Abstract
A real-time PCR assay was developed to identify varicella-zoster virus (VZV) and herpes simplex virus (HSV) DNA in clinical specimens from subjects with suspected herpes zoster (HZ; shingles). Three sets of primers and probes were used in separate PCR reactions to detect and discriminate among wild-type VZV (VZV-WT), Oka vaccine strain VZV (VZV-Oka), and HSV DNA, and the reaction for each virus DNA was multiplexed with primers and probe specific for the human β-globin gene to assess specimen adequacy. Discrimination of all VZV-WT strains, including Japanese isolates and the Oka parent strain, from VZV-Oka was based upon a single nucleotide polymorphism at position 106262 in ORF 62, resulting in preferential amplification by the homologous primer pair. The assay was highly sensitive and specific for the target virus DNA, and no cross-reactions were detected with any other infectious agent. With the PCR assay as the gold standard, the sensitivity of virus culture was 53% for VZV and 77% for HSV. There was 92% agreement between the clinical diagnosis of HZ by the Clinical Evaluation Committee and the PCR assay results.
Keywords: zoster vaccine, Shingles Prevention Study, clinical trial, diagnosis of herpes zoster, VZV ORF 62
INTRODUCTION
Herpes zoster (HZ; shingles) results from reactivation and replication of varicella-zoster virus (VZV) that has remained latent in sensory ganglia following varicella (chickenpox). A randomized double-blind placebo-controlled trial with 38,546 subjects 60 years of age and older, VA Cooperative Study #403: “The Shingles Prevention Study” (hereafter referred to as “the Study”), demonstrated that a live attenuated Oka/Merck zoster vaccine provided protection against HZ and postherpetic neuralgia [PHN; Oxmanet al., 2005a]. The validity of any efficacy trial or natural history study depends upon the evaluation of proven cases of the disease. The diagnosis of HZ in this trial was determined primarily (>93%) by a real-time PCR assay, developed and validated for the Study, that distinguished among wild-type strains of VZV (VZV-WT), the Oka/Merck vaccine strain (VZV-Oka), and herpes simplex virus (HSV).
Since all subjects enrolled in the Study were VZV seropositive and latently infected with VZV-WT[Oxman et al., 2005a], it was not expected that the attenuated Oka/Merck VZV would establish latency and reactivate to cause HZ. Therefore, the PCR assay’s primary objectives were high sensitivity and specificity for VZV-WT, in order to accurately diagnose HZ. This was important because the threshold for the clinical diagnosis of HZ was intentionally low in order to capture mild, atypical and potentially vaccine-modified cases of HZ. Consequently, it was crucial to determine whether or not “suspected cases of HZ” [unilateral rashes without alternative clinical diagnoses; Oxman et al., 2005a] that were diagnosed clinically were actually cases of HZ. Thus, the PCR assay was required to differentiate low virus-positive samples from true virus-negatives, which was achieved by minimizing the risk of cross-contamination during reagent, sample, and assay preparation. To ensure the integrity of each Study specimen, temporal and spatial separation of procedural steps and unidirectional work flow were implemented to minimize the risk of contamination. While the potential for contamination is considerably less with real-time PCR, which is performed in a closed system, than with conventional PCR[Niesters, 2002], specimens containing large amounts of viral DNA (≥108 genomes) were processed together with virus-negatives during DNA extraction, introducing the risk of cross-contamination. Thus, negative controls, including virus-negative clinical specimen submitted by Study sites and labeled as specimens from suspected cases of HZ, were introduced at different stages throughout the assay procedure.
In addition to providing a definitive diagnosis of HZ, it was also necessary to discriminate between VZV-Oka and VZV-WT in clinical specimens because of the potential confusion between vaccine-associated rashes and mild or vaccine-modified HZ in the period immediately after vaccination, as well as the unlikely possibility that the attenuated Oka/Merck VZV would establish latency and subsequently reactivate to cause HZ. A number of nucleotide polymorphisms have been described that differentiate VZV-Oka from VZV-WT strains circulating in the United States and Australia [Martin et al., 1982; Adams et al., 1989; LaRussa et al., 1992, 1998]. However, since VZV-Oka was derived from a Japanese wild-type strain, these polymorphisms fail to distinguish VZV-Oka from some Japanese VZV-WT strains. More extensive analyses have identified a number of polymorphisms that distinguish VZV-Oka from Japanese VZV-WT strains [Takada et al., 1995; Argaw et al., 2000; Gomi et al., 2000]. The T to C base change in open reading frame (ORF) 62 at position 106262 (in the SmaI restriction site) in VZV-Oka is absent in all known VZV-WT strains, thus providing a useful marker that differentiates the VZV-Oka vaccine from VZV-WT strains [Loparev et al., 2000a]. This single nucleotide polymorphism is one of three within ORF 62 (at positions 106262, 107252, 108111) identified by comparative analyses of sequence variations among VZVisolates from post-vaccination varicelliform rashes, from episodes of HZ, and from vaccine preparations that distinguish VZV-Oka from all VZV-WT strains [Quinlivan et al., 2004; Loparev et al., 2007; Breuer and Schmid, 2008].
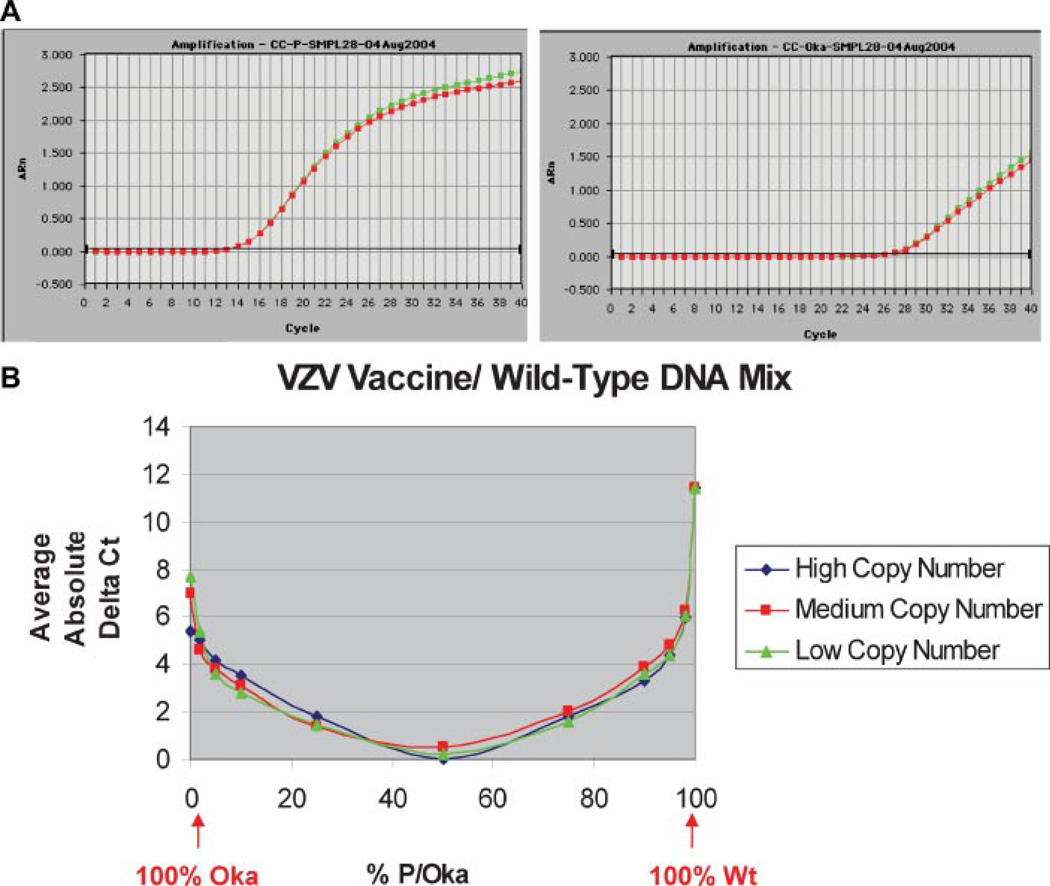
The real-time PCR assay developed for the Study used two sets of homologous primers and a common probe to detect and distinguish between VZV-WT (or the Oka parent strain, VZV-P) and the attenuated Oka vaccine strain, VZV-Oka, on the basis of the single nucleotide polymorphism at position 106262 in ORF 62. Clinical specimens were collected from Study subjects with suspected cases of HZ, and extracted DNA from these specimens was assayed for VZV-WT and VZV-Oka in two separate PCR reactions. The VZV-Oka primers also amplified VZV-WT DNA and vice versa, but with lower efficiency than the homologous target DNA (Fig. 1A). A third PCR reaction tested clinical specimens for the presence of HSV DNA. Each of these three PCR reactions was multiplexed with primers and probe for human β-globin to assess the presence of host cell DNA and thus assess specimen adequacy.
