Abstract
The mucosal immune system of the intestine is separated from a vast array of microbes by a single layer of epithelial cells. Cues from the commensal microflora are needed to maintain epithelial homeostasis, but the molecular and cellular identities of these cues are unclear. Here we provide evidence that signals from the commensal microflora contribute to the differentiation of a lymphocyte population coexpressing stimulatory natural killer cell receptors and the transcription factor RORγt that produced interleukin 22 (IL-22). The emergence of these IL-22-producing RORγthiNKp46+NK1.1int cells depended on RORγt expression, which indicated that these cells may have been derived from lymphoid tissue–inducer cells. IL-22 released by these cells promoted the production of antimicrobial molecules important in the maintenance of mucosal homeostasis.
The intestinal mucosal immune system is the body’s largest compilation of immune cells. It is unique in that it develops in very close proximity to a vast array of microbes. Most lymphocytes in the intestinal epithelium and lamina propria are separated from the commensal microflora by only a single layer of epithelial cells. Such proximity allows the development of a close symbiotic relationship among the cells of the immune system, the intestinal epithelium and the commensal microflora1,2. Immunological recognition of commensal bacteria, possibly involving Toll-like receptors, is required for the homeostasis and efficient repair of intestinal epithelial cells. It is believed that intestinal lymphocytes are involved in this homeostatic balance between the commensal microflora and intestinal epithelial cells3–5. Such interaction between lumenal microflora and intestinal epithelial cells has been demonstrated in studies involving animals raised in a sterile environment (such as ‘germ-free’mice) showing that the expression of antimicrobial proteins depends on signals from the lumenal microflora6. However, the cells involved in the maintenance of intestinal homeostasis and the molecules that mediate intestinal homeostasis have yet to be identified.
The organized lymphoid structures in the lamina propria of the intestine are the cryptopatches, the Peyer’s patches of the small intestine and the isolated lymphoid follicles of the colon1. The biological function of cryptopatches, which contain a uniform population of immature (c-Kit+) lymphocytes that lack expression of markers associated with mature hematopoietic lineages (lineage negative (Lin−)), is poorly understood7. Most lymphocytes in the cryptopatches express the transcription factor RORγt (A002302)8. RORγt is required for the development of lymphoid tissue–inducer cells (LTi cells), and mice lacking RORγt fail to develop lymph nodes and cryptopatches9,10. Thus, it has been argued that cryptopatches might be the lymph node anlagen of the mucosa11. Cryptopatches and isolated lymphoid follicles are surrounded by dendritic cells that can extend their dendrites through the epithelial cell layer into the intestinal lumen and transmit innate immune signals to the lymphocytes in the cryptopatches or isolated lymphoid follicles12. These dendritic cells might link lumenal bacteria and the lymphoid structures of the intestinal lamina propria13. Cryptopatches are situated in close proximity to the crypts of the epithelium, which contain the two cell types widely believed to be central for epithelial homeostasis and protection: epithelial stem cells and Paneth cells, respectively. Thus, the organized lymphoid structures of the intestinal lamina propria might integrate signals derived from the lumenal microflora and from immune cells to regulate epithelial function.
Natural killer (NK) cells are lymphocytes of the innate immune system that produce proinflammatory cytokines (such as interferon-γ (IFN-γ)) and mediate cellular cytotoxicity14. NK cells develop in the bone marrow, express invariant stimulatory and inhibitory receptors that regulate their state of activation and are found in secondary lymphoid organs, peripheral blood and peripheral organs, including liver and lungs. Although NK cell–mediated ‘natural cytotoxicity’ has been detected in lamina propria lymphocyte preparations from humans and mice15–17, detailed characterization of NK cell populations in the intestinal mucosa has not yet been done.
Interleukin 22 (IL-22), a cytokine of the extended IL-10 family18–20, is produced by IL-17–producing CD4+ T cells (TH-17 cells), whereas T helper type 1 and T helper type 2 cells produce only small amounts of this cytokine21–23. IL-23 enhances the release of IL-22 by TH-17 cells22,23. Although resting NK cells do not express IL-22 mRNA, small amounts of IL-22 mRNA have been detected in human NK cells stimulated with a combination of IL-2 and IL-12 (ref. 24). In the intestine, IL-22 is produced in an IL-23-dependent way during inflammation and promotes the expression of antimicrobial proteins and molecules involved in tissue repair (such as RegIIIβ, RegIIIγ, S100A8 and S100A9)25. However, the cellular sources of IL-22 remain unidentified. Notably, the heterodimeric IL-22 receptor is expressed exclusively by epithelial cells24. Such data indicate that IL-22 might be an important factor produced by lymphocytes to regulate epithelial homeostasis, protection and repair.
Inflammatory bowel disease is characterized by inappropriate immune stimulation caused by disturbances in the homeostatic crosstalk among the indigenous microflora, epithelium and the mucosal immune system26. Thus, understanding of the molecular and cellular requirements for mucosal homeostasis would be useful. Here we report the identification of an IL-22-producing lymphocyte population in the intestinal lamina propria that coexpressed NK cell– recognition receptors (such as NKp46, NKG2D and NK1.1) and RORγt. The appearance of these cells depended on RORγt and the presence of commensal microflora. Our data indicate that cues from lumenal bacteria ‘instruct’ the differentiation of a population of RORγthi lymphocytes that coexpress NK cell–recognition receptors and might be derived from LTi cells. IL-22 produced by this lymphocyte population contributed to epithelial homeostasis by regulating genes involved in tissue repair and antimicrobial defense.
RESULTS
Immature phenotype of mucosal NKp46+ cells
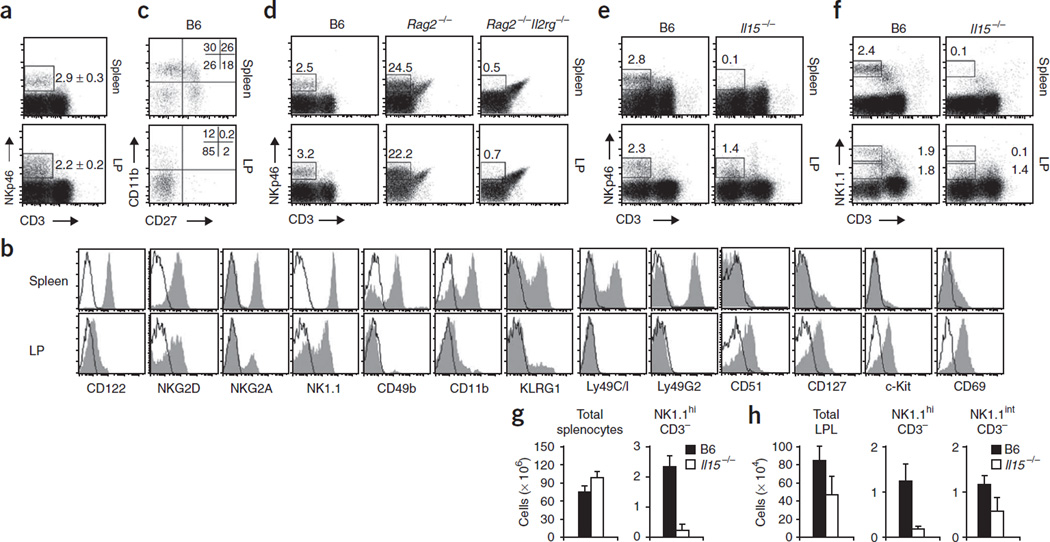
To study the function of NK cells during mucosal immune responses, we analyzed the presence and phenotype of NK cells in the lamina propria of the gut. We identified NK cells as NKp46+CD3− cells, because NKp46 is a marker shown to be expressed only by NK cells27,28. A sizeable fraction of lymphocytes of the lamina propria of the small intestine (Fig. 1a) and colon (data not shown) expressed NKp46. Notably, lamina propria NKp46+ cells coexpressed the IL-2– IL-15 receptor β-chain (CD122), which is the earliest marker of committed NK cell precursors and is expressed throughout differentiation of the NK cell lineage29 (Fig. 1b). Similar to splenic NK cells, lamina propria NKp46+ cells also expressed the stimulatory NK cell receptor NKG2D and the inhibitory receptor NKG2A (Fig. 1b). Whereas splenic NK cells had uniformly high expression of NK1.1, mucosal NKp46+ cells of the small intestine could be further subcategorized into NK1.1hi and NK1.1int populations (Fig. 1b). Further analysis of these mucosal NKp46+CD3− cells showed they had an unusual phenotype. Mucosal NKp46+ cells did not have appreciable expression of CD49b (DX5; Fig. 1b), which is expressed by most mature NK cells in the spleen30, and they lacked expression of NK cell maturation markers such as KLRG1 and CD11b31. Costaining of NK cells with antibody to CD27 (anti-CD27) and anti-CD11b identifies NK cell populations with different maturation, migratory and functional properties32. Mucosal NKp46+CD3− cells included an unusually large population of CD27−CD11b− cells not present in the spleen and other peripheral organs32 (Fig. 1c). Notably, lamina propria NKp46+CD3− cells lacked expression of the receptors Ly49A, Ly49C/I, Ly49D, Ly49H and Ly49G2 (Fig. 1b and data not shown). The surface phenotype of these cells resembled that of immature NK (iNK) cells of the bone marrow, an observation supported by the finding that NKp46+CD3− cells expressed CD51 (integrin αV), a marker believed to be expressed by iNK cells and NK cell precursors29,31 (Fig. 1b). Further phenotypic analysis showed that mucosal NKp46+ cells expressed the markers of common lymphoid progenitors, such as the IL-7R α-chain (CD127) and c-Kit (CD117), and had an activated phenotype, as determined by their expression of the activation marker CD69 (Fig. 1b). Thus, mucosal NKp46+CD3− cells have a phenotype resembling that of iNK cells of the bone marrow29.
