Abstract
Exosomes, a group of small vesicles (30–100 nm), originate when the inward budding of the endosomal membrane forms multivesicular bodies (MVBs). Exosomes are released into the extracellular space when the MVBs fuse with the plasma membrane. Numerous studies have indicated that exosomes play critical roles in mediating cell-to-cell communication. Also, exosomes are believed to possess a powerful capacity in regulating cell survival/death, inflammation and tumor metastasis, depending on the particular array of molecules contained within a particular population of exosomes. This mini-review will summarize dual roles of exosomes derived from different types of cells (i.e. endothelial cells, tumor cells, platelets, bone-marrow stem cells, cardiomyocytes, myocardial progenitor cells and among others) in endothelial cell proliferation, migration and tube-like formation. In particular, this review will focus on the therapeutic potential of exosomes as a natural nano-particle for delivering pro-/anti-angiogenic factors (proteins, mRNAs and microRNAs) into endothelial cells.
Keywords: Angiogenesis, cardiovascular disease, endothelial cells, exosomes, multivesicular bodies
INTRODUCTION
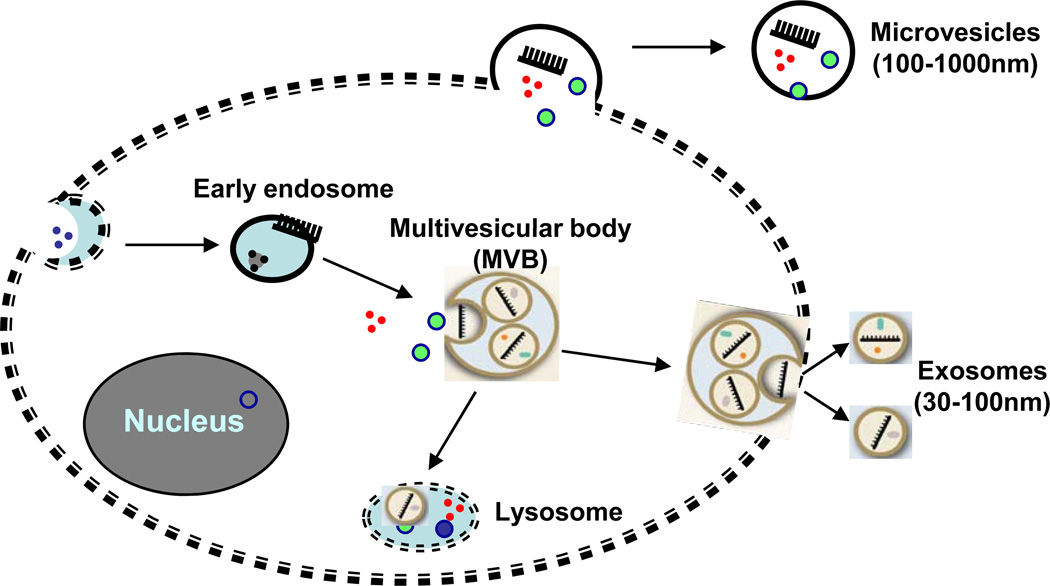
Angiogenesis is the process by which new capillaries arise from the preexisting vasculature. This process is controlled by multiple growth factors and signaling pathways, and depends upon the balance of pro-angiogenic and antiangiogenic factors. Recent studies have indicated that angiogenesis can also be modulated by cell-derived microparticles (microvesicles and exosomes) [1–3]. The biogenesis and properties of microvesicles are different from those of exosomes (Fig. 1). Microvesicles comprise a heterogeneous population of 100–1000nm particles. They are formed by a regulated reverse budding mechanism where the plasma membrane blebs outward through reorganization of the underlying cortical actin cytoskeleton [4, 5]. This results in the direct detachment of plasma membrane buds into the extracellular space (Fig. 1). In contrast, exosomes are small membrane vesicles with a lipid bilayer secreted by many, if not all, living cells [6–9]. These 30–100 nm exocytosed internal vesicles of endosomal origin have a cup-shaped morphology when imaged by electron microscopy analysis after differential centrifugation. Endosomes formation begins with the inward budding of the cell membrane. This initial step is followed by invagination of the limiting membrane of late endosomes to from multivesicular bodies (MVBs). Fusion of these multivesicular bodies with the plasma membrane results in the release of the internal vesicles, referred to as exosomes (Fig. 1).
