Abstract
The microtubule inhibitor colchicine is cardiotoxic and is suggested to impair impulse formation and conduction. However, little is known about the electrocardiographic (ECG) changes induced by colchicine in experimental animals and the detailed pathogenesis of its cardiotoxicity. Therefore, we analyzed cardiotoxicity in colchicine-treated rats using electrocardiographic, histopathological and blood-chemistry approaches. A telemetry device for transmitting ECG data was implanted into male Crl:CD(SD) rats, and ECG tracings were obtained. At 6 weeks of age, 1.25 mg/kg colchicine was injected intravenously once daily for 2 consecutive days, and ECG waveforms and heart rate variability were analyzed. Furthermore, 1.25 mg/kg colchicine or vehicle was injected for 1 or 2 consecutive days in other rats at 6 weeks of age. One day after the final dosing, heart and blood samples were taken for histopathological and bloodchemical examination. ECG analysis revealed a prolonged RR interval, QRS duration, PR interval and QT interval. Heart rate variability analysis showed an increase in high frequency (HF) components as an index of parasympathetic nervous activity. In blood chemical examinations, colchicine induced high levels of parameters of cardiac injury and low levels and/or variations in Ca, inorganic phosphorus, potassium and chloride. Histopathologically, colchicine-treated rats showed eosinophilic granular degeneration and cytoplasmic vacuolation of ventricular myocardial cells but no remarkable change in the atrioventricular node. Not only blood chemical and histopathological changes but also ECG changes were induced in colchicine-treated rats, which indicated a decrease in myocardium excitability and conductivity, and these changes might be related to increased parasympathetic nervous activity and low blood Ca levels.
Keywords: colchicine, cardiotoxicity, electrocardiogram, heart rate variability, parasympathetic nerve, rat
Colchicine is a microtubule disassembling agent that has been known for a long time. As this historic agent inhibits polymerization of α, β-tubulin heterodimers of microtubules1, colchicine has many microtubule-related medicinal virtues. For example, colchicine has been appreciated as a medical drug for treating gout2, 3, pericarditis4, 5, and dermatological disorders6 and as an immune suppressant7. Moreover, drugs related to colchicine are anticipated to be chemotherapeutic agents, because they inhibit cell division and vascularization of tumors8.
At the same time, the cardiotoxicity of colchicine and other microtubule disassembling agents has been recognized in situations of unexpected use for medical treatment9, acute poisoning by ingesting plants containing microtubule disassembling agents10, 11 and side-effects of chemotherapeutic use12,13,14. Moreover, it has been suggested that colchicine may have a toxic effect on cardiac impulse generation and conduction in humans10. Experimental studies have demonstrated that colchicine induces impairment of the intrinsic contractility of the myocardium15 and histopathologic changes in the heart16, 17.
As cardiotoxicity could directly lead to mortality, colchicine and related drugs require especially careful empirical study in nonclinical studies for pharmaceutical development. In such a case, analysis of the rat electrocardiogram (ECG) might provide valuable data about the quality and magnitude of cardiotoxicity. Indeed, the utility of rat ECG analysis in toxicology has been claimed in the last few years18. Nevertheless, few in vivo experimental studies have been reported, and less is known about the electrocardiographic changes induced by colchicine in experimental animals. Therefore, we examined the acute cardiotoxicity of colchicine in rats by ECG analysis, in addition to blood chemical and histopathological analyses.