Fig. 1.
Shingles Prevention Study PCR assay specificity for VZV-WT and VZV-Oka DNA. A: VZV amplification plots for a typical VZV-WT positive clinical specimen: The specimen was assayed in duplicate using VZV-WT (left) and VZV-Oka (right) primer-probe sets. With VZV-WT primers and probe, the fluorescence signal crosses the threshold at approximately cycle 13. With VZV-Oka primers and probe, the fluorescence signal crosses the threshold at approximately cycle 26. B: Difference in the average of replicate Ct values for mixtures of purified VZV-WT and VZV-Oka viral DNA with the following VZV-WT to VZV-Oka viral DNA ratios: 100/0, 98/2, 95/5, 90/10, 75/25, 50/50, 25/75, 10/90, 5/95, 2/98, and 0/100. These data indicate that when the Ct for VZV-WT is 5 cycles lower than the Ct for VZV-Oka, there is 97.5% confidence that ≥95.9% of the VZV DNA present is VZV-WT. Conversely, when the Ct for VZV-Oka is 5 cycles lower than the Ct for VZV-WT, there is 97.5% confidence that ≥93.9% of the VZV DNA present is VZV-Oka.
MATERIALS AND METHODS
PCR Primers and Probes
All primers and probes were designed using Primer Express software, Version 1.0 (Applied Biosystems, Foster City, CA). The real-time PCR assay for VZV DNA used two sets of primers and a single probe to detect and discriminate between VZV-WT and VZV-Oka DNA (Table I). Another set of primers and probe (Table I), targeting a conserved region of the HSV DNA polymerase gene, was used to detect both HSV-1 and HSV-2. VZV and HSV TaqMan fluorescent probes were labeled with the reporter dye FAM.
TABLE I.
Sequences of Primers and Probes Used for the Detection of VZV-WT, VZV-Oka, HSV, and β-Globin DNA
| Primers | |
| VZV-WT (VZV-P) gene 62-5′ | 5′-CAA AGC GGG TCC ATC CCTa-3′ |
| VZV-Oka gene 62-5′ | 5′-AA AGC GGG TCC ATC CCCa-3′ |
| VZV gene 62-3′ | 5′-ACC TGG CCT TTG CCG G-3′ |
| HSV-5′ | 5′-GAT CAA GCT CGA GTG CGA A-3′ |
| HSV-3′ | 5′-CCG GAT ACG GTA TCG TCG TAA-3′ |
| β-Globin-5′ | 5′-TTG CTT CTG ACA CAA CTG TGT TCA CTA GC-3′ |
| β-Globin-3′ | 5′-ACA GGG CAG TAA CCG CAG ACT TCT C-3′ |
| Probes | |
| VZV gene 62 | 6FAM-CCG TCG AGT ATC TAG GCT CGC GGT T-TAMRA |
| HSV | 6FAM-CCA AGC TGC TGC TCA TCG CCA AGA-TAMRA |
| β-Globin | VIC-ACC TCA AAC AGA CAC CAT GGT GCA CCT GAC TCC-TAMRA |
Polymorphism (T/C) in ORF 62 at position 106262 allows distinction between VZV-WT strains, including the Oka parent strain (VZV-P), and the VZV-Oka vaccine strain.
The separate PCR reactions used to detect each viral DNA were each multiplexed with primers and probe for human β-globin DNA to detect and quantify host cell DNA, and thus assess specimen adequacy. The β-globin TaqMan fluorescent probe was labeled with the reporter dye VIC.
Preparation of DNA Standards
Plasmid DNA standards for each viral target and for β-globin were included in each PCR assay at concentrations of 10, 100, 1,000, 10,000, and 100,000 copies per 50 µl reaction to verify assay sensitivity. The VZV-WT and VZV-Oka plasmids contained a 248 base pair insert amplified from VZV gene 62 of the Oka-parent strain (VZV-P) and VZV-Oka, respectively, cloned into pUC18. The HSV plasmid contained a 352 base pair insert from a conserved region of the HSV DNA polymerase gene cloned into pUC18. The viral DNA inserts included a BglI (in VZV-WT and VZV-Oka) or a SmaI restriction site (in HSV) engineered within the amplified segment to permit the identification of any suspected contamination of samples by positive controls. The β-globin plasmid contained a 237 base pair insert amplified from the human β-globin gene from MRC5 fibroblasts, which was subcloned into a pCR2.1 vector. The DNA concentration of each stock solution of plasmid DNA standard was quantified using the Quant-iT Pico Green dsDNA Assay Kit (Molecular Probes, Eugene, OR).
Specimen Lysis and DNA Extractions
Specimens were lysed and DNA extracted using the QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA) as specified by the manufacturer. Lysis yielded two 200 µl aliquots of lysate, one of which was stored below −80°C. The other aliquot was adjusted to 600 µl (with 200 µl of AL buffer + 200 µl of 100% ethanol) and applied to the QIAamp spin column. The DNA was eluted in a volume of 150 µl, yielding three 50 µl aliquots of extracted DNA per 200 µl sample of lysate. Extracted DNA was stored below −15°C until assayed.
Some clinical specimens from subjects with HZ or with HSV infections were virus DNA-positive with very high copy numbers, while others from rashes not caused by VZV or HSV were virus DNA-negative. To assure the integrity of each specimen and to minimize the potential for cross-contamination, lysis and DNA extraction procedures were spatially and temporally separated from each other and from sample loading and PCR assay. Each clinical specimen was lysed individually in a dedicated biosafety cabinet with disinfection of the workspace between samples. DNA extraction of specimen lysates was carried out in batches of ≤3 samples in a dedicated biosafety cabinet, accompanied by one mock specimen (PBS) control to monitor for cross-contamination. Mock specimen control samples were PCR assayed for all three viral DNA targets prior to assaying the associated clinical specimens to verify the absence of cross-contamination during DNA extraction. Disposable gloves, which were used during every step of specimen handling, were changed between samples, and the workspace was disinfected between extraction runs. A separate Clean Room, equipped with a dedicated biosafety cabinet, was used to store PCR reagents and to add PCR reaction mixes with the appropriate primer-probe sets to 96-well reaction plates. Personnel and specimen flow was always unidirectional from the Clean Room to rooms and biosafety cabinets used for sample receiving, preparation and lysis, DNA extraction or the addition of extracted DNA to reaction plates.
Real-Time PCR Assay for Viral and β-Globin DNA
Extracted DNA from each specimen was subjected to three separate PCR assays for VZV-WT, for HSV and for VZV-Oka DNA, each multiplexed with primers and probe for human β-globin DNA. Extracted DNA from Study specimens, control extracts, and negative amplification controls, as well as plasmid DNA standards, were added in a volume of 5 µl in duplicate to 45 µl of PCR reaction mixture in 96-well optical plates (Applied Biosystems), using separate plates for each viral DNA target. Final assay concentrations were 600 nM each of forward and reverse primers for the viral DNA targets (Baron Biotech, Milford, CT), 50 nM each of forward and reverse primers for β-globin DNA (Baron Biotech), 100 nM FAM-labeled viral fluorescent probe (Applied Biosystems), and 50 nM VIC-labeled β-globin fluorescent probe (Applied Biosystems) in 1× TaqMan Universal Master Mix (Applied Biosystems). Negative amplification controls included four replicates containing salmon testes DNA as template (No Template Control [NTC]) and six replicates containing PBS buffer as template (PBS controls) in each 96-well reaction plate. Up to 11 mock specimen controls were assayed prior to PCR assay of the up to 33 corresponding samples of extracted DNA from Study specimens. Serial dilutions of viral and β-globin plasmid DNA standards were assayed as positive amplification controls to verify assay sensitivity. Study specimens also included negative clinical control specimens submitted by the Study sites and labeled as specimens from suspected cases of HZ. One complete assay of DNA extracts from 33 Study specimens for the three viral DNA targets consisted of a total of 432 reaction wells (including the separately assayed mock specimen controls), 234 (54%) of which were occupied by negative and positive amplification controls, including serial dilutions of plasmid DNA standards. After sample loading, the 96-well optical plates were centrifuged for 1 min at 500 rpm and then incubated in a Prism® 7700 Sequence Detection System (Applied Biosystems) at 50°C for 2 min, and 95°C for 10 min, followed by 40 cycles of 94°C for 30 sec and 66°C (60°C for HSV target) for 75 sec. After completion of thermal cycling, the raw data were analyzed with a threshold setting of 0.05 for all DNA targets.
