Abstract
The last 2–3 years have seen numerous relationships develop between organometallic chemists, fluorine chemists and PET Centers around the world. These collaborations have led to the development of many new strategies for the late-stage introduction of fluorine-18 into complex bioactive molecules. In this perspective we highlight recent developments and key milestones since 2011.
Introduction
Positron emission tomography (PET) imaging is a non-invasive form of molecular imaging that allows quantitation of biochemical pathways and physiological processes following administration of a radiopharmaceutical (bioactive molecule tagged with a positron emitting radioisotope) to a patient.1 While the technique itself has been around for decades, the true potential of PET has only begun to be realized recently with approval of [18F]fludeoxyglucose ([18F]FDG) by the US Food and Drug Administration (FDA) and subsequent agreement to cover reimbursement by the Centers for Medicare and Medicaid Services (CMS) in the 1990s.2 A more formal regulatory environment for PET, both in approval process and Good Manufacturing Practices (cGMP) for PET drugs (e.g. 21CFR212),2c have led to the FDA approval of several additional PET radiopharmaceuticals ([11C]choline, [18F]AMYViD, [18F]Vizamyl, [18F]Neuraceq, [18F]sodium fluoride and [13N]ammonia).
In order for the potential impact of PET imaging on drug discovery and personalized medicine to be fully realized, there remains an urgent need to develop new radiopharmaceuticals for targets that cannot currently be imaged. When coupled with point-of-care PET scanners, these novel radiopharmaceuticals will provide clinicians with bedside access to the full picture of relevant disease markers on a patient-by-patient basis. The development of new radiopharmaceuticals requires extensive collaboration between physicians, chemists, radiochemists, physicists, biologists and others, to decide which molecules to radiolabel (e.g. small molecule, peptide, protein, antibody), and how best to incorporate the radionuclide. New radiopharmaceuticals continue to be prepared using radiochemistry developed in the four decades since [18F]fludeoxyglucose was first administered to a patient, and this work has been extensively reviewed.3 Despite these successes, radiochemistry has limitations such that new radiopharmaceuticals represent those scaffolds that can be readily radiolabeled regardless of whether or not they are best in class, or the most fit for purpose (based upon pharmacology, pharmacokinetics, etc.). A related and equally frustrating problem concerns other tantalizing radiopharmaceuticals, such as 6-[18F]fluoroDOPA, that have been prepared in quantities suitable for pre-clinical and even limited clinical evaluation (e.g. a few mCi) and shown enormous promise, but that have never matriculated to widespread clinical use because they cannot be synthesized in large enough quantities (e.g. >50 mCi). To prepare radiopharmaceuticals with potential for the largest impact in a clinical setting, the radiochemist should have the luxury of being able to radiolabel any scaffold. Therefore, in addition to development of new radiopharmaceuticals, there remains a concomitant need for a battery of radiochemical reactions that enable the ready incorporation of PET radionuclides into any bioactive molecules. Development of new PET radiochemical reactions present unique challenges however, and for such reactions to be useful they should meet each of the following requirements:
Reaction stoichiometry has to be compatible with the very small amounts of radionuclide (nano-mol);
Precursors for radiolabeling should be bench stable for months to years;
Synthetic transformations should ideally be possible under ambient conditions;
Radiochemical reaction times must be short (typically 3 – 30 min) due to the short half-lives of PET radionuclides (11C = 20 min, 18F = 110 min).
Radiochemical yields (RCY) of the radiopharmaceutical must be high enough that after completion of quality control testing (20 min – 1 hr) and transport of the dose(s) to the PET imaging center (minutes to hours) there is a dose remaining to administer to the patient(s);
Purification must be rapidly achievable using HPLC and/or solid-phase extraction (SPE) cartridges, followed by sterile filtration, to provide formulated products that are sterile, isotonic and suitable for human use;
Radiation safety concerns necessitate that reaction and purification steps be fully automated using specialized equipment;
The reaction profile should be sufficient to enable regulatory approval for clinical radiopharmaceutical manufacture including: RCY, purity, acceptable amounts of non-toxic by-products, automatable, adaptable to single-use cassettes manufactured according to cGMP etc.;
Methods should generate radiopharmaceuticals in high specific activity (>1 Ci/μmol). This is readily achievable using high specific activity nucleophilic fluoride, but cannot be achieved using electrophilic 18F-19F gas as the fluoride source.
Finally a PET radiochemistry method should be operationally simple and readily translatable to PET Centers all over the world for use by non-experts, as many such Centers do not have the luxury of trained organic chemists on staff.
Numerous academic groups are exploring development of new radiochemistry methodology and we have previously highlighted the impressive range of radiochemical reactions using carbon-11 and fluorine-18 that were reported between 2005 and 20113a,b However, in the case of fluorine-18 radiochemistry, certain long-standing issues still remain, including a scarcity of suitable labeling precursors for the generation of [18F]fluoro(hetero)arenes, difficulties in synthesizing high specific activity alkyl and aryl [18F]trifluoromethyl groups, and a lack of ability to prepare chiral radiopharmaceuticals as a single enantiomer via asymmetric radiofluorination reactions. Since the publication of our last highlight in 2012,3b momentum in the development of new reactions utilizing fluorine-18 has only increased, and has seen cutting-edge fluorine chemistry become highly tuned to the needs of the fluorine-18 radiochemistry community. The result has been the introduction of a remarkable selection of new fluorine-18 reactions, collectively grouped under the term “late-stage fluorination,” that address many of these longstanding problems associated with the synthesis of [18F]radiopharmaceuticals. This article provides perspective regarding key developments and milestones in the field of late-stage [18F]fluorination since 2011, and highlights challenges that still remain. In particular, we emphasize new transition metal-catalyzed reactions that are expected to change the way that fluorine-18 radiochemistry is conducted.
