Abstract
To assess the relevance and availability of subtotal nephrectomized common marmoset monkeys as a chronic renal failure (CRF) model, we observed for 26 weeks the pathophysiological condition of female marmosets subjected to five-sixth surgical nephrectomy (5/6Nx) by a two-step surgical method. The 5/6Nx marmosets showed a significant increase in serum levels of urea nitrogen, creatinine and cystatin-C immediately after 5/6Nx surgery. These renal disorder parameters subsequently tended to decrease with the passage of time but remained higher than the control levels by the end of the study. Hyperplastic parathyroid glands, a high turnover state of osteodystrophy in the femoral bone with higher serum ALP activity and anemia with hypocellularity of bone marrow were evident. The 5/6Nx marmosets showed a stable CRF condition for a long time and some characteristic disorders similar to those observed in CRF patients. These diagnostic aspects might be a species-specific anatomical and physiological signature, reflecting the nutritional condition. The CRF model using 5/6Nx marmosets might become a useful method of evaluating the unique mechanism of CRF development.
Keywords: common marmoset, 5/6 nephrectomy, chronic renal failure, bone, anemia
Introduction
The remnant kidney model has been a mainstay of experimental studies of chronic renal failure (CRF). This model has been produced mostly by unilateral nephrectomy and either partial infarction or amputation of the poles of the remaining kidney1,2,3. Rodent models remain essential tools that are widely employed by nephrological investigators. Rat models are usually able to faithfully recapture the major pathological manifestations of human diseases, whereas mice are notorious for resistance to development of clinical lesions after injury4, 5. Several critical factors, such as animal species, strain, gender, age and body weight, may play a decisive role in determining the outcome of a particular experiment5,6,7,8,9.
Nonhuman primates are used when they represent a well-established model of a compound class or when they are the relevant species (especially for biotechnology-derived products) for detecting known side effects. On the other hand, with the increase of biotechnology-derived pharmaceuticals, the use of nonhuman primates has also increased, as much as to raise public concern.
The common marmoset, a new world primate, is the shortest-lived of the primates, with an average lifespan of nine to 13 years and a maximum life span of 22 years10. Due to its small size and its relatively easy adaptation to laboratory conditions, the marmoset is increasingly used in many fields of biomedical research, including fundamental biology, pharmacology and toxicology studies11, 12. In addition, regulatory authorities worldwide recognize the use of marmosets in nonclinical development, and as they are one of the more primitive, nonhuman primate species and the most phylogenetically distant from humans, their use requires less ethical justification than the larger ‘‘old world monkey’’13. The marmoset has been used successfully by a number of pharmaceutical and biotechnology companies to support clinical trials and for product registration with the United States (US) Food and Drug Administration (FDA) and the European Medicines Agency (EMA)14, 15. In addition, the common marmoset shows a higher incidence of renal lesions including glomerulo- and tubulointerstitial nephritis or progressive nephropathy16,17,18,19,20,21 compared with that in the cynomolgus monkey22, 23.
The aim of this study was to investigate the relevance and availability of the subtotal nephrectomized common marmoset as a CRF model. In a previous study, we carried out five-sixth surgical nephrectomy (5/6Nx) on female marmosets and acquired data regarding the parameters of the renal disorder and the pathological changes of the remnant kidneys up to 13 weeks after the operation24. The 5/6Nx marmosets showed an increase in urine volume and elimination of urinary protein immediately after nephrectomy, but there was no progressive increase in the elimination of urinary protein (proteinuria). In the remnant kidney in 5/6Nx marmosets, slight degenerative glomerular abnormalities and interstitial fibrosis with inflammatory mononuclear cells were observed. There was no further progression of the glomerular changes over time24. In this study, we focused on the longitudinal course of serum biochemical, hematological and histopathological changes in 5/6Nx female marmosets for up to 26 weeks after the surgical operation.
Materials and Methods
Animals and surgical procedure
Common marmoset monkeys were obtained at one or two years old from CLEA Japan Inc. (Kawasaki, Japan). One or two animals were housed in a steel cage (width 390 × depth 550 × height 700 mm; Shin Toyo Seisaku-sho Ltd., Saitama, Japan) with environmental enrichments, generally a roost, an aerial bar and a table tennis ball. The animal room was kept under controlled conditions of temperature (24−30°C), relative humidity (30−70%) and ventilation (air exchange rate of 15 to 17 times/hr) with a 12-hour light/12-hour dark cycle. They were allowed free access to a commercial diet (CSM-1M, CLEA Japan Inc.) and tap water throughout both the acclimation and experimental periods. Additional supplements (dry fruits, boiled quail eggs or milk powder) were provided as considered appropriate. During the acclimation period, animals were checked daily by cage-side observation. Detailed clinical observation and body weight measurement were conducted weekly. At the end of the acclimation period, 24 female marmosets aged 40 to 63 months old were selected. Ten animals were allocated into the non-treated group, and 14 animals were allocated into the 5/6Nx group. The individual data for the animals are presented in Table 1. The experiment and care of the animals were conducted in accordance with the guiding principles for “The Care and Use of Laboratory Animals of Kyowa Hakko Kirin Co, Ltd”.
Table 1. Individual Data of the Marmosets.
Each marmoset in the 5/6Nx group underwent 5/6Nx by a two-step surgery25. In the first operation, marmosets were anesthetized by inhalation of isoflurane (Mylan Seiyaku, Tokyo, Japan) and held in a lateral position. The surgical site was shaved free of hair and disinfected with povidone-iodine and 70% ethanol. After identification of the left kidney by palpation, the skin, muscle and peritoneum in the region were dissected about 1 to 1.5 cm in length. The kidney was exteriorized by pressing the abdomen with the fingers, and then held by clipping the proximal ureter and renal vessels with a hemoclipper. The upper and lower poles of the left kidney were resected with a pair of surgical scissors, leaving one-third of the renal parenchyma and renal hilum intact. An ablation hemostatic sheet (SURGICEL, Ethicon Inc., NJ, USA) was used for hemostasis by covering the ablation surface. A few minutes later, the hemostat clipping was removed, and the ablation surface was checked for bleeding. The renal remnant was returned to the abdomen cavity. A continuous suturing method was used for suture of the abdominal muscle and peritoneum, and a buried suture method was used for suture of skin. The suture site was disinfected with povidone-iodine. In the second operation, the right kidney was removed after ligation of the proximal ureter and renal vessels with silk thread. After each operation, the animals were gently laid to rest on a hot plate kept at 38°C until awaking from anesthesia. The animals were then kept in an incubator set at 38°C. After checking their health status, animals in good condition were returned to the animal feeding cages in the animal room. In addition, all operated animals received a daily intramuscular dose of about 10 mg/kg lincomycin hydrochloride (Lincocin®, Pfizer Inc.) and about 0.3 mg/kg of meloxicam (Metacam®, Boehringer Ingelheim Vetmedica, Inc.) for 3 or 4 days after each operation.
Clinical signs
Clinical signs were recorded by daily cage-side monitoring of behavioral changes (apathy, loss of appetite).
Serum biochemistry, hematology and histopathology
At 5, 13 and 26 weeks after the 5/6Nx operation, 10 non-treated control and 11 5/6Nx animals were sacrificed. Under deep anesthesia with isoflurane, blood samples were collected from the abdominal vein of animals for selected blood chemistry and hematological examinations, and then the animals were euthanized by exsanguination to prevent them from suffering. The animals were then dissected for pathological observation.