Electrocardiographic experiments were performed using 3 male Crl:CD(SD) rats (Charles River Laboratories Japan, Kanagawa, Japan). At 5 weeks of age, a small telemetry device (weight = 3.9 g, volume = 1.9 cc; TA10ETA-F20, Data Sciences International, New Brighton, MN, USA) for transmitting ECG data was implanted into the dorsal subcutaneous region under systemic anesthesia with pentobarbital sodium. Paired wire electrodes that came with the telemetry device were placed under the skin of the dorsal and ventral thorax to record the apex-base (A-B) lead ECG. One week after the surgery, ECG signals were recorded from each rat in a cage that had been placed on a signalreceiving board (RA1610, Data Sciences International, New Brighton, MN, USA). ECG data were continuously sampled at 1 msec intervals, and all data analysis of ECG-wave components was performed using an ECG processor analyzing system (SRV2W, Softron, Tokyo, Japan) equipped on a personal computer in series with an analog-digital converter; the ECG data were stored on an external hard disk. During the period of ECG recording, 1.25 mg/kg colchicine (Wako Pure Chemical Industries, Osaka, Japan) dissolved in 5% glucose at a volume of 1 mL/kg was administered intravenously into the rats once daily for 2 consecutive days. This dosing schedule for colchicine is known to induce histopathological changes in the rat heart based on our previous study17. The ECG-wave components (RR interval, QRS duration, PR interval and QT interval) were analyzed in 10 consecutive beats, and power spectral analysis of heart rate variability was performed at 23, 21, 18 and 12 hours before the first injection; 1, 3, 6, 12 and 23 hours after the first injection; and 1, 3, 6 and 12 hours after the second injection. The frequency component of the RR interval on ECG was analyzed based on the Cooley-Tukey Fast Fourier Transform algorithm19. Two major spectral components, low frequency (LF: 0.1 – 1.0 Hz) and high frequency (HF: 1.0 – 3.0 Hz) power, were detected, and then the HF power was used as an index of parasympathetic nervous activity; the LF/HF ratio was used as an index of balance between sympathetic and parasympathetic nervous activity20.
Histopathological and blood chemical experiments were performed using 6 male Crl:CD(SD) rats (Charles River Laboratories Japan, Kanagawa, Japan). At 6 weeks of age, colchicine (Sigma-Aldrich, Tokyo, Japan) or vehicle (5% glucose) was administered (n = 3/group) once daily for 2 consecutive days. On the day after the last administration, 18-hour fasted animals were given pentobarbital anesthesia, and blood samples were collected from the abdominal aorta. Blood samples were treated with heparin to obtain plasma, and aspartate aminotransferase (AST), lactate dehydrogenase (LDH1–5), creatine kinase (CK-MM, MB, BB and m-CK), calcium (Ca), inorganic phosphorus (InP), Na+, K+ and Cl- were analyzed. Moreover, cardiac troponin T (cTnT) in heparinized whole blood was measured, and a semiquantitative result was obtained according to an earlier report21 with a Trop T sensitive kit (Roche Diagnostics, Tokyo, Japan), a monoclonal antibody-based immunoassay kit. After collection of whole blood, the heart, gastrocnemius muscle, liver, kidneys and stomach were extracted and immediately fixed in 10% neutral phosphate-buffered formalin. Also, hearts were cross-sectioned at one point in atria with the atrioventricular node and two points in ventricles as described in our previous report17. Fixed organs were embedded in paraffin and sectioned at a thickness of 4–6 μm. The specimens were stained with hematoxylin and eosin (HE) or subjected to immunohistochemical staining with anti-cleaved caspase-3 rabbit polyclonal antibody (Cell Signaling Technology, Danvers, MA, USA) followed by horseradish peroxidase-labeled goat antibody against rabbit Ig (Dako, Tokyo, Japan), and visualized with 3,3’-diaminobenzidine. Observation of these specimens was performed using a light microscope (BX51, Olympus Corporation, Tokyo, Japan). In addition, blood chemical experiments for the rats administered 1.25 mg/kg colchicine a single time intravenously (n = 3) were performed separately 24 hours after injection in a similar way.
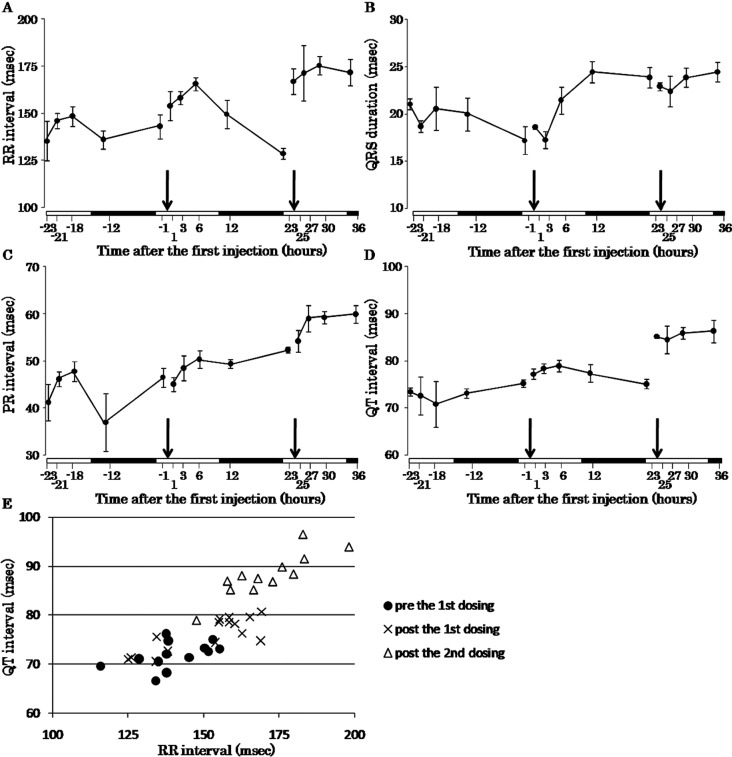
Electrocardiographic analysis revealed that the RR interval and QT interval were prolonged at 1 hour after the second injection of colchicine (Fig. 1A, D, E). The QRS duration and PR interval became gradually prolonged beginning at the first injection of colchicine (Fig. 1B, C). The results of power spectral analysis showed that the LF/HF ratio, as an index of balance between sympathetic and parasympathetic nervous activity, decreased at 6 hours after the second injection (Fig. 2A). The HF power, as an index of parasympathetic nervous activity, increased at 1 hour after the second injection of colchicine (Fig. 2B).
