directly adjust the HT-hip fracture result with the information on adherence to other medications given that only a small proportion of women were taking these other medications. However, to demonstrate how one might adjust for the healthy adherer effect in the analysis of the effect of HT on hip fracture, we used external adjustment methods. The crude hazard ratio for the association between high adherence to HT and hip fracture was 0.43 (95% CI 0.28 – 0.66). After controlling for factors described in the footnote of Table 2, the adjusted hazard ratio associated with high vs. low HT adherence was 0.54 (95% CI 0.34–0.84). Based on the observed prevalence of low adherence to osteoporosis medication of 50% and 30% among women with low and high placebo adherence, results in Table 4 yielded a more fully-adjusted hazard ratio of 0.62. This change in the hazard ratio from 0.54 to 0.62 is consistent with the external adjustment procedure providing some additional control for confounding related to the healthy adherer effect. Similar results were observed applying the differential prevalence of low adherence to statins.
Table 4.
Fully-Adjusted Association between High Adherence to Hormone Therapy and Hip Fracture after External Adjustment for Low Adherence Behaviors
| Formula | RRplacebo | RRfull | |
|---|---|---|---|
| 1 / 0.50 = 2.0 | 0.62* | ||
| 1 / 0.33 = 3.03 | 0.68 | ||
| 1 / 0.78 = 1.28 | 0.57 |
example calculation per (28),
RRadj=0.54 (observed adjusted association between high adherence to HT and hip fracture)
Ph=0.30 (prevalence of poor adherence behavior among women with high adherence to placebo, i.e., prevalence of low/missing persistence with osteoporosis medications from table 3)
Pl=0.50 (prevalence of poor adherence behavior among women with low adherence to placebo, i.e., prevalence of low/missing persistence with osteoporosis medications from table 3)
RRplacebo=0.50 (95%CI=0.33–0.78), inverted observed adjusted association between poor adherence to placebo and hip fracture, observed in Table 2
RRfull=fully adjusted association between HT and hip fracture, after controlling for residual confounding (adherence behavior identified by adherence to osteoporosis pharmacotherapy).
Applying differential adherence to statins identifies similar externally-adjusted results, i.e., RR=0.63, with point estimates for the RR ranging from 0.57 to 0.69 depending on the true value of RRplacebo
Discussion
Among postmenopausal women randomized to placebo in the two randomized HT trials of the WHI, we found a strong and significant inverse association between adherence to placebo and hip fracture, CHD, invasive breast cancer, cancer death, and all-cause mortality. The magnitude of this effect was greatest for hip fracture. Based upon these results, the healthy adherer effect may be an important factor that could confound observational analyses, leading to over-estimation of medication benefits. Analyzing a clinical trial only among the subgroup of adherent patients, rather than by intent-to-treat, may likewise be biased.
The association between placebo adherence in randomized controlled trials and mortality risk has been examined in at least eight previous reports. As summarized in a recent meta-analyses, high adherence to placebo was associated with lower all-cause mortality (HR 0.45, 95% CI 0.38–0.54) (12). Although the eight trials included enrolled 1167 participants with 636 deaths, only 240 women were included, among whom 19 deaths occurred. In one of these trials, adherence was measured by self report only, and adherence in a second was based on clinician’s impression. Objective measurement of adherence may differ from self reports or subjective assessments (30). Even despite some methodologic heterogeneity in how adherence was assessed, these prior reports support an association with placebo adherence and lower mortality in men participating in drug therapy trials following MI. Only limited evidence is available regarding placebo adherence in healthier populations and on other clinical outcomes including mortality. Among the few studies conducted in women and consistent with our findings, an analysis of adherence to placebo in postmenpausal women participating in an osteoporosis clinical trial suggested a lower rate of hip fracture associated with placebo adherence, but there were few hip fracture events and results were not statistically significant (31).
