Abstract
HIV enters the brain soon after seroconversion and can cause HIV associated neurocognitive disorders (HAND). While the more severe and progressive forms of HAND are less prevalent due to combination antiretroviral therapy (cART), ~ 40% of HIV-infected (HIV+) patients continue to have cognitive impairment. Some HIV+ individuals who have effective plasma HIV-1 RNA suppression with cART still develop HAND. It is often difficult to diagnose HAND in the outpatient setting as detailed neuropsychological performance testing is required.
Additional biomarkers that are relatively easy to obtain and clinically relevant are needed for assessing HIV associated neuropathologic changes. Recently developed non-invasive magnetic resonance imaging (MRI) techniques have great potential to serve as biomarkers. We review the application of some of these neuroimaging techniques [magnetic resonance spectroscopy (MRS), volumetric MRI, diffusion tensor imaging (DTI), functional MRI (fMRI)] in HIV+ individuals. Each of the neuroimaging methods offers unique insight into mechanisms underlying neuroHIV, could monitor disease progression, and may assist in evaluating the efficacy of particular cART regimens. It is hoped that considerable progress will continue to occur such that some of these neuroimaging methods will be incorporated across multiple sites and included in future HAND guidelines.
Keywords: HIV, neuroimaging, magnetic resonance spectroscopy, volumetrics, diffusion tensor imaging, functional MRI
Introduction
Human immunodeficiency virus (HIV) affects more than 1 million individuals In the United States (US) and over 40 million people worldwide1. Advances in combination antiretroviral treatment (cART) have transformed HIV from a rapidly fatal disease to a manageable chronic condition2–4. The proportion of older HIV-infected (HIV+) individuals is rapidly growing. More than half of all HIV+ individuals in the US are expected to be greater than 50 years old by 20155. HIV infected (HIV+) individuals receiving cART can now expect to live almost as long as HIV-uninfected (HIV−) individuals6.
Despite these advances, eradication of HIV from the brain has not occurred. The prevalence of HIV associated neurocognitive disorders (HAND) has remained constant (~ 40%) despite more available and effective antiretrovirals7,8. Soon after seroconversion, HIV rapidly spreads throughout the brain. Some HIV+ individuals who have effective plasma HIV-1 RNA suppression with cART still develop HAND 9. The continued presence of HAND in the cART era may result from non-mutually-exclusive factors including irreversible injury prior to initiating cART; persistent HIV-1 RNA in the central nervous system (CNS) compartment10, antiretroviral toxicities11–13, and/or persistent low level inflammation in the CNS14. A major effort has begun to optimize therapy for HAND by addressing persistent HIV reservoirs and immunologic activation in the brain.
HAND is often difficult to characterize in the typical outpatient visit (15–30 minutes). Multiple connections throughout the brain are often affected leading to the complex series of clinical signs and symptoms 15. Recent criteria have subdivided HAND into three categories: asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV associated dementia (HAD)8. These definitions are based upon an individual’s performance on neuropsychological performance (NP) testing and self-reported activities of daily living. However, limitations exist with the current HAND criteria16. Often NP testing (~3 hours) is performed in research setting at certain sites8. A continuum of HAND may occur instead of set distinctions17. Unlike other neurodegenerative disorders (i.e. Alzheimer’s disease)18 additional biomarkers (cerebrospinal fluid (CSF) or neuroimaging) have not been included in the HAND diagnosis. Biomarkers of HAND that are both easy to perform and clinically relevant remain an unmet need.
Neuroimaging techniques may therefore have increased utility in the diagnosis and management of HAND. A variety of novel non-invasive neuroimaging techniques have been developed and hold great promise as they often can be added to conventional sequences. Of note, three magnetic resonance imaging (MRI) techniques have been used in the neuroHIV research setting: metabolic (magnetic resonance spectroscopy (MRS)), structural (MRI volumetrics and diffusion tensor imaging (DTI), and functional (functional MRI (fMRI)). This review is not meant to be a comprehensive review of all MRI techniques and does not focus on other neuroimaging modalities (e.g. positron emission tomography 19).
Cerebral metabolite imaging using magnetic resonance spectroscopy (MRS)
MRS has been one of the most consistently used neuroimaging methods during the pre and post cART eras 20–23. A current PubMed search reveals greater than 75 articles that have used this technique to detect HIV-associated changes in cerebral metabolites (key search terms: “MRS”, “brain”, and “HIV”). Please see Table 1 for a select list of MRS studies performed in HIV+ patients. MRS detects the signal produced by protons of specific molecules within a volume of brain. Signal amplitude of a particular molecule X (AX) of interest is proportional to the number of moles of X (NX) with the brain volume (VB) interrogated. Typical molecules measured include: 1) N-acetyl aspartate (NAA)- a neuronal marker, 2) choline (Cho)- a marker of cellular proliferation and inflammatory response, 3) creatine (Cr)- a measure of brain energy metabolism and reference marker, 4) myo-inositol (MI)- a marker of gliosis, and 5) glutamine (Gln)/glutamate (Glu)- measures of neurotoxicity due to excess n-methyl-d-aspartate (NMDA) receptor activation.
Table 1.