The serum biochemical analyses were performed with an automatic analyzer (H7170S, Hitachi Ltd.) and an automated electrolyte analyzer (PVA-EXII, A&T Corporation). Hematological examination was performed using an automatic hematological analyzer (ADVIA 120, Siemens Healthcare Diagnostics). However, since this system could not clearly fractionate neutrophils and eosinophils in marmoset peripheral blood, the total number of both cell types was used as the granulocyte number. Blood coagulation parameter analyses were performed using an automated blood coagulation analyzer (CA-1500, Sysmex).
All major organs were collected and fixed in 10% neutral buffered formalin, embedded, sectioned at 4 to 6 μm, and stained using hematoxylin and eosin (H-E). The bone tissues were decalcified with 10% EDTA solution. The kidney sections were also stained with periodic acid-Schiff (PAS) reaction and Masson’s trichrome. These preparations were observed by light microscopy.
Statistics
Data were expressed as means ± SD. All parameters were evaluated with the Student’s t-test and an unpaired t-test with Welch’s correction. All p values resulted from two-sided statistical tests, and p<0.05 was considered to be significant.
Results
All animals in the non-treated control group survived to the end of study, whereas three animals in the 5/6Nx group died or were moribund. One animal (No. 101) was moribund on day 10, and another animal (No. 112) died on day 16 after the 2nd surgery. The moribund animal (No. 101) showed a marked change in renal functional parameters (an increase in serum urea nitrogen (UN), creatinine, cystatin-C (Cys-C)), electrolytes (an increase in inorganic phosphorus and potassium and a decrease in sodium and chloride), lipids (a decrease in total cholesterol, triglycerides and phospholipids), liver function (an increase in aspartate aminotransferase (AST) and choline esterase), and an increase in alkaline phosphatase (ALP) with severe anemia and thrombopenia. These animals were considered to be at the onset of the acute renal failure state. Another animal (No. 114) showed emesis and low appetite on day 94, loss of appetite and defecation, incomplete opening of the eyelids, and a decrease in locomotor activity on day 96. This animal showed hydronephrosis in the remnant kidney, alveolar hemorrhage in the lungs, parathyroid gland hyperplasia, an increase of adipose tissue in the bone marrow, and mineralization at renal tubules, tracheal cartilage and aortic tunica media. Hence this animal was considered to have been in a severe renal failure state due to a shift to hydronephrosis in the remnant kidney. Tremors and bradykinesia were observed in one animal (No. 111) of the 5/6Nx group. Reduction of appetite was also observed in some animals in the 5/6Nx group. These changes diminished within one or two weeks after surgery.
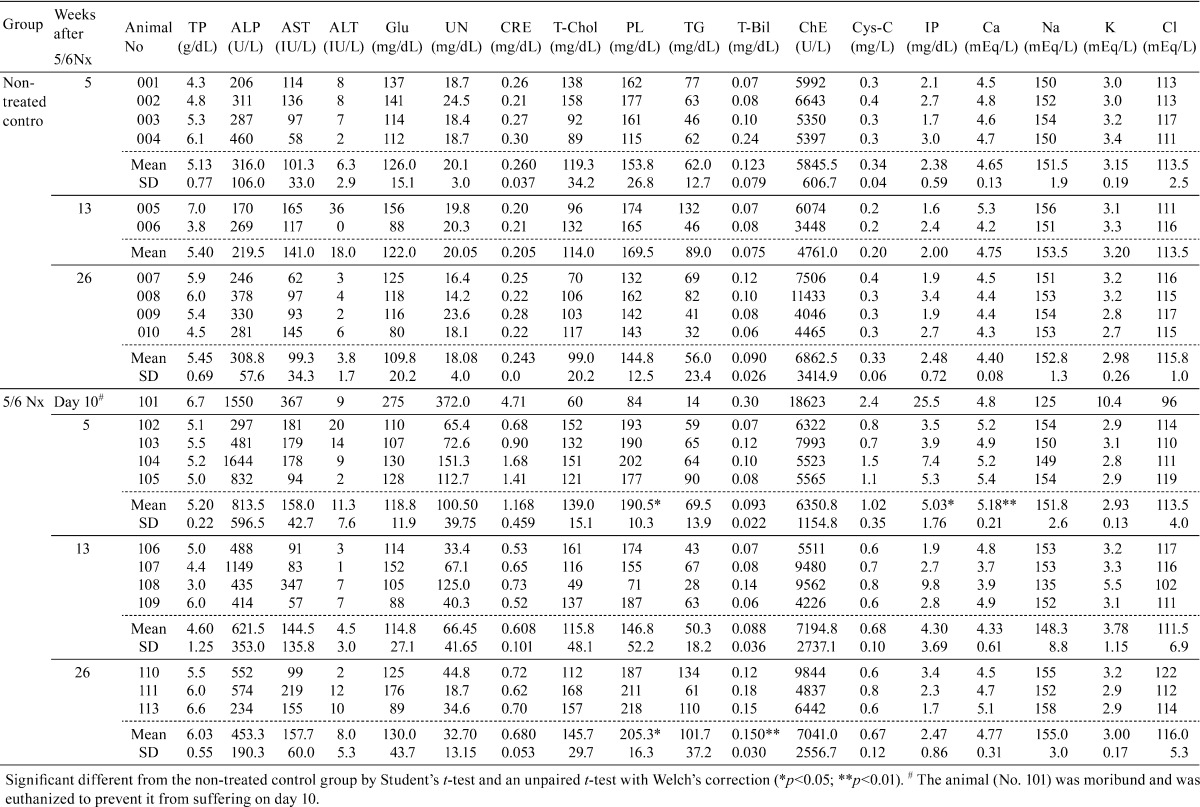
In the serum blood chemistry, the 5/6Nx marmosets showed a marked increase in the serum UN, creatinine and Cys-C levels at week 5. These levels subsequently tended to decrease with the passage of time, but the serum creatinine and Cys-C levels were sustained at higher levels at weeks 13 and 26 in the 5/6Nx marmosets. The serum calcium and inorganic phosphorus levels were significantly higher than the control level at week 5. The calcium and inorganic phosphorus levels were restored to normal levels at weeks 13 and 26. In one 5/6Nx animal (No. 108) at week 13, a higher level of inorganic phosphorus was observed. This animal revealed not only higher values of renal disorder parameters (serum UN, creatinine and Cys-C) but also a higher value of potassium and lower values of sodium, chloride and lipids (total cholesterol, triglyceride and phospholipids). The serum ALP levels were also increased at week 5, and the higher levels continued at weeks 13 and 26 in most of the 5/6Nx animals (except one case). The serum phospholipids concentration of the 5/6Nx marmosets was significantly higher than the control value at weeks 5 and 26. The mean serum total cholesterol, triglyceride and total bilirubin levels at week 26 also tended to be higher in the 5/6Nx group than in the control group (Table 2).
Table 2. Individual Serum Chemistry Data.