Our study was not designed to elucidate behaviors and other risk factor for which medication adherence may be a proxy, but factors associated with adherence to calcium and Vitamin D in the WHI has been described (32). However, factors related to adherence with these supplements may differ compared to adherence with prescription medications like HT. Nevertheless, we can offer some observations about behaviors that did not account for the healthy adherer effect we observed. The healthy adherer adjusted results were minimally different than the unadjusted results for most outcomes, suggesting that none of the baseline factors we controlled for accounted for the healthy adherer effect; these included age, race, income, education, marital status, occupation, health insurance, health care seeking behavior, preventive services utilization, health behaviors like smoking and alcohol, exercise, diet, medical conditions and medications, and depression
Although it is possible that the healthy adherer effect may be a proxy for unmeasured behaviors and health habits that WHI did not collect or that varied substantially over time, these effects may not affect all outcomes. For example, despite the strong association seen with placebo adherence and hip fracture, there was no similar inverse relation between adherence and wrist fractures or colon cancer. Wrist fractures have a weaker association with osteoporosis than hip and vertebral fractures (33) and different risk factors (34); wrist fractures typically occur in healthier, more active women. It is also possible that the differential association between adherence and hip versus wrist fracture may be related to major changes in health state (i.e. a ‘sick stopper’ effect), whereby declining health, worsening comorbidities, and an associated competing focus on other health issues results in patients changing patterns of medication use, perhaps becoming less adherent (35). These changes in health status may be more strongly associated with certain outcomes (e.g. hip fracture) than others (e.g. wrist fracture). The importance and magnitude of the healthy adherer effect also may vary by patient population. For example, an observational study of patients registered in a large database of post-MI patients in Ontario did not find evidence that outcome benefits were mediated by “healthy adherer” behavioral attributes (36).
Additional examination of time-varying confounders may be fruitful to better understand the pathway by which medication adherence as a behavior mediates its protective effect. We were unable to pursue this possibility because repeated measures of baseline factors were not made very frequently (i.e. every 1–3 years). Future studies that can link clinical trials or observational registries to administrative claims data, where data capture is essentially continuous, may yield a better understanding of the healthy adherer effect. Such a linkage with administrative data could allow better understanding of major and rapid changes in health state (e.g. new comorbidities, recent hospitalizations) and minimize loss to follow-up, although administrative data may be somewhat limited in providing clinically rich information.
Despite our lack of understanding of the pathway by which the ‘healthy adherer’ effect operates, it may nevertheless be possible to at least partially control for its effect using external adjustment (37, 38). In our study, the unmeasured confounder was adherence behavior. This information was used to adjust the association between high adherence to HT and hip fracture (HR = 0.54) to yield a healthy adherer-adjusted hazard ratio of 0.62. Depending on the ‘true’ value of the association of placebo adherence and hip fracture risk, the externally-adjusted HT-hip fracture hazard ratio may have ranged from 0.57 to as high as 0.68. Compared to our initially adjusted results, this result was closer to the result from the WHI HT RCT, which in the estrogen arm was 0.61 (0.41–0.91) (29) and in the estrogen+progestin arm was 0.67 (95% CI 0.47–0.96) (18, 20). Without the adjustment for the healthy adherer effect, the benefit of HT was over-estimated. External adjustment may provide an approach to controlling for the healthy adherer effect, independent of drug effect, and should be further examined in future studies. Of potential importance, adherence to hyperlipidemia and osteoporosis medications in WHI was self-reported, and some of the adherence data for these medications was missing (up to 15% of women). We categorized these missing data as non-persistence, given that the occurrence of missing data was strongly associated with non-adherence to placebo study medication. Additionally, our estimates of adherence to hyperlipidemia and osteoporosis medications were from women taking these at baseline, many of whom were likely to have been longstanding, prevalent users. A more robust measure of association of adherence to these medications, studying new users and deriving more precise information from a data source such as a pharmacy claims database, might allow for better estimation of adherence behavior and thus permit more complete adjustment.