The PCR assay results for Study specimens were determined according to the criteria listed in Table II, which incorporated the results of assay validation. For assays to be valid, both replicates of any tested specimen were required to be either positive or negative for the target virus DNA. Specimens that were negative for viral DNA but positive for β-globin DNA were scored as virus-negative; specimens negative for both viral and β-globin DNA were considered to be inadequate specimens. If the first Study specimen from a suspected case of HZ was negative or inadequate, all other specimens from the same episode were assayed.
TABLE II.
Criteria for the Determination of the Final PCR Result
| VZV-WT | VZV-Oka | HSV | β-Globin | No template control |
PBS controls |
Target DNA standards |
Result |
|---|---|---|---|---|---|---|---|
| ++ | + | − | + or − | − | − | + | VZV-WTa,b |
| + | ++ | − | + or − | − | − | + | VZV-Okab,c |
| − | − | + | + or − | − | − | + | HSV |
| − | − | − | + | − | − | + | Negatived (assay all other specimens from the same suspected case of HZ) |
| − | − | − | − | − | − | + | Inadequated (assay all other specimens from the same suspected case of HZ) |
If the Ct value for VZV-WT is at least 5 cycles lower than the Ct value for VZV-Oka, the specimen is defined as positive for VZV-WT.
If the specimen is positive for both VZV-WT and VZV-Oka, but there is less than 5 cycles difference between the two Ct values, the specimen is defined as VZV positive, but of indeterminate VZV strain.
If the Ct value for VZV-Oka is at least 5 cycles lower than the Ct value for VZV-Wt, the specimen is defined as positive for VZV-Oka.
The presence or absence of host cell (β-globin) DNA was assessed in the PCR reaction wells in which the β-globin primers and probe were multiplexed with the VZV-WT primers and probe.
Assay Validation
Peripheral blood mononuclear cells (PBMC; used in lieu of keratinocytes) were prepared at high (105 PBMC/extraction) and low (200 PBMC/extraction) concentrations and “spiked” with 0, 10, 100, 1,000, and 10,000 copies of each plasmid viral DNA standard prior to lysis and extraction to determine assay precision and percent recovery. During this phase of validation, three different lots of TaqMan Universal Master Mix and three different lots of the QIAamp DNA Mini Kit were tested in a total of nine assays to determine assay ruggedness.
Twenty-six specimens, consisting of infectious agents other than HSV-1, HSV-2, or VZV that might be present in HZ skin lesions or in skin lesions resembling HZ were tested for all three viral DNA targets to assess assay specificity. These consisted of diploid human fibroblasts (DHF) infected with Cytomegalovirus AD169; concentrated virus stocks of Epstein Barr Virus strains B95-8 and HH514–16; lymphocytes infected with Human Herpesvirus (HHV)-6A, HHV-6B, HHV-7, and HHV-8 and tested at 1:1 and 1:100 dilutions; primary Rhesus monkey kidney cells infected with prototype Coxsackievirus A7 and Enterovirus 71 and tested at dilutions of 1:1 and 1:100; primary Rhesus monkey kidney cells infected with prototype Coxsackieviruses A9, A16, B2, B5 and Echoviruses 11 and 71 and tested at a 1:1 dilution; and concentrated partially purified Human Papillomavirus-6. Other microorganisms tested consisted of log phase cultures of primary clinical isolates of Staphylococcus aureus, S. epidermidis, S. xylosus, Escherichia coli, Diphtheroids, Group A Streptococcus, Pseudomonas aeruginosa, and Candida albicans. In addition, two specimens from non-infectious skin lesions, that is, a swab specimen from folliculitis and a crust from a traumatic contusion, were tested.
Mixtures of purified viral DNA from VZV-WT and VZV-Oka grown in DHF were prepared containing the following ratios of VZV-WT to VZV-Oka DNA: 100/0, 98/2, 95/5, 90/10, 75/25, 50/50, 25/75, 10/90, 5/95, 2/98, and 0/100. The DNA concentration of the purified viral DNA was quantified using the Quant-iT Pico Green dsDNA Assay Kit (Molecular Probes). Mixtures were tested at high (~107), medium (~105), and low (~103) copy number. Three sets at each copy number were prepared independently, added directly to PCR mixtures, and assayed.
DHF infected with VZV-WT, VZV-Oka, HSV-1 or HSV-2 were enumerated by direct fluorescent antibody staining. Constructed samples containing known numbers of infected and uninfected DHF at total concentration of 5,000 cells/75 µl were prepared, stored with and without cotton-tipped swabs, and then lysed, extracted and assayed.
Samples used to assess concordance between the two PCR laboratories (Merck PCR Laboratory in West Point, PA and the CSP #403 Central Diagnostic PCR Laboratory at the VA Medical Center in San Diego, CA) consisted of two separate sets of 33 individual samples for each viral target (a total of 198 samples). Each set of 33 concordance samples (assayed for VZV-WT, VZV-Oka, or HSV DNA) consisted of nine virus-negative patient samples, nine typical virus-positive samples, and three sets of five 4-fold serial dilutions of lysate from constructed swab samples containing 10 virus-infected and 5,000 uninfected DHF. Virus-positive samples were either patient samples proven by virus culture to be VZV or HSV-positive, or constructed swab samples containing 1,000 DHF infected with VZV-WT or HSV-2. All VZV-Oka virus-positive samples were constructed swab samples containing 1,000 VZV-Oka infected DHF. All concordance samples and all clinical specimens were received or constructed, stored, and lysates prepared at the San Diego PCR Laboratory. One 200 µl aliquot of the lysate was shipped to the Merck Laboratory for DNA extraction and the remaining 200 µl aliquot was extracted at the San Diego site. DNA extractions were performed in both laboratories in groups of three, with a mock specimen control extracted with each group of three samples.
For clinical validation, 56 negative control clinical specimens from subjects with rashes on the torso below the neck and above the waist, which were considered by the Study physician not to be caused by VZV or HSV (negative control Study specimens), were collected by Study sites and shipped to the San Diego PCR Laboratory in a manner indistinguishable from specimens from suspected cases of HZ. In addition, 10 VZV-positive clinical specimens were collected from patients with culture-proven HZ who were not participants in the Study. Four lysates of diagnostic specimens from patients with VZV-Oka-positive rashes [confirmed by restriction fragment length polymorphism analysis of PCR products; LaRussa et al., 1992], 7 samples of extracted DNA from VZV-Oka positive diagnostic specimens [confirmed by melting curve analysis [Loparev et al., 2000b], and one clinical swab specimen from a child with a rash due to VZV-Oka were tested to further investigate assay specificity.
Clinical Specimens
Swab specimens from vesicular or pustular lesions and crusts or skin scrapings from maculopapular lesions in Study subjects with suspected cases of HZ (Study specimens) were collected at Study sites and shipped frozen overnight to the PCR Laboratory in San Diego. Specimen collection kits that included instructions, specimen containers, pre-evaluated cotton swabs, and stainless steel scalpel blades were sent to all study sites. Most specimens were swabs from the base of unroofed vesicles or pustules; a minority were crusts or scrapings from maculopapular lesions. A total of 68 negative control Study specimens from 48 subjects were randomly interspersed with ordinary Study specimens from suspected cases of HZ to monitor in real time for possible cross-contamination. They were not identified as negative controls to the PCR laboratories.
All clinical specimens examined were obtained from subjects who provided written informed consent or were aliquots of diagnostic specimens from patients who were not Study subjects, which had been de-identified. The Study protocol and consent documents were approved by the VA Cooperative Studies Human Rights Committee and local Institutional Review Boards.
RESULTS
Assay Validation
The PCR assay was validated as a qualitative assay. Results were reported as Ct (cycle threshold) values, that is, the amplification cycle number at which the fluorescence of the PCR product exceeded the detection threshold. Initial validation experiments consisted of 27 PCR assay runs designed to evaluate the cutoff Ct value for sample positivity, the limits of detection (LOD) for the three viral and one β-globin DNA targets, assay precision, percent recovery from samples “spiked” with varying levels of virus, and the effect of extraneous uninfected cells on viral DNA recovery. In order to generate Ct cutoff and LOD values that reflected the clinical performance of the assay, additional validation runs assessing assay specificity were carried out with 56 virus-negative and ten VZV-WT-positive clinical specimens. A common Ct cutoff value for all four DNA targets was chosen to simplify the assay procedure and minimize errors in evaluation of assay results. The Ct cutoff value was established by subtracting three Standard Deviations of all 10 copy number plasmid DNA standards (where variability was greatest within and between assays) from the maximum Ct value of 40. This resulted in a Ct cutoff value of 36.35 for all DNA targets. Using this cutoff value, the LOD (defined as the lowest DNA copy number that has a 95% probability of producing a Ct value below the cutoff for the assay) was calculated to be 13 copies of VZV-WT plasmid DNA, 13 copies of VZV-Oka plasmid DNA, 31 copies of HSV plasmid DNA, and 93 copies of β-globin plasmid DNA. Because of the diploid nature of ORF 62 (duplicated as ORF 71), the assay’s LOD for VZV-WT and VZV-Oka viral DNA is 7 genomes. Samples with Ct values greater than 36.35 were scored as negative. Serial dilutions of lysates from samples constructed with 10 VZV-WT infected DHF showed that the LOD for VZV-WT corresponded to approximately 1/2,000 of the VZV-WT DNA in a single VZV-infected DHF.