Fig. (1).
Microvesicles are directly generated from outward budding of the plasma membrane, whereas exosomes are generated from inward budding of the cell membrane to form endosomes, followed by invagination of the limiting membrane of late endosomes to from multivesicular bodies. Fusion of the multivesicular bodies with the plasma membrane results in the release of the internal vesicles, then called exosomes.
The concept of exosomes initially appeared with the description of the shedding process of the transferrin receptor by maturing reticulocytes [10]. Recently, numerous studies have identified that exosomes can be released from various cell types including dendritic cells, B lymphocytes, tumor cell lines, platelets, cardiomyocytes, endothelial cells, stem cells and among others [11–18]. These nano-vesicles contain major histocompatibility complex class I and II molecules, cytosolic chaperone proteins, subunits of trimeric G proteins, cytoskeletal proteins, annexins, integrins, enzymes, and elongation factors [19]. These exosomal proteins have known functions in cell fusion, adhesion and biosynthetic processes, but most have yet to be assigned specific roles in exosome formation and function. Accumulating evidence has recently revealed that exosome content exchange may represent a novel pathway of intercellular communication by delivery of functional RNAs/microRNAs and proteins [5–9]. For example, exosomes secreted by platelets contain a tissue factor involved in coagulation events [16]. Dendritic cell-derived exosomes containing major histocompatibility complex molecules able to activate T cells [20]. The influence of exosomes on cell membrane potential and on developmental tissue patterning has been suggested to be related to their transport of morphogens and RNAs [21]. Circulating exosomes obtained from plasma of glioma patients were confirmed as positive for the mutant/variant mRNA of epidermal growth factor receptor (EGFRvIII), which defines a clinical subtype of glioma [22]. More interestingly, these exosomes display pro-angiogenic properties, indicating that glioma-derived exosomes play a role in initiating angiogenesis [22].
As noted above, in this review, we will focus on exosomes that modify the pro- or anti-angiogenic program of endothelial cells through the release of pro- or anti-angiogenic factors. We will also explore the possibility that exosomes might be useful therapeutic tools for regulating angiogenesis.
ENDOTHELIAL CELL-DERIVED EXOSOMES MIGHT HAVE AUTOCRINE/PARACRINE POTENTIALS
Endothelial cells can release different types of membrane vesicles, including microvesicles, exosomes and apoptotic bodies, in response to cellular activation or apoptosis [1–3]. These different vesicles are distinguished from one another on the basis of subcellular origin, size, content, and the mechanism(s) leading to their formation [1–3]. Most importantly, they have both salutary and deleterious effects on coagulation, inflammation, endothelial function, and angiogenesis, depending on their composition (see reviews elsewhere) [1–3]. Importantly however, a significant number of earlier studies need to be revisited, as they attributed biological effects rather non-specifically to either exosomes, microvesicles, or apoptotic bodies, without validating the purity of the membrane vesicle preparation used.
The paradoxical functions of endothelial cell-derived exosomes in maintaining vascular homeostasis is perhaps not that surprising when one considers that different patho/physiological conditions will cause endothelial cells to produce exosomes with distinctive composition (proteins and RNAs). A recent study by Sheldon et al. [23] has shown that endothelial exosomes might be involved in vascular development as they incorporate and transfer Delta-like ligand 4 (Dll4; Delta 4) protein to neighboring endothelial cells, leading to an inhibition of Notch signaling and an increased capillary-like structure formation in vitro and in vivo. This suggests that the Delta like ligand/Notch pathway does not require direct cell–cell contact but rather that exosomes to expand the range of cell signaling potential for angiogenesis regulation. Other studies have also shown that endothelial-derived exosomes contain proteins which can be implicated in their pro-angiogenic potential. For example, Taraboletti et al. [24] have reported that matrix metalloproteinases harbored by exosomes from endothelial cells are functionally active and lead to endothelial cell invasion and capillary- like formation.