Study strengths include a large, ethnically diverse study population of well characterized postmenopausal women at a wide range of ages from 40 U.S. centers. Adherence to trial medication or placebo was rigorously determined using a prospectively defined procedure and not based on self report. Clinical outcomes were rigorously ascertained. Even for women who withdrew from the WHI, follow-up on the mortality endpoint was available in 98% of participants. Additionally, we were able to adjust for a broad and comprehensive set of healthy behaviors that have been postulated to explain at least some of the ‘healthy adherer’ effect, although many of these factors were measured only at baseline. Despite these strengths, our study has some potential limitations. Small number of events for some outcomes (e.g. colorectal cancer) required collapsing adherence categories into 2 levels, a convention we followed for all outcome analyses for consistency. Women were censored at the time they did not return for any further WHI study visits. If one assumes that these women were non-adherent, then it is likely that our results are conservative compared to the estimates we would obtain if we imputed non-adherence for these women and allowed them to remain in the analysis. Additionally, and despite the diverse nature of the more than 13,000 WHI clinical trial participants represented in our analysis, our findings may have limited generalizability to non-trial settings, although this would not compromise the internal validity of our results. Finally, adherence to a medication provided by a study like WHI may be different, and may be associated with different outcomes, than adherence to a medication prescribed for any other reason, although the direction of any potential bias is difficult to predict.
In conclusion, we report that adherence to placebo was associated with improved clinical outcomes and lower all-cause mortality, suggesting that the healthy adherer effect is important across a broader set of outcomes than previously reported, and is relevant for community-dwelling women as well as men. This work underscores the importance of developing, as part of a future research agenda, a better understanding of the healthy adherer effect, both in terms of the behaviors or factors that mediate the observed beneficial effects, and as well as how adherence changes over time in relation to changes in health states. In the meantime, we have presented one approach to adjusting for this healthy adherer effect to provide more valid estimates of benefits due to the medication rather than factors solely associated with adherence itself.
Acknowledgements
Dr. Curtis receives support from the National Institutes of Health (AR 053351). M. Alan Brookhart is supported by a career development award from the National Institute on Aging (AG027400). Dr. Cadarette holds a Canadian Institutes of Health Research New Investigator Award in the Area of Aging and Osteoporosis. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
References
- 1.Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21(9):1453–1460. doi: 10.1185/030079905X61875. [DOI] [PubMed] [Google Scholar]
- 2.Cramer JA, Silverman S. Persistence with bisphosphonate treatment for osteoporosis: finding the root of the problem. Am J Med. 2006;119(4 Suppl 1):S12–S17. doi: 10.1016/j.amjmed.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int. 2006;17(11):1645–1652. doi: 10.1007/s00198-006-0179-x. [DOI] [PubMed] [Google Scholar]
- 4.Briesacher B, Andrade S, Fouayzi H, Chan K. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437–443. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perreault S, Lamarre D, Blais L, Dragomir A, Berbiche D, Lalonde L, et al. Persistence with treatment in newly treated middle-aged patients with essential hypertension. Ann Pharmacother. 2005;39(9):1401–1408. doi: 10.1345/aph.1E548. [DOI] [PubMed] [Google Scholar]
- 6.Parris ES, Lawrence DB, Mohn LA, Long LB. Adherence to statin therapy and LDL cholesterol goal attainment by patients with diabetes and dyslipidemia. Diabetes Care. 2005;28(3):595–599. doi: 10.2337/diacare.28.3.595. [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165(20):2414–2419. doi: 10.1001/archinte.165.20.2414. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JR, Westfall AO, Cheng H, Lyles K, Saag KG, Delzell E. The Benefit of Adherence with Bisphosphonates Depends on Age and Fracture Type: Results From an Analysis of 101,038 New Bisphosphonate Users. J Bone Miner Res. 2008;23(9):1435–1441. doi: 10.1359/JBMR.080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81(8):1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 10.Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18(3):271–277. doi: 10.1007/s00198-006-0230-y. [DOI] [PubMed] [Google Scholar]