Fig. 1.
Time-response curves of the RR interval (A), QRS duration (B), PR interval (C), QT interval (D) and QT-RR plot (E) before and after injections of colchicine. Arrows indicate time points of colchicine injection. White bars and filled bars on horizontal axes indicate the light period and dark period, respectively. Error bars indicate the SE.
Fig. 2.
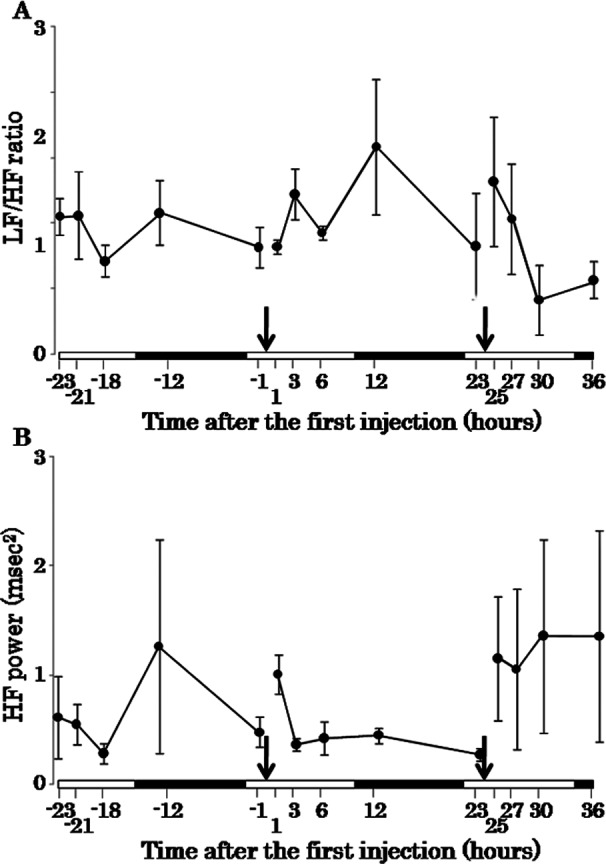
Time-response curves of the LF/HF ratio (A) and HF power (B) before and after injections of colchicine. The LF/HF ratio is an index of balance between sympathetic and parasympathetic nervous activity, and HF power is an index of parasympathetic nervous activity. Arrows indicate time points of colchicine injection. White bars and filled bars on horizontal axes indicate the light period and dark period, respectively. Error bars indicate the SE.