Assay ruggedness with respect to different lots of TaqMan Universal Master Mix and of extraction kits was evaluated by testing each combination of three master mix lots and three extraction kit lots across a total of nine assays. No significant differences due to Master Mix lot or extraction kit lot were observed, regardless of virus DNA or PBMC concentration, indicating that the assay is rugged with respect to both (data not shown).
The same data used to assess ruggedness were employed to estimate assay precision. The overall precision estimates across different concentrations of PBMC and expected copy numbers was 22.5% for VZV-WT, 46.0% for VZV-Oka, and 33.9% for HSV. When the expected copy number was 10 and the PBMC concentration was low, the percent recovery was between 53% and 76% for each virus DNA. However, the percent recovery was low (between 5% and 15%) when the expected copy number was 10 and the PBMC concentration was high, suggesting amplification competition by the β-globin multiplex reaction. When the expected copy number was ≥100, the percent recovery was generally ≥80%. Typically, samples with higher copy numbers of viral DNA had higher percent recovery, whereas in samples with lower copy numbers of viral DNA, the percent recovery was dependent on PBMC concentration.
The second phase of assay validation assessed specificity. For this purpose, samples of other infectious agents that might be present in skin lesions resembling those of HZ and samples from non-infectious lesions that might be confused with HZ were tested. None of these were amplified by the primer-probe sets designed to detect DNA from VZV-WT, VZV-Oka, or HSV. In addition, constructed swab samples with known numbers of uninfected DHF and DHF infected with VZV-WT, VZV-Oka, or HSV were assayed to assess the assay’s specificity for these three viral DNA targets. The results demonstrated that the PCR assay distinguished among the three targets without exception.
DNA sequencing of the amplicons from PCR assays of three VZV-WT and three VZV-Oka specimens showed the predicted nucleotide sequences, which were identical, except at position 106262, where the “T” in the VZV-WT amplicon was replaced by a “C” in the VZV-Oka amplicon. Sequencing of the amplicons from PCR assays of three HSV specimens showed the expected nucleotide sequences for the conserved region of the HSV DNA polymerase gene selected for the PCR assay (data not shown).
In addition, the incidence of cross-contamination during DNA extraction was evaluated. Thirty-two (32) mock specimen control samples, each extracted together with three virus-positive specimens, were all negative for all viral DNA targets and for β-globin DNA, demonstrating the absence of cross-contamination during DNA extraction (data not shown).
Discrimination of VZV-WT DNA From VZV-Oka DNA in Mixed Samples
The distribution of the average replicate Ct values for samples with different ratios of VZV-WT to VZV-Oka viral DNA at different total copy numbers is shown in Figure 1B. These data indicate that when the Ct value for VZV-WT is 5 cycles lower than the Ct value for VZV-Oka, there is 97.5% confidence that ≥95.9% of the VZV DNA present is VZV-WT. Conversely, when the Ct value for VZV-Oka is 5 cycles lower than the Ct value for VZV-WT, there is 97.5% confidence that ≥93.9% of the VZV DNA present is VZV-Oka. Based on these findings, we defined clinical specimens with a Ct value ≥5 cycles lower for VZV-WT than for VZV-Oka as positive for VZV-WT viral DNA and vice versa. We also defined clinical specimens with a difference in Ct value of less than 5 cycles between the VZV-WT and VZV-Oka reactions as positive for VZV-DNA, but of indeterminate virus strain.
Specificity of the PCR Assay Evaluated With Constructed Samples
When constructed samples (swabs and solutions) prepared with known numbers of VZV-WT or VZV-Oka infected and uninfected DHF were assayed, amplification with the homologous primers and probe was always greater than amplification with the heterologous primers and probe (Table III). With individual VZV-WT samples, Ct differences in amplification by the VZV-WT and heterologous VZV-Oka primers and probe ranged from 5.00 to 8.52 cycles, with an average of 6.11 cycles. With individual VZV-Oka samples, the Ct value difference with the homologous and heterologous primers and probe ranged from 1.92 to 3.63 cycles, with an average of 2.94 cycles.
TABLE III.
Average Ct Values for Extracted DNA From Constructed Samples (Swab and Non-Swab) With Different Ratios of VZV-WT Infected and Uninfected Cells (A) and VZV-Oka Infected and Uninfected Cells (B)
| Specimen | VZV-WT infected cells |
Uninfected cells |
Mean VZV-WT Ct valuea |
SD | Mean VZV-Oka Ct valuea |
SD | Mean Ct difference Oka-WT |
| (A) VZV-WT | |||||||
| Swab | 10 | 5,000 | 28.33 | 0.81 | 34.24 | 0.43 | 5.91 |
| Non-swab | 10 | 5,000 | 28.13 | 0.87 | 33.98 | 0.71 | 5.85 |
| Swab | 100 | 0 | 24.08 | 0.33 | 31.11 | 0.86 | 7.03 |
| Non-swab | 100 | 0 | 23.87 | 0.26 | 30.85 | 1.31 | 6.98 |
| Swab | 100 | 4,900 | 23.77 | 0.23 | 30.42 | 0.53 | 6.65 |
| Non-swab | 100 | 4,900 | 24.04 | 0.24 | 29.54 | 0.31 | 5.50 |
| Swab | 1,000 | 4,000 | 19.90 | 0.44 | 25.76 | 0.77 | 5.86 |
| Non-swab | 1,000 | 4,000 | 20.09 | 0.19 | 25.19 | 0.24 | 5.10 |
| Specimen | VZV-Oka infected cells |
Uninfected cells |
Mean VZV-WT Ct valuea |
SD | Mean VZV-Oka Ct valuea |
SD | Mean Ct difference WT-Oka |
| (B) VZV-Oka | |||||||
| Swab | 10 | 5,000 | 32.29 | 0.26 | 29.15 | 0.30 | 3.14 |
| Non-swab | 10 | 5,000 | 32.11 | 0.36 | 29.67 | 0.41 | 2.44 |
| Swab | 100 | 0 | 29.04 | 0.23 | 25.74 | 0.41 | 3.30 |
| Non-swab | 100 | 0 | 28.33 | 0.31 | 25.45 | 0.16 | 2.88 |
| Swab | 100 | 4,900 | 28.80 | 0.22 | 25.52 | 0.16 | 3.28 |
| Non-swab | 100 | 4,900 | 28.36 | 0.46 | 25.60 | 0.51 | 2.76 |
| Swab | 1,000 | 4,000 | 25.05 | 0.52 | 22.15 | 0.33 | 2.90 |
| Non-swab | 1,000 | 4,000 | 24.36 | 0.21 | 21.56 | 0.30 | 2.80 |
Mean Ct value for DNA extracted from four constructed samples per specimen.
Concordance Between Laboratories
Prior to testing Study specimens, concordance between the Merck and the San Diego VA PCR Laboratories was assessed (data not shown). There was no cross-contamination of the negative samples in either laboratory. The positive and negative PCR results for detection of viral target DNA in adequate negative and adequate positive samples were 100% concordant between the two laboratories. In addition, the two laboratories demonstrated comparable sensitivity (within fourfold) with samples containing low concentrations of each of the three target DNAs, that is, with samples having Ct values in the region of the assay cutoff.