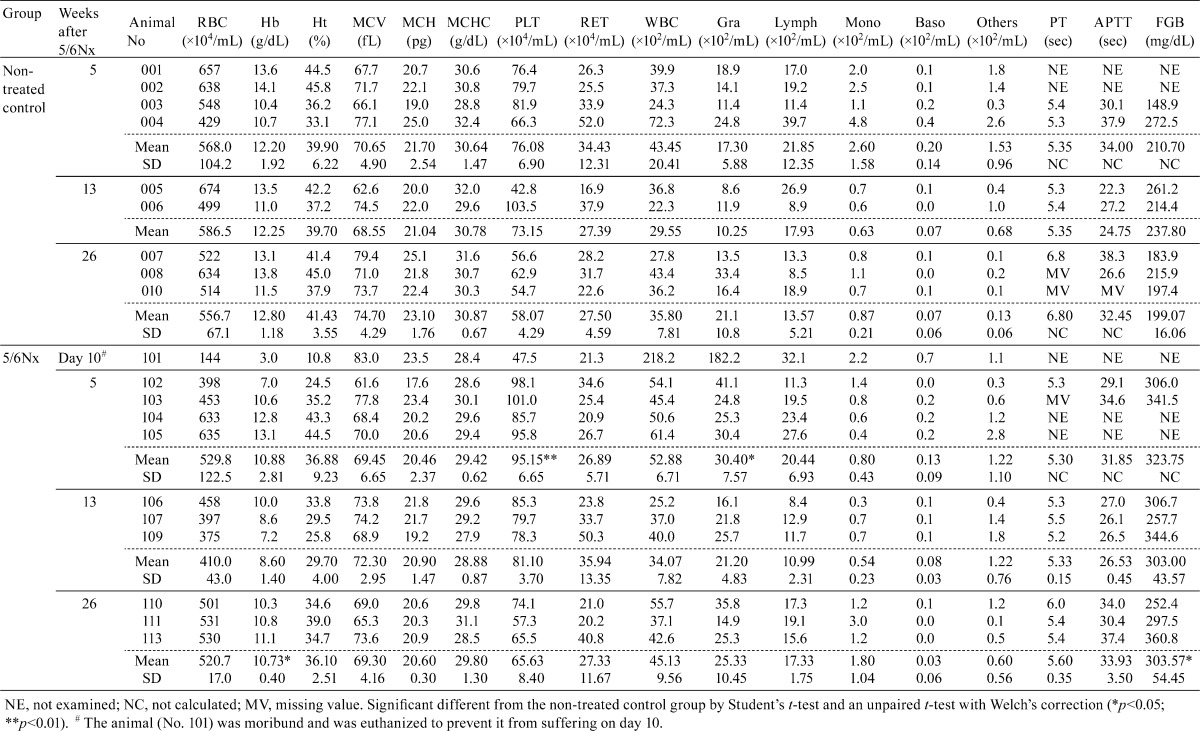
Mean red blood cell counts, and hemoglobin, hematocrit and MCH levels decreased in the 5/6Nx marmosets. The grade of these changes was marked at week 13 after surgery. Regarding the reticulocyte counts, one 5/6Nx animal (No. 104) showed a lower level at week 5, whereas another animal (No. 109) showed a higher level at week 13. Mean granulocyte and platelet counts increased significantly at week 5 in the 5/6Nx marmosets. These parameters were similar to the control values at the end of the study. The mean serum concentration of fibrinogen in the 5/6Nx marmosets increased and continued to remain higher than the control level until the end of the study (Table 3).
Table 3. Individual Hematology Data.
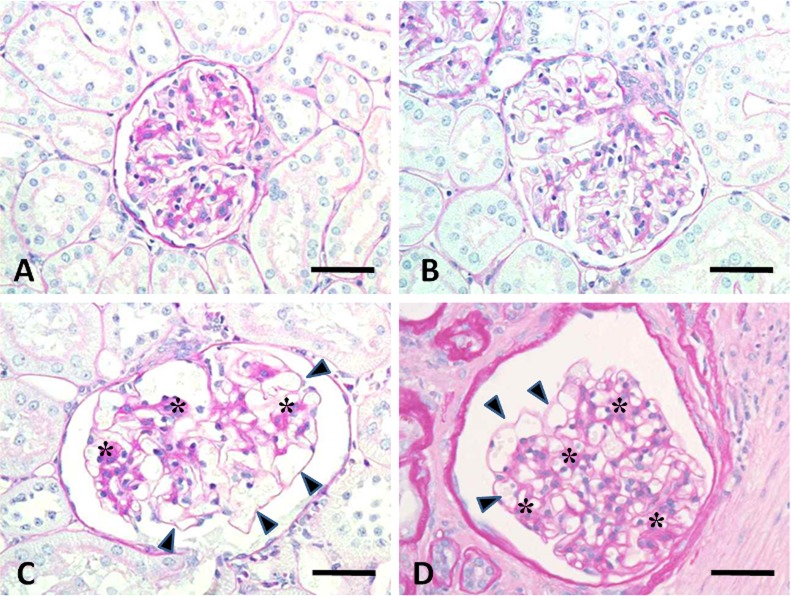
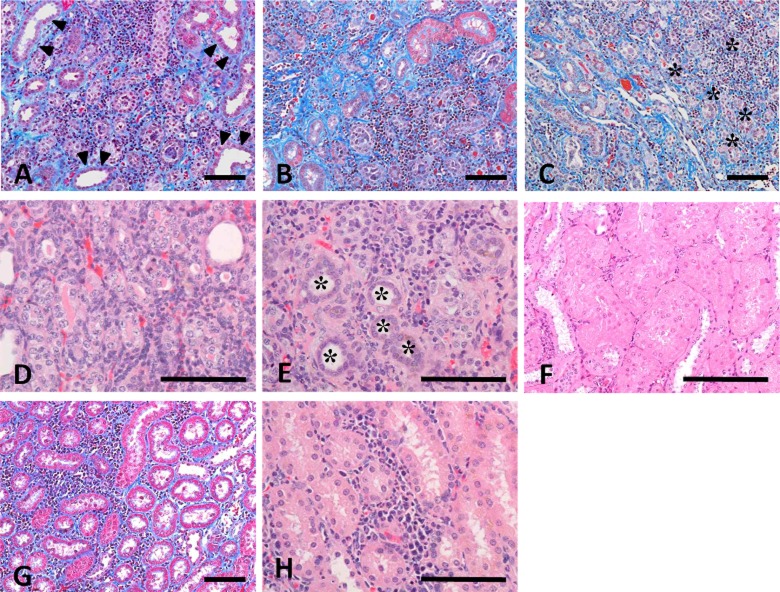
The histopathological changes in the 5/6Nx marmosets are presented in Table 4. The remnant kidneys from 5/6Nx marmosets at week 5 revealed hypertrophic glomeruli with an increase in size of the Bowman’s capsule (Fig. 1B) and degenerative and/or regenerative renal tubules with interstitial fibrosis and inflammatory or mononuclear cell infiltration (Fig. 2A, D). In addition, atrophic or regenerative renal tubules and dilated lumens of distal and/or collecting tubules were also observed. At week 13, hypertrophic glomeruli showed increased dilatation of glomerular capillaries, but there was slight if any mesangial change (Fig. 1C). There were only a few degenerative glomeluri containing swollen glomerular epithelium and nectrotic mesangial cells. Atrophic renal tubules surrounded by thickened basement membrane in the fibrosis area with prominent infiltration of mononuclear cells were apparent (Fig. 2B, E). At week 26, the glomerular changes disappeared except in one 5/6Nx animal (Fig. 1D). The interstitial mononuclear cell infiltration remained at week 26 (Fig. 2C). In one 5/6Nx marmoset, hypertrophic renal tubules were evident at week 26 (Fig. 2F). On the other hand, most of the kidneys in non-treated control marmosets revealed slight to moderate interstitial inflammatory and/or mononuclear cell infiltration. Normal or degenerative renal tubules were surrounded by infiltrated mononuclear cells, but interstitial fibrosis was not apparent (Fig. 2G, H).