Aromatic Fluorination with Fluorine-18
A longstanding challenge in the radiochemistry community is the lack of ready access to reactions that enable the late-stage fluorination of electron-rich or electron-neutral (hetero)arenes using nucleophilic [18F]fluoride. Historically, direct [18F]fluorination of activated aromatic rings containing electron-withdrawing groups and a suitable leaving group (-NMe3+, -NO2, F, Cl, -SAr2+ etc.) has been possible using SNAr reactions.3d,e In contrast, the nucleophilic fluorination of electron-neutral and electron-rich rings has been highly limited using traditional fluorine-18 radiochemistry. A number of groups have focused upon addressing this need, and the first approach involves radiofluorination of potent electrophiles such as diaryliodonium salts.4a For example, treatment of (2-thienyl)(aryl)iodonium salts 1 with [18F]KF selectively affords [18F]fluoroarenes. In these transformations, the highly electron-rich 2-thienyl group directs radiofluorination to the other aryl group on iodine with reasonable selectivity (Scheme 1a).4b However, the starting materials can be challenging to prepare and often have modest shelf lives. Furthermore, radiofluorination of electron-neutral or -rich substrates, often require extreme temperatures (≥150 °C) and provide modest regioselectivity. Because of the harsh conditions, these protocols provide low to modest radiochemical yields and demonstrate limited tolerance to common functional groups. While such precursors have been used effectively,4c the issues outlined above have limited their utility, and there remain major opportunities for improvement.
Scheme 1.
Reactions of Aryl Iodonium Salts and Iodonium Ylides with Fluorine-18.4b,5b,c
One approach to improving this chemistry has been the introduction of iodonium ylides, which have recently been demonstrated as substrates that enable fluorination of electron rich arenes.5 The first example was reported by Coenen and colleagues, who used iodonium ylide precursors 2 derived from Meldrum’s acid (building on promising initial work patented by Satyamurthy and Barrio5a) to synthesize fluorophenoxyethers 3, which are potential radioligands for PET imaging of the serotonin and norepinephrine (reuptake) transporters (Scheme 1b).5b The concept was expanded by Vasdev, Liang and coworkers who introduced spirocyclic iodonium ylides 4 to address precursor stability issues of the earlier ylides.5c The spirocyclic iodonium ylides are stable crystalline materials that are reactive towards fluorine-18. They have been used to synthesize [18F]fluoro(hetero)arenes bearing electron donating or electron withdrawing groups in moderate to good radiochemical yields. This method has also been applied to other highly functionalized molecules and existing PET radiopharmaceuticals (Scheme 1c).
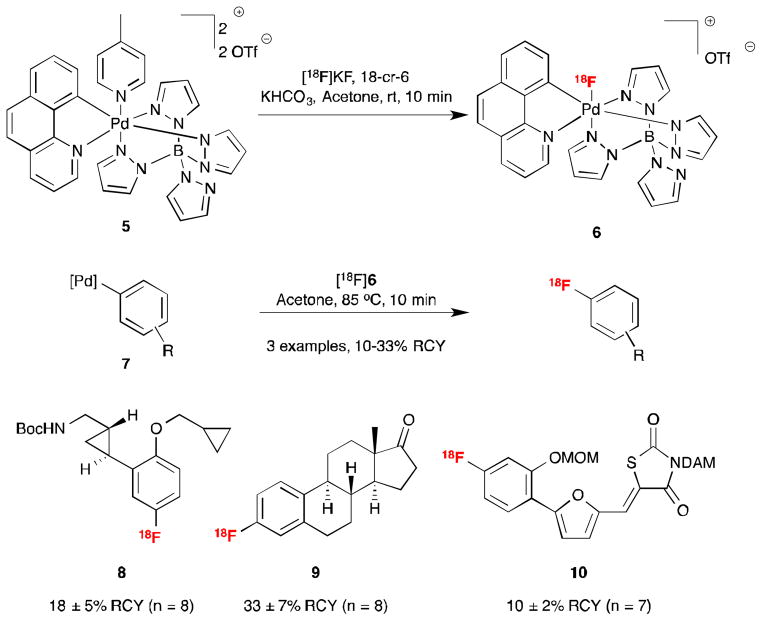
A second recently developed strategy for the nucleophilic [18F]fluorination of electron-rich aromatic substrates with [18F]fluoride employs transition metal catalysts (and/or reagents). Transition-metal catalysis offers opportunities to accelerate the rate of radiofluorination reactions. In addition, it could also enable enhancements in reactivity, selectivity and substrate scope. For example, Hooker and Ritter have demonstrated radiofluorination of arenes using Pd complexes.6 Treatment of the in situ generated fluorophilic palladium(IV) species 5 with [18F]KF generates 6 (Scheme 2). The newly formed [18F]6 can react with palladium(II) aryl compounds 7 to form Pd(IV) aryl fluoride complexes, which then undergo C–F coupling to yield the radiotracer (for a detailed discussion of the reaction mechanism, see reference 6c). This two-step 18F-labeling process gives reasonable yields of several target compounds (e.g. 8 – 10). The development of 6, given the practicality and wider availability of [18F]fluoride versus [18F]F2, which would historically have been employed in such transformations, provides the radiochemist with access to an alternative source of electrophilic [18F]fluorine.
Scheme 2.
Palladium-catalyzed [18F]Fluorination6
Despite these advantages, the need for a two-step procedure and the sensitivity of compound 6 to air and moisture, which necessitates azeotropic drying of the [18F]fluoride, has complicated transferal of this palladium-mediated process to the automated synthesis modules used in most radiochemistry laboratories. The authors have commented on difficulties in translation and this has hampered widespread adoption by the radiochemistry community to date.6d To address these limitations, Hooker and Ritter followed up this work with a method for [18F]fluorination of arylnickel complexes 11 (Scheme 3a) that offers a one-step, rapid oxidative fluorination of a range of arenes using aqueous [18F]fluoride and oxidant 12.7 Despite the relatively wide substrate scope of this reaction, Hooker and Ritter noted two key limitations in this method: (1) increasing the volume of aq. [18F]fluoride to the Ni-mediated reaction (>1% v/v) degraded the Ni complex and oxidant and (2) classical azeotropic drying of the [18F]fluoride resulted in solutions that were too basic and thus resulted in low RCY.