Taken together this information argues that endothelial cell-derived exosomes containing proteins and RNAs/microRNAs which may function as paracrine or autocrine factors have the potential to facilitate angiogenesis and metastasis. A recent study by Halkein et al. [25] reported that the 16-kDa N-terminal prolactin fragment (16K-PRL) stimulated EC to release miR-146a-loaded exosomes, which were absorbed by cardiomyocytes, leading to a subsequent decrease in metabolic activity and decreased expression of Erbb4, Notch1, and Irak1. However, it remains unclear whether such miR-146a-loaded exosomes enter to neighbor ECs and attenuate angiogenesis. Future studies will be needed to determine whether miRNAs or other factors packaged in endothelial exosomes can initiate, inhibit or modulate angiogenesis.
TUMOR CELL-DERIVED EXOSOMES PROMOTE ANGIOGENESIS
The microcirculation is essential for tissue homeostasis by balancing supply of oxygen and nutrients and removing products of cellular metabolism in a manner that supports tissue homeostasis. In a similar way, cancer (solid tumor) progression can only occur when angiogenesis occurs simultaneously. These new blood vessels supply nutrients, oxygen, and growth factors to facilitate the growth of the tumor and promote formation of metastases [26, 27]. Therefore, in development of a tumor, the local release of angiogenic factors by tumor cells is required to activate an otherwise quiescent microvasculature.
Earlier studies identified tumor antigens and MHC class I molecules loaded with tumor peptides in exosomes derived from tumor cells [7]. Recently, the recognition that exosomes modulate the cancer angiogenic process has been expanding [28, 29]. For example, it has been shown that exosomes from LAMA84 chronic myeloid leukemia (CML) cells affect vascular remodeling in vitro through an IL-8 mediated activation of VCAM-1 [29]. While the mechanisms of interaction of CML exosomes with endothelial cells have not been elucidated, exosomes are known to interact with target cells in three specific ways: binding to cell surface receptors, fusion with the plasma membrane, or internalization [8]. The ability of exosomes to interact with and stimulate endothelial cells suggests exosomes as a new target for CML therapy. For example, a recent study reported that cells treated with non-toxic concentrations of both imatinib and dasatinib, two chemotherapy drugs for CML, effectively reduced exosome release by more than 55% [29]. This study identifies exosome release and uptake as a potential new therapeutic target for the treatment of CML. Furthermore, Umezu et al. [30] recently found that miRNA-enclosed exosomes have a critical role in mediating leukemia cell-to-endothelial cell communication. They observed that exosomes, collected from miR-92a-overexpressing leukemia cells (K562 cells), did enter into endothelial cells, resulting in an enhanced migration and tube formation, albeit did not affect EC proliferation. Together, these studies support the idea that exosomes play an important role in neoplasia-to-endothelial cell communication.
In addition, glioblastoma cell-derived exosomes have been shown to interact with endothelial cells and thereby stimulate endothelial cell proliferation [31, 32]. For instance, Kucharzewska et al. (2013) [32] observed that exosomes derived from glioblastoma multiforme (GBM) cells grown at hypoxic compared with normoxic conditions significantly stimulated angiogenesis ex vivo and in vitro. Interestingly, those GBM cell-derived hypoxic exosomes induced endothelial cells to secrete several potent growth factors/cytokines and to stimulate pericyte PI3K/AKT signaling activation and migration. This study provides evidence that exosomes can be potentially targetable driver of hypoxia-dependent intercellular signaling during tumor development. Moreover, King et al. [33] reported that hypoxia promoted the release of exosomes from breast cancer cells, and the hypoxically regulated miR-210 was presented at elevated levels in hypoxic exosomes. Other investigators have also reported that exosomes released by a pancreatic cell line transfected with D6.1A tetraspanin stimulate endothelial tubulogenesis [34]; and heparanase has been shown to not only drive exosome secretion from tumor cells, but also impact exosome protein cargo as reflected by higher levels of syndecan-1, VEGF, and hepatocyte growth factor in exosomes secreted by heparanase-overexpressing cells than those of heparanase-reduced cells [35]. Squamous carcinoma and colorectal cancer cells can secret exosomes enriched in proteins and cell cycle-related mRNAs that can facilitate angiogenesis and metastasis [36, 37]. Taken together, these observations suggest that tumor cell-derived exosomes may serve a critical role in promoting angiogenesis and thereby enabling tumor growth and metastatic proliferation.