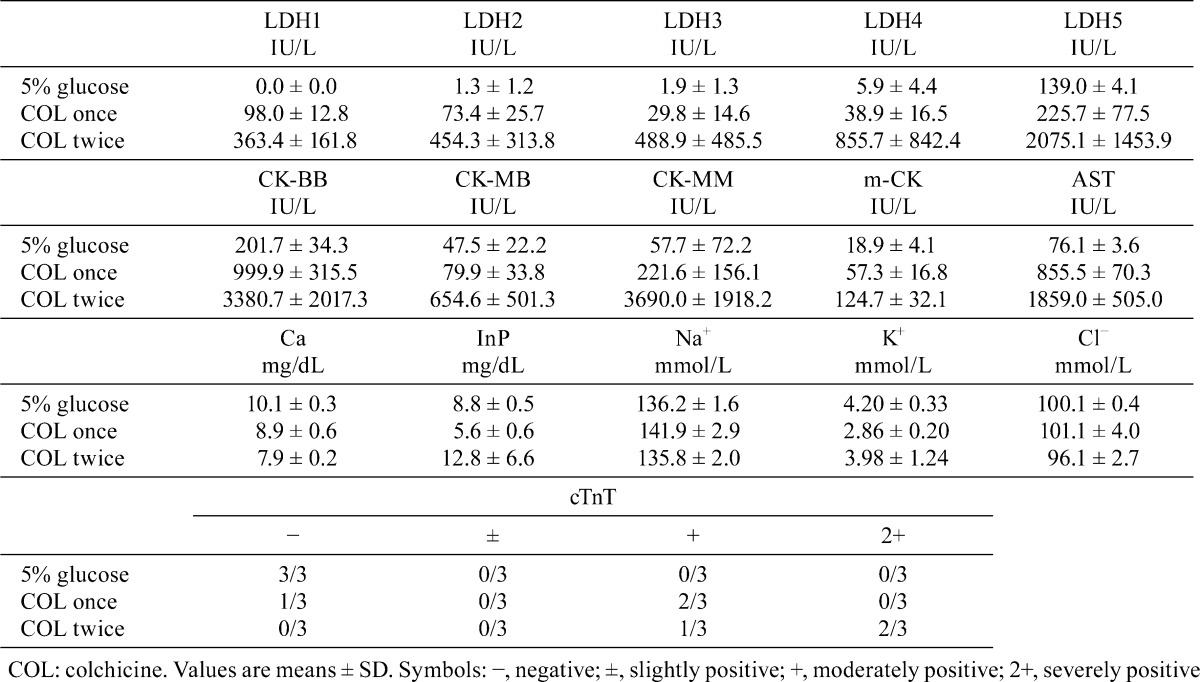
Regarding the blood chemical data, high levels of AST, LDH (1–5), CK (CK-BB, CK-MB, CK-MM and m-CK) and cTnT and a low level of Ca were detected in rats treated with colchicne once (Table 1). Moreover, low and varying levels of InP, K+ and Cl− in plasma were detected (Table 1). These changes were more clear in rats treated with colchicine twice (Table 1).
Table 1. Blood Chemical Data of Rats Administered Colchicine or 5% Glucose.
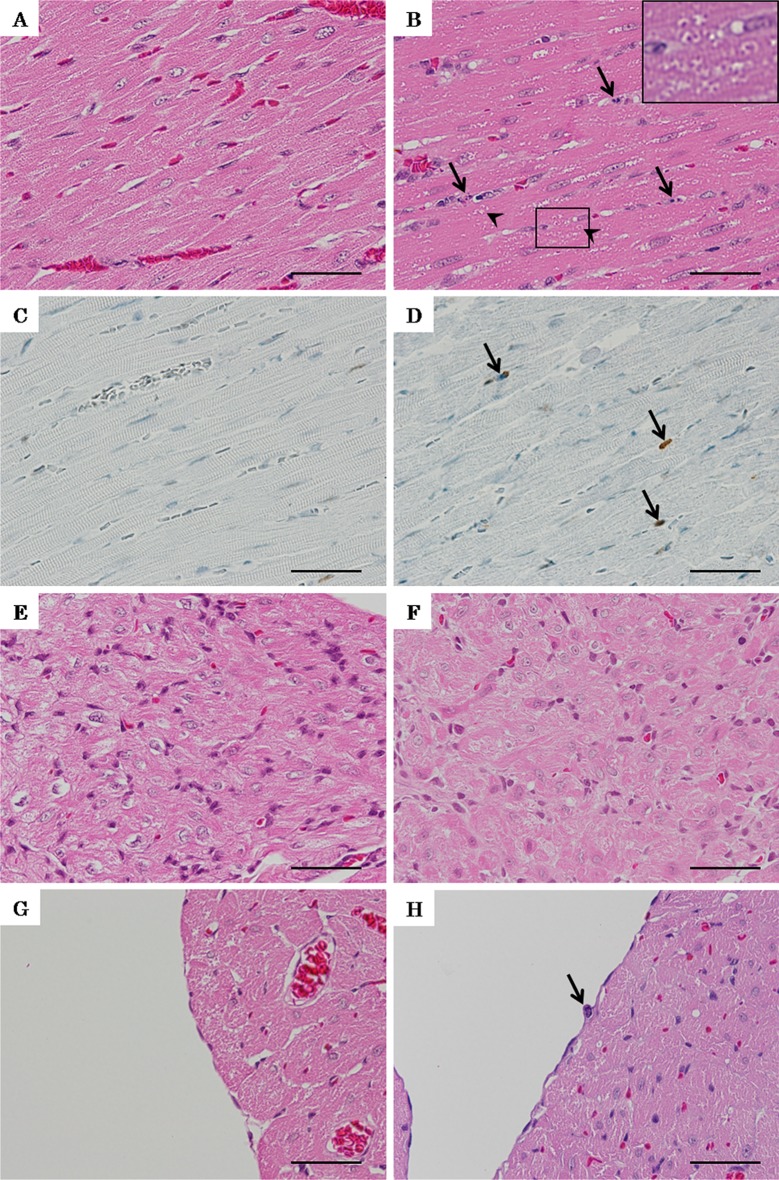
Histopathologically, colchicine-treated rats showed degeneration, with eosinophilic granules and vacuolation in the sarcoplasm of myocardial cells (Fig. 3B). These lesions were diffused and extended throughout the ventricles but were most prominent around the interventricular septum and left ventricular wall of the cardiac apex. Moreover, pyknosis, karyorrhexis and mitotic figures of interstitial cells and endocardial cells, which were positive for cleaved caspase-3, were observed (Fig. 3B, D and H). The atrioventricular nodes of colchicine-treated rats showed no remarkable findings (Fig. 3F).
Fig. 3.
Micrograph of lesions in the hearts of rats treated with colchicine or 5% glucose twice A: Normal left ventricular wall of a rat given 5% glucose. B: Degeneration with eosinophilic granules and vacuolation in sarcoplasm (arrowheads) of myocardial cells and pyknosis, karyorrhexis and mitotic figures (white