Scheme 3.
Nickel-mediated Oxidative [18F]Fluorination.7
Efforts to improve the chemistry were therefore undertaken.7b Elution of the [18F]fluoride from the ion exchange cartridge with tetrabutylammonium bicarbonate (TBAB) (to produce [18F]TBAF) was adventitious, as was the addition of pyridinium p-toluenesulfonate (PPTS) to produce a bicarbonate buffer that was compatible with oxidant 12. In a pre-clinical application of the chemistry, [18F]MDL100907 16, a radiotracer for the 5HT2a receptor, was synthesized and evaluated in non-human primates.7b [18F]MDL100907 has been synthesized previously via [18F]fluorophenethyl bromide.8 Traditional syntheses of [18F]fluorophenethyl bromide require the initial radiosynthesis of [18F]fluoroacetophenone (usually from the corresponding nitro-precursor), subsequent bromination to yield 2-bromo-4′-[18F]fluoroacetophenone and final reduction to give [18F]fluorophenethyl bromide. While yields can be moderate (2.5 – 60% RCY over 3-steps), the procedure is complicated, difficult to automate and the bromination step suffers from reproducibility issues.8 Hooker and Ritter looked to simplify this process by applying their nickel chemistry to the radiosynthesis of [18F]MDL100907. Thus, [18F]fluorination of nickel-complex 13 using the optimized conditions described above generated [18F]fluorophenethyl bromide 14 in one step (35% radiochemical conversion), and this could subsequently be coupled with chiral amine 15 in the same pot to generate [18F]MDL100907 16 in 3% RCY over 2-steps (Scheme 3b).7b While radiochemical yields of [18F]MDL100907 were comparable to those previously reported,8a and the new method still requires the synthesis of arylnickel complex 13 and radiolabeled prosthetic group 14 (i.e. not true “late-stage fluorination”), the operational simplicity of accessing [18F]fluorophenethyl bromide in one-step is attractive from an automation perspective and should find utility in the field.
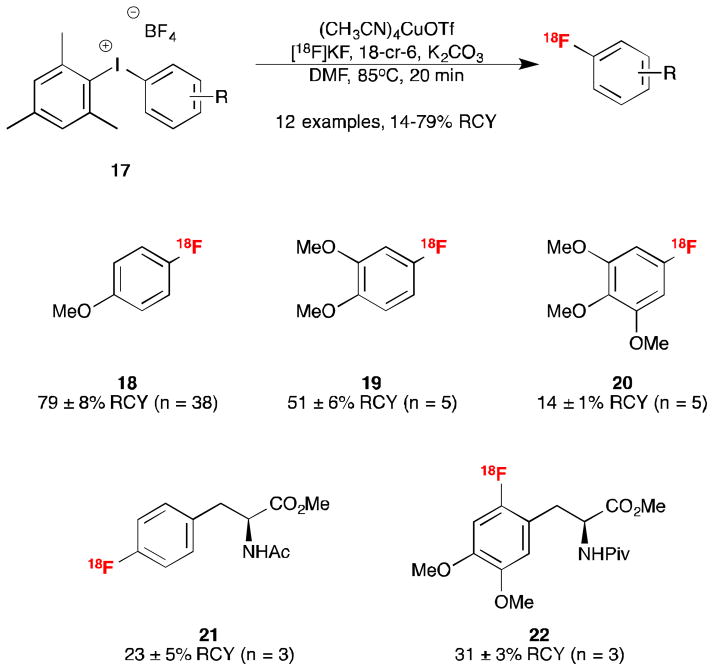
Fluorination of arenes can be promoted by copper catalysis.9,10 Sanford and Scott sought to develop a general, mild and high-yielding procedure for the radiofluorination of diverse aromatic substrates through a synergistic merger of transition-metal catalysis and the fluorination of diaryliodonium salts.9 Building on initial work from Sanford’s group showing that Cu salts catalyze the fluorination of stable and synthetically accessible mesityl-substituted diaryliodonium salts,9a they developed a rapid Cu-catalyzed radiofluorination of (mesityl)(aryl)iodonium salts 17 (precursors are shelf-stable under ambient conditions).9b In the initial work with fluorine-19, copper (II) catalysts provided the highest yields of fluorinated arenes. However, because of the different stoichiometry involved in fluorine-18 radiochemistry, the catalysts were re-screened and copper (I) catalysts were found to provide higher yields of [18F]fluorinated arenes in the 20 min time constraint of the radiosynthesis. This is attributed to the rate of the reaction being higher for copper (I) catalysts, making them more suitable for use with fluorine-18. In contrast, copper (II) catalysts give higher yields over longer reaction times (due to less side reactions) making them more compatible with fluorine-19. Further studies into the reaction mechanism are underway and will be reported in due course. Optimal conditions for the radiofluorination were found to be [18F]KF-18-crown-6, commercially available (MeCN)4CuOTf and [Ar–I–Mes]BF4 substrates.9b The latter is significant because no detrimental effect on specific activity was observed using BF4 salts suggesting that isotopic exchange is not an issue under the mild conditions and short reaction times employed. One attractive feature of this work is that the RCY was found to be very reproducible on the model 4-methoxy system – typically not varying more than 5% even over >35 trials on different days using different solvent sources. The method is very general, and a single set of reaction parameters (conducted under ambient conditions) enables high specific activity radiofluorination of electron-rich, -neutral, and -deficient arene substrates. Proof-of-concept was demonstrated through the synthesis of a range of fluorinated arenes such as anisoles 18 – 20, including the highly electron-rich [18F]trimethoxy fluorobenzene. The methodology was then applied to the synthesis of bioactive molecules of interest such as protected precursors to 4-[18F]-fluorophenylalanine 21 and 6-[18F]fluoroDOPA 22 (Scheme 4). Current limitations of this method are that multi-step synthesis can be required to access diaryliodonium precursors. Additionally, the automated synthesis procedures (e.g. of 21 and 22) still require optimization, as the isolated radiochemical yield was low (1% in the case of 22).