EXOSOMES GENERATED FROM PLATELETS EXERT DUAL EFFECTS ON ENDOTHELIAL CELL APOPTOSIS AND PROLIFERATION
Experimental and clinical data suggest that activated platelets play a crucial role in the pathophysiology of tissue injury and organ dysfunction. For example, in the early stages of sepsis, platelets are strongly activated and hyper-adhesive to the vascular wall which consequently promotes leukocyte accumulation, migration, and activation [38]. Recent studies provide support for the notion that, in sepsis, both increased generation of NO and the presence of LPS can trigger the release of platelet-derived exosomes, whereas thrombin or TNF-α induces the generation of phosphatidylserine-rich microparticles [39]. Furthermore, Janiszewski et al. [40] reported that platelet-derived exosomes from septic patients contain the p22phox and gp91phox subunits of the NADPH oxidase. They found that incubation of these sepsis-related exosomes with endothelial cells induces caspase-3 activation and apoptosis of target endothelial cells through active ROS/RNS generation by NADPH oxidase and NO synthase type II. In addition, platelet exposure to LPS or NO in vitro may be a valuable model for the generation of exosomes involved in redox signaling [39]. Collectively, these studies provide evidence that exosomes and their contents might be the source of platelet-induced septic vascular dysfunction. Future studies will be needed to explore whether exosomes could be a novel target or tool for the treatment of vascular dysfunction related to diabetes, hypertension, or sepsis.
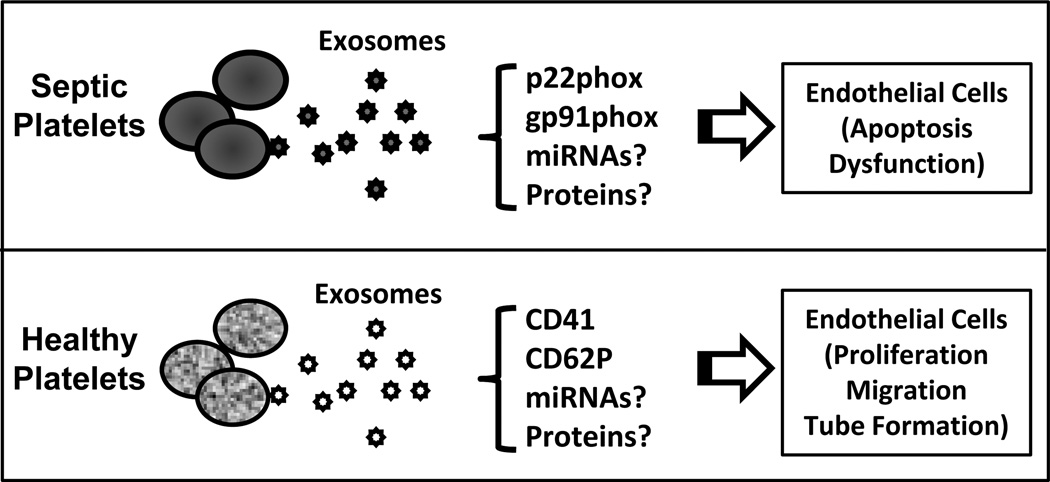
Whereas platelet-derived exosomes from septic patients displayed pro-apoptotic property for endothelial cells, Janowska-Wieczorek et al. [41] have shown that exosomes released from healthy human platelet α-granules could contribute to tumor metastasis and angiogenesis (Note: in this paper, authors referred to exosomes as smaller microvesicles). They observed that these exosomes transferred the platelet-derived integrin CD41 to most of the lung cancer cell lines tested and stimulated the phosphorylation of mitogen-activated protein kinase p42/44 and serine/threonine kinase as well as the expression of membrane type 1-matrix metalloproteinase (MT1-MMP). Importantly, these exosomes stimulated mRNA expression for angiogenic factors such as MMP-9, vascular endothelial growth factor, interleukin-8 and hepatocyte growth factor, consequently, induce angiogenesis in lung cancer, suggesting an implication in metastasis. The dual effects of platelet-derived exosomes in endothelial cell apoptosis and proliferation might be attributed to the different method of platelet activation to obtain exosomes and consequently their different contents (Fig. 2).
Fig. (2).
Exosomes generated from platelets present dual effect on endothelial cell survival and angiogenesis. Platelet-derived exosomes from human septic patients contains p22phox and gp91phox which induce endothelial cell apoptosis and dysfunction (anti-angiogenesis); whereas exosomes isolated from healthy human platelets carry with CD41 and CD62P which promote endothelial cell proliferation, migration and tube formation (pro-angiogenesis).