Select citations of magnetic resonance spectroscopy (MRS) in HIV+ patients
| Reference | Subjects | % HIV+ on cART | HAND classification | Field strength | Regions of Interest | Major Findings |
|---|---|---|---|---|---|---|
| Cysique et al, PLoS One 2013 | 92 HIV+ (56 yo), 30 HIV− (55 yo) 100% male | 100% | NA | 3T | FWM, caudate, parietal cortex | HIV+ had lower NAA and increased MI in FWM, lower NAA in caudate, and higher Cho/NAA and MI in parietal cortex. HIV and aging interaction in NAA FWM |
| Valcour et al, PLoS One 2013 | 61 acute HIV+ (35 yo), no HIV− | 0% | 14 ANI 8 MND 6 HAD |
1.5T | FGM, FWM, BG, occiptal gray matter | Blood CD14+ associated with lower NAA and higher MI in FGM, FWM and BG |
| Sailasuta et al, PLoS One 2012 | 31 acute HIV+ (30 yo) 26 chronic HIV+ (34 yo) 10 HIV− (36 yo) |
0% Primary HIV+ not on cART, 100% chronic HIV+ | NA | 1.5 T | FWM, FGM, BG, occipital gray matter | Acute HIV+ had elevated Cho/Cr in BG and occipital gray matter compared to chronic HIV+ and HIV−. cART in acute HIV+ led to normalization of Cho/Cr in BG |
| Valcour et al, JID 2012 | 20 acute HIV+ (31 yo, 90% male, no HIV− | 0% | NA | 1.5T | FGM, FWM, occiptal gray matter, BG | Acute HIV+ had elevated Cho/Cr in occipital gray matter. Higher CSF neopterin was associated with elevated Cho/Cr in occipital gray matter and elevated MI/Cr in FWM. |
| Harezlak et al, AIDS 2011 | 240 HIV+ (47 yo) 28 HIV− (53 yo) |
100% | 124 ADC 0 66 ADC 0.5 60 ADC >1 |
Not stated | BG, FWM, FGM | Increased MI/Cr and Cho/Cr in BG, FGM, FWM in all HIV+ groups compared to HIV− Decreased Glu/Cr in FWM of ADC 0. Decreased NAA/Cr in ADC >1 compared to other HIV+ groups. |
| Lentz et al, J Neurovirology 2011 | 9 primary HIV+ (39 yo) 9 HIV− (31 yo) 100% male |
Baseline: 22.2% 2-months: 44.4% 6-months: 55.6% |
All asymptomatic | 1.5T | FGM, FWM, and BG | Cho/Cr increased in FWM and FGM at 2- and 6-months in primary HIV+. Higher levels of peripheral CD16+ monocytes were associated with lower NAA and higher Cho. |
| Letendre et al, J Neurovirology 2011 | 129 HIV+ (42 yo, 89% male), no HIV− | 91% | 30 ADC 0 83 ADC 1 14 ADC 2 2 ADC 3 |
1.5 T | Parietal cortex, WM (including FWM), BG | Higher CSF IP-10 correlated with lower NAA/Cr in FWM and higher MI/Cr in the parietal cortex, FWM, and BG. Higher CSF MCP-1 correlated with lower NAA/Cr in FWM and parietal cortex. |
| Ernst et al, J MRI 2010 | 45 HIV+ (46 yo, 93% male) 46 HIV− (43 yo, 80% male) |
100% | 27 cognitively normal 6 ANI 10 MND 2 HAD |
3T | BG, FGM, FWM, parietal cortex | Lower Glu in parietal GM in HIV+ with cognitive deficits. Lower Glu in BG in HIV+ with no cognitive deficits. |
| Mohamed et al, MRI 2010 | 86 HIV+ (47 yo, 69 % male), no HIV− | 100% | 21 ADC 0 31 ADC 0.5 24 ADC > 1 |
3T | FWM and BG | Patients with ADC >1 had decreased Glu, Glu/Cr and increased MI, MI/Cr in FWM and decreased NAA in the BG. |
| Paul et al, J of the International Neuropsychological Society 2008 | 22 HIV+ (38 yo, 86% male) 20 HIV− (35 yo, 47% male) |
> 50% | 6 ADC 0 16 ADC 1 |
1.5T | BG | Cho/Cr higher and NAA/Cr lower in HIV+ vs. HIV−. MRS measures correlated with NP testing |
| Schweinsburg et al, J Neurovirology 2005 | 18 HIV+ (32 yo), 17 HIV− (28 yo) | 67% | NA | 1.5T | FGM and FWM | HIV+ patients on NRTIs had lower NAA in the FWM compared to HIV−. HIV+ patients not receiving NRTIs had intermediate NAA levels. |
| Chang et al, NeuroImage 2004 | 100 HIV+ (40 yo) 37 HIV− (34 yo) Cohort 77% male |
100% | 61 cognitively normal 39 congitively impaired | 1.5T | FWM, BG, parietal cortex | MI/Cr increased in WM of cognitively normal HIV+ MI/Cr Increased in WM and BG of cognitively impaired HIV+ Cho/Cr Increased in WM and BG of cognitively impaired HIV+ NAA/Cr decreased in WM and BG of cognitively impaired HIV+ CSF viral load correlated with increase MI/Cr and Cho/Cr in WM and decreased NAA/Cr in parietal cortex Aging and HIV infection have additive effect on increased MI/Cr and Cho/Cr in BG and WM |
| Yiannoutsos, NeuroImage 2004 | 100 HIV+, no HIV− | NA | NA | 1.5T | FWM, FGM, BG, parietal cortex | Three metabolic patterns: 1) inflammatory (elevated MI/Cr in all regions and elevated Cho/Cr in the FWM and parietal cortex); 2) basal ganglia (elevated NAA/Cr and Cho/Cr); 3) neuronal (reduced NAA/Cr in FGM and parietal cortex). |
Abbreviations: NA= not available, ADC= AIDS dementia complex, FWM= frontal white matter, WM= white matter, FGM= frontal grey matter, BG= basal ganglia, NAA= N-Acetyl Aspartate, Cho=Choline, Cr=Creatine, MI= Myo-inositol, Gln=Glutamine, Glu= Glutamate, cART= combination antiretroviral therapy
In general, MRS can be performed on conventional MRI scanners but technical assistance is needed to insure good quality scans are obtained. MRS studies should be carefully performed to insure homogeneity of the magnetic field and suppression of the water signal24. Depending on both the institution and time available for scanning, single or multi-voxel MRS have been acquired using a variety of acquisition techniques to yield qualitative vs. semi- quantitative vs. quantitative values. Due to quantification limitations, calibration is often performed using a phantom or an internal signal [e.g. water (H2O) or Cr]. This can result in metabolite ratios rather than absolute concentrations (e.g. NAA/Cr).
Though often limited to certain brain regions (e.g. frontal gray, frontal or parietal white matter, and basal ganglia), MRS provides key insights into the dynamic changes in the brain metabolic profile from primary (≤ 1 year since seroconversion) to chronic (> 1 year since seroconversion) infection. Soon after seroconversion, MRS metabolites have been shown to be affected21,25–27. HIV+ subjects scanned during the first year of infection have increased Cho/Cr in the frontal and white matter21 compared to HIV− controls. A subsequent study confirmed these findings with primary HIV+ individuals having higher Cho/Cr in the basal ganglia compared to HIV− controls25. Observed MRS changes are correlated with markers of CNS infection and inflammation (detectable HIV-1 RNA and chemokines)25 and neuronal injury (neurofilament light chain)27. Within chronically infected patients, brain metabolite changes are also evident. Many studies have often observed reductions in NAA and concomitant increases in Cho and MI 22,28–31. More recent MRS studies performed at higher magnetic fields using newer analysis methods have demonstrated reductions in Glu32 and Gln29. Observed MRS changes in chronically infected HIV+ patients are proportional to the degree of cognitive impairment22,29. While increases in MRS markers of inflammation (Cho and MI) are seen in cognitively normal HIV+ patients, greater changes in inflammation (Cho and MI) and neuronal loss (NAA/Cr and Glu/Cr) are observed in HAND patients22,29,32
The introduction of cART has dramatically reduced the more severe forms of HAND and can also lead to improvements, but not normalization, of brain metabolites31,33,34. Early treatment with cART may be neuroprotective and mitigate the early inflammatory changes seen in primary HIV+ patients. Commencement of therapy soon after diagnosis normalizes Cho/Cr in the basal ganglia within 6 months26. A number of clinical trials have started to include MRS markers to evaluate the efficacy of adjunctive therapy for HAND35. This technique may have great potential in future early prevention studies.