arrows) of interstitial cells in left ventricular wall of a rat given colchicine. A high magnification view of the eosinophilic granules and vacuolation in sarcoplasm enclosed by a black rectangle is shown in the upper right. C: Almost no cleaved caspase-3-positive cells in the normal myocardium of a rat given 5% glucose. D: Cleaved caspase-3-positive cells (arrows) in the intersititial cells of the myocardium, which showed pyknosis, karyorrhexis and mitotic figures. E: Normal atrioventricular node of a rat given 5% glucose. F: Atrioventricular node of a rat given colchicine with no remarkable findings. E: Normal endocardial cells of a rat given 5% glucose. H: Pyknosis, karyorrhexis and mitotic figures (arrow) of endocardial cells of a rat given colchicine. A, B and D–H: H-E staining, bars = 40 µm. C and D: Immunohistochemical staining for cleaved caspase-3, bars = 40 µm.
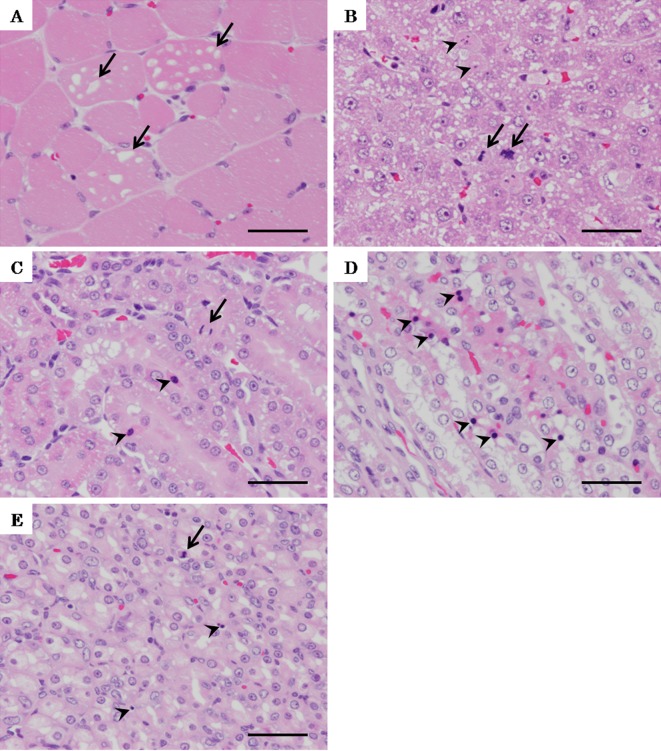
Skeletal muscle of colchicine-treated rats showed vacuolation in the sarcoplasm (Fig 4A). The livers of colchicine-treated rats showed single-cell necrosis and an increase in mitotic figures in midlobular hepatocytes (Fig. 4B). The kidneys of colchicine-treated rats showed pyknosis and an increase in mitotic figures in the tubular epithelium and collecting ducts (Fig. 4C, D). The stomach of a rat treated with colchicine showed pyknosis and an increase in mitotic figures in the mucosal epithelium (Fig. 4E).