BONE MARROW STEM CELL-DERIVED EXOSOMES STIMULATE ANGIOGENESIS
The use of bone marrow-derived stem cells such as hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) to repair cardiac tissues was predicated on the hypothesis that these cells could differentiate into cardiomyocytes and other supporting cell types. However, careful rodent experimentation has demonstrated that few of the transplanted bone marrow cells engraft and survive, and fewer cells differentiate into cardiomyocytes or supporting cells [42–44]. In spite of this, transplantation of bone marrow stem cells improves some cardiac functions in animal models and patients, and this has been largely attributed to paracrine factors (i.e. VEGF, FGF, HGF and IGF) secreted from implanted stem cells [37–39].
Interestingly, several recent studies indicated that MSC-conditioned culture medium contains a significant amount of exosomes [18, 45–48]. Moreover, these nano-vesicles can mediate protection against ischemia/reperfusion-induced kidney [46] and cardiac injury [18]. One might speculate that the major mechanism underlying exosome-mediated protective effects in these model systems might be associated with their pro-angiogenic ability. For example, Sahoo et al. [48] demonstrated that exosomes collected from the conditioned media of mobilized human CD34+ cells had the characteristic size (40 to 90 nm; determined by dynamic light scattering) and cup-shaped morphology (electron microscopy). In addition, these exosomes expressed exosome-marker proteins such as CD63, phosphatidylserine (flow cytometry) and TSG101 (immunoblotting), as well as CD34+. In vitro, CD34+- exosomes replicated the angiogenic activity of CD34+ cells by increasing endothelial cell viability, proliferation, and tube formation on Matrigel. In vivo, the CD34+-exosomes stimulated angiogenesis in Matrigel plug and corneal assays. Notably, exosomes from human CD34+ stem cells but not those obtained from CD34+-depleted stem cells had angiogenic activity. Notably, the exosome-depleted conditioned media which should have contained both the supplemental growth factors and any secreted soluble proteins did not have angiogenic effects. In aggregate, the results of this study support the idea that CD34+-exosomes are the key paracrine vector for CD34+ cell-induced vessel growth.
While stem cell-derived exosomes have been shown to promote angiogenesis, the underlying mechanisms are not completely understood. It is plausible that exosomes can stimulate both receptor-mediated and genetic signaling pathways by transferring exosomal proteins, RNAs or microRNAs into the cytoplasm of endothelial cells. Indeed, Sahoo et al. [48] determined that CD34+-exosomes are enriched with pro-angiogenic microRNAs (i.e. miR-126 and miR-130a). Nonetheless, the repertoire of specific molecules transported by stem cell-derived exosomes remains to be fully characterized.
CARDIOMYOCYTE- AND MYOCARDIAL PROGENITOR CELL-DERIVED EXOSOMES PROMOTE ANGIOGENESIS
Myocardial progenitor cells (CMPCs) have been shown to be a very promising cell source for the treatment of the diseased myocardium, however, the engraftment of progenitor cells and the number of newly generated cardiomyocytes and vascular cells are in many cases too low to explain the improved cardiac function and morphology [49, 50]. Vrijsen et al. [51] recently reported that CMPCs release exosomes into their environment, and that exosomes from CMPCs are able to stimulate migration of endothelial cells in an in vitro scratch wound assay. They also showed that CMPC-exosomes contain matrix metalloproteinases (MMPs) and extracellular matrix metalloproteinase inducer (EMMPRIN). Thus, CMPC-derived exosomes themselves are able to breakdown the extracellular matrix or activate MMPs. This study provides evidence that exosomes, released by CMPCs upon transplantation, might be involved in the activation of endothelial cells and thereby result in increased capillary density. However, it remains unclear at present whether CMPC-derived exosomes can affect proliferation, survival and differentiation of cardiomyocytes and myofibroblasts. In addition, future research is required to determine whether CMPC- and other progenitor cell-derived exosomes have the potential in reprogramming adult cells into progenitor cells.
Regarding cardiomyocytes, we recently observed that cardiomyocyte-derived exosomes contains a large amount of Hsp20 [17]. As a result, the exosomal Hsp20 remarkably promotes HUVEC promotion, migration and tube-like formation. Mechanistically, the exosomal Hsp20 physically interacts with VEGFR2 and activates its downstream Akt/ERK signaling cascade. Our study suggests that cardiomyocytes may have pro-angiogenic property through releasing Hsp20-incorporated exosomes.