Scheme 4.
Copper-Catalyzed [18F]Fluorination of (Mesityl)(Aryl)Iodonium Salts.9b
Prior work in the Sanford group has also shown that aryl fluorides can be generated from a range of different aryl boron reagents (e.g. potassium aryl- and heteroaryltrifluoroborates, aryl boronic acids, aryl pincol boronate esters) in the presence of a copper catalyst.10a Gouverneur and co-workers very recently extended this work to fluorine-18 radiochemistry,10b showing that pinacol-derived aryl- 23a and heteroaryl-boronic esters 23b can be radiofluorinated in good radiochemical yields using [Cu(OTf)2(py)4] as the copper source (Scheme 5). Gouverneur discovered that RCY was affected by the boronic ester to [Cu(OTf)2] ratio. The best results were obtained by reducing the amount of Cu complex relative to boronic ester (ratio boronic ester:[Cu(OTf)2] = 10:1). Interestingly, drying the fluoride used in the reaction under N2 led to decreased RCYs and encouraged the formation of 1,1′-biphenyl (by protodeboronation), indicating that the presence of O2 was beneficial for the reaction. Consequently, the reaction vial was purged with air after drying [18F]fluoride, which increased the RCY. All ligands tested in the reaction had a negative impact on the RCY except pyridine, which led the team to test [Cu(OTf)2(py)4] and identify it as the optimum catalyst for the reaction. Precursors with unprotected alcohol or amine functionalities could be fluorinated, but in lower radiochemical yields (<10 %) presumably because of competitive C-O or C-N coupling. With optimized conditions in hand, the methodology was applied to the synthesis of several radiotracers of interest, including 6-[18F]fluoroDOPA 24 and [18F]DAA1106 25.
Scheme 5.
[18F]Fluorination of Aryl and (Hetero)aryl Boronic Esters.10b
Both copper-mediated fluorination reactions have the advantage of using commercially available air stable copper catalysts, as well as precursors that are stable for extended periods of time under ambient conditions. Scott and Sanford automated the radiofluorination of (mesityl)(aryl)iodonium salts using a GE TRACERlab synthesis module, although this procedure still requires optimization. Gouverneur’s laboratory has not yet reported the suitability of the radiofluorination of pinacol (hetero)aryl-boronic esters for automated synthesizers. Both the iodonium salt and boronic ester precursors can be readily prepared by organic chemists, but accessing such precursors could prove synthetically challenging to non-experts. This is especially true for more complicated instances such as the preparation of the precursor to 22, which requires a multi-step synthesis. Therefore widespread adoption of these methods for established radiopharmaceuticals such as 6-[18F]fluoroDOPA will require global access to the precursors through commercial avenues. The stability and long shelf life of both the iodonium salt and boronic ester precursors should facilitate this requirement.
Finally, radiochemists frequently have a need to radiolabel phenols. Historically, this has been achieved by radiofluorination of the corresponding aldehyde (or ketone), subsequent Baeyer-Villiger oxidation to the ester (the more electron-rich aromatic ring migrates) and final saponification of the ester to yield the [18F]phenol.11a,b This is a complex and low yielding transformation involving two additional steps after introduction of the fluorine-18, leading to a total of three steps that require automation. In an effort to simplify this transformation and the synthesis of such [18F]phenolic derivatives, Gouverneur reported a metal-free oxidative [18F]fluorination of phenols 26.11c The reaction is based upon phenol umpolung under oxidative conditions (phenyliodine diacetate (PIDA)), and direct nucleophilic fluorination with [18F]TBAF (Scheme 6a). In the case of bromophenol 27, subsequent Suzuki–Miyaura coupling and reductive debromination were demonstrated to yield 28 and 29, respectively (Scheme 6b). The one-step fluorination procedure as well as the excellent radiochemical yields and tolerance (necessity) for a free phenol functionality are all attractive features, as they facilitate translation of the methodology to automated radiochemistry synthesis modules, with proof-of-concept being demonstrated with an Advion Nano-Tek microfluidic system.
Scheme 6.
Metal-free Oxidative [18F]Fluorination of Phenols.11c
This impressive new series of reactions enables the radiofluorination of electron-rich, -neutral, and -deficient arene substrates, overcoming issues that have made accessing such products extremely challenging using more traditional strategies for [18F]fluorinating (hetero)arenes. Many of the reported radiosyntheses are being used already to prepare radiopharmaceuticals for pre-clinical evaluation and development. Additionally, radiochemical yields and specific activities are high enough to envisage using these methods to prepare doses for clinical PET imaging studies in the future. We (and the other groups highlighted herein) are currently developing these methodologies for clinical use. Key to this is determination of the compatibility of each of the methods with different functionality and/or protecting groups. Reasonable functional group tolerance has been demonstrated in the initial reports, but more thorough systematic studies are needed in each case to establish which methods will perform in the more complex settings of clinically relevant scaffolds. In particular, protecting groups are used for amines and alcohols in most reported examples, and future work should evaluate the necessity of these groups (which necessitate undesirable deprotection steps). Where protecting groups are needed, those that can be removed with mild acid or base (rather than, for example, conc. HI or conc. HBr) are most desirable, as these conditions can be easily adapted for automated synthesis modules. Additionally, the radiofluorination of a broader scope of heteroarenes is also of great interest, as these appear commonly in biologically active molecules. Moreover, the need for operational simplicity (including use of simple commercially available catalysts and precursors) and automation using existing radiochemistry synthesis module platforms should be reiterated. Ultimately, for widespread adoption by the radiochemical community, which can be considered one hallmark of a successful radiochemical methodology, new methods should be compatible with the single use cassettes used in cGMP compliant radiopharmaceutical manufacture in most PET Centers around the world. Clinical radiopharmaceutical doses prepared using these new methods should be suitable for human use, meaning that they should meet all of the standard regulatory requirements.2a In addition, there will be specific quality control requirements for using these methods such as confirming that purification (HPLC and/or SPE) provides doses that are free of residual metals, ligands, solvents, precursors and any other method-specific contaminants or by-products.