Increasing evidence has also suggested that certain antiretrovirals may cause mitochondrial toxicity and lead to neuronal loss36,37. Chronically infected HIV+ patients on cART regimens that included nucleotide reverse transcriptase inhibitors (NRTIs) had significant reductions in NAA in the frontal white matter compared to HIV− controls. HIV+ individuals receiving alternative cART regimens that did not include NRTIs exhibited intermediate decreases in NAA38. A more recent study has observed that HIV+ patients receiving NRTIs had reductions in parietal and frontal gray matter Glu that were predictive of worse cognitive performance.32
With a larger proportion of HIV+ growing older with the disease, a number of studies have started to investigate the interaction between HIV and aging using MRS. HIV+ patients have been shown to have significant reductions in Glu to levels equivalent to those in HIV− controls a decade older.32 Another study confirmed these findings by demonstrating that HIV+ patients exhibited age dependent declines in NAA and Gln, such that the metabolic profile of a 30 year old HIV+ subject was equivalent to a 56 year-old HIV− control.22 In both instances, while HIV and aging effects were observed, no interaction was present.
Overall, MRS offers a valuable method for monitoring HIV associated neuropathologic changes. Observed MRS changes may be more sensitive than conventional MRI alone and could augment current neuroimaging protocols. MRS measures may detect subtle early changes associated with HIV infection, and concentrations or ratios of cerebral metabolites measured by MRS could be used as a quantitative indicator of cerebral involvement. In addition, MRS could be used to evaluate the efficacy of therapeutics directed against HIV infection within the CNS during early stages of infection. Some limitations exist in the current MRS HIV research literature, including mostly cross-sectional studies, as well as analyses restricted to specific regions of interest. However, MRS results suggest that contributions of inflammation, aging, and drug toxicity could all contribute to the continued prevalence of HAND. Additional studies that include more HIV− controls are needed. Longitudinal studies, with a focus on repeated imaging of HIV+ patients as they transition through different stages of infection, as well as prior to and after stable cART, are needed. In addition, the impact of co-morbidities (e.g. hepatitis, substance abuse, etc.) on MRS measurements should be more fully characterized in HIV+ patients.
Structural Neuroimaging
Volumetrics Analysis of MRI
Volumetric MRI examines particular regions of interest and assesses if abnormal structural changes are present in affected individuals compared to healthy controls 39. This method provides a useful tool to rule out alternate etiologies and can support a diagnosis of HAND. Specific structures or general brain regions (e.g. white and grey matter) are analyzed 40. A PubMed search using keyword search terms “MRI”, “volume”, “brain”, and “HIV” identifies more than 60 articles. Please see Table 2 for a select list of MRI volumetric studies performed in HIV+ patients. Typically, higher field MRI (initially 1.5T and now 3T) has been used to acquire high resolution T1-weighted images. In particular, a magnetization prepared rapid acquisition gradient echo (MPRAGE) image provides the greatest contrast for segmenting grey matter, white matter, and CSF. While not typically acquired with conventional imaging sequences, the MPRAGE sequence can be obtained on most MRI scanners.
Table 2.
Select citations of magnetic resonance imaging (MRI) of volumetrics in HIV+ patients
| Reference | Subjects | % HIV+ on cART | HAND classification | Field strength | Regions of interest | Major Findings |
|---|---|---|---|---|---|---|
| Bonnet et al, AIDS 2013 | 400 HIV+ (47 yo, 79% male), no HIV− | 89% | 21% ANI 31% MND 7% HAD |
1.5T | GM and WM | MND or HAD had lower WM and GM than ANI. |
| Fennema-Nostestine et al, J Neurovirol 2013 | 75 HIV+ (45 yo, 83% male), no HIV− | 79% | NA | 1.5T | Total cerebral WM, abnormal WM, subcortical and cortical GM, and ventricular and sulcal CSF | Greater plasma CD4 recovery associated with increased abnormal WM and subcortical GM volumes. Virologic suppression was independently associated with increased GM volume. |
| Kallianpur et al, Neurology 2013 | 135 HIV+ (54 yo, 91% male), 12 HIV−(54 yo, 100% male) | 100% | NA | 3T | Caudate, amygdala, hippocampus, thalamus, nucleus accumbens, putamen, globus pallidus, subcortical and cortical GM, cerebral WM, cerebellar GM, cerebellar WM, brainstem, lateral ventricles | HIV+ subjects with detectable viral load in the periphery had decreases in cerebellar and subcortical GM compared to HIV+ subjects with undetectable viral load. HIV + subjects with detectable viral load had ventricular enlargement and reduction of caudate, putamen, thalamus, hippocampus, nucleus accumbens, brainstem, total cortical GM, and cerebral WM compared to HIV− |
| Ances et al, J Acquir Immune Defic Syndr 2012 | 52 HIV+ (36 yo, 91% male), 26 HIV− (35 yo, 77% male) | 50% | NA | 3T | Amygdala, caudate, thalamus, hippocampus, putamen, corpus callosum, cerebral GM and WM | HIV+ subjects had reduction in amygdala, caudate, and corpus callosum compared to HIV−. Both HIV and aging were independently associated with volume reductions |
| Becker et al, Neurology 2012 | 84 HIV+ (38 yo) 76 HIV− (39 yo) 100% male |
NA | NA | 3T | GM and WM | HIV+ subjects had greater GM loss in posterior and inferior temporal lobe, parietal cortex, and cerebellum compared to HIV−. Both aging and HIV affect WM and GM volumes. Cardiovascular disease risk factors were not associated with brain volume loss. |
| Pfefferbaum et al, Biol Psychiatry 2012 | 127 HIV+,(45 yo, 70% male), 218 HIV− (47 yo, 69% male) | 87% | NA | 1.5T | Lateral frontal, medial frontal, temporal, parietal, occipital, caudate, putamen, globus pallidus, hippocampus, amygdala, thalamus | HIV+ subjects had reduced thalamic and frontal volumes compared to HIV−. Volume loss correlated with CD4 nadir and history of AIDS. |
| Ragin et al, Neurology 2012 | 43 HIV+ (33 yo, 88% male) 21 HIV− (31 yo, 76% male) |
47% | NA | 3T | Total brain volume, cortical and subcortical GM, WM, ventricular volume | HIV+ subjects had reductions in total and cortical GM and increase in the ventricular volume compared to HIV−. |