MOST IMPORTANT QUESTIONS AND PROBLEMS FOR THE THERAPEUTIC APPLICATION OF EXOSOMES
It is now well established that exosomes can carry and transfer proteins and mRNAs/microRNAs to and into target cells and these proteins can consequently modify target cell phenotype. With this in mind it would seem reasonable to speculate that exosomes may represent a new way to deliver either pro- or anti-angiogenic signaling molecules into endothelial cells and thereby, enhance or impair angiogenesis, respectively. As a result, we could overexpress proangiogenic exosomal proteins (e.g., Dll4) or microRNAs (e.g., miR-126 and miR-130a, see reviews elsewhere [52]) in bone marrow-derived stem cells, and follow this by collecting culture supernatants for isolation of pro-angiogenic exosomes. Similarly, we might prepare anti-angiogenic exosomes by uploading anti-angiogenic factors (e.g., miR-320 [53]). These exosomes would be expected to interact with endothelial cells through endocytosis, phago-cytosis and membrane fusion [54]. Whether exosomes internalized by the endothelium is related to caveolae remains unknown. After internalization in endothelial cells, exosomes are preferentially fused to endosomes and lysosomes, thereby releasing pro- or anti-angiogenic contents.
Exosomes, as in vivo delivery tools, are argued to have many potential advantages over the typical virus vectors, lipid nanoparticles (liposomes) and polycationic agents [45, 55–60]. Firstly, exosomes are naturally occurring biological entities with low inherent toxicity and minimal immune response. Secondly, they are relatively stable in the circulation as they avoid opsonins and coagulation factors. Thirdly, their small size (30–100nm) allows them to avoid phagocytosis by the mononuclear cell system (which prefers particles >100nm in size). Fourthly, their membrane structure allows them to easily pass their content across the cell membrane into the cytosol of recipient cells. Finally, exosomes are more convenient to manipulate than intact cells, since they are not “alive”, but rather are metabolically inactive nanovesicles. This makes exosomes extremely durable, allowing them to be stored at −80°C for over two years without detectable loss of their biological activities. Nonetheless, there are still many concerns that must be addressed before their use in targeted clinical trials. For example: How efficiently and specifically can exosomes deliver proteins/RNAs into target tissues/cells? How can we regulate the loading of specific contents into exosomes? How can large amounts of clinical-grade exosomes be collected and/or generated? While the study of exosomes as a natural nano-delivery device for the treatment of human disease is just emerging, we believe that, with the advance of new bioengineering and cellular modification techniques, engineering or modification of the exosome surface antigen and internal content will enable their use to target angiogenesis-related diseases with even more specificity than is now achievable (see reviews elsewhere [55–60]).
In addition, future investigations should address the combined beneficial effects of microvesicles and exosomes, because of their complementarity in the regulation of angiogenesis. Another important aspect is related to the complex composition of microvesicles and exosomes. In this regard, proteomic analyses are needed to identify all components of these cell-derived vesicles to provide extensive evidence about their side effects. Overall, exosomes are complex cellular entities that display a large number of activities affecting cells involved in angiogenesis. Therefore, caution must be exercised in the utilization of exosomes as autologous therapeutic tools in diseases associated with altered angiogenesis.