Fig. 4.
Micrograph of lesions in the gastrocnemius muscle, liver, kidney and stomach of rats treated with colchicine twice. A: Vacuolation in sarcoplasm (arrows) of the gastrocnemius muscle of a rat treated with colchicine. B: Single-cell necrosis (arrowheads) and increase in mitotic figures (arrows) of midlobular hepatocytes of a rat treated with colchicine. C: Pyknosis (arrowheads) and increase in mitotic figures (arrows) of the tubular epithelium in the kidney of a rat treated with colchicine. D: Pyknosis (arrowheads) of the collecting duct in the kidney of a rat treated with colchicine. E: Pyknosis (arrowheads) and increase in mitotic figures of the mucosal epithelium of the stomach of a rat treated with colchicine. A–E: H-E staining, bars = 40 µm. C and D: Immunohistochemical staining for cleaved caspase-3, bars = 40 µm.
In this study, colchicine exhibited cardiotoxicity, in addition to myotoxicity, hepatotoxicity, nephrotoxicity and gastrointestinal toxicity, which was consistent with reports in humans22. Not only high levels of cardiac injury-related blood chemical parameters, i.e., AST, LDH 1, LDH 2, CK-MB and cTnT, and histopathological changes in the heart, i.e., degeneration with eosinophilic granules and vacuolation in the sarcoplasm of myocardial cells, but also electrocardiographic changes, i.e., prolongation of the RR interval, QRS duration, PR interval and QT interval, were detected. Prolongation of the RR interval suggested a decrease in heart excitability induced by disturbance of impulse initiation. This change is usually produced by alteration of the sinoatrial rhythm23. Prolongation of the QRS duration and PR interval suggested abnormality of the atrioventricular and ventricular conduction system. These decreases in excitability and conductivity of the heart were considered to be related to colchicine-induced bradycardia and atrioventricular block in humans.
Moreover, it is suggested that the increase in parasympathetic nervous activity affected prolongation of the RR interval and QT interval because these changes occurred at the same time as the increase in HF power. Prolongation of the RR interval induced by parasympathetic nervous activity may affect prolongation of the QT interval. The reason for the hyperactivity of the parasympathetic nerve after the second injection remains to be elucidated. The LF/HF ratio was not decreased at 1–3 hours after the second injection in spite of an increase in HF power after 1 hour. So, it is likely that sympathetic nervous activity also increased slightly at 1–3 hours after the second injection but calmed down until 6 hours after. The effect of the sympathetic nerve on the ECG data was thought to be little because any changes in the ECG waveform corresponded to this sympathetic nervous change. As for prolongation of the QT interval, a factor other than prolongation of the RR interval affected this change because the QT interval after the second injection was prolonged compared with that after the first injection even though the RR intervals in both cases were similar in length. The low level of Ca in blood induced by colchicine might affect this change, especially after the second injection, in addition to prolongation of the RR interval. It has been reported that a 20% decrease in the blood Ca level induced prolongation of the QT interval24. In this study, the blood Ca level of the rats treated with colchicine twice decreased by about 20%. However, this effect of a low Ca level is not severe because the QT interval at 24 hours after the first injection did not prolong remarkably when the blood Ca level decreased by about 10%. The cause of the low blood Ca level was considered to be related to impaired bone cell calcium homeostasis in colchicine-treated rats25.
The decrease in conductivity was suggested to be induced by not only the increase in parasympathetic nervous activity, because prolongation of the QRS duration and PR interval occurred earlier than the increase in HF power. Degeneration of ventricular myocardial cells was slight, and no change in the atrioventricular node was observed. So it might be difficult to consider histopathological change in the heart as the main preceding cause of the decrease in conductivity.