| Thames et al, Neuropsychologia 2012 | 20 HIV+ (53 yo, 60% male), no HIV− | 100% | 50% had HAND | 1.5T | Putamen and caudate | Impaired word generation significantly predicted reduction in caudate volume |
| Towgood et al, Cortex 2012 | 40 HIV+ (47 yo), 42 HIV− (45 yo) 100% male |
100% | NA | 3T | Total GM and WM | HIV+ subjects had reduced GM volume in the medial and superior frontal gyri compared to HIV−. HIV and aging were independently associated with reduced frontotemporal GM and WM volumes. |
| Jernigan et al, J Neurovirol 2011 | 251 HIV+ (44 yo, 82% male), no HIV− | 76% | 37% neurocognitively impaired | 1.5T | Total WM, abnormal WM, subcortical and cortical GM | Lower CD4 nadir and higher current CD4 cell counts were associated with lower total WM and subcortical GM volumes. Detectable HIV in the CSF correlated with total WM volume loss. Longer cART exposure correlated with reduced WM volumes and increased ventricular volumes. |
| Ragin et al, J Neurovirol 2011 | 8 HIV+ (51 yo, 75% male), no HIV− | 100% | NA | 3T | Cerebral cortex, cerebral WM, caudate, putamen, pallidum, accumbens, amygdala, hippocampus, thalamus, cerebellar cortex, cerebellar WM | MMP-7 significantly correlated with brain atrophy in multiple brain regions. |
| Cohen et al, J Neurovirol 2010 | 82 HIV+ (46 yo, 52% male), no HIV− | 80% | 16% ADC >1 | 1.5T | Cortical and subcortical GM; WM; total ventricular volume; frontal, parietal, temporal, and occipital lobes | HIV+ subjects with cognitive impairment had greater reductions in GM and parietal cortical volumes and increased ventricular volumes. Nadir CD4 and duration of infection correlated with volumetric reductions in WM, GM, parietal, temporal, and frontal lobes; and hippocampal volume. |
| Cardenas et al, J Neurovirology 2009 | 39 HIV+ (45 yo), 30 HIV− (42 yo) 100% male |
100% | NA | 1.5T | Total, frontal, temporal, parietal, and occipital WM and GM | HIV+ subjects had greater WM volume loss than SN controls. Greater rates of WM and GM volume loss were found in HIV+ subjects with detectable plasma HIV viral loads. |
| Castelo et al, Arch Neurology 2007 | 22 HIV+ (43 yo) 22 HIV− (43 yo) |
77% | NA | 3T | Hippocampus, caudate nucleus, putamen, globus pallidus | HIV+ subjects had putamen hypertrophy compared to HIV−. Enlarged putamen correlated with increased CD4 cell counts. |
| Thompson et al, PNAS 2005 | 26 HIV+ (47yo, 96% male) 14 HIV− (38 yo, 57% male) |
50% | NA | 1.5T | Cortical GM | HIV+ patients had volumetric loss within primary sensory, motor, and premotor cortices compared to HIV−. Volumetric loss in frontopolar areas correlated with nadir CD4+ cell counts. |
| Patel et al, AJNR 2002 | 20 HIV+, NAS (36 yo, 77% male), no HIV− | 94% | 8 ADC 0 5 ADC 0.5 2 ADC 1 |
1.5T | Total GM volume | Greater cognitive impairment was associated with greater volume loss. |
Abbreviations: NA= not available, ADC= AIDS dementia complex, HAND= HIV associated neurocognitive disorders, ANI= asymptomatic neurocognitive impairment, MND= mild neurocognitive disorder, HAD= HIV associated dementia, WM= white matter, GM= grey matter, cART= combination antiretroviral therapy
Early volumetric work concentrated on measuring ratios of subcortical (e.g. caudate) to intraventricular volumes. This technique could not isolate the location of atrophy and missed brain regions not within the field of view 41. Semi or fully automated methods have been developed for segmenting the brain based on voxel signal intensity properties of tissues 42–44. Currently, a variety of pre-processing programs are available but some experience is needed for analysis.
In the pre and early antiretroviral era, significant volume loss was observed in the basal ganglia, posterior cortex, and total white matter of HIV+ patients compared to age matched HIV− individuals45–47. Atrophy was greatest in more advanced stages of infection but changes were seen even in cognitively normal HIV+ individuals48. Subsequent studies in the cART era have demonstrated subcortical and cortical atrophy in HIV+ patients43,49–51 (See Figure 1). HIV+ individuals, especially those with an AIDS-defining event, have thinner cortical thickness (primary sensory, and motor),50 smaller cortical volumes,28,43,50,52,53 and larger total ventricular size50,53. Ongoing brain volume loss occurs despite initiation of cART28,51. Changes in brain volume may commence early as cortical atrophy and expansion of the third ventricle are observed in primary HIV infection52.
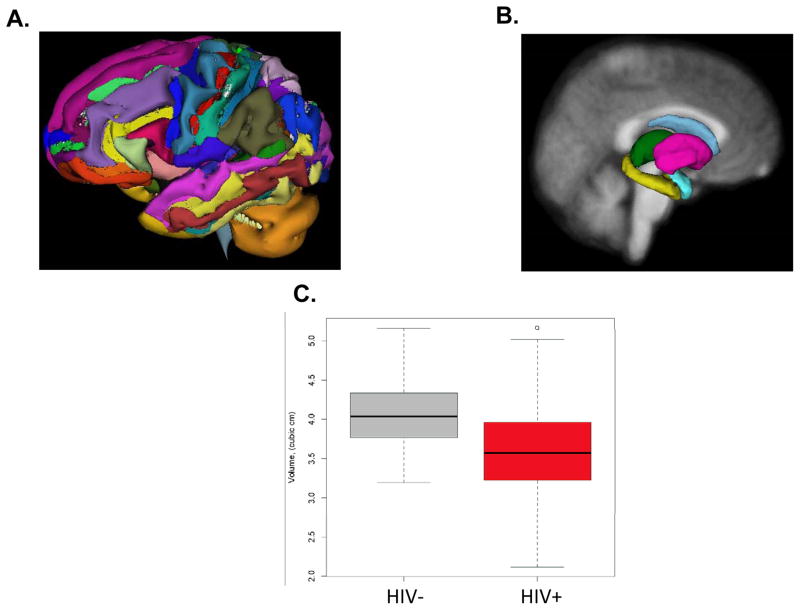
Figure 1.
Freesurfer segmentation of cortical (A) and subcortical (B) regions of the brain. (C) Comparison of volumes from the right caudate for HIV− controls and HIV+ patients. Overall, HIV+ patients had significantly smaller volumes (p < 0.05)
Volumetric changes also correlate with NP testing and clinical measures. A number of studies have reported structure-function relations with poorer cognitive or motor performance associated with smaller brain volumes30,50,54–61. Both greater viral burden (plasma HIV-1 RNA, cerebrospinal fluid HIV-1 RNA, peripheral monocyte DNA) and immune response to the virus (nadir CD4+ T lymphocyte counts) are associated with greater volume.28,43,53,55,56,61–63
Common co-morbidities may also contribute to volume abnormalities in HIV+ patients. Hepatitis C co-infection,43 alcoholism,56 cigarette smoking,64 and small-vessel disease65 may exacerbate brain atrophy in the setting of HIV-infection. Furthermore, characteristic volume loss associated with aging may independently affect certain brain structures in older HIV+ individuals51,56,66,67. Older HIV+ individuals suffering from multiple co-morbidities may experience greater cumulative volume losses, increasing their risk for HIV-induced neurocognitive impairment.68
Overall, MRI volumetric analysis demonstrates that brain structure abnormalities begin early and progress throughout the course of HIV infection. Brain structural integrity in HIV likely reflects dynamic effects of current immune status and active viral replication, superimposed on possible residual effects associated with severe prior immunosuppression and other comorbidities. Though most MRI volumetric studies have been performed cross-sectionally, additional longitudinal studies could assess for risk factors for developing HAND and response to therapy. Future studies should include more HIV− controls for comparison.