Results of the PCR Assay of Study Specimens
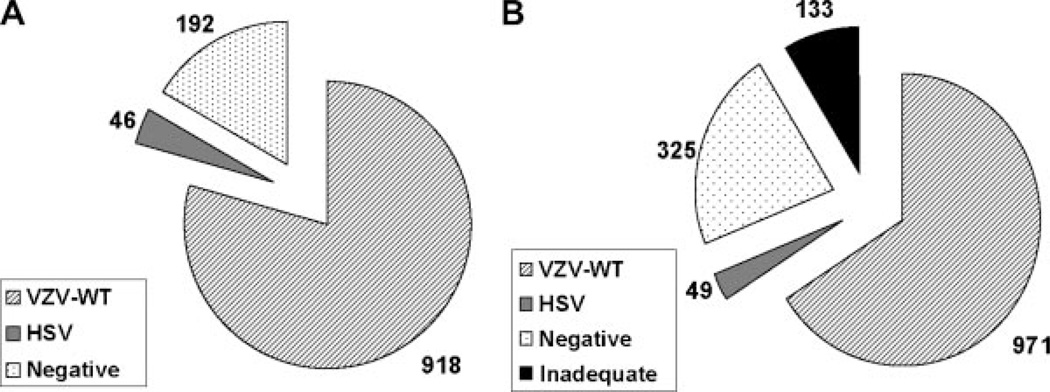
One or more clinical specimens were received from 1,220 of the 1,308 suspected cases of HZ in the Study, and the diagnosis in 1,156 (88.4%) of the 1,308 suspected cases of HZ was determined by PCR assay [Oxman et al., 2005a]. The case distribution is shown in Figure 2A. A total of 1,479 clinical specimens from 1,220 of the 1,308 suspected cases of HZ were assayed by PCR (Fig. 2B). Of these, 971 (66%) were positive for VZV-WTDNA, 49 (3%) were positive for HSV DNA, 325 (22%) were negative for virus DNA (but positive for β-globin DNA, indicating that the specimens were adequate for PCR diagnosis) and 133 (9%) were inadequate (negative for both virus and β-globin DNA). One specimen was positive for both HSV and VZV-WT DNA. This specimen, which contained a large amount of VZV-WT DNA and a very small amount of HSV DNA(Ct values of 16.68 and 34.50, respectively), was from a subject with a unilateral facial rash, which might have reflected overlapping episodes of facial HZ and herpes labialis, a rare event in immunocompetent patients [Giehl et al., 2008]. No Study specimen contained VZV-Oka DNA or DNA from an indeterminate VZV strain.
Fig. 2.
Shingles Prevention Study PCR assay results. A: PCR Assay Results for Suspected Cases of HZ in the Shingles Prevention Study. Of a total of 1,308* suspected cases of HZ, 1,156 (88.4%) were diagnosed by PCR assay. Nine hundred eighteen cases (79%) were positive for VZV-WT, 46 (4%) positive for HSV DNA and 192 (17%) were negative for both VZV and HSV DNA. No cases were positive for VZV-Oka DNA or of indeterminate VZV strain. (B) PCR Assay Results for Shingles Prevention Study Specimens. A total of 1,479 clinical specimens from 1,220 of the 1,308* suspected cases of HZ in the Shingles Prevention Study were assayed for viral and cellular DNA. Nine hundred seventy-one (66%) of the clinical specimens were positive for VZV-WT and 49 (3%) were positive for HSV DNA. One specimen (0.1%) was positive for both VZV-WT and HSV DNA. 325 (22%) of the clinical specimens were negative for virus DNA and 133 (9%) were inadequate specimens (negative for viral and β-globin DNA). No specimens were positive for VZV-Oka DNA or of indeterminate VZV strain. *Includes two PCR-positive cases unrecognized clinically.
None of the negative control samples extracted with each group of ≤3 Study specimens showed any amplification with any of the four primer-probe sets, with one exception: During the first extractions of Study specimens by a new technician, three of the mock specimen control samples showed some amplification with the VZV-WT primers and probe. Investigation revealed that the technician had not changed gloves between each sample during the DNA extraction procedure as specified in the protocol. With subsequent strict adherence to the extraction protocol no additional negative control samples showed any amplification for the duration of the Study. In the case of the nine Study specimens associated with the three mock specimen controls described above, which were suspect for cross-contamination during DNA extraction, DNA was extracted individually (to avoid the possibility of cross-contamination) from the second retained aliquot of each specimen lysate and subsequently assayed per protocol.
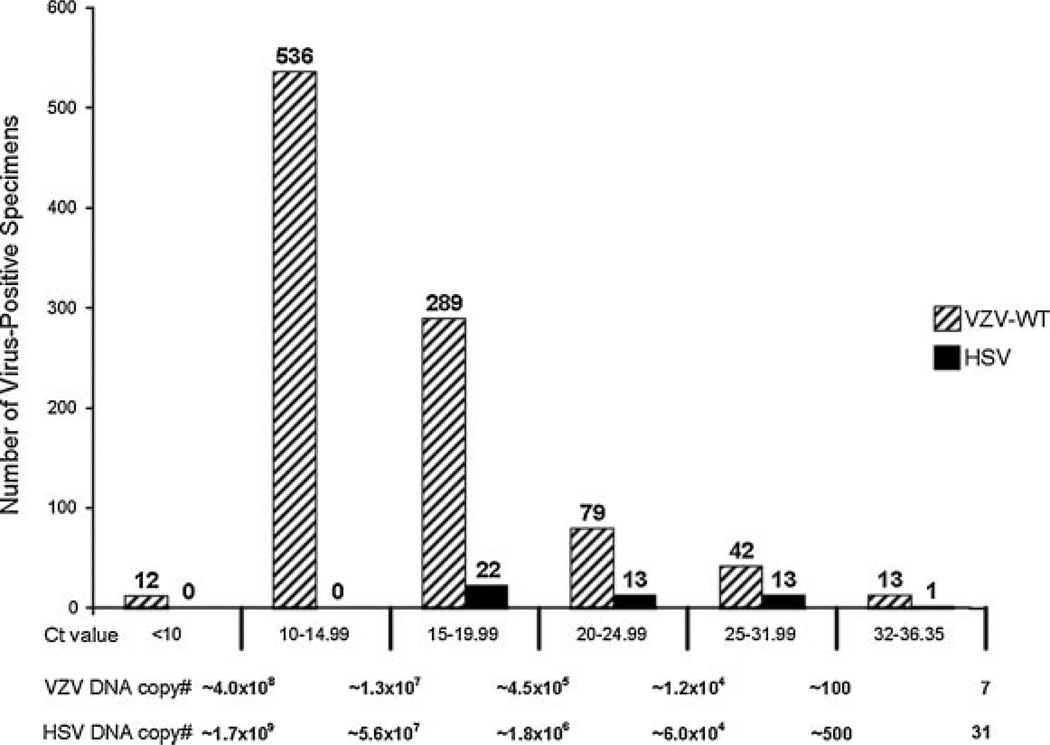
Virus-positive Study specimens with Ct values between 36.35 and 32 were considered to be “low virus positive.” Although the assay was not intended to be quantitative, serial dilutions of each plasmid DNA standard were incorporated in each assay; a Ct value within this range would correspond to between 7 and 100 copies of VZV DNA, or 31–300 copies of HSV DNA. Only 13 (1.3%) of the VZV-WT positive Study specimens were low virus-positives, with Ct values between 36.35 and 32.00. Over 86% of the VZV-WT positive Study specimens had Ct values under 20, corresponding to >400,000 copies of VZV-WT DNA/reaction (Fig. 3). Of the 49 HSV positive Study specimens, 1 was low virus-positive, and 22 (44.9%) had Ct values below 20 (Fig. 3). None of 68 negative control Study specimens from 48 subjects with rashes considered by the Study physician not to be caused by VZV or HSV and submitted as specimens from suspected cases of HZ were virus DNA-positive by PCR assay.
Fig. 3.
Average Ct values for virus positive clinical specimens. The VZV-WT and HSV positive Shingles Prevention Study specimens were grouped according to mean Ct value. Low virus positive specimens are defined as those with Ct values between the cutoff of 36.35 and 32. There were only 13 specimens that tested low positive for VZV-WT DNA and 1 specimen that tested low positive for HSV DNA.
Among the VZV-WT DNA-positive Study specimens, the average Ct difference between VZV-WT and VZV-Oka primer-probe sets was 12 cycles (range: 5.27–24.47 cycles) with 82.8% of all samples showing a Ct difference ≥10 cycles (see Fig. 1A for the VZV-WT and VZV-Oka amplification plots from a typical clinical specimen).
PCR Assay Results for Non-Study Specimens From Rashes Caused by VZV-Oka
In clinical specimens from rashes caused by VZV-Oka obtained from non-Study patients, the average Ct value difference between VZV-Oka and VZV-WT primer-probe sets was 5.60 (range: 3.41–7.50; Table IV). Two of these specimens (18%) were low virus-positive for VZV-Oka DNA, and three (27%) had Ct values below 20 cycles. The remaining six specimens (55%) had Ct values between 20 and 31.99 cycles (Table IV). The single clinical specimen from a child with a rash due to VZV-Oka processed and assayed in the San Diego PCR Laboratory showed a Ct value difference between VZV-Oka and VZV-WT primer-probe sets of 5.41 with a Ct value of 18.06 with the VZV-Oka primer probe set, corresponding to >1.5 million genomes of VZV-Oka DNA per 5 µl (Table IV).