Figure 1.
Lamina propria NKp46+ cells have an immature phenotype. (a,b) Flow cytometry of lamina propria (LP) lymphocytes from the small intestine and spleen cells stained with anti-CD3 and anti-NKp46. (a) Numbers adjacent to outlined areas indicate percent NKp46+CD3− cells (mean ± s.e.m.). (b) Gating on NKp46+CD3− cells: filled histograms, specific staining (antibodies to markers below plots); open histograms, isotype-matched control antibody. Data are representative of at least eight independent experiments. (c) Flow cytometry of splenocytes and lamina propria cells stained with anti-NKp46, anti-CD3, anti-CD11b and anti-CD27, gated on NKp46+CD3− cells. Numbers in quadrants indicate percent cells in each. Data are representative of three independent experiments. (d) Flow cytometry of lamina propria cells and splenocytes (mouse strains, above plots) stained with anti-NKp46 and anti-CD3. Numbers above outlined areas indicate percent NKp46+CD3− cells. Data are representative of three independent experiments. (e,f) Flow cytometry of B6 and Il15−/− lamina propria cells stained with anti-CD3 and anti-NKp46 (e) or anti-NK1.1 (f). Numbers adjacent to outlined areas indicate percent cells in gate. Data are representative of four independent experiments. (g,h) Absolute numbers of various cell populations (above graphs) in the spleen (g) or intestinal lamina propria (h). LPL, lamina propria lymphocytes. Data are representative of three independent experiments (error bars, s.d.; n = 6 mice).
Developmental requirements of mucosal NKp46+ cells
Because the mucosal NKp46+CD3− cells expressed markers of NK cell progenitors as well as some markers of common lymphoid progenitors, we analyzed the molecular pathways required for the development of mucosal NKp46+ cells. As with splenic NK cells, the development of mucosal NKp46+CD3− cells proceeded normally in recombination activation gene 2–deficient (Rag2−/−) mice but was considerably impaired in Rag2−/− mice also deficient in the common γ-chain (Il2rg−/−), which lack all lymphocytes33 (Fig. 1d). Thus, similar to splenic NK cells, mucosal NKp46+CD3− cells belong to a lymphoid lineage that does not require somatic recombination of immune receptors.
Reflective of the involvement of IL-15 in promoting the development and survival of NK cells, Il15−/− mice have fewer NK cells in the periphery34. However, in the lamina propria, a fraction of NKp46+CD3− cells developed independently of IL-15 (Fig. 1e). Further analysis of CD3− lamina propria lymphocytes showed that the NK1.1hi subset was developmentally dependent on IL-15 (Fig. 1f) and, similar to their number of splenic NK cells (Fig. 1g), Il15−/− mice had a much lower absolute number of lamina propria NK1.1hiCD3− cells (Fig. 1h). Notably, the absolute number of lamina propria lymphocytes was also lower in Il15−/− mice (Fig. 1h), which suggests that IL-15 is important for the homeostasis of lamina propria lymphocytes. In contrast to NK1.1hiCD3− cells, the NK1.1intCD3− subset was retained in Il15−/− mice (Fig. 1f) and their absolute numbers were diminished proportionally to the overall lower number of lamina propria lymphocytes (Fig. 1h). These data demonstrate that mucosal NKp46+NK1.1hi cells have developmental requirements similar to those of peripheral NK cells and might therefore be part of the NK cell lineage. In contrast, NKp46+NK1.1int cells develop in the absence of IL-15 and follow a developmental program different from that of conventional NK cells.
NKp46+ cells localize in cryptopatches
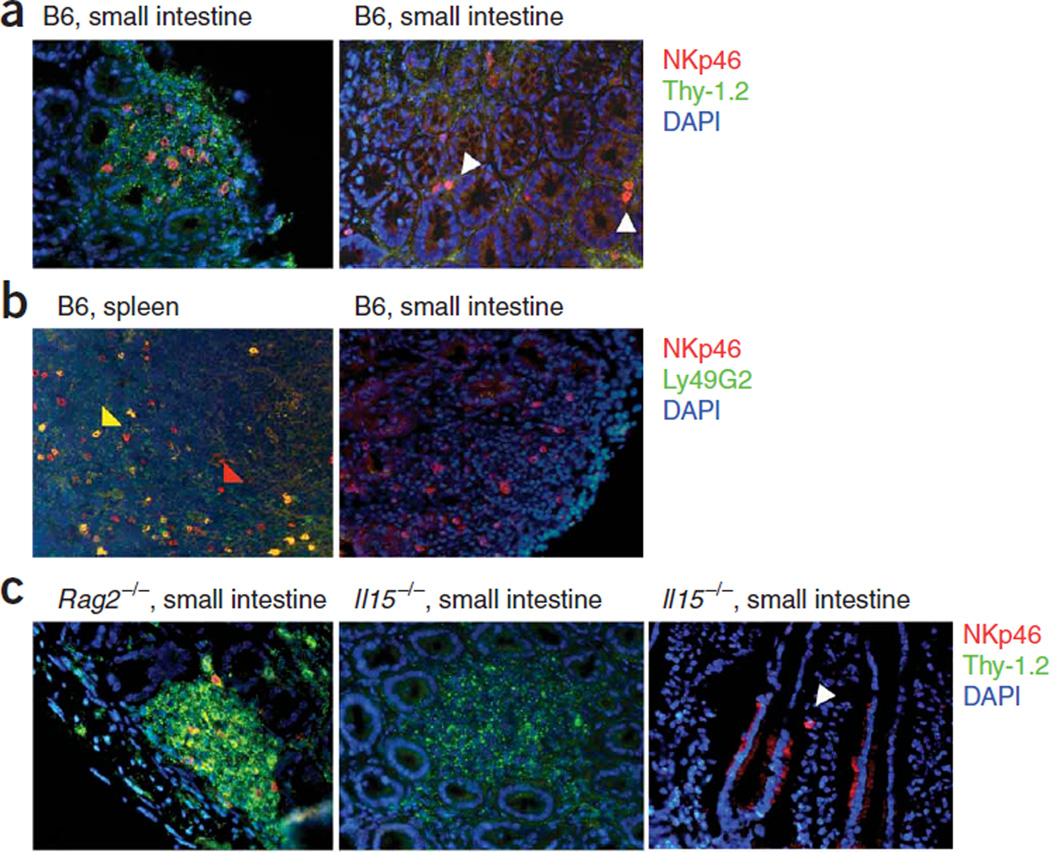
We also analyzed the localization of mucosal NKp46+ cells in the mucosa. In this context, the coexpression of CD127, c-Kit and Thy-1.2 (CD90.2) by lamina propria NKp46+ cells (Fig. 1b and data not shown) is notable, as it has been shown that a homogenous population of Lin−CD127+c-Kit+Thy-1.2+ lymphocytes populates the cryptopatches of the intestine7. Approximately 20% of the NKp46+ cells localized in the cryptopatches of the intestine (Fig. 2a, left), and we found single NKp46+ cells scattered throughout the lamina propria (Fig. 2a, right). Approximately 20–30% of all cells in the cryptopatches expressed NKp46 (Fig. 2a). To extend our flow cytometry data (Fig. 1b), we confirmed by immunofluorescence staining of tissue sections that mucosal NK cells did not express Ly49 receptors (Fig. 2b, right), whereas a substantial fraction of splenic NK cells expressed Ly49 receptors (Fig. 2b, left). Rag2−/− mice had normal cryptopatch development7 and normal numbers of NKp46+ cells inside and outside the cryptopatches (Fig. 2c), which confirmed our flow cytometry data (Fig. 1d). Although Il15−/− mice lacked the NK1.1hi population of NKp46+CD3− cells (Fig. 1e,f), they had normal cryptopatch architecture (Fig. 2c, middle) and had NKp46+ cells scattered throughout the lamina propria (Fig. 2c, right) and in cryptopatches (data not shown), albeit at lower numbers (Fig. 1h).