Diffusion Tensor Imaging
More recently, diffusion tensor imaging (DTI) has become a popular method for studying white matter structural integrity.60,69–71 A current PubMed search including the following keywords: “DTI”, “brain”, and “HIV” identifies more 30 articles. Please see Table 3 for a select list of DTI studies performed in HIV+ patients. DTI measures the diffusion of water molecules in white matter. Movement of water can be anisotropic with diffusion greater along the length of the fiber (longitudinal direction) than perpendicular to it (radial or transverse direction), as myelin may restrict diffusion72. For each voxel, a tensor is calculated that describes the 3-dimensional shape of diffusion of water. The fiber direction is indicated by the tensor’s main eigenvector. Diffusion along the major axis is assumed to reflect diffusivity parallel to the white matter tract. Mean diffusivity (MD) reflects the average diffusion in the major axis and the two minor axes. Fractional anisotropy (FA) is a value between zero and one and provides a measure of the general shape of the ellipsoid73.
Table 3.
Select citations of diffusion tensor imaging (DTI) in HIV+ patients
| Reference | Subjects | % on cART | HAND classification | Field strength | Findings |
|---|---|---|---|---|---|
| Zhu et al., J Neurovirol 2013 | 50 HIV+ (48 yo, 65% male) 13 HIV − (51 yo, 23% male) |
100% | 86% neurologically asymptomatic 14% mild cognitive impairment |
1.5T | Compared to controls, HIV+ neurologically asymptomatic individuals showed increased MD in the posterior hemispheres. HIV+ individuals with mild cognitive impairment showed additional increased MD in prefrontal areas and decreased FA compared to HIV− controls and HIV+ neuroasymptomatic individuals. These findings correlated with duration of infection and multiple cognitive domains. |
| Du et al., Psychiatry Res. 2012 | 10 HIV+ (53 yo, 80% male), 24 HIV− (49 yo, 67% male) | 100% | NA | 1.5T | FA was affected most in HIV+ patients compared to HIV− controls |
| Hoare et al., Metab Brain Dis. 2012 | 128 HIV+ (29 yo, 33% male), 32 HIV− (25 yo, 39% male) | 0% | NA | 3T | Lower FA values correlated with poorer prospective memory performance in HIV+ individuals |
| Stubbe-Drger et al., BMC Neurol. 2012 | 19 HIV+ (41 yo, 100% male) 19 HIV− (41 yo, 79% male) |
68% | NA | 3T | FA was reduced in HIV+ subjects compared to HIV− controls |
| Gongvatana et al., J Neurovirol. 2011 | 85 HIV+ (45 yo, 67% male), no HIV− | 81% | 48% neurocognitively impaired | 3T | Higher current CD4 cell count correlated with higher FA in parietal lobes. Initiation of cART correlated with higher FA in temporal lobes. |
| Muller-Oehring et al., Neuropsychologia 2010 | 21 HIV+ (43 yo, 76% male) 19 HIV− (42 yo, 58% male) |
71.00% | NA | 1.5T | HIV+ participants showed poor fiber integrity in posterior portion of the corpus callosum compared to HIV− controls In HIV+ subjects compromised callosal fiber integrity was correlated with poorer neuropsychological performance |
| Pfefferbaum et al., AIDS 2009 | 42 HIV+ (43 yo, 69% male) 88 HIV− (45 yo, 48% male) |
79% | NA | 1.5T | HIV+ participants exhibited higher MD than HIV− controls in the posterior corpus callosum, internal and external capsules, and superior cingulate bundles. |
| Chen et al., Neuroimage 2009 | 29 HIV+ (35 yo, 75% male) 18 HIV− (40 yo, 50% male) |
62% | 28% HAD | 3T | HIV+ participants with HAD exhibited significantly MD in the parietal white matter. Widespread FA and MD abnormalities were present in HIV+ patients compared to HIV− controls. |
| Stebbins et al., J Acquir Immune Defic Syndr. 2007 | 30 HIV+ (45 yo, 53% male), 30 HIV− (41 yo, 43% male) | 77% | NA | 1.5T | HIV+ patients had lower whole brain FA and higher whole brain MD compared to HIV− controls. Whole-brain FA and MD did not significantly correlate with cognitive performance measures in HIV+ patients. |
| Pfefferbaum et al., Brain 2009 | 94 HIV+ (43 yo, 74% male) 130 HIV− (45 yo, 54% male) |
68% | NA | NA | HIV+ subjects with a history of an AIDS-defining event, CD4+ cell count <200, or alcoholism had greater abnormalities in MD and FA compared to HIV− controls. |
| Wu et al, AJNR 2006 | 11 HIV+ (49 yo, 82% male) 11 HIV− (42 yo, 82% male) |
91% | 6 MSK 0.5 4 MSK 1 1 MSK 2 |
1.5T | HIV+ patients exhibited significantly reduced splenium FA values compared to HIV− controls. Changes in FA in HIV+ patients correlated with neuropsychological performance. Increases in MD in the splenium in HIV+ patients correlated with neuropsychological performance. |
| Thurnher et al., AJNR 2005 | 60 HIV+ (42 yo, 80% male) 30 HIV− (40 yo, 73% male) |
NA | NA | 1.5T | In HIV+ patients FA was significantly decreased in the genu of the corpus callosum compared to HIV− controls. |
| Ragin et al., J Neurovirol 2005 | 11 HIV+ (49 yo, 82% male), 11 HIV− (42 yo, 82% male) | 91% | NA | 1.5T | HIV+ subjects had increased MD in the putamen and centrum semiovale compared to HIV− controls. MD values in putamen correlated with neuropsychological performance in HIV+ patients. |
| Pomara et al., Psychiatry Res 2001 | 6 HIV+ (40 yo, 67% male), 9 HIV− (43 yo, 78% male) | 83% | NA | NA | In HIV+ subjects, FA was increased in the internal capsule and decreased in the FWM compared to HIV− controls. |
Abbreviations: NA= not available, ADC= AIDS dementia complex, HAND= HIV associated neurocognitive disorders, ANI= asymptomatic neurocognitive impairment, MND= mild neurocognitive disorder, HAD= HIV associated dementia, MSK= Memorial Sloan Kettering scale, FA= fractional anisotropy, MD+ mean diffusivity, cART= combination antiretroviral therapy
In general, DTI can be performed on conventional MRI scanners but technical assistance is required. Depending on both the institution and time available for scanning, DTI with either a single or multiple diffusion sensitivity parameters (“b values”) can be performed. A minimum of 6 directions are acquired. Conventional preprocessing packages exist but experience is required for analysis.