Aliphatic Fluorination with Fluorine-18
Metal-catalyzed C-sp3 radiofluorination is also receiving growing attention. One approach focuses upon fluorination of allylic substrates.12 For example, Gouverneur and co-workers described a protocol for palladium-catalyzed allylic fluorination. Cinnamyl carbonates and cinnamyl halides 30 were converted to their corresponding allylic fluorides 31 (Scheme 7a).12a Nguyen and co-workers introduced an iridium-catalyzed allyl fluorination of trichloroacetimidates 32,12b and demonstrated compatibility with fluorine-18 to generate allylic fluoride 33 (Scheme 7b). Gouverneur, building upon her earlier palladium-mediated allylic fluorination, also reported that a similar iridium catalytic system was capable of radiofluorinating allylic carbonates 34 and 36 to obtain either the linear 35 (Scheme 7c) or branched 37 (Scheme 7d) products, respectively.12c All of these reactions demonstrate proof-of-concept, but the allylic fluoride unit is not particularly common and to the best of our knowledge these approaches have yet to be used to synthesize a radiopharmaceutical.
Scheme 7.
Metal-catalyzed Allylic Fluorination Reactions.12
The first example of an enantioselective radiosynthesis was recently reported by Doyle and colleagues, and was focused upon the synthesis of radiopharmaceuticals containing the [18F]fluorohydrin moiety 39.13a Historically, such radiotracers have been prepared from the corresponding cyclic sulfonates,13b or by selective displacement of differentially protected diols.13c Both approaches are effective, but require separation of regioisomers and/or stereochemistry to be set prior to labeling if the radiolabeled product is required as a single enantiomer.13d Doyle’s approach overcomes these issues by treating racemic epoxides 38 with a chiral transition metal [18F]fluoride catalyst ([18F](Salen)CoF) to generate radiopharmaceuticals as single enantiomers. In an important new contribution to the field of fluorine-18 radiochemistry, Doyle showed that [18F](R,R)-(Salen)CoF could be prepared by elution of [18F]fluoride from a quaternary ammonium (QMA) ion-exchange cartridge using (R,R)-(salen)CoOTs in a process that is directly analogous to the preparation of [18F]KF and can be carried out under air without the use of rigorously dried solvents or glassware. The subsequent radiofluorination reaction proceeds under mild conditions, and has been used to access research imaging agents as well as clinical radiopharmaceuticals such as [18F]THK-5105 (40, tau) and [18F]FMISO (41, tumor hypoxia) in moderate to high radiochemical yields with excellent control of stereochemistry (Scheme 8). This work sets the stage for further work in the area of asymmetric fluorination reactions with fluorine-18, including expanding the substrate scope beyond [18F]fluorohydrins.
Scheme 8.
Enantioselective Radiosynthesis of Radiotracers Containing [18F]Fluorohydrins.13a
High throughput screening (HTS) to identify scaffolds with affinity for a given target is used in developing radiopharmaceuticals, just like it is used in medicinal chemistry. The difference in the PET field is that it is currently not feasible to accurately predict whether a given radiolabeled molecule will have all of the necessary requirements (pharmacokinetics, metabolism profile, high specific binding and low non-specific binding, blood-brain barrier permeability etc.) to make a successful radiopharmaceutical. Therefore a precursor for each needs to be prepared and then the molecule needs to be radiolabeled and evaluated. This can be a very time consuming endeavor and so methods that simplify this process are in demand. One possibility is the development of strategies for C-H fluorination that would allow easy radiolabeling of candidates identified from the HTS directly without the need for complex precursor syntheses. With this in mind, the first example of such a C-H radiofluorination reaction was recently reported by Hooker and Groves.14 The method utilizes Mn(salen)OTs as a catalyst and enables rapid benzylic C-H radiofluorination in the presence of PhIO, K2CO3 and [18F]fluoride under an atmosphere of air (Scheme 9). Notably, like Doyle’s work described above, the catalyst itself could be used to directly elute [18F]fluoride from the ion exchange cartridge, which eliminated the need to azeotropically dry the fluoride and shortened the synthesis time. The team reported [18F]labeling of the benzylic position in a range of substrates 42 to generate benzyl fluorides 43 in average to good radiochemical yields. High functional group tolerance was demonstrated (e.g. 44 – 46), and the methodology was applied to the radiolabeling of several bioactive molecules to generate benzyl fluoride analogs, including drug molecules of interest across medicinal chemistry space such as PDE10A inhibitor 47 and ACE inhibitor 48. The method allows ready radiolabeling of a series of complex molecules that would otherwise by inaccessible by other methods. Purification of the radiopharmaceutical from the precursor is an important facet of radiochemistry method development because doses cannot (typically) be contaminated with residual unreacted precursor. As C-H radiofluorination develops beyond this initial report, it will be critical to have strategies for the (potentially) problematic separation of the C-H precursors from the related C-F products.
Scheme 9.
Late-stage Benzylic C-H Fluorination.14
Much like their aromatic counterparts described above, if the novel methods for aliphatic fluorination are to find widespread use in PET Centers around the world, they should be readily automatable and also provide radiopharmaceutical doses that are free of contaminants (particularly residual metals for each of the described processes) and acceptable for human use. One additional concern of alkyl fluorides is their known propensity to undergo dehydrofluorination in vivo.15 This can be particularly problematic for activated positions, and should be accounted for when developing new radiotracers.