Figure 2.
Mucosal NKp46+ cells localize in cryptopatches. (a–c) Immunofluorescence staining of sections from the small intestines and spleens of mice (strains, above images). White arrowheads indicate scattered NKp46+ cells outside cryptopatches in B6 mice (a) and Il15−/− mice (c); yellow and red arrowheads indicate splenic NKp46+Ly49G2+ and NKp46+Ly49G2− NK cells, respectively (b). Markers (and staining color), right margins. Original magnification, ×40. Data are representative of five independent experiments.
RORγt+ subset of mucosal NKp46+ cells
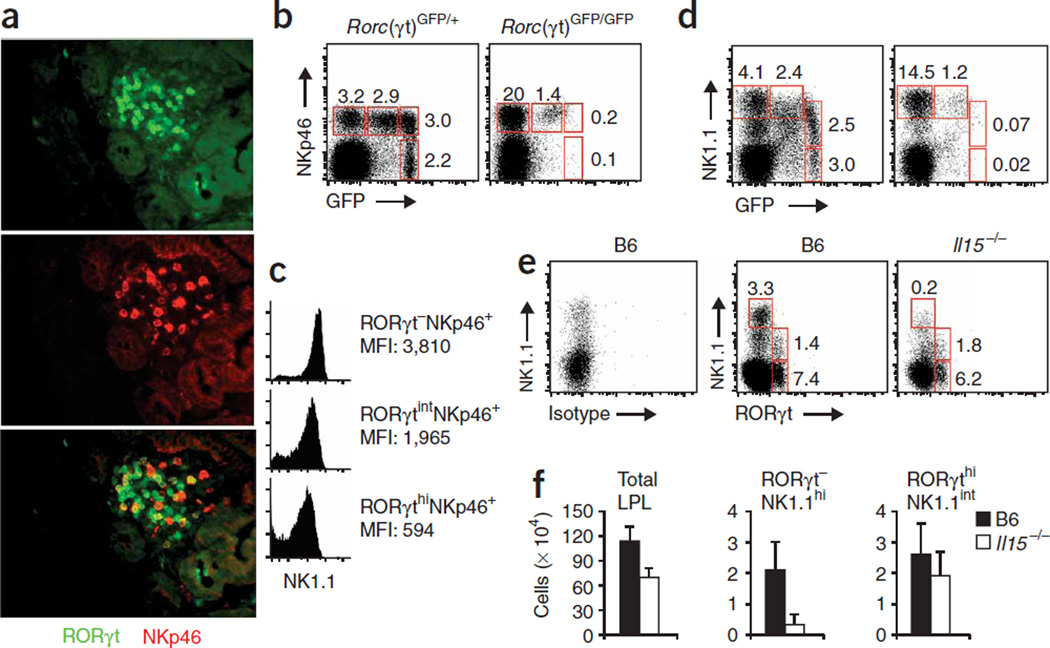
Initial data indicated that cryptopatches might be organs for the extrathymic development of mucosal lymphocytes35,36, and subsequent data have shown that most cells in cryptopatches express RORγt8. RORγt is indispensable for the lineage commitment of LTi cells, which are required for the development of cryptopatches, isolated lymphoid follicle and lymph nodes8–10. We used mice with a green fluorescent protein (GFP) reporter cassette ‘knocked into’ the gene encoding RORγt (Rorc (called ‘Rorc(γt)’ here); Rorc(γt)GFP/+ and Rorc(γt)GFP/GFP mice) to investigate expression of RORγt by mucosal NKp46+ cells37. By immunofluorescence staining of tissue sections, we identified RORγt+NKp46+ cells, RORγt+NKp46− cells and a few RORγt−NKp46+ cells in the cryptopatches (Fig. 3a). Whereas RORγt+NKp46+ and RORγt−NKp46+ cells were also present outside cryptopatches scattered throughout the lamina propria, RORγt+NKp46−CD3− cells were rare outside cryptopatches (data not shown).
Figure 3.
NKp46+ cells of the lamina propria express RORγt. (a) Sections from the small intestines of Rorc(γt)GFP/+ mice, stained with anti-GFP (green; RORγt) or anti-NKp46 (red). Original magnification, ×40. Data are representative of four independent experiments. (b–d) Flow cytometry of Rorc(γt)GFP/+ and Rorc(γt)GFP/GFP lamina propria lymphocytes stained with anti-CD3, anti-NKp46 and anti-NK1.1 and gated on CD3− cells. (b,d) Numbers adjacent to outlined areas indicate percent cells in gate. (c) NK1.1 expression by various cell populations (right margin). MFI, mean fluorescence intensity. Data are representative of five independent experiments. (e) Flow cytometry of B6 and Il15−/− lamina propria lymphocytes stained with anti-CD3 anti-NK1.1 and a monoclonal antibody specific for RORγt, or with isotype-matched control antibody and gated on CD3− cells. Numbers adjacent to outlined areas indicate percent cells in gate. Data are representative of three independent experiments. (f) Absolute numbers of various lamina propria cell populations (above graphs). Data are representative of three independent experiments (error bars, s.d.; n = 4 mice).