Variable results have been observed when DTI has been used to study the effects of HIV on white matter integrity.61,67,69,74–84 In general many studies have shown that HIV leads to an increase in MD and a decrease in FA within white matter tracts [including the corpus callosum (CC) and centrum semiovale (CSO)] (See Figure 2). However, subtle differences exist in the location of these changes depending on the study.85–88 For example, Filippi and colleagues showed a decrease in FA and an increase in MD in the genu and splenium of the CC of HIV+ patients81. Thurnher and colleagues observed a reduction in FA within the genu of the CC of HIV+ patients76. Wu and colleagues reported a reduction in FA within the splenium of the CC in HIV+ individuals. This reduction in FA was associated with worsening motor speed performance75. However, Wright and colleagues observed a reduction in MD throughout the CC and CSO of HIV+ patients compared to HIV− controls 89. Instead of region of interest analyses, a voxelwise analysis can also be performed. Gongvtana and colleagues showed significantly higher MD and lower FA throughout the white matter of HIV+ individuals compared to HIV− controls.74
Figure 2.
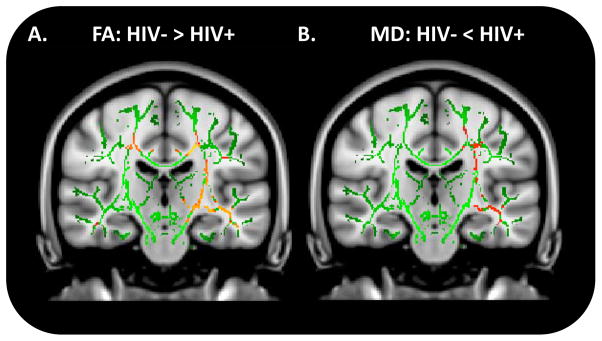
Voxelwise comparisons for fractional anisotropy (FA) (A) and mean diffusivity (MD) (B) between HIV− controls and HIV+ patients using Tract-Based Spatial Statistics (TBSS). Red: p=0.05; Orange: p=0.03; Yellow: p=0.01.
Typically, comparisons have been performed between HIV+ and HIV− controls. HIV+ individuals receiving cART (HIV+/cART+) and those naïve to cART (HIV+/cART−) have often been merged into a single group. The few studies that have investigated the effects of cART on DTI parameters in HIV+ individuals have shown conflicting results. Pffeferbaum and colleagues demonstrated that HIV+/cART− individuals had significantly higher MD values in the inferior cingulate bundle, occipital forceps and superior longitudinal fasciculus compared to HIV− controls or HIV+/cART+84. However, Chen and colleagues noted no significant differences in DTI parameters between HIV+/cART− and HIV+/cART+ patients90. A decrease in FA was seen in the temporal lobes of HIV+/cART+ compared to HIV+/cART− individuals74 suggesting possible neurotoxicity. More recently, Wright and colleagues demonstrated that initiation of cART led to significant increases in MD but not FA in the CC and CSO of HIV+ subjects89.
In summary, DTI may be a more sensitive method than conventional T2 imaging for detecting subtle changes despite the presence of normal appearing white matter in HIV+ patients. Most DTI studies have been cross sectional, and studied changes in chronic or advanced HIV infection. The effects of early HIV infection or of cART initiation on the white matter have not been systematically assessed by DTI. Furthermore, few DTI studies have included enough HIV− controls. Additional studies comparing DTI parameters to CSF biomarkers and assessing the potential impact of co-morbidities need to be performed.
Functional magnetic resonance imaging (fMRI)
A nascent literature has started to develop utilizing blood oxygen level dependent (BOLD) fMRI to investigate the effects of HIV on brain function 91. A PubMed search using “fMRI”, “BOLD”, and “HIV” as keyword search terms yielded 9 articles. While the BOLD sequence can be performed on conventional MRI scanners additional technical assistance is required for designing functional task paradigms. Preprocessing programs are available but significant experience is needed.
Fluctuations in the BOLD response within specific brain regions indirectly reveal the coupling between changes in neuronal activity and cerebral blood flow for a particular stimulus 92. Increases or decreases in brain activation during a task, as compared to rest or a neutral task, are assumed to be related to the cognitive function that is under investigation 93. HIV+ patients have greater parietal activation for a simple attention task and greater frontal and parietal activation during more complex attention tasks.94 These BOLD changes in HIV+ patients may reflect increased recruitment of additional areas to meet cognitive demands 32,33,57,94–102 A recent systematically meta-analysis of BOLD fMRI studies using various functional tasks in HIV+ patients was performed using an activation likelihood estimation (ALE). HIV+ patients had greater functional activation within the left inferior frontal gyrus and caudate nucleus compared to HIV− controls 103. Dysfunction in this fronto– striatal network was qualitatively related to neurocognitive impairment. When assessed at rest, functional connections between brain networks may be compromised in HAND, in ways that are similar to aging 104.
To date most BOLD fMRI studies have been performed in a limited number of HIV+ patients with most receiving cART. Only a few studies have started to assess the impact of co-morbidities such as methamphetamine use. A common task paradigm has not been developed across studies or sites. Additional BOLD fMRI studies are needed to evaluate the efficacy of novel therapies.
The future of advanced neuroimaging
Considerable progress has been made in applying MRI methods to understand neuroHIV. However, most studies have compared HIV+ individuals to HIV− controls with secondary comparisons concentrating on HAND diagnosis or certain laboratory measures or co-morbidities. Further studies that investigate the pathophysiology of spread of the disease throughout the brain are needed. These studies could help predict which HIV+ patients are at increased risk for developing HAND.
For neuroimaging to take the next step, these techniques need to be included not only within research criteria for HAND but also in the evaluation of therapeutics. This can be accomplished by using a common protocol at multiple research sites. This protocol should include multiple MRI modalities. A first attempt has been made by the AIDS Clinical Trial Group (ACTG) with multiple sites scanning HIV+ patients using the same imaging paradigm. Results from this pilot study were encouraging and it is hoped that a similar protocol can be rolled out to more sites. Cross modality comparisons within the same HIV+ individual will provide us a more complete understanding of the HIV pathophysiology.
Acknowledgments
This work was supported by grants R01NR014449, R01NR012657, R01NR012907, R21MH099979, and the Alzheimer’s Association (BMA).