In contrast to the results of our present study, several in vitro studies have revealed that colchicine stimulates beating cardiac myocytes26 and enhances calcium and sodium currents in cardiac myocytes27, 28 at 1–15 μM. The Cmax of rats administered 1.5 mg/kg colchicine intravenously was estimated to be approximately 3 μM29. So, it is likely that the exposure level in the current experiment (1.25 mg/kg intravenously) reached the concentration at which colchicine affects beating, Ca and Na currents of cardiac myocytes, at least, instantaneously, suggesting that colchicine might induce an increase in excitability and conductivity in the present experiment. The reason for this difference between the previous results in vitro and the current results in vivo was not clarified in this study. Also, prolongation of the QRS duration and PR interval persisted over 12 hours after the injections even though the half-life in blood of colchicine is short. This might be associated with secondary causes such as the low level of Ca and variation of electrolytes in plasma, which are thought to be related to injuries of the bone, kidney and gastrointestinal tract. It has been reported that in the case of colchicine poisoning by oral ingestion in humans, serious cardiac events occurred at 24–36 hours after ingestion. So, cardiac events of colchicine poisoning are thought to be induced by not only direct effects on cardiac myocytes but also secondary causes such as changes in autonomic nervous activity and/or changes in body fluid composition. The results of this study concerning the autonomic nerve and biochemistry may suggest this secondary cause. In addition, the cardiotoxicity of the metabolites of colchicine must be discussed. However, the toxicity of the metabolites of colchicine is unknown. In order to further elucidate the relation between decreases in excitability and conductivity, the changes in electrolytes and metabolites of colchicine, time-response changes and dose-response changes of electrolytes in blood, colchicine metabolism and ECG parameters must be assessed in detail.
In conclusion, electrocardiographic changes indicating decreased excitability and conductivity of the myocardium, in addition to blood chemical and histopathological changes, were detected in rats intravenously administered colchicine. It is thought that decreased excitability and conductivity of the myocardium were related to an increase in parasympathetic nervous activity and a low level of blood Ca.
Declaration of Conflicting Interests: We have no financial relationships to disclose.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Morris PG, and Fornier MN. Microtubule active agents: beyond the taxane frontier. Clin Cancer Res. 14: 7167–7172. 2008. [DOI] [PubMed] [Google Scholar]
- 2.Davis JC., JrA practical approach to gout. Current management of an ‘old’ disease. Postgrad Med. 106: 115–116, 119–123. 1999. [DOI] [PubMed] [Google Scholar]
- 3.Lascaratos J. ‘Arthritis’ in Byzantium (AD 324–1453): unknown information from non-medical literary sources. Ann Rheum Dis. 54: 951–957. 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oakley CM. Myocarditis, pericarditis and other pericardial diseases. Heart. 84: 449–454. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tingle LE, Molina D, and Calvert CW. Acute pericarditis. Am Fam Physician. 76: 1509–1514. 2007. [PubMed] [Google Scholar]
- 6.Sullivan TP, King LE, Jr , and Boyd AS. Colchicine in dermatology. J Am Acad Dermatol. 39: 993–999. 1998. [DOI] [PubMed] [Google Scholar]
- 7.Simkin PA, and Gardner GC. Colchicine use in cyclosporine treated transplant recipients: how little is too much? J Rheumatol. 27: 1334–1337. 2000. [PubMed] [Google Scholar]
- 8.Tozer GM, Prise VE, Wilson J, Locke RJ, Vojnovic B, Stratford MRL, Dennis MF, and Chaplin DJ. Combretastatin A-4 phosphate as a tumor vascular-targeting agent: early effects in tumors and normal tissues. Cancer Res. 59: 1626–1634. 1999. [PubMed] [Google Scholar]
- 9.Mullins ME, Carrico EA, and Horowitz BZ. Fatal cardiovascular collapse following acute colchicine ingestion. J Toxicol Clin Toxicol. 38: 51–54. 2000. [DOI] [PubMed] [Google Scholar]