By flow cytometry, we identified three distinct populations of NKp46+CD3− lymphocytes of the intestinal lamina propria in terms of RORγt expression: RORγt−NKp46+, RORγtintNKp46+ and RORγthiNKp46+ (Fig. 3b). These three populations all expressed the receptor NKG2D (data not shown) and could be further subcategorized by their surface expression of NK1.1 (Fig. 3c). The RORγt− NKp46+ and RORγtintNKp46+ populations had high expression of NK1.1, whereas the RORγthiNKp46+ cells had intermediate expression of NK1.1 (Fig. 3c). These populations were also readily detectable during analysis of the expression of NK1.1 and RORγt by CD3− lamina propria cells (Fig. 3d). All NK1.1-expressing lamina propria lymphocytes expressed similar amounts of NKp46 (data not shown). We have called these populations of lamina propria lymphocytes RORγt−NKp46+NK1.1hi, RORγtintNKp46+NK1.1hi and RORγthi NKp46+NK1.1int cells here. We confirmed the data from Rorc(γt)GFP/+ mice using intracellular staining to detect RORγt in mucosal cells from C57BL/6 (B6) mice (Fig. 3e). Although the intracellular staining procedure allowed us to distinguish between RORγthi and RORγt− cell populations, we could not detect RORγtdim cells.
The data reported above documenting cell populations coexpressing RORγt and NK cell markers contrasts with data generated by genetic fate–mapping studies, which indicate that true NK cells of the spleen do not express RORγt at any time during their development8. In this context, the intermediate expression of the NK1.1 marker by RORγthiNKp46+ cells was notable, as the development of NK1.1intCD3− cells of the lamina propria was independent of IL-15 (Fig. 1f,h). We investigated the development of mucosal NK1.1+ RORγt-expressing lymphocytes in mice lacking RORγt (Rorc(γt)GFP/GFP). A larger fraction of RORγt−NKp46+NK1.1hi NK cells was present in the lamina propria of RORγt-deficient Rorc(γt)GFP/GFP mice, whereas this population was smaller in terms of relative and absolute numbers in the lamina propria of Il15−/− mice (Fig. 3b–f). In contrast, RORγthiNKp46−NK1.1− cells and RORγthiNKp46+NK1.1int cells were absent from RORγt-deficient mice but were present in Il15−/− mice (Fig. 3b–f). These data demonstrate that like LTi cells, the RORγthiNKp46+NK1.1int subpopulation depends on RORγt expression for development and/or homeostasis. Published data indicate a close developmental relationship between LTi cells and NK cells38,39. Thus, the RORγt-dependent population of RORγthiNKp46+NK1.1int lymphocytes is either derived from RORγthiNKp46−NK1.1− LTi-like cells of the lamina propria or represents a unique RORγt-dependent lineage. Further experiments are needed to distinguish between these two models.
Mucosal NKp46+ cells lack NK functions
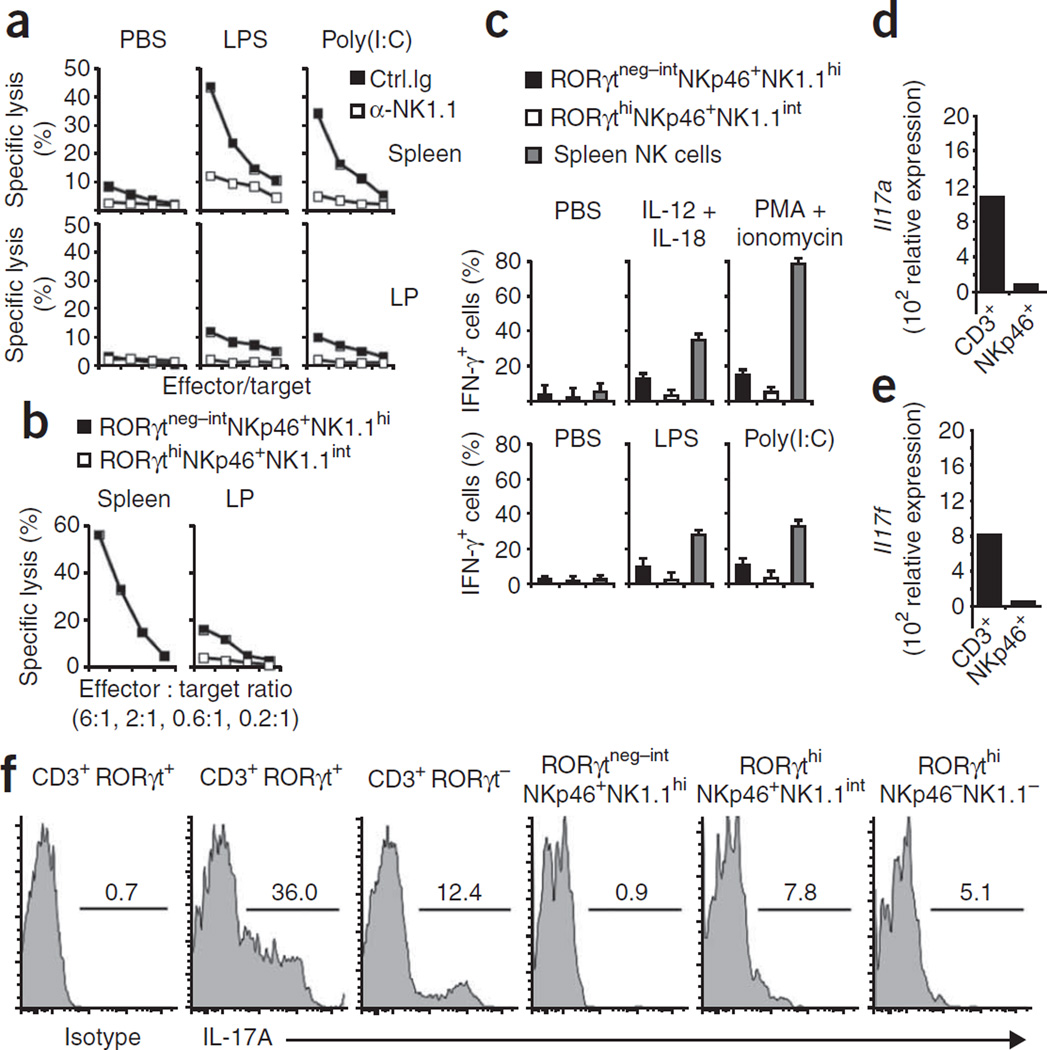
To assign a function to RORγtneg–int NKp46+NK1.1hi and RORγthiNKp46+ NK1.1int cells, we investigated their ability to kill NK target cells. After being primed with lipopolysaccharide or polyinosinic-polycytidylic acid, bulk lamina propria NK cells had only marginal natural killing activity relative to that mediated by splenic NK cells (Fig. 4a). Although specific lysis of various tumor targets was low, mucosal NK cells showed some cell-mediated cytotoxicity, as depletion of all mucosal NK cells with a monoclonal antibody specific for NK1.1 abolished cytotoxicity (Fig. 4a). Next we analyzed the cytotoxicity of purified RORγthi NKp46+NK1.1int and RORγtneg–intNKp46+NK1.1hi cells. Notably, RORγthiNKp46+NK1.1int mucosal cells showed almost no cytotoxicity, whereas RORγtneg–intNKp46+NK1.1hi NK cells showed some killing activity, albeit much less than that of splenic NK cells stimulated in the same conditions (Fig. 4b).
Figure 4.