Approaches for [18F]Trifluoromethylation
Finally, owing to its prevalence in drugs and potential radiotracers, the trifluoromethyl (-CF3) group is also an attractive target for radiolabeling with fluorine-18. Historically, [18F]trifluoromethyl groups have been prepared by reaction of [18F]fluoride with, for example, -CF2Br precursors.16 However, separation of the radiolabeled product from the precursor can be challenging leading to low specific activity radiotracers. With the goal of developing methods that enable production of high specific activity trifluoromethyl groups, new approaches to both [18F]alkyl- and [18F]aryl-CF3 have been reported recently.17,18 The former can be accessed from gem-difluoro enol ethers using chemistry initially reported by Riss and colleagues.17a,b They developed a simple and efficient procedure for the preparation of 2-[18F]fluoro-2,2-difluoroethyltosylate 50, beginning from difluorovinylsulfonate 49 (Scheme 10a), and used it as a prosthetic group for radiolabeling bioactive molecules 51. This approach has been refined by Scott and co-workers, who extended the concept to prepare complex radiopharmaceuticals directly in a one-step late-stage fluorination without the need for a two-step prosthetic group approach, and also found that radiochemical yields improved upon addition of proton sources of lower pKa than previously reported (e.g. NH4Cl). They accessed precursor 52 from commercially available lansoprazole, and used this methodology to prepare [18F]N-methyl lansoprazole 53 (Scheme 10b), a radiotracer with sub-nanomolar affinity for aggregated tau found in Alzheimer’s disease and Progressive Supranuclear Palsy that is currently being translated into clinical imaging trials.17c Importantly, using a perfluorophenyl-capped semi-preparative HPLC column, trifluoromethyl product 53 was readily separated from the gem-difluoro enol ether precursor 52, as well as the [18F]gem-difluoro enol ether by-product (not shown) that was thought to be formed through an addition-elimination type mechanism. This allows isolation of [18F]trifluoromethylated products in good specific activity (up to 1 Ci/μmol).
Scheme 10.

New Strategies for Alkyl [18F]Trifluoromethylation.17
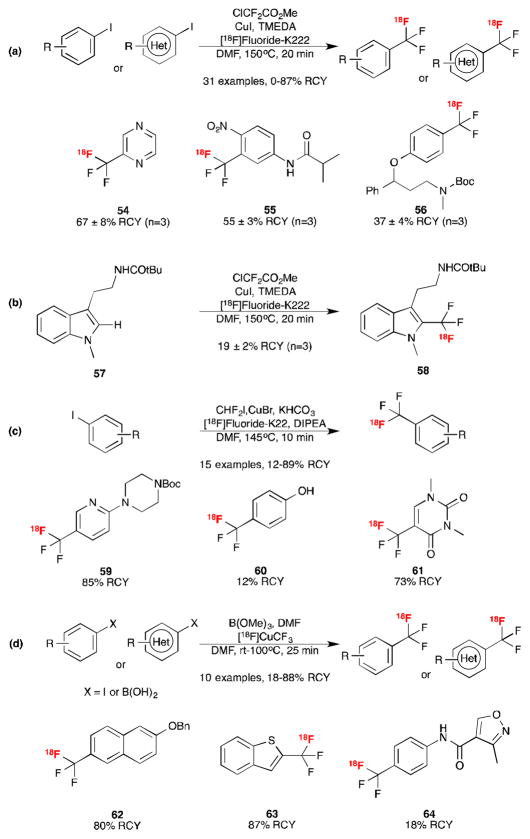
[18F]Trifluoromethyl arenes are also of interest to the fluorine-18 radiochemistry community. Reflecting this, several groups have explored copper-mediated [18F]trifluoromethylation of arene and heteroarene precursors using nucleophilic fluoride recently.18 The protocols are related, initially generating difluorocarbene, and then reacting it with [18F]fluoride in the presence of a copper catalyst to prepare [18F]CuCF3. This reactive species can then undergo cross-coupling with aryl iodides18 or aryl boronic acids.18c The first example was reported by Gouverneur, who showed that [18F]CuCF3 could be prepared from methyl chlorodifluoroacetate using CuI, N,N,N′,N′-tetramethylethylenediamine (TMEDA) and [18F]fluoride-krytpofix-2.2.2 (K222) in DMF.18a The resulting [18F]CuCF3 species underwent cross-coupling with a range of aryl and heteroaryl iodides in moderate to high radiochemical yields (e.g. 54 – 56, Scheme 11a). This radiochemistry does not require the synthesis of complex organometallic precursors and can be performed with commercially available reagents in a reaction vessel exposed to air, all of which should promote automation and widespread use in the radiochemistry community. A direct C-H oxidative [18F]trifluoromethylation of N-methyl indole 57 was also demonstrated using this methodology to yield 58 (Scheme 11b), offering scope for further development as it could allow easy access to [18F]radiopharmaceuticals for screening purposes like the C-H benzylic fluorination from Hooker and Groves described above.14
Scheme 11.
New Strategies for [18F]Trifluoromethylation of Arenes.18
The Riss group showed that the [18F]CuCF3 species could be generated by treating difluoroiodomethane with potassium bicarbonate, DIPEA and [18F]fluoride-K222 in DMF. They then coupled this reagent with a range of aryl iodides to generate [18F]trifluoromethyl arenes (e.g. 59) in good radiochemical yields (Scheme 11c).18b The methodology was tolerant of a free phenolic functionality on the aryl iodide, although the corresponding [18F]trifluoromethylated product 60 was formed in lower radiochemical yield (12%). Both Riss (61) and Gouverneur’s (not shown) one-pot methods have also been used to [18F]trifluoromethylate pyrimidine-2,4(1H,3H)-diones.18a,b
Lastly, Ivashkin et al. showed that [18F]CuCF3 could be prepared by treating [18F]fluoroform (accessed via difluorocarbene from a difluoromethylsulfonium salt) with the copper catalyst (CuI or CuCl) and potassium t-butoxide.18c Reaction of [18F]CuCF3 with aryl iodides or aryl boronic acids in DMF (trimethylborate was also included to neutralize any excess potassium t-butoxide) provided the trifluoromethylated products in generally high radiochemical yields (Scheme 11d). In addition to studies with model test compounds, all of these methods have been employed to prepare bioactive compounds of possible interest to the PET imaging community.