Table 4. Individual histopathological findings of the kidney, parathyroid gland, lung, trachea, aorta, bone and bone marrow.
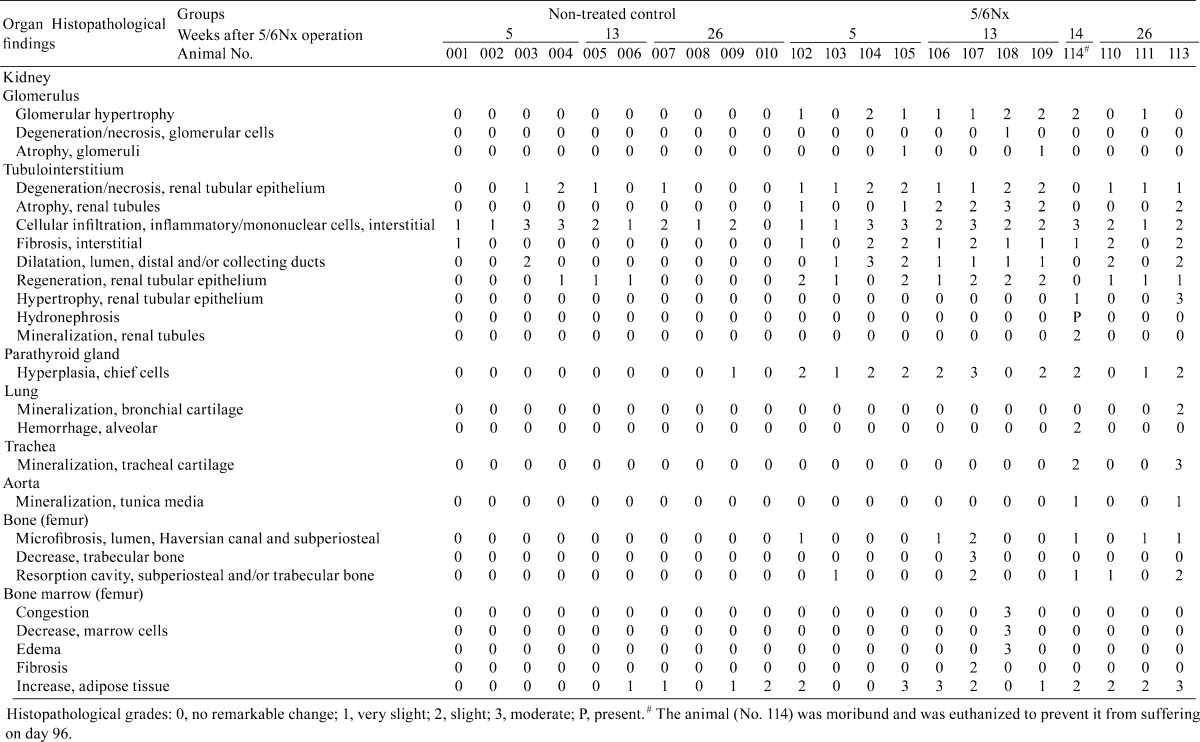
Fig. 1.
Photomicrograph of glomerular changes in kidneys from a non-treated control marmoset (A) and 5/6Nx marmosets at weeks 5 (B), 13 (C) and 26 (D). Hypertrophic glomeruli show dilatation of glomerular capillaries (arrowheads) and an increase in the size of the Bowman’s capsule. A few glomeluli show a very slight increase in mesangial area (asterisks) . Animal Nos.: A, 001; B, 104; C, 109; D, 111. PAS reaction. Scale bar = 50 μm
Fig. 2.
Photomicrograph of tubulointerstitial lesions in kidneys from 5/6Nx marmosets at weeks 5 (A and D), 13 (B and E) and 26 (C and F) and a non-treated control marmoset at week 5 (G and H). A-C: Interstitial fibrosis with inflammatory or mononuclear cells in the remnant kidney of 5/6Nx marmosets at weeks 5 (A) and 13 (B). The interstitial mononuclear cell infiltration remained at week 26 (C). Dilated lumens of distal and/or collecting tubules (arrowleads) and atrophic renal tubules (asterisks) are observed. D: Degenerative, necrotic and regenerative renal tubules at week 5. E: Atrophic renal tubules (asterisks) surrounded by thickened basement membrane in the fibrosis area with mononuclear cell infiltration at week 13. F: Hypertrophic renal tubules in one 5/6Nx animal at week 26. G and H: Interstitial mononuclear cell infiltration without interstitial fibrosis in the kidney of a non-treated animal. Animal Nos.: A, 104; B and E, 109; C and F, 111; D, 105; G and H, 003. A-C and G, Masson’s trichrome stain; D-F and H, H-E stain. Scale bar = 100 μm
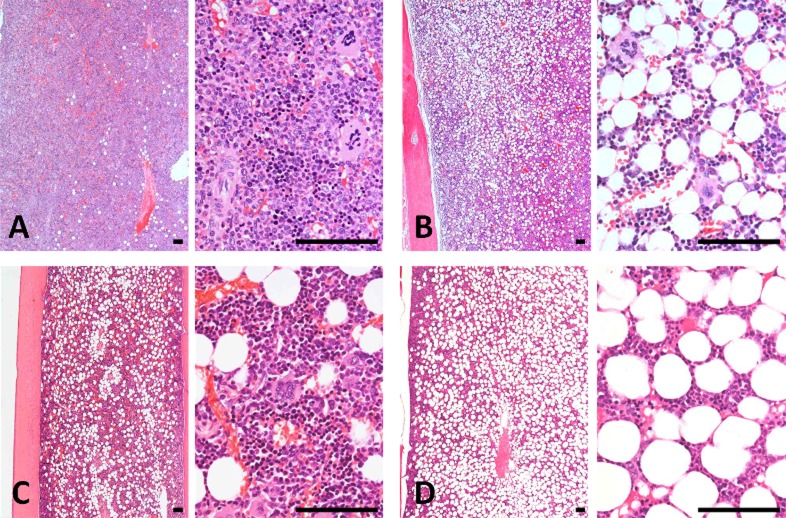
Hyperplasia of the chief cells in the parathyroid glands was observed in the 5/6Nx marmosets at weeks 5, 13 (Fig. 3) and 26. One animal with parathyroid hyperplasia (No. 113) showed mineralization at bronchial and tracheal cartilage (Fig. 4A) and aortic tunica media (Fig. 4B). Multiple site of mineralization were also observed in a moribund animal at week 14 (No. 114). The 5/6Nx marmosets showed bone resorptive changes in the lumens of Haversian canals (Fig. 5B, C) with microfibrosis (Fig. 5D) and in the subperiosteal cortical bone (Fig. 5F) in the femur. In one 5/6Nx animal (No. 107), trabecular bone decreased and was surrounded by fibrous tissue (Fig. 5H). This animal revealed moderate parathyroid hyperplasia (Fig. 3B). In addition, an increase in adipose tissue with a decrease in marrow cells in femoral bone marrow appeared in the 5/6Nx marmosets at weeks 13 (Fig. 6B) and 26 (Fig. 6D) as compared with the levels in non-treated control animals at weeks 13 (Fig. 6A) and 26 (Fig. 6C), respectively.
Fig. 3.