Mucosal NKp46+ cells have only weak NK cell effector functions. (a) Cytotoxicity of lamina propria lymphocytes or splenocytes from B6 mice injected twice with anti-NK1.1 or isotype-matched control antibody and, 1 d later, with PBS, lipopolysaccharide (LPS) or polyinosinic-polycytidylic acid (Poly(I:C)), assessed 24 h later as killing of YAC-1 mouse lymphoma target cells at effector/target ratios of 6:1, 2:1, 0.6:1 and 0.1:1. (b) Cytotoxicity of sorted lamina propria lymphocyte populations (RORγtneg–intNKp46+NK1.1hi and RORγthiNKp46+NK1.1int) or splenocyte cells (RORγt−NKp46+NK1.1+) from Rorc(γt)GFP/+ mice injected with polyinosinic-polycytidylic acid, assessed 1 d later as killing of YAC-1 target cells. (c) IFN-γ-producing cells among Rorc(γt)GFP/+ lamina propria lymphocyte and splenocyte populations (key) incubated for 6 h in the presence of brefeldin A with various stimuli (above graphs) and then stained with anti-IFN-γ (top row), and IFN-γ-producing cells among lamina propria and spleen cells from Rorc(γt)GFP/+ mice injected with various Toll-like receptor ligands (above graphs) and, 1 d later, incubated for 6 h with YAC-1 cells in the presence of brefeldin A and then stained with anti-IFN-γ (bottom row). (d,e) Quantitative RT-PCR analysis of the expression of IL-17A mRNA (Il17a; d) and IL-17F mRNA (Il17f; e) in sorted lamina propria CD3+ and NKp46+CD3− (NKp46+) cells. (f) Flow cytometry of Rorc(γt)GFP/+ lamina propria cells stained with anti-CD3, anti-NK1.1 or anti-IL-17 or isotype-matched control antibody, gated on subsets above plots. Numbers above lines indicate percent IL-17+ cells. Data are representative of four (a), three (b–e) or five (f) independent experiments.
Another important function of NK cells is production of the proinflammatory cytokine IFN-γ during early stages of infection. Although mucosal RORγtneg–intNKp46+NK1.1hi NK cells produced IFN-γ after stimulation with a combination of IL-12 and IL-18 or with phorbol 12-myristate 13-acetate in combination with the calcium ionophore ionomycin, RORγthiNKp46+NK1.1int cells did not (Fig. 4c, top). We obtained similar data when analyzing IFN-γ production by these lamina propria cell populations after in vivo priming of NK cell function (Fig. 4c, bottom).
RORγthiNKp46+NK1.1int cells produce IL-22 but not IL-17
As RORγt is required for the differentiation of TH-17 cells, which produce IL-17 and IL-22, we analyzed the production of these cytokines by the various NKp46+CD3− lymphocyte populations in the lamina propria. By quantitative RT-PCR, we detected very small amounts of mRNA transcripts encoding IL-17A and IL-17F in sorted NKp46+CD3− cells from the intestinal lamina propria, whereas mRNA for both cytokines was easily detectable in mucosal CD3+ T cells that included a substantial fraction of mucosal TH-17 cells40 (Fig. 4d,e). We confirmed those data by intracellular cytokine staining. Whereas a large fraction of RORγt+ mucosal T cells readily expressed IL-17A and IL-17F protein, only a small but reproducible fraction of RORγthi NKp46+NK1.1int cells produced IL-17 (Fig. 4f and data not shown).
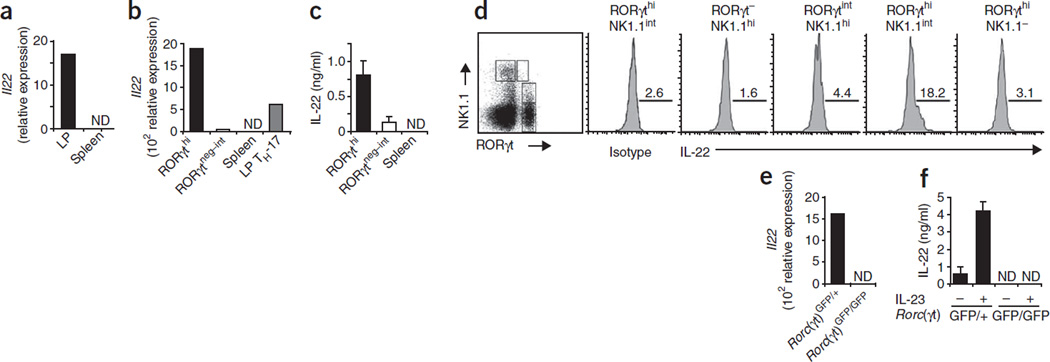
Quantitative RT-PCR data with highly purified cell populations showed that mucosal but not splenic NKp46+CD3− cells expressed IL-22 mRNA (Fig. 5a). To assess the IL-22 mRNA production in the various NKp46+ lymphocyte subpopulations, we highly purified mucosal RORγthiNKp46+NK1.1int and RORγtneg–intNKp46+NK1.1hi NK cells from Rorc(γt)GFP/+ mice. RORγthiNKp46+NK1.1int cells had high expression of IL-22 mRNA (Fig. 5b) and produced IL-22 protein, as determined by enzyme-linked immunosorbent assay (ELISA; Fig. 5c). Notably, RORγthiNKp46+NK1.1int cells reproducibly expressed two- to threefold more IL-22 mRNA than did mucosal TH-17 cells (CD4+CD3+RORγt+ cells; Fig. 5b). In contrast, RORγtneg–intNKp46+NK1.1hi NK cells lacked IL-22 mRNA and protein (Fig. 5b,c). We further confirmed those data by intracellular cytokine staining with a monoclonal antibody specific for IL-22 (ref. 23; Fig. 5d). Notably, RORγthiNKp46−NK1.1− LTi-like cells did not produce IL-22 (Fig. 5d), which suggested that RORγt expression by itself is not sufficient to support IL-22 production.
Figure 5.
Lamina propria RORγthiNKp46+NK1.1int cells produce IL-22. (a) Quantitative RT-PCR analysis of IL-22 mRNA expression (Il22) in splenic and lamina propria NKp46+CD3− cells sorted from B6 mice. Data are representative of three experiments. (b,c) Quantitative RT-PCR analysis of IL-22 mRNA expression (b) and ELISA of IL-22 protein production (c) by sorted splenic NKp46+CD3− cells (Spleen) and of RORγthiNKp46+NK1.1int (RORγthi), RORγtneg–intNKp46+NK1.1int (RORγtneg–int) and CD4+RORγtint (LP TH-17) lamina propria cells sorted from Rorc(γt)GFP/+ mice. Data are representative of three experiments. (d) Flow cytometry of B6 lamina propria cells stained with anti-NK1.1 and anti-RORγt (far left), or with anti-IL-22 or an isotype-matched control antibody and gated on subsets above plots (right). Numbers above lines indicate percent IL-22+ cells. Data are representative of four experiments. (e,f) Quantitative RT-PCR analysis of IL-22 mRNA expression (b) and ELISA of IL-22 protein production (c) by RORγthiNKp46+NK1.1int cells sorted from Rorc(γt)GFP/+ mice and RORγtintNKp46+NK1.1hi cells from Rorc(γt)GFP/GFP mice; for ELISA, cultures were stimulated for 72 h with IL-23. ND, not detectable. Data are representative of three independent experiments.
In agreement with the requirement of RORγt for the differentiation of the IL-22-producing RORγthiNKp46+NK1.1int population (Fig. 3b,d), IL-22 mRNA and protein was absent from highly purified RORγtintNKp46+NK1.1hiCD3− cells from mice lacking RORγt (Fig. 5e). We obtained similar results with lamina propria NKp46+CD3− cells from RORγt-deficient mice (data not shown). Notably, sorted lamina propria NKp46+CD3− cells or sorted RORγtint NKp46+NK1.1hiCD3− cells from mice lacking RORγt failed to produce IL-22 even after in vitro culture with IL-23, which strongly enhanced IL-22 production by NKp46+CD3− cells or RORγthiNKp46+NK1.1int cells of RORγt-sufficient mice (Fig. 5f and data not shown). Thus, RORγthiNKp46+NK1.1int cells are a source of IL-22 during homeostasis, and their differentiation requires RORγt.