Abbreviations
- ANI
asymptomatic neurocognitive impairment
- cART
combination antiretroviral therapy
- CC
corpus callosum
- Cho
choline
- CNS
central nervous system
- Cr
creatine
- CSF
cerebrospinal fluid
- CSO
centrum semiovale
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- fMRI
functional magnetic resonance imaging
- Gln
glutamine
- Glu
glutamate
- HAD
HIV associated dementia
- HAND
HIV associated neurocognitive disorders
- HIV
Human immunodeficiency virus
- HIV+
HIV-infected
- HIV−
HIV-uninfected
- MD
mean diffusivity
- MI
myoinositol
- MND
mild neurocognitive disorder
- MPRAGE
magnetization prepared rapid acquisition gradient echo
- MRS
magnetic resonance spectroscopy
- NAA
n-acetyl aspartate
- NMDA
n-methyl-d-aspartate
- NRTIs
nucleoside reverse transcriptase inhibitors
- NP
neuropsychological performance
References
- 1.Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Topics in HIV medicine: a publication of the International AIDS Society, USA. 2010 Apr-May;18(2):45–55. [PMC free article] [PubMed] [Google Scholar]
- 2.Justice AC. HIV and aging: time for a new paradigm. Current HIV/AIDS reports. 2010 May;7(2):69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 3.Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. Journal of neurovirology. 2012 Aug;18(4):291–302. doi: 10.1007/s13365-012-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009 Sep 1;49(5):651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 5.Luther VP, Wilkin AM. HIV infection in older adults. Clinics in geriatric medicine. 2007 Aug;23(3):567–583. vii. doi: 10.1016/j.cger.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013 Feb 1;62(2):149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010 Dec 7;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007 Oct 30;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spudich S. HIV and neurocognitive dysfunction. Current HIV/AIDS reports. 2013 Sep;10(3):235–243. doi: 10.1007/s11904-013-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002 Jun;59(6):923–928. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- 11.Liner KJ, 2nd, Ro MJ, Robertson KR. HIV, antiretroviral therapies, and the brain. Curr HIV/AIDS Rep. 2010 May;7(2):85–91. doi: 10.1007/s11904-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 12.Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009 Jul 17;23(11):1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012 Oct;18(5):388–399. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mind Exchange Working G. Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the mind exchange program. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013 Apr;56(7):1004–1017. doi: 10.1093/cid/cis975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Current opinion in neurology. 2011 Jun;24(3):275–283. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valcour V, Paul R, Chiao S, Wendelken LA, Miller B. Screening for cognitive impairment in human immunodeficiency virus. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011 Oct;53(8):836–842. doi: 10.1093/cid/cir524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011 May;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sathekge M, Goethals I, Maes A, van de Wiele C. Positron emission tomography in patients suffering from HIV-1 infection. European journal of nuclear medicine and molecular imaging. 2009 Jul;36(7):1176–1184. doi: 10.1007/s00259-009-1126-9. [DOI] [PubMed] [Google Scholar]
- 20.Cysique LA, Moffat K, Moore DM, et al. HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: a (1)H MRS study. PloS one. 2013;8(4):e61738. doi: 10.1371/journal.pone.0061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lentz MR, Kim WK, Kim H, et al. Alterations in brain metabolism during the first year of HIV infection. Journal of neurovirology. 2011 Jun;17(3):220–229. doi: 10.1007/s13365-011-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. Aids. 2011 Mar 13;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Descamps M, Hyare H, Stebbing J, Winston A. Magnetic resonance imaging and spectroscopy of the brain in HIV disease. J HIV Ther. 2008 Sep;13(3):55–58. [PubMed] [Google Scholar]
- 24.Kantarci K. Proton MRS in mild cognitive impairment. Journal of magnetic resonance imaging: JMRI. 2013 Apr;37(4):770–777. doi: 10.1002/jmri.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. The Journal of infectious diseases. 2012 Jul 15;206(2):275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sailasuta N, Ross W, Ananworanich J, et al. Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PloS one. 2012;7(11):e49272. doi: 10.1371/journal.pone.0049272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iannucci G, Rovaris M, Giacomotti L, Comi G, Filippi M. Correlation of multiple sclerosis measures derived from T2-weighted, T1-weighted, magnetization transfer, and diffusion tensor MR imaging. AJNR. American journal of neuroradiology. 2001 Sep;22(8):1462–1467. [PMC free article] [PubMed] [Google Scholar]
- 28.Cardenas VA, Meyerhoff DJ, Studholme C, et al. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. Journal of neurovirology. 2009 Jul;15(4):324–333. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed MA, Barker PB, Skolasky RL, et al. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magnetic resonance imaging. 2010 Nov;28(9):1251–1257. doi: 10.1016/j.mri.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul RH, Ernst T, Brickman AM, et al. Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. Journal of the International Neuropsychological Society: JINS. 2008 Sep;14(5):725–733. doi: 10.1017/S1355617708080910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yiannoutsos CT, Nakas CT, Navia BA. Assessing multiple-group diagnostic problems with multi-dimensional receiver operating characteristic surfaces: application to proton MR Spectroscopy (MRS) in HIV-related neurological injury. NeuroImage. 2008 Mar 1;40(1):248–255. doi: 10.1016/j.neuroimage.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. Journal of magnetic resonance imaging: JMRI. 2010 Nov;32(5):1045–1053. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang L, Ernst T, Leonido-Yee M, et al. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology. 1999 Sep 11;53(4):782–789. doi: 10.1212/wnl.53.4.782. [DOI] [PubMed] [Google Scholar]
- 34.Tarasow E, Wiercinska-Drapalo A, Jaroszewicz J, et al. Antiretroviral therapy and its influence on the stage of brain damage in patients with HIV - 1H MRS evaluation. Medical science monitor: international medical journal of experimental and clinical research. 2004 Jun;10(Suppl 3):101–106. [PubMed] [Google Scholar]
- 35.Schifitto G, Yiannoutsos CT, Ernst T, et al. Selegiline and oxidative stress in HIV-associated cognitive impairment. Neurology. 2009 Dec 8;73(23):1975–1981. doi: 10.1212/WNL.0b013e3181c51a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson KR, Su Z, Margolis DM, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010 Apr 20;74(16):1260–1266. doi: 10.1212/WNL.0b013e3181d9ed09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009 Jul 17;23(11):1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweinsburg BC, Taylor MJ, Alhassoon OM, et al. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. Journal of neurovirology. 2005 Aug;11(4):356–364. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]
- 39.Klauschen F, Goldman A, Barra V, Meyer-Lindenberg A, Lundervold A. Evaluation of automated brain MR image segmentation and volumetry methods. Human brain mapping. 2009 Apr;30(4):1310–1327. doi: 10.1002/hbm.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fjell AM, Westlye LT, Amlien I, et al. High consistency of regional cortical thinning in aging across multiple samples. Cerebral cortex. 2009 Sep;19(9):2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dal Pan GJ, McArthur JH, Aylward E, et al. Patterns of cerebral atrophy in HIV-1-infected individuals: results of a quantitative MRI analysis. Neurology. 1992 Nov;42(11):2125–2130. doi: 10.1212/wnl.42.11.2125. [DOI] [PubMed] [Google Scholar]