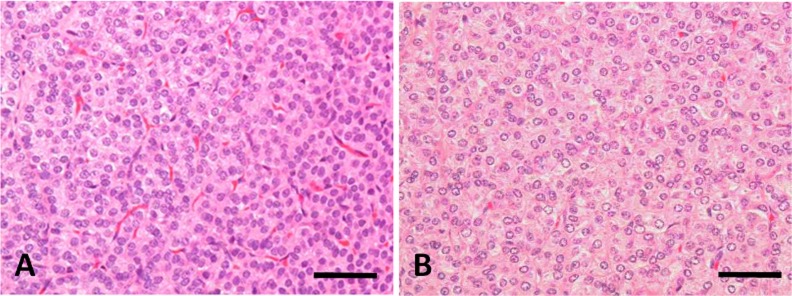
Photomicrograph of parathyroid glands from a non-treated control marmoset (A) and a 5/6Nx marmoset (B) at week 13. Hyperplasia of the chief cells appeared in the 5/6Nx animal. Animal Nos.: A, 006; B, 107. H-E stain. Scale bar = 100 μm
Fig. 4.
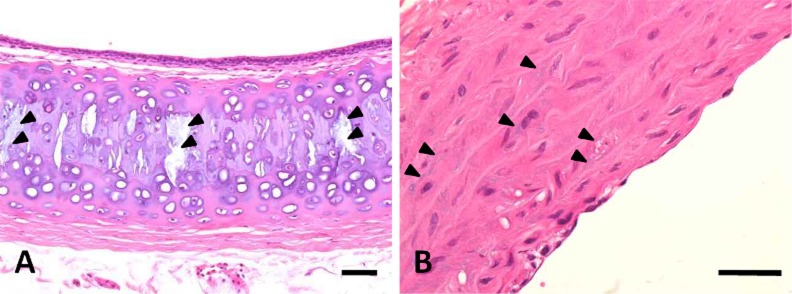
Photomicrograph of mineralization in the trachea (A) and aorta (B) in a 5/6Nx marmoset at week 26. Arrowheads show mineralization in tracheal cartilage and aortic tunica media. Animal No. 113. H-E stain. Scale bar = 100 μm
Fig. 5.
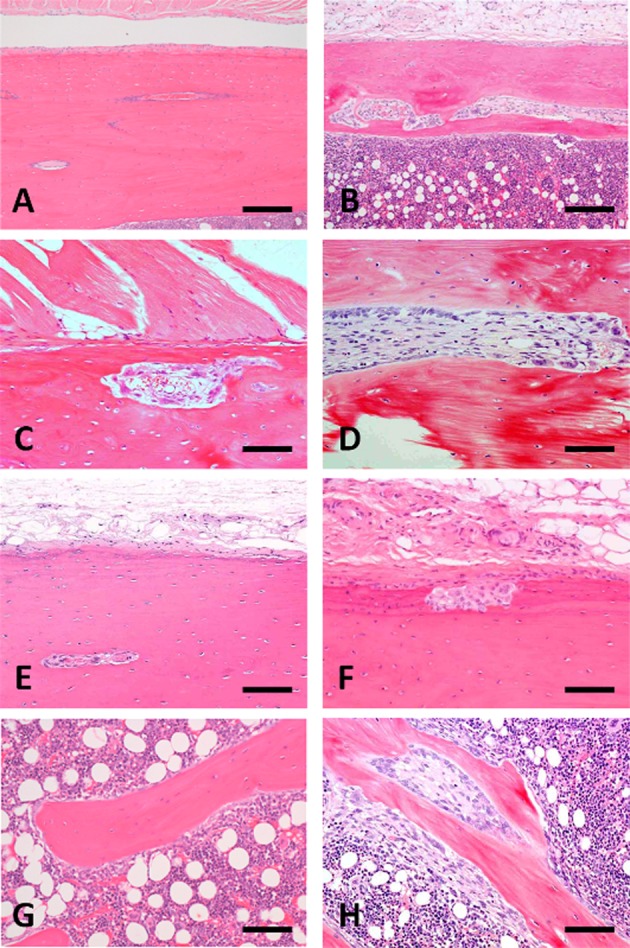
Photomicrograph of femoral bone changes in non-treated control marmosets at weeks 13 (A and G) and 26 (E) and the 5/6Nx marmosets at weeks 13 (B, C, D and H) and 26 (F). Histological structure of femoral cortical bone at week 13(A) and 26 (E) and trabecular bone at week 13 (G) in non-treated control animals. Bone resorptive change (B and C) and microfibrosis (D) in the lumens of Haversian canals of cortical bone and a decrease in trabecular bone with surrounding fibrous tissue (H) present in 5/6Nx animals at week 13. Resorptive changes appear in the subperiosteal cortical bone (F) of 5/6Nx animals at week 26. Animal Nos.: A and G, 005; B, C, D and H, 107; E, 007; F, 113. H-E stain. Scale bar = 100 μm
Fig. 6.
Photomicrograph of femoral bone marrow changes in non-treated control marmosets at weeks 13 (A) and 26 (C) and 5/6Nx marmosets at weeks 13 (B) and 26 (D). An increase in adipose tissue with a decrease in marrow cells appeared at week 13 (B) and 26 (D) in the 5/6Nx animals compared with each of the histological structures in non-treated control animals, respectively. Animal Nos.: A, 006; B, 107; C, 009; D, 113. H-E stain. Scale bar = 100 μm
Discussion
The most widely studied model for the pathophysiology of CRF is the 5/6Nx or the remnant kidney model. Five-sixth experimental nephrectomy has been shown to cause systemic hypertension, proteinuria, a decrease in renal function and progressive glomerlosclerosis26,27,28.
In the 5/6Nx marmosets, the urine volume and elimination of urinary protein increased immediately after nephrectomy, and recovery was slower than that in 5/6Nx rats24. The serum UN, creatinine and Cys-C levels remained higher than those of the control until the end of the study, suggesting that the uremic condition persisted for a long time in the 5/6Nx marmosets.
The remnant kidney of 5/6Nx marmosets showed hypertrophic glomeruli at weeks 5 and 13. The change almost diminished at week 26. The dilatation of glomerular capillaries and increase in size of the Bowman’s capsule might be caused by glomerular hyperperfusion and hyperfiltration depending on a reduction in renal mass29. There were no progressive degenerative or sclerotic glomerular changes in the remnant kidney from the 5/6Nx marmosets until week 26. We previously reported that the thick glomerular basement membrane of marmosets might resist glomerular hyperfiltration and prevent glomerular injury24. In addition, elevated systemic pressure is accompanied by elevated glomerular capillary pressure in several experimental models of hypertensive renal disease, including renal ablation30, 31. In a two-kidney one-clip renal hypertension model, common marmosets showed high blood pressure and plasma renin activity during the first 3 to 5 weeks after renal arterial clipping and return to the control level within 10 weeks32, while rats showed renal hypertension at 4 months after clipping33. Hence, the recovery of systemic blood pressure in marmosets after renal nephrectomy may be correlated with the disappearance of the glomerular hypertrophic changes, which consequently prevent further progressive glomerular injury or prominent proteinuria in the remnant kidney of 5/6Nx marmosets.