TABLE IV.
PCR Assay Results for Non-Study Clinical Cases Positive for VZV-Oka DNA: Ct Values for Duplicate Samples of Extracted DNA Assayed Using VZV-Oka and VZV-WT Primer-Probe Sets
| Sample ID | VZV Oka | VZV Wild-Type | Ct difference: WT-Oka |
||||
|---|---|---|---|---|---|---|---|
| Ct no. 1 | Ct no. 2 | Ct mean | Ct no. 1 | Ct no. 2 | Ct mean | ||
| Oka GS5005a (NY) | 16.77 | 16.72 | 16.75 | 21.35 | 21.51 | 21.43 | 4.68 |
| Oka GS5008a (NY) | 22.06 | 22.02 | 22.04 | 27.73 | 28.04 | 27.89 | 5.85 |
| Oka GS5010a (NY) | 18.99 | 18.79 | 18.89 | 22.13 | 22.47 | 22.30 | 3.41 |
| Oka GS5013a (NY) | 31.96 | 31.89 | 31.93 | 35.75 | 35.72 | 35.74 | 3.81 |
| Oka#1b (St.L.) | 19.51 | 19.85 | 19.68 | 24.62 | 24.43 | 24.53 | 4.85 |
| Oka#3b (St.L.) | 28.88 | 25.75 | 27.32 | 34.76 | 34.78 | 34.77 | 7.45 |
| Oka#4b (St.L.) | 35.81 | 30.67 | 33.24 | 40.00 | 39.02 | 39.51 | 6.27 |
| Oka#5b (St.L.) | 20.48 | 20.19 | 20.34 | 26.44 | 29.24 | 27.84 | 7.50 |
| Oka#7b (St.L.) | 34.76 | 34.34 | 34.55 | 40.00 | 40.00 | 40.00 | 5.45 |
| Oka#8b (St.L.) | 21.22 | 21.10 | 21.16 | 27.70 | 27.70 | 27.70 | 6.54 |
| Oka#9b (St.L.) | 25.20 | 24.52 | 24.86 | 28.87 | 32.71 | 30.79 | 5.93 |
| 400120c (SD) | 18.02 | 18.10 | 18.06 | 23.41 | 23.53 | 23.47 | 5.41 |
Aliquots of extracted DNA from specimen lysates from rash lesions caused by VZV-Oka in susceptible recipients of Oka/Merck varicella vaccine.
Aliquots of DNA extracted from rash lesions caused by VZV-Oka.
Extracted DNA from a clinical specimen from a child with a rash caused by live attenuated Oka/Merck varicella vaccine, processed in the San Diego Central PCR Laboratory.
Concordance of PCR and Virus Culture Results
Although local virus culture was not required by the Study protocol, some Study sites obtained viral cultures from subjects with suspected HZ when possible. A total of 470 of the 918 suspected cases of HZ that were PCR-positive for VZV-WT DNA were also cultured for VZV and HSV. Of the 470 VZV-WT DNA positive cases, 250 (53.2%) were VZV culture-positive. In one case (0.2%) the culture result was reported to be HSV. Viral culture results for the remaining 219 (46.6%) of the VZV-WT DNA positive cases were virus culture-negative. No case evaluated by both PCR and virus culture was culture-positive and PCR negative.
Virus culture results were available for 26 of the 46 cases with PCR specimens that contained HSV DNA. Twenty (76.9%) of these 26 cases were HSV-positive by virus culture and 6 (23.1%) were HSV culture-negative.
Concordance of the Clinical Diagnoses of the Clinical Evaluation Committee With the PCR Assay Results
The Study’s Clinical Evaluation Committee (CEC), which consisted of five physicians (LDG, MJL, MNO, KES, SES) with expertise in HZ, adjudicated every suspected case of HZ without knowledge of treatment or laboratory results. Each CEC member provided an independent individual clinical diagnosis for each suspected case of HZ, and all non-unanimous cases were discussed and voted upon, with three or more votes required to establish a clinical diagnosis of HZ; otherwise the case of suspected HZ was classified as “not a case of HZ” [Oxman et al., 2005a]. Under the Study’s rules of operation, “indeterminate” cases were scored as “not a case of HZ” for the zoster vaccine efficacy analysis. To avoid potential bias in favor of zoster vaccine that might be introduced if mild or atypical vaccine-modified cases of HZ were missed, the index of suspicion was high and the threshold for diagnosing HZ was intentionally low, both at the 22 Study sites and during CEC adjudication. HSV was not a diagnostic choice, and the CEC members were instructed to score as HZ cases suspected of being HSV in which there was no history of prior recurrences of a similar rash in the same dermatome. According to the hierarchical algorithm used in the Study, PCR results, where available, had priority over the CEC’s clinical diagnosis in determining “confirmed cases of HZ” for the zoster vaccine efficacy analysis [Oxman et al., 2005a]. Consequently, the diagnosis in more than 93% of the confirmed cases of HZ was determined by the PCR assay [Oxman et al., 2005a]. Comparison of the CEC’s clinical diagnoses with the PCR assay results (Table V) shows that there was a high degree of agreement. Without incorporating “indeterminate” cases and without including the cases that were HSV DNA positive by PCR, the CEC’s sensitivity in diagnosing HZ [true positives (N = 844)/(true positives + false negatives (N = 870))] was 97.0%, and the CEC’s specificity [true negatives (N = 105)/(true negatives + false positives (N = 158))] was 66.5%. With the PCR assay as the gold standard, the CEC’s overall accuracy in making the correct diagnosis [(true positives (N = 844) + true negatives (N = 105))/all cases (N = 1,028)] was 92.3%.
TABLE V.
Comparison of the Clinical Diagnoses of the Clinical Evaluation Committee (CEC) With the PCR Assay Results
| PCR result | CEC diagnosis | |
|---|---|---|
| VZV DNA positive (916) | Herpes zoster | 844 (92.1%) |
| Indeterminate | 46 (5.0%) | |
| Not Herpes zoster | 26 (2.8%) | |
| Negative for VZV (and HSV) DNA (192) | Herpes zoster | 53 (27.6%) |
| Indeterminate | 34 (17.7%) | |
| Not Herpes zoster | 105 (54.7%) | |
| HSV Positive (46)a | Herpes zoster | 14 (30.4%) |
| Indeterminate | 11 (23.9%) | |
| Not Herpes zoster | 21 (45.7%) | |
HSV was not a diagnostic option for the CEC. Furthermore, to ensure that mild, vaccine-modified or atypical cases of HZ were evaluated and assessed for pain and discomfort, and for interference with activities of daily living and quality of life, the CEC intentionally had a low threshold for diagnosing HZ. CEC members were also instructed to score as HZ cases suspected of being HSV, but lacking a history of prior recurrences in the same dermatome.
DISCUSSION
Traditionally, VZV has been diagnosed in the laboratory by shell vial culture and direct fluorescent antibody staining of the infected cells. During the last decade, however, diagnostic procedures involving PCR have increasingly replaced these conventional diagnostic techniques for the laboratory diagnosis of viral infections [reviewed by Espy et al., 2006]. The advantage of real-time PCR techniques is their high sensitivity and specificity.
The use of real-time PCR assays for the identification and discrimination of VZV-WT and VZV-Oka avoids the post-amplification manipulations of restriction fragment polymorphism analysis [LaRussa et al., 1992; Loparev et al., 2000a; Takayama and Takayama, 2004] and results in higher detection rates while minimizing cross-contamination with amplified DNA [Loparev et al., 2000b; Tipples et al., 2003; Campsall et al., 2004]. A recently described real-time PCR assay for the qualitative detection and differentiation of VZV-WT and Oka vaccine strains uses the Invader-Plus system [Allawi et al., 2006; Tang et al., 2007], which accurately and specifically identifies VZV strains with single-base changes using a multiplex reaction. The two detection probes for VZV-WT and VZV-Oka, respectively, are configured to report to fluorescent resonance energy transfer cassettes with different 5′ fluorescent dyes [Tang et al., 2007].
The real-time PCR assay developed and validated for the Shingles Prevention Study is highly sensitive and specific for the detection and discrimination of VZV-WT, VZV-Oka, and HSV in clinical specimens. The assay employs two sets of primers to discriminate between VZV-WT and the Oka vaccine strain on the basis of the single nucleotide polymorphism at position 106262 in ORF 62, which is known to distinguish the Oka vaccine strain from all known wild-type strains of VZV, including Japanese isolates and the Oka parent strain [Quinlivan et al., 2005; Loparev et al., 2007]. A single fluorescent probe that hybridizes with a conserved region within the amplicon is used to detect and quantify amplification of both VZV-WT and VZV-Oka DNA by homologous and heterologous primer pairs. The HSV primers and probe identify a conserved region of the DNA polymerase gene, thus detecting both HSV-1 and HSV-2 DNA. The assay reliably detects as few as 7 copies of the VZV-WT or the VZV-Oka genome, and as few as 31 copies of the HSV genome, and has high specificity. The amplification reaction for each viral DNA target is multiplexed with primers and probe that detect human β-globin DNA with a LOD of 93 DNA copies.