The ready access that these methods provide to high specific activity [18F]trifluoromethyl groups using standard starting materials and reagents, and the prominent role of the CF3 group in drug development, means these methods should find widespread use in the synthesis of [18F]radiopharmaceuticals, and some indeed already have. The straightforward processes are suitable for use with automated synthesis modules and microfluidic devices.
Conclusions
The last few years have seen the formation of a number of multidisciplinary teams composed of PET radiochemists, fluorine chemists and organometallic chemists around the world. The synergism of these collaborations is already apparent, having led to the development of many new strategies for the late-stage introduction of fluorine-18 into complex bioactive molecules, including strategies for enantioselective radiofluroination and C-H fluorination. Such approaches have enabled pre-clinical (and, with further development, it is anticipated eventual clinical) application of radiopharmaceuticals previously inaccessible via traditional fluorine-18 radiochemistry, and also offer tools that could improve employment of techniques such as HTS in the development of new radiopharmaceuticals. Key to successful translation of the described methods into widespread use is completion of detailed studies, many of which are already ongoing, to establish which methods will be compatible with the challenges of cGMP compliant radiopharmaceutical production on a daily basis.
Supplementary Material
Acknowledgments
We acknowledge the NIH (GM073836 (MSS) and T32-EB005172 (PJHS)) for financial support
Notes and references
- 1.a) for a general overview of PET imaging see: Ametamey SM, Honer M, Schubiger PA. Chem Rev. 2008;108:1501. doi: 10.1021/cr0782426.for a review of the applications of PET imaging in drug discovery see: Matthews PM, Rabiner EA, Passchier J, Gunn RN. Br J Clin Pharmacol. 2012;73:175. doi: 10.1111/j.1365-2125.2011.04085.x.for an overview of the role of PET imaging in personalized medicine see: Pither R. Expert Rev Mol Diagn. 2003;3:703. doi: 10.1586/14737159.3.6.703.
- 2.for an overview of PET drug approval, reimbursement and regulatory requirements see Schwarz SW, Dick D, VanBrocklin HF, Hoffman JM. J Nucl Med. 2014;55:1132. doi: 10.2967/jnumed.113.132472.Wagner HN., Jr J Nucl Med. 2007;48:495. doi: 10.2967/jnumed.48.4.495.07.Bietendorf J. J Nucl Med Technol. 2004;32:33.
- 3.Scott PJH. Angew Chem. 2009;121:6115.Angew Chem Int Ed. 2009;48:6001.Littich R, Scott PJH. Angew Chem. 2012;124:1132;. doi: 10.1002/anie.201106785.Angew Chem Int Ed. 2012;51:1106.for a comprehensive review of PET radiochemistry see: Miller PW, Long NJ, Vilar R, Gee AD. Angew Chem. 2008;120:9136. doi: 10.1002/anie.200800222.Angew Chem Int Ed. 2008;47:8998. doi: 10.1002/anie.200800222.Cole EL, Stewart MN, Littich R, Hoareau R, Scott PJH. Current Top Med Chem. 2014;14:875. doi: 10.2174/1568026614666140202205035.For a dedicated review on [18F]fluoroaryl synthesis, see: Tredwell M, Gouverneur V. Angew Chem, Int Ed. 2012;51:11426. doi: 10.1002/anie.201204687.Cai L, Lu S, Pike VW. Eur J Org Chem. 2008;17:2853.Schirrmacher R, Wängler C, Schirrmacher E. Mini Rev Org Chem. 2007;4:317.
- 4.for a review of radiochemistry with diaryliodonium salt and iodonium ylide precursors see: Yusubov MS, Svitich DY, Larkina MS, Zhdankin VV. ARKIVOC. 2013:364.Ross TL, Ermert J, Hocke C, Coenen HH. J Am Chem Soc. 2007;129:8018. doi: 10.1021/ja066850h.DiMagno S. 2010/048170A2. Patent, WO. 2010
- 5.a) Satyamurthy N, Barrio JR. 2010/117435 A2. Patent, WO. 2010; b) Cardinale J, Ermert J, Humpert S, Coenen HH. RSC Adv. 2014;4:17293. [Google Scholar]; c) Rotstein BH, Stephenson NA, Vasdev N, Liang SH. Nat Commun. 2014 doi: 10.1038/ncomms5365. [DOI] [PubMed] [Google Scholar]
- 6.(a) Lee E, Kamlet AS, Powers DC, Neumann CN, Boursalian GB, Furuya T, Choi DC, Hooker JM, Ritter T. Science. 2011;334:639. doi: 10.1126/science.1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kamlet AS, Neumann CN, Lee E, Carlin SM, Moseley CK, Stephenson N, Hooker JM, Ritter T. PLoS One. 2013;8:e59187. doi: 10.1371/journal.pone.0059187. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Brandt JR, Lee E, Boursalian GB, Ritter T. Chem Sci. 2014;5:169. doi: 10.1039/C3SC52367E. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Halford B. Chem Eng News. 2014;92(28):33. [Google Scholar]