- 11.McDermott MM, Schmitt B, Wallner E. Impact of medication nonadherence on coronary heart disease outcomes. A critical review. Arch Intern Med. 1997;157(17):1921–1929. [PubMed] [Google Scholar]
- 12.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366(9502):2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz RI, Viscoli CM, Berkman L, Donaldson RM, Horwitz SM, Murray CJ, et al. Treatment adherence and risk of death after a myocardial infarction. Lancet. 1990;336(8714):542–545. doi: 10.1016/0140-6736(90)92095-y. [DOI] [PubMed] [Google Scholar]
- 15.Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med. 1980;303(18):1038–1041. doi: 10.1056/NEJM198010303031804. [DOI] [PubMed] [Google Scholar]
- 16.Brookhart MA, Patrick AR, Dormuth C, Avorn J, Shrank W, Cadarette SM, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166(3):348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 17.Dormuth CR, Patrick AR, Shrank WH, Wright JM, Glynn RJ, Sutherland J, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009;119(15):2051–2057. doi: 10.1161/CIRCULATIONAHA.108.824151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA. 2003;290(13):1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 19.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 20.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 21.Jamal SA, Browner WS, Bauer DC, Cummings SR. Warfarin use and risk for osteoporosis in elderly women. Ann Intern Med. 1998;128:829–832. doi: 10.7326/0003-4819-128-10-199805150-00006. [DOI] [PubMed] [Google Scholar]
- 22.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 23.Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122(2 Suppl):S3–S13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 25.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 26.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 27.Hosmer JDW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. Wiley Series in Probability and Statistics: John Wiley and Sons. 1999 [Google Scholar]
- 28.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 29.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 30.Wu JR, Moser DK, Chung ML, Lennie TA. Objectively measured, but not self-reported, medication adherence independently predicts event-free survival in patients with heart failure. J Card Fail. 2008;14(3):203–210. doi: 10.1016/j.cardfail.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtis JR, Delzell E, Chen L, Black D, Ensrud K, Judd S, et al. The relationship between bisphosphonate adherence and fracture: Is it the behavior or the medication? results from the placebo arm of the fracture intervention trial. J Bone Miner Res. 2010 doi: 10.1002/jbmr.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner R, Dunbar-Jacob J, Leboff MS, Granek I, Bowen D, Snetselaar LG, et al. Predictors of adherence in the Women's Health Initiative Calcium and Vitamin D Trial. Behav Med. 2009;34(4):145–155. doi: 10.3200/BMED.34.4.145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18(11):1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 34.Kelsey JL, Browner WS, Seeley DG, Nevitt MC, Cummings SR. Risk factors for fractures of the distal forearm and proximal humerus. The Study of Osteoporotic Fractures Research Group. Am J Epidemiol. 1992;135(5):477–489. doi: 10.1093/oxfordjournals.aje.a116314. [DOI] [PubMed] [Google Scholar]
- 35.Glynn RJ, Knight EL, Levin R, Avorn J. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology. 2001;12(6):682–689. doi: 10.1097/00001648-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 37.Sturmer T, Glynn RJ, Rothman KJ, Avorn J, Schneeweiss S. Adjustments for unmeasured confounders in pharmacoepidemiologic database studies using external information. Med Care. 2007;45(10 Supl 2):S158–S165. doi: 10.1097/MLR.0b013e318070c045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneeweiss S, Glynn RJ, Tsai EH, Avorn J, Solomon DH. Adjusting for unmeasured confounders in pharmacoepidemiologic claims data using external information: the example of COX2 inhibitors and myocardial infarction. Epidemiology. 2005;16(1):17–24. doi: 10.1097/01.ede.0000147164.11879.b5. [DOI] [PubMed] [Google Scholar]