Commensals promote RORγt+NKp46+ differentiation
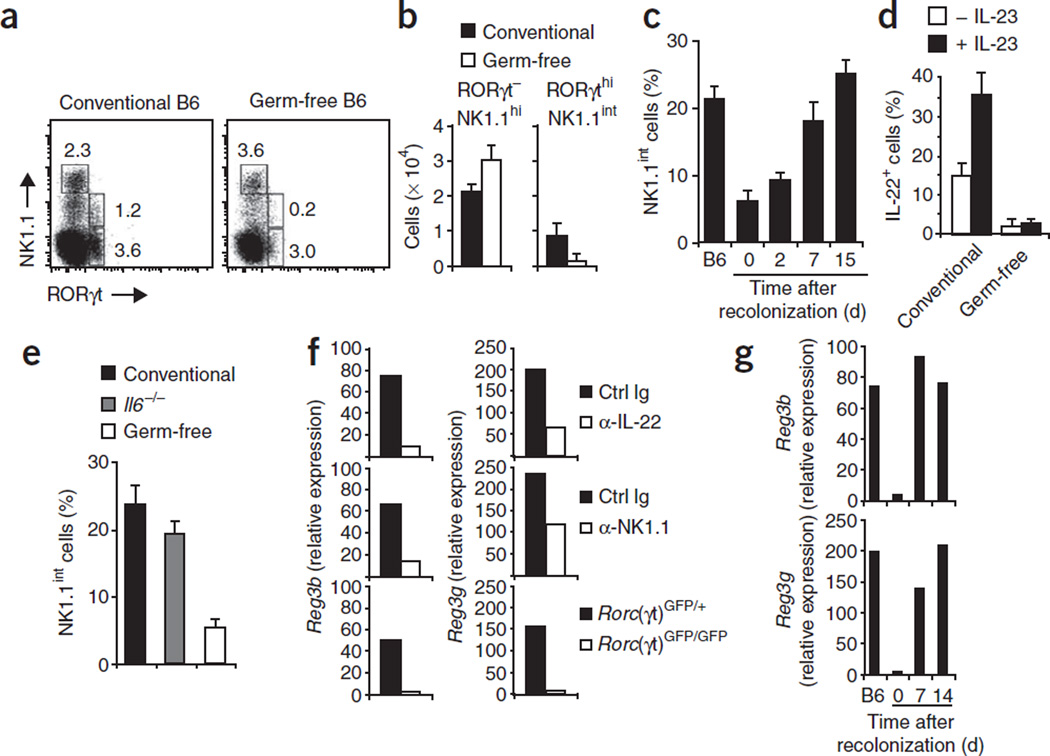
Expression of the IL-22 receptor is restricted to epithelial cells24. Treatment of colon explants with IL-22 leads to the upregulation of antimicrobial proteins and genes involved in tissue regeneration25. Because it has been shown that the lumenal microflora promotes epithelial expression of a very similar set of genes6, we hypothesized that commensal bacteria might ‘instruct’ the differentiation and/or homeostasis of IL-22-producing RORγthiNKp46+NK1.1int cells. To test that, we investigated the expression of RORγt and NK cell receptors in germ-free mice. RORγtneg–intNKp46+NK1.1hi NK cells and RORγthi NKp46−NK1.1− LTi-like cells were normally represented in the mucosa of germ-free mice (Fig. 6a). In addition, the function of these LTi-like cells in cryptopatch formation was not altered in germ-free mice7 (data not shown). In contrast, absolute and relative numbers of RORγthi NKp46+NK1.1int cells were lower in germ-free mice (Fig. 6a,b). Although we noted some variation in the fraction of RORγthiCD3− lymphocytes in the lamina propria of normal mice, the ratio of RORγthiNK1.1int cells to RORγthiNK1.1− lymphocytes remained consistent. In germ-free mice, this ratio was much lower but returned to normal after recolonization of the germ-free mice with normal microbial flora (Fig. 6c). Although fewer RORγthiNKp46+NK1.1int were still present in germ-free mice, only a very small fraction produced IL-22, and IL-22 production could not be induced by stimulation with IL-23 (Fig. 6d).
Figure 6.
Germ-free mice lack IL-22-producing RORγthiNKp46+NK1.1int cells. (a) Flow cytometry of lamina propria lymphocytes from conventional and germ-free B6 mice, stained with anti-NK1.1 and anti-RORγt. Numbers adjacent to outlined areas indicate percent cells in each gate. Data are representative of three independent experiments with a total of 20 conventional mice and 24 germ-free mice. (b) Absolute numbers of lamina propria cell populations. Data are representative of three independent experiments (error bars, s.d.; n = 4 mice). (c) NK1.1int cells among all CD3−RORγthi cells in the lamina propria of conventional B6 mice, of germ-free B6 mice and of germ-free mice recolonized with normal microflora (time, horizontal axis). Data are representative of three independent experiments (error bars, s.d.; n = 3 mice). (d) IL-22-producing RORγthiNK1.1int cells among lamina propria cells from conventional or germ-free B6 mice, incubated for 6 h with IL-23 in the presence of brefeldin A, then stained with anti-NK1.1, anti-RORγt and anti-IL-22. Data are representative of two independent experiments with ten mice per group. (e) NK1.1int cells among all CD3−RORγthi cells in the lamina propria of conventional B6 mice, germ-free B6 mice and Il6−/− mice. Data are representative of three independent experiments (error bars, s.d.; n = 3 mice). (f) Quantitative RT-PCR analysis of RegIIIβ (Reg3b) and RegIIIγ (Reg3g) mRNA in epithelial cells purified from B6 mice (top row) or Rag2−/− mice (middle row) injected three times every other day with 200 µg anti-IL-22 (α-IL-22; B6) or 200 µg anti-NK1.1 (α-NK1.1; Rag2−/−) or with isotype-matched control antibody (Ctrl Ig), analyzed 2 d after the final injection, or from Rorc(γt)GFP/+ and Rorc(γt)GFP/+ mice (bottom row). (g) Quantitative RT-PCR analysis as in f of epithelial cells from conventional B6 mice (B6) and germ-free mice recolonized with normal microflora (time, horizontal axis). Data are representative of three experiments (f,g).
Studies have shown that RORγt mRNA expression is lower in intestinal CD4+ T cells obtained from Il6−/− mice40. It is believed that IL-6 produced under the influence of the intestinal commensal flora is an important signal that promotes RORγt expression in intestinal TH-17 cells. Although Il6−/− mice did indeed have fewer intestinal TH-17 cells (CD4+RORγtdim T cells; data not shown), the ratio of RORγthiNK1.1int cells to RORγthiNK1.1− lymphocytes was normal in Il6−/− mice (Fig. 6e). These data collectively show that signals from the commensal microflora are required for the emergence of IL-22-producing RORγthiNKp46+NK1.1int cells in the intestinal lamina propria.
We investigated the contribution of IL-22 produced by RORγthi NKp46+NK1.1int cells to epithelial homeostasis by analyzing the expression by intestinal epithelial cells of genes encoding the IL-22-response molecules RegIIIβ and RegIIIγ, which are involved in tissue repair and antimicrobial responses41. Neutralization of IL-22 led to much less epithelial expression of RegIIIβ and RegIIIγ (Fig. 6f, top). To determine the function of IL-22-producing RORγthiNKp46+ NK1.1int cells in the epithelial expression of these genes, we assessed RegIIIβ and RegIIIγ mRNA in epithelium isolated from mice lacking all T cells and B cells (Rag2−/− mice) that were depleted of mucosal NK1.1+ cells, including the IL-22-producing RORγthiNKp46+NK1.1int subset (data not shown). Rag2−/− control mice expressed amounts of epithelial RegIIIβ and RegIIIγ mRNA similar to that of normal B6 mice (Fig. 6f, middle). Notably, depletion of all NK1.1+ cells led to much lower expression of RegIIIβ and RegIIIγ (Fig. 6f, middle), which indicated that RORγthiNKp46+NK1.1int cells are an important source of IL-22. In line with our findings that the differentiation of RORγthiNKp46+NK1.1int cells depended on commensal microflora and RORγt, RORγt-deficient and germ-free mice also showed impaired expression of RegIIIβ and RegIIIγ (Fig. 6f,g). Recolonization of germ-free mice normalized the expression of RegIIIβ and RegIIIγ (Fig. 6g). Our data demonstrate that signals from the commensal microflora ‘instruct’ the differentiation of RORγthiNKp46+NK1.1int cells that produce IL-22 and may promote epithelial homeostasis.