When VZV-WT and VZV-Oka DNA were mixed in varying ratios, a difference in Ct values of ≥5 cycles between amplification by the VZV-WT and the VZV-Oka primer-probe sets indicated that the specimen contained either wild-type or vaccine strain DNA with a high degree of confidence. Since the typical difference between amplification by the VZV-WT and VZV-Oka primer-probe sets was at least 10 cycles for assays of Study specimens, it is highly likely that all of these specimens contained solely VZV-WT DNA.
No Study specimen was found to contain VZV-Oka DNA. This was expected in the two-thirds of the cases of HZ that occurred in placebo recipients who had never been exposed to VZV-Oka. Furthermore, establishment of neuronal latency by the superinfecting VZV-Oka vaccine strain with subsequent reactivation to cause HZ was unlikely in the immunocompetent vaccine recipients who were already VZV seropositive and latently infected with VZV-WT as a result of prior varicella. The absence of VZV-Oka in samples from cases of HZ in zoster vaccine recipients indicates either that VZV-Oka rarely, if ever, establishes latency in sensory ganglia already latently infected with VZV-WT, or that if VZV-Oka does establish latent neuronal infections in VZV seropositive vaccine recipients, it rarely, if ever, reactivates to cause HZ. Moreover, if VZV-Oka did establish latent infections, it would be expected to do so in ganglia innervating the injection site and, upon reactivation, cause HZ in the corresponding dermatome. The rarity of cases of HZ involving the deltoid region in Study subjects (<1%; unpublished Shingles Prevention Study data), the absence of a greater proportion of such cases in the vaccine recipients than in the placebo recipients [Oxman et al., 2005b], and the failure to identify VZV-Oka in any case of HZ in the Study [this report; Oxman et al., 2005a] provide clinical evidence against the occurrence of HZ caused by VZV-Oka in Study vaccine recipients.
For the 11 specimens of VZV DNA from non-Study patients with rashes caused by VZV-Oka, the average Ct value difference between the homologous and heterologous VZV PCR primer-probe set was 5.61 (ranging from 3.41 to 7.50 cycles). While several of these specimens showed Ct differences in amplification by the two primer-probe sets below 5 cycles, amplification of the VZV-Oka DNA with the homologous primers and probe was still significantly higher than amplification with the heterologous primers and probe. Since these specimens were from vaccine-associated rashes in VZV-naive recipients of varicella vaccine, mixtures of VZV-WT and VZV-Oka would be unlikely. However, because of the cross-reactivity between VZV-WT and VZV-Oka in this assay, it is theoretically possible that a very small proportion of the DNA in a clinical specimen may be from the heterologous virus. Thus, in some situations, both laboratory and clinical (e.g., epidemiologic history) data may be required to achieve an accurate diagnosis. The single clinical specimen from a child with a rash caused by VZV-Oka that was processed and assayed in the San Diego PCR Laboratory using the same protocol and reagents used in the Study showed a Ct value difference between the homologous and heterologous VZV PCR primer-probe set of 5.41 cycles.
The 53.2% VZV isolation rate from VZV-WT DNA-positive clinical specimens and the 76.9% HSV isolation rate from HSV DNA-positive clinical specimens provide additional evidence that real-time PCR assays have substantially greater sensitivity than virus isolation for the diagnosis of VZV, as well as HSV infections [Sauerbrei et al., 1999; Espy et al., 2000].
The correlation between the CEC’s clinical diagnoses of HZ and the PCR assay results was high, with the CEC’s accuracy in diagnosing HZ being 92.3%. The CEC’s sensitivity in diagnosing HZ was 97%, but the specificity of the CEC’s clinical diagnosis of HZ was relatively low at 66.5%. Both the high sensitivity and the relatively low specificity of the CEC’s clinical diagnosis of HZ most likely resulted from their attempt to include mild and atypical cases that might have been vaccine-modified HZ.
Despite the intentionally low threshold for the clinical diagnosis of HZ, only 53 (5.8%) of the 911 suspected cases of HZ that were diagnosed as clinical cases of HZ by the CEC were PCR assay-negative for VZV or HSV DNA. Three of these showed some amplification, with Ct values between the cutoff value and 40, and fluorescent amplification curves that were consistent with amplification rather than background noise. This suggests that these three specimens may have contained VZV DNA in quantities below the 7 copy assay cutoff. Thus the sensitivity of the PCR assay using the 7 copy cut-off may not have been 100%. Nevertheless, the high sensitivity of the PCR assay, (LOD equivalent to approximately 1/2,000 of a VZV infected cell), makes it probable that the majority of these clinically diagnosed cases of HZ were false positives, a supposition reinforced by the observation that local virus cultures, performed in 25 (47%) of the 53 CEC-positive/PCR-negative cases were all negative, as were local PCR assays performed in 5 (9%) of the cases. Furthermore, the clinical features of the 53 CEC-positive/PCR-negative cases differed substantially from those of the average confirmed case of HZ in the Study: none developed PHN, fewer had prodromal pain, their average HZ Severity of Illness Score was lower, and the proportion that were vaccine recipients was 58%, compared with 33% among the confirmed cases of HZ. Nevertheless, the non-invasive method used to obtain clinical specimens from non-vesicular lesions might have sampled non-viable cornified epithelial cells on the surface of the stratum corneum without sampling viable cells in the underlying stratum spinosum and stratum granulosum that might have contained replicating VZV.
In summary, the PCR assay employed in the Shingles Prevention Study was sensitive and specific for the detection of VZV and HSV, and discriminated between wild-type and Oka vaccine strains of VZV. It provided a practical means for unequivocally establishing the diagnosis of HZ in the pivotal clinical trial that established the efficacy of live attenuated Oka/Merck zoster vaccine.
ACKNOWLEDGMENTS
The authors thank George Miller (Yale University) for kindly providing EBV 95-8 and EBV HH154-16; Mark Pallansch (CDC) for kindly providing prototype Cox-sackieviruses A7, A9, A16, B2, B5, Echoviruses 9 and 11, and Enterovirus 71; Philip Pellett (CDC) for kindly providing lymphocytes infected with HHV-6A, HHV-6B, HHV-7 and HHV-8, Richard Buller (St. Louis Children’s Hospital) for kindly providing DNA aliquots of clinical specimens from patients with rashes caused by VZV-Oka; Sharon Steinberg (Columbia University, New York) for kindly providing lysates of clinical specimens from patients with rashes caused by VZV-Oka; Leon J. Wopschall (Clinical Virology Laboratory, VA San Diego Healthcare System) for assistance with the preparation and assay of cells infected with VZV-WT, VZV-Oka, HSV-1, HSV-2, CMV and prototype Enteroviruses; Janice Caping (Clinical Microbiology Laboratory, VA San Diego Healthcare System) for kindly providing log phase bacterial cultures of Staphylococcus aureus, S. epidermidis, S. xylosus, Escherichia coli, Diphtheroids, Group A Streptococcus, Pseudomonas aeruginosa, and cultures of Candida albicans; David Looney and Werner Witke at the UCSD Center for AIDS Research (NIH/DAIDS 2 P30 AI36214-14, Richman DD) for DNA sequencing of VZV-WT, VZV-Oka, and HSV amplicons; Yathi Naidu for preliminary PCR assay experiments; Harold Stanley for advice and support throughout the study; Frank Taddeo for advice and helpful discussions regarding PCR assay techniques; and Anne Kendall for assistance with data verification.
Grant sponsor: Cooperative Studies Program, Department of Veterans Affairs, Office of Research and Development; Grant sponsor: Merck (to the Cooperative Studies Program); Grant sponsor: James R. and Jesse V. Scott Fund for Shingles Research (to Dr. Oxman).