ACKNOWLEDGEMENTS
The research in Dr. Guo-Chang Fan’s lab is supported by NIH grant 2R01-087861.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Martinez MC, Andriantsitohaina R. Microparticles in angiogenesis: therapeutic potential. Circ Res. 2011;109:110–119. doi: 10.1161/CIRCRESAHA.110.233049. [DOI] [PubMed] [Google Scholar]
- 2.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101:439–451. [PubMed] [Google Scholar]
- 3.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 4.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artifacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014–2018. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- 6.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Wieckowski E, Whiteside TL. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res. 2006;36:247–254. doi: 10.1385/IR:36:1:247. [DOI] [PubMed] [Google Scholar]
- 8.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 9.Zhu H, Fan GC. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis. 2011;1:138–149. [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn J, Johnstone RM. Origin of a soluble truncated transferrin receptor. Blood. 1993;81:2442–2451. [PubMed] [Google Scholar]
- 11.Théry C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 13.Ma B, Ren J, Jiang HF, Jia J. Antitumor activities against hepato-cellular carcinoma induced by bone marrow mesenchymal stem cells pulsed with tumor-derived exosomes. Beijing Da Xue Xue Bao. 2008;40:494–499. [PubMed] [Google Scholar]
- 14.Li QL, Bu N, Yu YC, Hua W, Xin XY. Ex vivo experiments of human ovarian cancer ascites-derived exosomes presented by dendritic cells derived from umbilical cord blood for immunotherapy treatment. Clin Med Oncol. 2008;2:461–467. doi: 10.4137/cmo.s776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu S, Liu C, Su K, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 16.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 17.Zhang X, Wang X, Zhu H, et al. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS ONE. 2012;7:e32765. doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett. 2003;89:125–131. doi: 10.1016/s0165-2478(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 21.Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18:199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Nedawi K, Meehan B, Micallef J, et al. J.Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 23.Sheldon H, Heikamp E, Turley H, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 24.Taraboletti G, D'Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halkein J, Tabruyn SP, Ricke-Hoch M, et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123(5):2143–2154. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 27.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 28.Taverna S, Flugy A, Saieva L, et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012;130:2033–2043. doi: 10.1002/ijc.26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mineo M, Garfield SH, Taverna S, et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 2012;15:33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umezu T, Ohyashiki K, Kuroda M, et al. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2012;32(22):2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 31.Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kucharzewska P, Christianson HC, Welch JE, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gesierich S, Berezovskiy I, Ryschich E, Zöller M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006;66:7083–7094. doi: 10.1158/0008-5472.CAN-06-0391. [DOI] [PubMed] [Google Scholar]
- 35.Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase Regulates Secretion, Composition, and Function of Tumor Cell-derived Exosomes. J Biol Chem. 2013;288:10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JE, Tan HS, Datta A, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong BS, Cho JH, Kim H, et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 39.Gambim MH, do Carmo Ade O, Marti L, Veríssimo-Filho S, Lopes LR, Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septicvascular dysfunction. Crit Care. 2007;11:R107. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janiszewski M, Do Carmo AO, Pedro MA, Silva E, Knobel E, Laurindo FR. Platelet-derived exosomes of septic individuals possess proapoptotic NADPH oxidase activity: A novel vascular redox pathway. Crit Care Med. 2004;32:818–825. doi: 10.1097/01.ccm.0000114829.17746.19. [DOI] [PubMed] [Google Scholar]
- 41.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Zhao T, Huang W, et al. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27:3021–3031. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen Z, Zheng S, Zhou C, Wang J, Wang T. Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction. J Cell Mol Med. 2011;15:1032–1043. doi: 10.1111/j.1582-4934.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 46.van Koppen A, Joles JA, van Balkom BW, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One. 2012;7:e38746. doi: 10.1371/journal.pone.0038746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu W, Huang L, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315:28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Sahoo S, Klychko E, Thorne T, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang XL, Rokosh DG, Guo Y, Bolli R. Cardiac progenitor cells and bone marrow-derived very small embryonic-like stem cells for cardiac repair after myocardial infarction. Circ J. 2010;74:390–404. doi: 10.1253/circj.cj-09-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, Dauwe D, Patel A, Janssens S. Role of stem and progenitor cells in postmyocardial infarction patients. Min Cardioangiol. 2009;57:219–231. [PubMed] [Google Scholar]
- 51.Vrijsen KR, Sluijter JP, Schuchardt MW, et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med. 2010;14:1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu H, Fan GC. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc Res. 2012;94:284–292. doi: 10.1093/cvr/cvr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2009;36:181–188. doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 54.Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111:488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 55.Seow Y, Wood MJ. Biological gene delivery vehicles: beyond viral vectors. Mol Ther. 2009;17:767–777. doi: 10.1038/mt.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 57.Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 59.Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11–15. doi: 10.1016/j.biocel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Fleissner F, Goerzig Y, Haverich A, Thum T. Microvesicles as novel biomarkers and therapeutic targets in transplantation medicine. Am J Transplant. 2012;12:289–297. doi: 10.1111/j.1600-6143.2011.03790.x. [DOI] [PubMed] [Google Scholar]