- 7.a) Lee E, Hooker JM, Ritter T. J Am Chem Soc. 2012;134:17456. doi: 10.1021/ja3084797. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ren H, Wey HY, Strebl M, Neelamegam R, Ritter T, Hooker JM. ACS Chem Neurosci. 2014;5:611. doi: 10.1021/cn500078e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Mühlhausen U, Ermert J, Herth MH, Coenen HH. J Label Compd Radiopharm. 2008;52:6. [Google Scholar]; b) Hwang DR, Dence CS, Gong J, Welch MJ. Appl Radiat Isot. 1991;42:1043. doi: 10.1016/0883-2889(91)90008-o. [DOI] [PubMed] [Google Scholar]; c) Dence CS, McCarthy TJ, Welch MJ. Appl Radiat Isot. 1993;44:981. doi: 10.1016/0969-8043(93)90054-e. [DOI] [PubMed] [Google Scholar]
- 9.a) Ichiishi N, Canty AJ, Yates BF, Sanford MS. Org Lett. 2013;15:5134. doi: 10.1021/ol4025716. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ichiishi N, Brooks AF, Topczewski JJ, Rodnick ME, Sanford MS, Scott PJH. Org Lett. 2014;16:3224. doi: 10.1021/ol501243g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Ye Y, Schimler SD, Hanley PS, Sanford MS. J Am Chem Soc. 2013;135:16292. doi: 10.1021/ja408607r. [DOI] [PubMed] [Google Scholar]; b) Tredwell M, Preshlock SM, Taylor NJ, Gruber S, Huiban M, Passchier J, Mercier J, Génicot C, Gouverneur V. Angew Chem Int Ed. 2014;53:7751. doi: 10.1002/anie.201404436. [DOI] [PubMed] [Google Scholar]
- 11.a) Chakraborty PK, Kilbourn MR. Appl Rad Isot. 1991;42:673. doi: 10.1016/0883-2889(91)90039-4. [DOI] [PubMed] [Google Scholar]; b) Ekaeva I, Barre L, Lasne MC, Gourand F. Appl Radiat Isot. 1995;46:777. [Google Scholar]; c) Gao Z, Lim YH, Tredwell M, Li L, Verhoog S, Hopkinson M, Kaluza W, Collier TL, Passchier J, Huiban M, Gouverneur V. Angew Chem Int Ed. 2012;51:6733. doi: 10.1002/anie.201201502. [DOI] [PubMed] [Google Scholar]
- 12.a) Hollingworth C, Hazari A, Hopkinson MN, Tredwell M, Benedetto E, Huiban M, Gee A, Brown JM, Gouverneur V. Angew Chem. 2011;123:2661;. doi: 10.1002/anie.201007307. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:2613. [Google Scholar]; b) Topczewski JJ, Tewson TJ, Nguyen HM. J Am Chem Soc. 2011;133:19318. doi: 10.1021/ja2087213. [DOI] [PubMed] [Google Scholar]; c) Benedetto E, Tedwell M, Hollingworth C, Khotavivattana T, Brown JM, Gouverneur V. Chem Sci. 2013;4:89. [Google Scholar]
- 13.a) Graham TJA, Lambert RF, Ploessl K, Kung HF, Doyle AG. J Am Chem Soc. 2014;136:5291. doi: 10.1021/ja5025645. [DOI] [PubMed] [Google Scholar]; b) Berridge MS, Franceschini MP, Rosenfeld E, Tewson TJ. J Org Chem. 1990;55:1211. [Google Scholar]; c) Okamura N, Furumoto S, Harada R, Tago T, Yoshikawa T, Fodero-Tavoletti M, Mulligan RS, Villemagne VL, Akatsu A, Yamamoto T, Arai H, Iwata R, Yanai K, Kudo Y. J Nucl Med. 2013;54:1420. doi: 10.2967/jnumed.112.117341. [DOI] [PubMed] [Google Scholar]; d) Limpachayaporn P, Wagner S, Kopka K, Hermann S, Schäfers M, Haufe G. J Med Chem. 2013;56:4509. doi: 10.1021/jm400257a. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Liu W, Ren H, Neelamegam R, Hooker JM, Groves JT. J Am Chem Soc. 2014;136:6842. doi: 10.1021/ja5039819. [DOI] [PubMed] [Google Scholar]
- 15.a) Pike VW. Trends Pharmacol Sci. 2009;30:431. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chun J-H, Pike VW, Lu S. Eur J Org Chem. 2011:4439. doi: 10.1002/ejoc.201100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilbourn MR, Pavia MR, Gregor VE. Appl Radiat Isot. 1990;41:823. doi: 10.1016/0883-2889(90)90059-p. [DOI] [PubMed] [Google Scholar]
- 17.a) Aigbirhio FI, Riss PJ. Chem Commun. 2011;47:11873. doi: 10.1039/c1cc15342k. [DOI] [PubMed] [Google Scholar]; b) Riss PJ, Ferrari V, Brichard L, Burke P, Smith R, Aigbirhio FI. Org Biomol Chem. 2012;10:6980. doi: 10.1039/c2ob25802a. [DOI] [PubMed] [Google Scholar]; c) Fawaz MV, Brooks AF, Rodick ME, Carpenter GM, Shao X, Desmond TJ, Sherman P, Quesada CA, Hockley BG, Kilbourn MR, Albin RL, Frey KA, Scott PJH. ACS Chem Neurosci. 2014;5 doi: 10.1021/cn500103u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Huiban M, Tredwell M, Mizuta S, Wan Z, Zhang X, Collier TL, Gouverneur V, Passchier J. Nat Chem. 2013;5:941. doi: 10.1038/nchem.1756. [DOI] [PubMed] [Google Scholar]; b) Rühl T, Rafique W, Lien VT, Riss PJ. Chem Commun. 2014;50:6056. doi: 10.1039/c4cc01641f. [DOI] [PubMed] [Google Scholar]; c) Ivashkin P, Lemonnier G, Cousin J, Grégoire V, Labar D, Jubault P, Pannecoucke X. Chem Eur J. 2014 doi: 10.1002/chem.201403630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.