DISCUSSION
Here we have phenotypically and functionally characterized NKp46+CD3− lymphocytes in the intestinal lamina propria. These cells lacked markers of mature NK cells and instead had a phenotype resembling that of iNK cells of the bone marrow. We were able to further distinguish three subpopulations on the basis of expression of RORγt and NK1.1. From these populations, the RORγt−NKp46+NK1.1hi and RORγtintNKp46+NK1.1hi cells had developmental requirements similar to those of conventional NK cells in that their development depended on IL-15 and the common γ-chain but not on RORγt or recombination-activation gene products. Together with data showing that RORγt-deficient mice have normal numbers of peripheral NK cells9 (unpublished data), these findings suggest that RORγt−NKp46+NK1.1hi and RORγtintNKp46+NK1.1hi cells are part of the NK cell lineage. Genetic fate–mapping experiments have shown that splenic NK cells do not express RORγt during their development8, which suggests that the acquisition of RORγt by the RORγtintNKp46+NK1.1hi NK cells of the intestinal lamina propria depends on signals from the intestinal environment or that these cells follow a distinct developmental program. Notably, like RORγtint intestinal TH-17 cells40, RORγtintNKp46+NK1.1hi NK cells were present in Rorc(γt)GFP/GFP mice. However, RORγtintNKp46+NK1.1hi NK cells did not produce any cytokines associated with TH-17 cells in the conditions tested here and thus their function remains to be identified.
The third subpopulation of mucosal NKp46+ lymphocytes had an RORγthiNK1.1int phenotype and was absent from mice genetically lacking RORγt but present in mice lacking IL-15. These findings suggest that mucosal RORγthiNKp46+NK1.1int cells are not part of the NK cell lineage. As RORγt is required for the development and lineage commitment of fetal LTi cells and adult LTi-like cells in the cryptopatches and other tertiary lymphoid organs8–10,37, RORγthiNKp46+ NK1.1int cells may be derived from RORγthiLin− LTi-like cells and may not represent authentic NK cells. That view is supported by data demonstrating that human CD127+RORγt+ fetal LTi cells can differentiate into CD56+CD127+RORγt+ NK-like cells42. Mucosal RORγthiNKp46+NK1.1int cells of mice expressed various stimulatory NK cell receptors but did not have conventional NK cell effector functions. Similar to the human CD56+CD127+RORγt+ cells, they produced homeostatic cytokines such as IL-22 and lacked expression of major histocompatibility complex class I–specific inhibitory receptors42. A close developmental relationship between the NK and LTi lineages has already been suggested by analysis of mice genetically deficient in the transcription factor Id2 that lack development of NK cells and LTi cells, as well as by data showing that LTi cells from newborn mice upregulate NK cell markers after in vitro culture in IL-2 (refs. 37–39). Such data collectively suggest that in the gut environment, RORγthi LTi-like cells may upregulate expression of NK cell–recognition receptors and produce homeostatic cytokines.
In contrast to RORγthiLin− LTi-like cells and RORγtneg–int NKp46+NK1.1hi NK cells, RORγthiNKp46+NK1.1int cells produce IL-22 (our data and ref. 43). This finding indicates that RORγt expression is not sufficient for IL-22 production. In germ-free mice, the absolute and relative numbers of RORγthiNKp46+NK1.1int cells producing IL-22 were lower, whereas the number of RORγthiNKp46− NK1.1− LTi cells was not altered much7. Thus, cues from the indigenous microflora might drive the differentiation of RORγthi NKp46−NK1.1− LTi cells into IL-22-producing RORγthiNKp46+ NK1.1int cells. Alternatively, RORγthiNKp46+NK1.1int cells may represent a lymphocyte lineage distinct from LTi cells and NK cells, which depend on RORγt and commensal microflora for their differentiation. It is unclear how signals from the lumenal microbes are communicated to RORγthi cells. Given published data documenting dendritic cell–mediated NK cell priming44, it is possible that dendritic cells surrounding the cryptopatches and extending their protrusions between the epithelium into the lumen might be key mediators in this process12. The expression of various stimulatory immunoreceptors by RORγthiNKp46+NKp46+NK1.1int cells is notable. Many of these receptors recognize ligands expressed by stressed and diseased cells45. Thus, RORγthiNKp46+NK1.1int cells might be sentinels that sense damaged cells, leading to the release of homeostatic cytokines (such as IL-22).
The sensing of commensal microflora is important for epithelial homeostasis and the expression of antimicrobial molecules3,5,6. However, the molecular program initiated by these signals and the cellular intermediaries remained unknown. IL-22 may be an important participant in this, as the IL-22 receptor is expressed by epithelial cells but not by most hematopoietic cells24. Gene array data from IL-22-treated colon explants show that IL-22 induces the expression of genes encoding molecules that promote epithelial homeostasis, such as small secreted C-type lectins of the RegIII family25. Our data have established an important link between commensal microflora and RORγthiNKp46+NK1.1int cells producing IL-22 that contributed to the homeostatic expression of IL-22-response genes.
On the basis of our data, we propose that cells in cryptopatches, whose function remains unknown, are more diverse than previously appreciated. Given their unusual location at the bottom of the crypts in direct proximity to epithelial stem cells and Paneth cells and their ability to produce homeostatic cytokines, IL-22-producing cells in cryptopatches may represent a central point of control of epithelial integrity. The mucosal immune system is evolutionarily ancient, and it was the autochthonous function of the immune system to protect surfaces in intimate contact to potentially hazardous pathogens before the development of organized secondary lymphoid organs. Our data have added to that perspective by showing that the mucosal immune system reacts to cues from the indigenous microflora, which ‘instruct’ the differentiation of IL-22-producing cells that express receptors able to sense cellular stress and epithelial damage.
METHODS
Mice
B6 and Rag2−/− mice were from Charles River Laboratories. Il15−/− mice and Rag2−/−Il2rg−/− mice on a B6 background were from Taconic Farms. Germfree B6 mice were generated by transfer of embryos at the two-cell stage in sterile conditions into germ-free ‘pseudopregnant’ recipient female NMRI mice. After being weaned, the mice were randomly bred and were maintained in positive-pressure plastic isolators and provided a γ-irradiated commercial diet and autoclaved water. Their germ-free status was verified by weekly culture of fecal samples. For recolonization experiments, germ-free mice were placed in cages with conventional B6 mice. Rorc(γt)GFP/+ mice37 (a gift from D. Littman) were backcrossed eight to ten generations onto B6 mice. Il6−/− mice46 on a B6 background were provided by M. Kopf. Mice were used at 8–16 weeks of age. All experiments were approved by and were in accordance with local animal care and use committees (Regierungspräsidium Freiburg).
Preparation of lamina propria lymphocytes and isolation of intestinal epithelial cells
Lamina propria lymphocytes were prepared according to a published protocol40 (detailed protocol, Supplementary Methods online). For preparation of intestinal epithelial cells, intestinal epithelium was removed from the underlying tissue by incubation for 10 min at 37 °C in 30 mM EDTA with calcium- and magnesium-free PBS, followed by vigorous shaking. Epithelial cells were collected by centrifugation and were washed. Multicellular epithelial aggregates were separated from contaminating cells and residual tissue by repeated differential sedimentation at 1g in ice-cold PBS47.