Footnotes
The Shingles Prevention Study was planned and/or administered by a Planning/Executive committee consisting of Michael N. Oxman (Chair), Robert D. Arbeit, Patricia Barry, Christopher E. Beisel, Kathy D. Boardman, Cindy L. Colling, Larry E. Davis, Lawrence D. Gelb, Anne A. Gershon, Anthony R. Hayward, Michael R. Irwin, Gary R. Johnson, Myron J. Levin, Peter N. Peduzzi, Kenneth E. Schmader, Michael S. Simberkoff, Stephen E. Straus (deceased), Adriana Weinberg, Heather M. Williams, Jeffrey L. Silber, Paula Annunziato, Christina Y. Chan, and Ivan S.F. Chan. Study Investigators include: L.E. Davis (Albuquerque, NM); C.A. Kauffman (Ann Arbor, MI); S.K. Keay (Baltimore, MD); A.R. Marques, N.E. Soto, and P. Brunell (Bethesda, MD), J.W. Gnann (Birmingham, AL); R. Serrao, D.J. Cotton, R.P. Goodman, and R.D. Arbeit (Boston, MA); C.T. Pachucki (Hines, IL); M.J. Levin (Denver, CO); K.E. Schmader (Durham, NC); W.A. Keitel (Houston, TX); R.N. Greenberg (Lexington, KY); V.A. Morrison (Minneapolis, MN); P.F. Wright and M.R. Griffin (Nashville, TN); M.S. Simberkoff (New York, NY); S.S. Yeh and Z. Lobo (Northport, NY); M. Holodniy and J. Loutit (Palo Alto, CA); R.F. Betts (Rochester, NY); L.D. Gelb (St. Louis, MO); G.E. Crawford (San Antonio, TX); J. Guatelli and P.A. Brooks (San Diego, CA); K.M. Neuzil (Seattle, WA); and J.F. Toney (Tampa, FL).
REFERENCES
- Adams SG, Dohner DE, Gelb LD. Restriction fragment differences between the genomes of the Oka varicella vaccine virus and American wild-type varicella-zoster virus. J Med Virol. 1989;29:38–45. doi: 10.1002/jmv.1890290108. [DOI] [PubMed] [Google Scholar]
- Allawi HT, Li H, Sander T, Aslanukov A, Lyamichev VI, Blackman A, Elagin S, Tang YW. Invader plus method detects herpes simplex virus in cerebrospinal fluid and simultaneously differentiates types 1 and 2. J Clin Microbiol. 2006;44:3443–3447. doi: 10.1128/JCM.01175-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw T, Cohen JI, Klutch M, Lekstrom K, Yoshikawa T, Asano Y, Krause PR. Nucleotide sequences that distinguish Oka vaccine from parental Oka and other varicella-zoster virus isolates. J Infect Dis. 2000;181:1153–1157. doi: 10.1086/315335. [DOI] [PubMed] [Google Scholar]
- Breuer J, Schmid DS. Vaccine Oka variants and sequence variability in vaccine-related skin lesions. J Infect Dis. 2008;197:S54–S57. doi: 10.1086/522140. [DOI] [PubMed] [Google Scholar]
- Campsall PA, Au NHC, Prendiville JS, Speert DP, Tan R, Thomas EE. Detection and genotyping of varicella-zoster virus byTaqMan allelic discrimination real-time PCR. J Clin Microbiol. 2004;42:1409–1413. doi: 10.1128/JCM.42.4.1409-1413.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy MJ, Ross TK, Teo R, Svien KA, Wold AD, Uhl JR, Smith TF. Evaluation of LightCycler PCR for implementation of laboratory diagnosis of herpes simplex virus infections. J Clin Microbiol. 2000;38:3116–3118. doi: 10.1128/jcm.38.8.3116-3118.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy MJ, Uhl JR, Sloan LM, Buckwalter SP, Jones MF, Vetter EA, Yao JDC, Wengenack NL, Rosenblatt JE, Cockerill FR, III, Smith TF. Real-time PCR in clinical microbiology: Applications for routine laboratory testing. Clin Microbiol Rev. 2006;19:165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl KA, Müller-Sander E, Rottenkolber M, Degitz K, Volkenandt M, Berking C. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. J Eur Acad Dermatol Venereol. 2008;22:722–728. doi: 10.1111/j.1468-3083.2008.02587.x. [DOI] [PubMed] [Google Scholar]
- Gomi Y, Imagawa T, Takahashi M, Yamanishi K. Oka varicella vaccine is distinguishable from its parental virus in DNA sequence of open reading frame 62 and its transactivation activity. J Med Virol. 2000;61:497–503. doi: 10.1002/1096-9071(200008)61:4<497::aid-jmv13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- LaRussa P, Lungu O, Hardy I, Gershon A, Steinberg SP, Silverstein S. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J Virol. 1992;66:1016–1020. doi: 10.1128/jvi.66.2.1016-1020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRussa P, Steinberg S, Arvin A, Dwyer D, Burgess M, Menegus M, Rekrut K, Yamanishi K, Gershon A. Polymerase chain reaction and restriction fragment length polymorphism analysis of varicella-zoster virus isolates from the United States and other parts of the world. J Infect Dis. 1998;178:S64–S66. doi: 10.1086/514267. [DOI] [PubMed] [Google Scholar]
- Loparev VN, Argaw T, Krause PR, Takayama M, Schmid DS. Improved identification and differentiation of varicella-zoster virus (VZV) wild-type strains and an attenuated varicella vaccine strain using a VZV open reading frame 62-based PCR. J Clin Microbiol. 2000a;38:3156–3160. doi: 10.1128/jcm.38.9.3156-3160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loparev VN, McCaustland K, Holloway BP, Krause PR, Takayama M, Schmid DS. Rapid genotyping of varicella-zoster virus vaccine and wild-type strains with fluorophore-labeled hybridization probes. J Clin Microbiol. 2000b;38:4315–4319. doi: 10.1128/jcm.38.12.4315-4319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loparev VN, Rubtcova E, Seward JF, Levin MJ, Schmid DS. DNA sequence variability in isolates recovered from patients with postvaccination rash or herpes zoster caused by Oka varicella vaccine. J Infect Dis. 2007;195:502–510. doi: 10.1086/510532. [DOI] [PubMed] [Google Scholar]
- Martin JH, Dohner DE, Wellinghoff WJ, Gelb LD. Restriction endonuclease analysis of varicella-zoster vaccine virus and wild-type DNAs. J Med Virol. 1982;9:69–76. doi: 10.1002/jmv.1890090110. [DOI] [PubMed] [Google Scholar]
- Niesters HGM. Clinical virology in real-time. J Clin Virol. 2002;25:S3–S12. doi: 10.1016/s1386-6532(02)00197-x. [DOI] [PubMed] [Google Scholar]
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan ISF, Wang WWB, Annunziato PW, Silber JL. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005a;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- Oxman MN, Williams HM, Levin MJ, Johnson GR, Zhang JH, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Annunziato PW, Chan ISF, Silber JL, Wang WWB Shingles Prevention Study Group. Efficacy of Zoster Vaccine According to Dermatome Region. Program and Abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19; Washington DC. Abstract G-839. [Google Scholar]
- Quinlivan ML, Gershon AA, Steinberg SP, Breuer J. Rashes occurring after immunization with a mixture of viruses in the Oka vaccine are derived from single clones of virus. J Infect Dis. 2004;190:793–796. doi: 10.1086/423210. [DOI] [PubMed] [Google Scholar]
- Quinlivan M, Gershon AA, Steinberg SP, Breuer J. An evaluation of single nucleotide polymorphisms used to differentiate vaccine and wild type strains of varicella-zoster virus. J Med Virol. 2005;75:174–180. doi: 10.1002/jmv.20253. [DOI] [PubMed] [Google Scholar]
- Sauerbrei A, Eichorn U, Schacke M, Wutzler P. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31–36. doi: 10.1016/s1386-6532(99)00042-6. [DOI] [PubMed] [Google Scholar]
- Takada M, Suzutani T, Yoshida I, Matoba M, Azuma M. Identification of varicella-zoster virus strains by PCR analysis of three repeat elements and a PstI-site-less region. J Clin Microbiol. 1995;33:658–660. doi: 10.1128/jcm.33.3.658-660.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama M, Takayama N. New method of differentiating wild-type varicella-zoster virus (VZV) strains from Oka varicella vaccine strain by VZV ORF 6-based PCR and restriction fragment length polymorphism analysis. J Clin Virol. 2004;29:113–119. doi: 10.1016/s1386-6532(03)00112-4. [DOI] [PubMed] [Google Scholar]
- Tang YW, Allawi HT, DeLeon-Carnes M, Li H, Day SP, Schmid DS. Detection and differentiation of wild-type and vaccine mutant varicella-zoster viruses using an Invader Plus method. J Clin Virol. 2007;40:129–134. doi: 10.1016/j.jcv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Tipples GA, Safronetz D, Gray M. A real-time PCR assay for the detection of varicella-zoster virus DNA and differentiation of vaccine, wild-type and control strains. J Virol Meth. 2003;113:113–116. doi: 10.1016/s0166-0934(03)00229-5. [DOI] [PubMed] [Google Scholar]