- 10.Mendis S. Colchicine cardiotoxicity following ingestion of Gloriosa superba tubers. Postgrad Med J. 65: 752–755. 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brvar M, Ploj T, Kozelj G, Mozina M, Noc M, and Bunc M. Case report: fatal poisoning with Colchicum autumnale. Crit Care. 8: R56–R59. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvo-Romero JM, Fernández-Soria-Pantoja R, Arrebola-Garcia JD, and Gil-Cubero M. Ischemic heart disease associated with vincristine and doxorubicin chemotherapy. Ann Pharmacother. 35: 1403–1405. 2001. [DOI] [PubMed] [Google Scholar]
- 13.Goli AK, Osman MN, Koduri M, Byrd RP, and Roy TM. A case report of vinorelbine monotherapy-related acute bronchospasm and non-ST elevation acute coronary syndrome. Tenn Med. 104: 47–48. 2011. [PubMed] [Google Scholar]
- 14.Subbiah IM, Lenihan DJ, and Tsimberidou AM. Cardiovascular toxicity profiles of vascular-disrupting agents. Oncologist. 16: 1120–113-. 2011. [DOI] [PMC free article] [PubMed]
- 15.Mery P, Riou B, Chemla D, and Lecarpentier Y. Cardiotoxicity of colchicine in the rat. Intensive Care Med. 20: 119–123. 1994. [DOI] [PubMed] [Google Scholar]
- 16.Mikaelian I, Buness A, de Vera-Mudry MC, Kanwal C, Coluccio D, Rasmussen E, Char HW, Carvajal V, Hilton H, Funk J, Hoflack JC, Fielden M, Herting F, Dunn M, and Suter-Dick L. Primary endothelial damage is the mechanism of cardiotoxicity of tubulin-binding drugs. Toxicol Sci. 117: 144–151. 2010. [DOI] [PubMed] [Google Scholar]
- 17.Tochinai R, Ando M, Suzuki T, Suzuki K, Nagata Y, Hata C, Uchida K, Kobayashi T, Kado S, and Kaneko K. Histopathological studies of microtubule disassembling agent-induced myocardial lesions in rats. Exp Toxicol Pathol. 65: 737–743. 2013. [DOI] [PubMed] [Google Scholar]
- 18.Farraj AK, Hazari MS, and Cascio WE. The utility of the small rodent electrocardiogram in toxicology. Toxicol Sci. 121: 11–30. 2011. [DOI] [PubMed] [Google Scholar]
- 19.Cooley JW, and Tukey JW. An algorism for the machine calculation of complex Fourier series. Math Comput. 19: 297–301. 1965. [Google Scholar]
- 20.Kuwahara M, Yayou K, Ishii K, Hashimoto S, Tsubone H, and Sugano S. Power spectral analysis of heart rate variability as a new method for assessing autonomic activity in the rat. J Electrocardiol. 27: 333–337. 1994. [DOI] [PubMed] [Google Scholar]
- 21.Kurata M, Iidaka T, Sasayama Y, Fukushima T, Sakimura M, and Shirai N. Correlation among clinicopathological parameters of myocardial damage in rats treated with isoproterenol. Exp Anim. 56: 57–62. 2007. [DOI] [PubMed] [Google Scholar]
- 22.Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov-Raz G, Pollak U, Koren G, and Bentur Y. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol (Phila). 48: 407–414. 2010. [DOI] [PubMed] [Google Scholar]
- 23.Berne RM, and Levy MN. Cardiovascular Physiology. In: Cardiovascular physiology, 7th ed. Mosby, MO, USA. 7–54. 1997. [Google Scholar]
- 24.Conrad LL, and Baxter DJ. Effect of calcium deficiency on Q-T interval and distribution of Ca-45 in rat heart. Am J Physiol. 210: 831–832. 1966. [DOI] [PubMed] [Google Scholar]
- 25.Ohya K, and Ogura H. The effects of colchicine or vinblastine on the blood calcium level in rats. Eur J Pharmacol. 248: 111–119. 1993. [DOI] [PubMed] [Google Scholar]
- 26.Lampidis TJ, Kolonias D, Savaraj N, and Rubin RW. Cardiostimulatory and antiarrhythmic activity of tubulin-binding agents. Proc Natl Acad Sci USA. 89: 1256–1260. 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gómez AM, Kerfant BG, and Vassort G. Microtubule disruption modulates Ca(2+) signaling in rat cardiac myocytes. Circ Res. 86: 30–36. 2000. [DOI] [PubMed] [Google Scholar]
- 28.Motlagh D, Alden KJ, Russell B, and García J. Sodium current modulation by a tubulin/GTP coupled process in rat neonatal cardiac myocytes. J Physiol. 540: 93–103. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bittner B, González RC, Walter I, Kapps M, and Huwyler J. Impact of Solutol HS 15 on the pharmacokinetic behaviour of colchicine upon intravenous administration to male Wistar rats. Biopharm Drug Dispos. 24: 173–181. 2003. [DOI] [PubMed] [Google Scholar]