Immunofluorescence staining and flow cytometry
For cell surface analysis, cell populations were stained with fluorescence-conjugated antibodies (complete list, Supplementary Methods). A FACSCanto II or a FACSCalibur (BD Biosciences) was used for flow cytometry and data were analyzed with FlowJo software (Tree Star). A MoFlo (DAKO Cytomation) was used for cellsorting experiments.
Intracellular staining
Intracellular cytokine staining was done as described44. For intracellular detection of RORγt, cells were made permeable with Foxp3 staining solution according to the protocol provided by the manufacturer (eBioscience) and were stained with Alexa Fluor 488–conjugated monoclonal anti-RORγt (B2D; eBioscience).
In vitro stimulation and ELISA
Cell populations were cultured in vitro in the presence of human IL-2 (500 ng/ml; Novartis Pharma) and mouse IL-15 (50 ng/ml; Peprotech) and were stimulated for various times with IL-23 (10 ng/ml; Peprotech). Supernatants of these cultures were removed at various time points and assessed for IL-22 protein content by ELISA with the Quantikine Mouse/Rat IL-22 Immunoassay kit according to the manufacturer’s protocol (R&D Systems).
Real-time PCR
RNA was isolated with the RNeasy Plus Mini kit (Qiagen). Total RNA was reverse-transcribed into cDNA with the High Capacity cDNA Archive kit (Applied Biosystems). An ABIPrism 7900 sequence detector (Applied Biosystems) was used for subsequent real-time PCR with TaqMan Universal Master Mix and the Assay-on-Demand system (Applied Biosystems), which include forward and reverse primers with a 5-carboxyfluorescein-labeled probe for the target gene (assays, Supplementary Methods). Expression of mRNA was calculated with SDS 2.1 software (Applied Biosystems). The amount of mRNA was normalized to that of the ‘housekeeping’ gene Hprt1 (encoding hypoxanthine guanine phosphoribosyl transferase), and expression was calculated as the ‘n-fold’ difference relative to Hprt1 according to the cycling threshold (CT) formula 2−ΔCT, where ΔCT = CT (target) − CT (endogenous control).
Immunofluorescent staining of tissue sections
Small intestines and colon were fixed for 2 h on ice in a 4% (wt/vol) solution of paraformaldehyde. Excess paraformaldehyde was removed by incubation in fresh PBS. Fixed tissues were incubated overnight at 4 °C in 30% (wt/vol) sucrose. The next day, samples were embedded in optimum cutting temperature compound (Sukara Finetech), were frozen in liquid nitrogen and were stored at −80 °C until use. Cryostat sections 5 µm in thickness were collected on frosted slides and were rehydrated for 5 min with PBS. Sections were blocked and were made permeable in PBS containing 0.1% (vol/vol) Triton X-100, 0.1% (wt/vol) BSA, 20% (vol/vol) FCS and 10% (vol/vol) goat serum, followed by an avidin-biotin step to block endogenous biotin according to the manufacturer’s protocol (Dako Cytomation). Slides were then stained with the appropriate antibodies (Supplementary Methods). Sections were washed three times for 5 min each and were mounted with mounting medium containing the DNA-intercalating dye DAPI (4,6-diamidino-2-phenylindole; Vectashield; Vector Laboratories).
In vitro assay of NK cell function
NK cell function was primed in vivo by injection of mice with 150 µg polyinosinic-polycytidylic acid (Sigma) or 100 ng lipopolysaccharide (Invivogen) as described44. The cytotoxic activity of NK cells toward target cells was determined with a standard 4-hour 51Cr-release assay. Before the cytotoxicity assay, the percentage of NK1.1+CD3− cells in the lymphocyte population was determined and lymphocyte numbers were adjusted to contain the same number of NK cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Kist for support; M. Lucas, H. Pircher, W. Schachterle and C. Vonarbourg for critical comments on the manuscript; M. Schnare for discussions; K. Geiger and M. Follo for cell sorting; D. Littman (New York University) for Rorc(γt)GFP/+ mice; B. Stockinger (National Institute for Medical Research Mill Hill) for support and reagents; M. Kopf (Swiss Federal Institute of Technology Zürich) for Il6−/− mice on a B6 background; J.-C. Renauld (Ludwig Institute for Cancer Research Brussels) for anti-IL-22; and N. Goeppert for technical assistance. Supported by Deutsche Forschungsgemeinschaft (Di 764/ 2-2, GRK1104 (A.M.) and SFB620).
Footnotes
Accession codes. UCSD-Nature Signaling Gateway (http://www.signaling-gate way.org): A002302.
Note: Supplementary information is available on the Nature Immunology website.
AUTHOR CONTRIBUTIONS
S.L.S., V.L.B., A.M., K.O. and C.H. did and analyzed the experiments; C.J. generated and provided the germ-free mice and contributed to the experimental design; and A.D. and S.L.S. designed the experiments and wrote the paper.
References
- 1.Newberry RD, Lorenz RG. Organizing a mucosal defense. Immunol. Rev. 2005;206:6–21. doi: 10.1111/j.0105-2896.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 2.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 3.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Zaph C, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 5.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 6.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanamori Y, et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+IL-7R+Thy1+ lympho-hemopoietic progenitors develop. J. Exp. Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberl G, Littman DR. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 9.Sun Z, et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 10.Kurebayashi S, et al. Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl. Acad. Sci. USA. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat. Rev. Immunol. 2005;5:413–420. doi: 10.1038/nri1600. [DOI] [PubMed] [Google Scholar]
- 12.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 14.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 15.Tagliabue A, Befus AD, Clark DA, Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J. Exp. Med. 1982;155:1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan PG, Hapel AJ, Doe WF. Lymphokine-activated and natural killer cell activity in human intestinal mucosa. J. Immunol. 1985;135:1731–1738. [PubMed] [Google Scholar]
- 17.Gibson PR, Jewell DP. The nature of the natural killer (NK) cell of human intestinal mucosa and mesenteric lymph node. Clin. Exp. Immunol. 1985;61:160–168. [PMC free article] [PubMed] [Google Scholar]
- 18.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J. Immunol. 2000;164:1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 19.Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc. Natl. Acad. Sci. USA. 2000;97:10144–10149. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie MH, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2–4 and IL-22R. J. Biol. Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 21.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 23.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 24.Wolk K, et al. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 26.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Walzer T, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sivori S, et al. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J. Exp. Med. 1997;186:1129–1136. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu. Rev. Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 30.Arase H, Saito T, Phillips JH, Lanier LL. Cutting edge: the mouse NK cellassociated antigen recognized by DX5 monoclonal antibody is CD49b (α2 integrin, very late antigen-2) J. Immunol. 2001;167:1141–1144. doi: 10.4049/jimmunol.167.3.1141. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 32.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 33.DiSanto JP, Müller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito H, et al. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity. 2000;13:691–702. doi: 10.1016/s1074-7613(00)00068-6. [DOI] [PubMed] [Google Scholar]
- 37.Eberl G, et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 38.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 39.Yokota Y, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 40.Ivanov II, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 41.Graf R, et al. Exocrine meets endocrine: pancreatic stone protein and regenerating protein–two sides of the same coin. J. Surg. Res. 2006;133:113–120. doi: 10.1016/j.jss.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Cupedo T, et al. Human fetal lymphoid tissue–inducer cells are interleukin 17–producing precursors to RORC+ CD127+ natural killer cells. Nat. Immunol. advance. 2008 Nov 23; doi: 10.1038/ni.1668. online publication. [DOI] [PubMed] [Google Scholar]
- 43.Luci C, et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 2008 Nov 23; doi: 10.1038/ni.1681. advance online publication, [DOI] [PubMed] [Google Scholar]
- 44.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diefenbach A, Raulet DH. Innate immune recognition by stimulatory immunoreceptors. Curr. Opin. Immunol. 2003;15:37–44. doi: 10.1016/s0952-7915(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 46.Kopf M, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 47.Lotz M, et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.