Loss of renal function is also associated with development of interstitial fibrosis, which is characterized by loss of the renal parenchyma, tubular atrophy and accumulation of extracellular matrix proteins34, 35. In the 5/6Nx marmosets, higher serum creatinine and Cys-C levels, which are renal functional disorder parameters, were sustained until week 26, and the damaged renal tubules surrounded by thickened basement membrane or interstitial fibrosis with infiltrated mononuclear cells remained at week 26. Plasma fibrinogen, which is a marker of acute inflammation, also continued to be detected at a high level for a long time in the 5/6Nx marmosets, suggesting that the inflammation should continue in the bodies of these animals. In recent years, many researchers have suggested that inflammation has deleterious effects on the progression of renal disease, including diabetes nephropathy36, 37. Continuous renal non-resolving inflammation might be correlated with the formation of tubulointerstitial lesions in the remnant kidney38, 39 and sustain the chronic renal failure condition for a long time in the 5/6Nx marmosets.
Spontaneous progressive nephropathy dominated by glomerular lesions in common marmosets has been reported20, 21. The historical renal lesion in common marmosets is characterized by glomerular lesions with an increase in mesangial matrix, which progresses with aging, and secondary tubulointerstitial lesions, including tubular hyperplasia20. In our study, two non-treated control animals showed remarkable tubulointerstitial nephritis without any glomerular changes and interstitial fibrosis. Both animals were about 42 months old at necropsy and revealed no changes in renal disorder parameters. Tubulointerstitial inflammation is primarily a visible lesion in young marmosets. The intensity of spontaneous glomerular changes with deposition of immunoglobulin might be higher in marmosets over 7 years old29. The present study used young animals that were about 3.5 to 5 years old. When older animals are used, the renal changes with spontaneous glomerular lesions in the remnant kidney may develop into progressive lesions showing glomerular- and tubulointerstitial nephropathy.
Renal osteodystrophy is an almost universal consequence in CRF patients. Two major subtypes of renal osteodystrophy can be distinguished in humans on the basis of histology: lesions characterized by high bone turnover and low bone turnover40. High-turnover renal osteodystrophy is related to a marked and persistent increase in circulating levels of parathyroid hormone (PTH) with parathyroid cell hyperplasia41 and shows characteristic bone histology, including an increase in number and size of osteoclasts, increased osteoblastic activity, enhanced resorption and osteoid surfaces. The 5/6Nx marmosets showed higher serum ALP activity and parathyroid cell hyperplasia throughout the study period. In addition, a transient increase of serum calcium and inorganic phosphorus levels was noted. Some of the 5/6Nx animals had bone resorptive changes with microfibrosis in the lumens of Haversian canals and subperiosteum in cortical bone and trabecular bone. An animal with a marked bone lesion also had higher levels of serum ALP activity and a higher grade of hyperplastic lesion of parathyroid. Hence the bone in the 5/6Nx marmosets might be in a high turnover state. On the other hand, 5/6Nx rats previously showed an increase of PTH and bone resorption but suppressed bone formation42. During progressive CRF development in 5/6Nx rats, the total plasma calcium and phosphate concentrations were almost unchanged and ALP levels were reduced43.
Calcitriol administration was associated with a significant suppression of bone resorption and a marked increase in all osteoid parameters in the CRF condition43, 44. The common marmoset has extremely high circulating levels of 1,25-(OH)2D3 without exhibiting hypercalcemia45, 46 and has an abnormal 1,25-(OH)2D3 receptor system, suggesting that the marmoset monkey has end-organ resistance to 1,25(OH)2D3. Common marmosets at one year of age with low serum 25-hydroxyvitamin D3 and 24R, 25-dihydroxyvitamin D3, low calcium levels and high ALP levels may exhibit osteomalacia-like bone changes resembling vitamin D-dependent rickets type II47,48,49. The incidence of bone abnormalities in marmosets dramatically declines, especially in young, growing animals. This may be due to the large quantities of vitamin D3 in the commercial diet (2750 IU of vitamin D3 in 100 g of CSM-1M pellets). The 5/6Nx marmosets in the present study showed a decrease in body weight immediately after 5/6Nx surgery; however, their body weights were stable, and feeding did not cause any marked difference between the 5/6Nx animals and control animals (data not shown). Hence, the osteodystrophy-like changes in the 5/6Nx marmosets were probably secondary to renal failure and not nutritional in origin, although feeding of a high vitamin D3 diet may have affected the grade of the bone lesions. Control of the vitamin D3 concentration in the diet may influence bone lesion progression in 5/6Nx marmosets.
In chronic kidney disease (CKD) patients, a relationship between disorders of mineral metabolism (elevated phosphorus levels), abnormal bone (osteodystrophy), and vascular calcification appeared50. In the 5/6Nx marmosets, a transient increase of serum calcium and inorganic phosphorus levels was noted, and the levels gradually returned to normal by the end of the study. Hence, most of the 5/6Nx animals at weeks 13 and 26 did not show systemic mineralization including the vascular system (excluding two animals). One 5/6Nx animal at week 26 and the moribund animal at week 14 showed mineralization in the medial artery and tracheal and/or bronchial cartilage with hyperparathyroid-related bone changes. The 5/6Nx animal at week 26 revealed hypercalcemia (5.1 mg/dL vs 4.3 to 4.5 mg/dL in control animals). On the other hand, the moribund animal might have been in a severe renal failure state due to a shift to hydronephrosis.
In the other biochemical parameters, the 5/6Nx marmosets demonstrated slight changes in liver function markers, showing higher values of AST and phospholipids at weeks 5 and 26 and higher values of total cholesterol, triglyceride and total bilirubin at week 26. Liver metabolism dysfunction could also play a role in the lipid profile changes encountered in the CRF rats: increased values of total cholesterol and tryglycerides51. Lipid abnormalities are observed in CKD patients52, 53 and are a risk factor for cardiovascular events in CKD patients54. The lipid changes encountered in CKD patients might be reproduced in 5/6Nx marmosets.
Anemia is a common complication of CKD. In the 5/6Nx marmosets, the red blood cell counts and hemoglobin and hematocrit values were lower at week 5. Moreover, the reticulocyte count was also lower, which suggests a failure of the erythropoietin (EPO) response mechanism due to putatively insufficient erythropoietin production associated with the renal mass reduction. At week 13, despite the increase in reticulocyte counts, cellularity in the femoral bone marrow was reduced at weeks 13 and 26. Mechanisms involved in the pathogenesis of renal anemia include deficiency of EPO, chronic inflammation, iron deficiency and a shortened half-life of erythrocytes. Although the prevalence of anemia increases with diminishing renal function, normochromic and normocytic anemia is observed at a relatively early stage of renal dysfunction. Hypoplasia of the erythroid precursors usually found in the bone marrow with normal leukopoiesis and megakaryocytopoiesis55. Renal EPO is mainly produced by the interstitial fibroblast in the deep cortex and outer medulla in the kidney56, 57. Patients with renal failure may develop anemia and refractory EPO because of mechanisms associated with chronic inflammation58. In addition, hepcidin that evolves as a potent regulator of the body’s iron distribution, is indicated as a potential of dysregulation of iron metabolism in CKD patients59, 60, and excessive hepcidin production contributes to the functional iron deficiency and associated anemia during inflammation states. Hence, the continuous chronic inflammation in the remnant kidney of 5/6Nx marmosets might contribute to the pathogenesis of renal anemia by decreasing EPO production and sensitivity or the available iron for hematopoiesis.
We performed 5/6Nx in female marmosets and observed the pathophysiological conditions of renal disorder and chronic renal failure for 26 weeks. The 5/6Nx marmosets showed a significant increase in serum parameters correlated with the renal disorder and renal failure immediately after the 5/6Nx surgery. The changes in these renal disorder parameters occurred slowly. In the remnant kidneys, tubulointerstitial changes comprised degenerative or atrophic renal tubules surrounded by an interstitial fibrosis area with mononuclear cell infiltration remained for a long time. There were no progressive glomerular changes including glomerulosclerosis. In addition, hyperplastic parathyroid glands, a high turnover state of osteodystrophy in the femoral bone with higher serum ALP activity and anemia with slight hypocellularity of bone marrow were evident. During the progression of CRF, the state, intensity or grade of complicating morbidity is influenced by many factors such as systemic hypertension, inflammation, uremic toxins, physical condition, aging and nutritional condition. By regulating or applying the loads of these factors, 5/6Nx marmosets may develop a pathophysiological condition more similar to the clinical state of human CRF patients.
The 5/6Nx marmosets showed a stable CRF condition for a long time and showed some characteristic disorders similar to those in human CRF patients. These diagnostic aspects might be a species-specific anatomical and physiological signature reflecting the nutritional condition. Although it is necessary to study and obtain more detailed information about biological features including age and gender, the CRF model using 5/6Nx marmosets might become a useful method of evaluating the unique mechanism of CRF development.
Declaration of Conflicting Interest: There are no conflicts of interest to declare or financial interests to disclose.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Morrison AB. Experimentally induced chronic renal insufficiency in the rat. Lab Invest. 11: 321–332. 1962. [PubMed] [Google Scholar]
- 2.Gretz N, Waldherr R, and Strauchi M. The remnant kidney model. In: Experimental and Genetic Rat Models of Chronic Renal Failure. N Gretz, and M Strauch (eds). Karger, Basel. 1–28. 1993. [Google Scholar]
- 3.Griffin KA, Picken M, and Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 4: 2023–2031. 1994. [DOI] [PubMed] [Google Scholar]
- 4.Breyer MD, Böttinger E, Brosius FC, 3rd , Coffman TM, Harris RC, Heilig CW, and Sharma K. AMDCC Mouse models of diabetic nephropathy. J Am Soc Nephrol. 16: 27–45. 2005. [DOI] [PubMed] [Google Scholar]
- 5.Eddy AA, López-Guisa JM, Okamura DM, and Yamaguchi I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr Nephrol. 27: 1233–1247. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, and Meldrum DR. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res. 67: 594–603. 2005. [DOI] [PubMed] [Google Scholar]
- 7.Korstanje R, and DiPetrillo K. Unraveling the genetics of chronic kidney disease using animal models. Am J Physiol Renal Physiol. 287: F347–F352. 2004. [DOI] [PubMed] [Google Scholar]
- 8.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, and Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes. 54: 2628–2637. 2005. [DOI] [PubMed] [Google Scholar]
- 9.Fleck C, Appenroth D, Jonas P, Koch M, Kundt G, Nizze H, and Stein G. Suitability of 5/6 nephrectomy (5/6NX) for the induction of interstitial renal fibrosis in rats—influence of sex, strain, and surgical procedure. Exp Toxicol Pathol. 57: 195–205. 2006. [DOI] [PubMed] [Google Scholar]
- 10.Nishijima K, Saitoh R, Tanaka S, Ohsato-Suzuki M, Ohno T, and Kitajima S. Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology. 13: 439–443. 2012. [DOI] [PubMed] [Google Scholar]
- 11.Mansfield K. Marmoset models commonly used in biomedical research. Comp Med. 53: 383–392. 2003. [PubMed] [Google Scholar]
- 12.Orsi A, Rees D, Andreini I, Venturella S, Cinelli S, and Oberto G. Overview of the marmoset as a model in nonclinical development of pharmaceutical products. Regul Toxicol Pharmacol. 59: 19–27. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Home-Office, Statistics of Scientific Procedures on Living Animals Great Britain. 2000.
- 14.Smith D, Trennery P, Farningham D, and Klapwijk J. The selection of marmoset monkeys (Callithrix jacchus) in pharmaceutical toxicology. Lab Anim. 35: 117–130. 2001. [DOI] [PubMed] [Google Scholar]
- 15.Zühlke U, and Weinbauer G. The common marmoset (Callithrix jacchus) as a model in toxicology. Toxicol Pathol. 31(Suppl): 123–127. 2003. [DOI] [PubMed] [Google Scholar]
- 16.Brack M, and Rothe H. Chronic tubulointerstitial nephritis and wasting disease in marmosets (Callithrix jacchus). Vet Pathol. 18(Suppl 6): 45–54. 1981. [DOI] [PubMed] [Google Scholar]
- 17.Brack M. IgM-mesangial nephropathy in callithricids. Vet Pathol. 25: 270–276. 1988. [DOI] [PubMed] [Google Scholar]
- 18.Kaspareit J, Friderichs-Gromoll S, Buse E, and Habermann G. Background pathology of the common marmoset (Callithrix jacchus) in toxicological studies. Exp Toxicol Pathol. 57: 405–410. 2006. [DOI] [PubMed] [Google Scholar]
- 19.David JM, Dick EJ, Jr , and Hubbard GB. Spontaneous pathology of the common marmoset (Callithrix jacchus) and tamarins (Saguinus oedipus, Saguinus mystax). J Med Primatol. 38: 347–359. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isobe K, Adachi K, Hayashi S, Ito T, Miyoshi A, Kato A, and Suzuki M. Spontaneous glomerular and tubulointerstitial lesions in common marmosets (Callithrix jacchus). Vet Pathol. 49: 839–845. 2012. [DOI] [PubMed] [Google Scholar]
- 21.Yamada N, Sato J, Kanno T, Wako Y, and Tsuchitani M. Morphological study of progressive glomerulonephropathy in common marmosets (Callithrix jacchus). Toxicol Pathol. 41: 1106–1115. 2013. [DOI] [PubMed] [Google Scholar]
- 22.Chamanza R, Marxfeld HA, Blanco AI, Naylor SW, and Bradley AE. Incidences and range of spontaneous findings in control cynomolgus monkeys (Macaca fascicularis) used in toxicity studies. Toxicol Pathol. 38: 642–657. 2010. [DOI] [PubMed] [Google Scholar]
- 23.Sato J, Doi T, Kanno T, Wako Y, Tsuchitani M, and Narama I. Histopathology of incidental findings in cynomolgus monkeys ( macaca fascicularis ) used in toxicity studies. J Toxicol Pathol. 25: 63–101. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki Y, Yamaguchi I, Onoda N, Saito T, Myojo K, Imaizumi M, Takada C, Kimoto N, Takaba K, and Yamate J. Differential renal glomerular changes induced by 5/6 nephrectomization between common marmoset monkeys (Callithrix jacchus) and rats. Exp Toxicol Pathol. 65: 667–676. 2013. [DOI] [PubMed] [Google Scholar]
- 25.Chanutin A, and Ferris EB. Experimental renal insufficiency produced by partial nephrectomy. Arch Intern Med. 49: 767–787. 1932. [Google Scholar]
- 26.Chow KM, Liu AC, and Chang TMS. Animal remnant kidney model of chronic renal failure revisited. Hong Kong J Nephrol. 5: 57–64. 2003. [Google Scholar]
- 27.Dai C, Kiss LP, and Liu Y. Animal models of kidney disease. In: Source of Models for Biomedical Research. PM Conn (ed). Humana Press, New Jersey. 657–664. 2008. [Google Scholar]
- 28.Fujihara CK, Antunes GR, Mattar AL, Malheiros DM, Vieira JM, Jr , and Zatz R. Chronic inhibition of nuclear factor-kappaB attenuates renal injury in the 5/6 renal ablation model. Am J Physiol Renal Physiol. 292: F92–F99. 2007. [DOI] [PubMed] [Google Scholar]
- 29.Arataki M. Experimental researches on the compensatory enlargement of the surviving kidney after unilateral nephrectomy (albino rat). Am J Anat. 36: 437–450. 1926. [Google Scholar]
- 30.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, and Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 241: F85–F93. 1981. [DOI] [PubMed] [Google Scholar]
- 31.Anderson S, Meyer TW, Rennke HG, and Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 76: 612–619. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood JM, Gulati N, Michel JB, and Hofbauer KG. Two-kidney, one clip renal hypertension in the marmoset. J Hypertens. 4: 251–254. 1986. [DOI] [PubMed] [Google Scholar]
- 33.Russell GI, Brice JM, Bing RF, Swales JD, and Thurston H. Haemodynamic changes after surgical reversal of chronic two-kidney, one-clip hypertension in the rat. Clin Sci (Lond). 61(Suppl 7): 117s–119s. 1981. [DOI] [PubMed] [Google Scholar]
- 34.Schainuck LI, Striker GE, Cutler RE, and Benditt EP. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1: 631–641. 1970. [DOI] [PubMed] [Google Scholar]
- 35.Mackensen S, Grund KE, Sindjić M, and Bohle A. Influence of the renal cortical interstitium on the serum creatinine concentration and serum creatinine clearance in different chronic sclerosing interstitial nephritides. Nephron. 24: 30–34. 1979. [DOI] [PubMed] [Google Scholar]
- 36.Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol. 24: 1445–1452. 2009. [DOI] [PubMed] [Google Scholar]
- 37.Wada J, and Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 124: 139–152. 2013. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 7: 684–696. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan C, and Ding A. Nonresolving inflammation. Cell. 140: 871–882. 2010. [DOI] [PubMed] [Google Scholar]
- 40.Sherrard DJ, Hercz G, Pei Y, Maloney NA, Greenwood C, Manuel A, Saiphoo C, Fenton SS, and Segre GV. The spectrum of bone disease in end-stage renal failure—an evolving disorder. Kidney Int. 43: 436–442. 1993. [DOI] [PubMed] [Google Scholar]
- 41.Naveh-Many T, Rahamimov R, Livni N, and Silver J. Parathyroid cell proliferation in normal and chronic renal failure rats. The effects of calcium, phosphate, and vitamin D. J Clin Invest. 96: 1786–1793. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moscovici A, Bernheim J, Popovtzer MM, and Rubinger D. Renal osteodystrophy in rats with reduced renal mass. Nephrol Dial Transplant. 11(Suppl 3): 146–152. 1996. [DOI] [PubMed] [Google Scholar]
- 43.Jablonski G, Klem KH, Attramadal A, Dahl E, Rønningen H, Gautvik KM, Haug E, and Gordeladze JO. Surgically induced uremia in rats. I: Effect on bone strength and metabolism. Biosci Rep. 13: 275–287. 1993. [DOI] [PubMed] [Google Scholar]
- 44.Jablonski G, Mortensen BM, Klem KH, Mosekilde L, Danielsen CC, and Gordeladze JO. Vitamin D3 analogs and salmon calcitonin partially reverse the development of renal osteodystrophy in rats. Calcif Tissue Int. 57: 385–391. 1995. [DOI] [PubMed] [Google Scholar]
- 45.Shinki T, Shiina Y, Takahashi N, Tanioka Y, Koizumi H, and Suda T. Extremely high circulating levels of 1 alpha,25-dihydroxyvitamin D3 in the marmoset, a new world monkey. Biochem Biophys Res Commun. 114: 452–457. 1983. [DOI] [PubMed] [Google Scholar]
- 46.Adams JS, Gacad MA, Braker AJ, Gonzales B, and Rude RK. Serum concentrations of 1,25-dihydroxyvitamin D3 in Platyrrhini and Catarrhini: a phylogenetic appraisal. Am J Primatol. 9: 219–224. 1985. [DOI] [PubMed] [Google Scholar]
- 47.Chalmers DT, Murgatroyd LB, and Wadsworth PF. A survey of the pathology of marmosets (Callithrix jacchus) derived from a marmoset breeding unit. Lab Anim. 17: 270–279. 1983. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi A, Kohno Y, Yamazaki T, Takahashi N, Shinki T, Horiuchi N, Suda T, Koizumi H, Tanioka Y, and Yoshiki S. Bone in the marmoset: a resemblance to vitamin D-dependent rickets, type II. Calcif Tissue Int. 39: 22–27. 1986. [DOI] [PubMed] [Google Scholar]
- 49.Flurer CI, Wetzel A, Rambeck WA, and Zucker H. Osteomalacia in marmosets: model of human vitamin D resistant rickets type 2. Adv Anim Physiol Anim Nutr. 20: 74–77. 1990. [Google Scholar]
- 50.Moe SM, and Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 95: 560–567. 2004. [DOI] [PubMed] [Google Scholar]
- 51.Garrido P, Reis F, Costa E, Teixeira-Lemos E, Parada B, Alves R, Piloto N, Sereno J, Figueiredo A, Pinto R, Carvalho L, Rocha-Pereira P, Belo L, Santos-Silva A, and Teixeira F. Characterization of a rat model of moderate chronic renal failure—focus on hematological, biochemical, and cardio-renal profiles. Ren Fail. 31: 833–842. 2009. [DOI] [PubMed] [Google Scholar]
- 52.Johnson DW, Armstrong K, Campbell SB, Mudge DW, Hawley CM, Coombes JS, Prins JB, and Isbel NM. Metabolic syndrome in severe chronic kidney disease: Prevalence, predictors, prognostic significance and effects of risk factor modification. Nephrology (Carlton). 12: 391–398. 2007. [DOI] [PubMed] [Google Scholar]
- 53.Tsimihodimos V, Dounousi E, and Siamopoulos KC. Dyslipidemia in chronic kidney disease: an approach to pathogenesis and treatment. Am J Nephrol. 28: 958–973. 2008. [DOI] [PubMed] [Google Scholar]
- 54.Chan CM. Hyperlipidaemia in chronic kidney disease. Ann Acad Med Singapore. 34: 31–35. 2005. [PubMed] [Google Scholar]
- 55.Nangaku M, and Eckardt KU. Pathogenesis of renal anemia. Semin Nephrol. 26: 261–268. 2006. [DOI] [PubMed] [Google Scholar]
- 56.Bachmann S, Le Hir M, and Eckardt KU. Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem. 41: 335–341. 1993. [DOI] [PubMed] [Google Scholar]
- 57.Obara N, Suzuki N, Kim K, Nagasawa T, Imagawa S, and Yamamoto M. Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood. 111: 5223–5232. 2008. [DOI] [PubMed] [Google Scholar]
- 58.Macdougall IC, and Cooper AC. Hyporesponsiveness to erythropoietic therapy due to chronic inflammation. Eur J Clin Invest. 35(Suppl 3): 32–35. 2005. [DOI] [PubMed] [Google Scholar]
- 59.Deicher R, and Hörl WH. New insights into the regulation of iron homeostasis. Eur J Clin Invest. 36: 301–309. 2006. [DOI] [PubMed] [Google Scholar]
- 60.Tomosugi N, Kawabata H, Wakatabe R, Higuchi M, Yamaya H, Umehara H, and Ishikawa I. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood. 108: 1381–1387. 2006. [DOI] [PubMed] [Google Scholar]