- 42.Fennema-Notestine C, Ellis RJ, Archibald SL, et al. Increases in brain white matter abnormalities and subcortical gray matter are linked to CD4 recovery in HIV infection. Journal of neurovirology. 2013 Aug;19(4):393–401. doi: 10.1007/s13365-013-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jernigan TL, Archibald SL, Fennema-Notestine C, et al. Clinical factors related to brain structure in HIV: the CHARTER study. Journal of neurovirology. 2011 Jun;17(3):248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dewey J, Hana G, Russell T, et al. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. NeuroImage. 2010 Jul 15;51(4):1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heindel WC, Jernigan TL, Archibald SL, Achim CL, Masliah E, Wiley CA. The relationship of quantitative brain magnetic resonance imaging measures to neuropathologic indexes of human immunodeficiency virus infection. Archives of neurology. 1994 Nov;51(11):1129–1135. doi: 10.1001/archneur.1994.00540230067015. [DOI] [PubMed] [Google Scholar]
- 46.Aylward EH, Henderer JD, McArthur JC, et al. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993 Oct;43(10):2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- 47.Aylward EH, Brettschneider PD, McArthur JC, et al. Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. The American journal of psychiatry. 1995 Jul;152(7):987–994. doi: 10.1176/ajp.152.7.987. [DOI] [PubMed] [Google Scholar]
- 48.Stout JC, Ellis RJ, Jernigan TL, et al. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Archives of neurology. 1998 Feb;55(2):161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- 49.Heaps JM, Joska J, Hoare J, et al. Neuroimaging markers of human immunodeficiency virus infection in South Africa. J Neurovirol. 2012 Jun;18(3):151–156. doi: 10.1007/s13365-012-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson PM, Dutton RA, Hayashi KM, et al. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences of the United States of America. 2005 Oct 25;102(43):15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012 Apr 15;59(5):469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ragin AB, Du H, Ochs R, et al. Structural brain alterations can be detected early in HIV infection. Neurology. 2012 Dec 11;79(24):2328–2334. doi: 10.1212/WNL.0b013e318278b5b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen RA, Harezlak J, Schifitto G, et al. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. Journal of neurovirology. 2010 Feb;16(1):25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel SH, Kolson DL, Glosser G, et al. Correlation between percentage of brain parenchymal volume and neurocognitive performance in HIV-infected patients. AJNR. American journal of neuroradiology. 2002 Apr;23(4):543–549. [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen RA, Harezlak J, Gongvatana A, et al. Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. Journal of neurovirology. 2010 Nov;16(6):435–444. doi: 10.3109/13550284.2010.520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfefferbaum A, Rosenbloom MJ, Sassoon SA, et al. Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biological psychiatry. 2012 Sep 1;72(5):361–370. doi: 10.1016/j.biopsych.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castelo JM, Courtney MG, Melrose RJ, Stern CE. Putamen hypertrophy in nondemented patients with human immunodeficiency virus infection and cognitive compromise. Archives of neurology. 2007 Sep;64(9):1275–1280. doi: 10.1001/archneur.64.9.1275. [DOI] [PubMed] [Google Scholar]
- 58.Thames AD, Foley JM, Wright MJ, et al. Basal ganglia structures differentially contribute to verbal fluency: evidence from Human Immunodeficiency Virus (HIV)-infected adults. Neuropsychologia. 2012 Feb;50(3):390–395. doi: 10.1016/j.neuropsychologia.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Becker JT, Sanders J, Madsen SK, et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain imaging and behavior. 2011 Jun;5(2):77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sullivan EV, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Pfefferbaum A. Pontocerebellar contribution to postural instability and psychomotor slowing in HIV infection without dementia. Brain imaging and behavior. 2011 Mar;5(1):12–24. doi: 10.1007/s11682-010-9107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C, Zhang X, Komery A, Li Y, Novembre FJ, Herndon JG. Longitudinal diffusion tensor imaging and perfusion MRI investigation in a macaque model of neuro-AIDS: a preliminary study. NeuroImage. 2011 Sep 1;58(1):286–292. doi: 10.1016/j.neuroimage.2011.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kallianpur KJ, Shikuma C, Kirk GR, et al. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology. 2013 May 7;80(19):1792–1799. doi: 10.1212/WNL.0b013e318291903f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ragin AB, D’Souza G, Reynolds S, et al. Platelet decline as a predictor of brain injury in HIV infection. Journal of neurovirology. 2011 Oct;17(5):487–495. doi: 10.1007/s13365-011-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Durazzo TC, Rothlind JC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Chronic cigarette smoking and heavy drinking in human immunodeficiency virus: consequences for neurocognition and brain morphology. Alcohol. 2007 Nov;41(7):489–501. doi: 10.1016/j.alcohol.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMurtray A, Nakamoto B, Shikuma C, Valcour V. Small-vessel vascular disease in human immunodeficiency virus infection: the Hawaii aging with HIV cohort study. Cerebrovascular diseases. 2007;24(2–3):236–241. doi: 10.1159/000104484. [DOI] [PubMed] [Google Scholar]
- 66.Becker JT, Maruca V, Kingsley LA, et al. Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology. 2012 Feb;54(2):113–121. doi: 10.1007/s00234-011-0854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Towgood KJ, Pitkanen M, Kulasegaram R, et al. Mapping the brain in younger and older asymptomatic HIV-1 men: frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex; a journal devoted to the study of the nervous system and behavior. 2012 Feb;48(2):230–241. doi: 10.1016/j.cortex.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Valcour VG. HIV, aging, and cognition: emerging issues. Topics in antiviral medicine. 2013 Jul-Aug;21(3):119–123. [PMC free article] [PubMed] [Google Scholar]
- 69.Tate DF, Sampat M, Harezlak J, et al. Regional areas and widths of the midsagittal corpus callosum among HIV-infected patients on stable antiretroviral therapies. Journal of neurovirology. 2011 Aug;17(4):368–379. doi: 10.1007/s13365-011-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christensen A, Russ S, Rambaran N, Wright SW. Patient perspectives on opt-out HIV screening in a Guyanese emergency department. International health. 2012 Sep;4(3):185–191. doi: 10.1016/j.inhe.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Turner MR, Modo M. Advances in the application of MRI to amyotrophic lateral sclerosis. Expert opinion on medical diagnostics. 2010 Nov;4(6):483–496. doi: 10.1517/17530059.2010.536836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychology review. 2010 Jun;20(2):209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wycoco V, Shroff M, Sudhakar S, Lee W. White matter anatomy: what the radiologist needs to know. Neuroimaging clinics of North America. 2013 May;23(2):197–216. doi: 10.1016/j.nic.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Gongvatana A, Cohen RA, Correia S, et al. Clinical contributors to cerebral white matter integrity in HIV-infected individuals. Journal of neurovirology. 2011 Oct;17(5):477–486. doi: 10.1007/s13365-011-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB. Diffusion alterations in corpus callosum of patients with HIV. AJNR. American journal of neuroradiology. 2006 Mar;27(3):656–660. [PMC free article] [PubMed] [Google Scholar]
- 76.Thurnher MM, Castillo M, Stadler A, Rieger A, Schmid B, Sundgren PC. Diffusion-tensor MR imaging of the brain in human immunodeficiency virus-positive patients. AJNR. American journal of neuroradiology. 2005 Oct;26(9):2275–2281. [PMC free article] [PubMed] [Google Scholar]
- 77.Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry research. 2001 Feb 28;106(1):15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- 78.Muller-Oehring EM, Schulte T, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Callosal degradation in HIV-1 infection predicts hierarchical perception: a DTI study. Neuropsychologia. 2010 Mar;48(4):1133–1143. doi: 10.1016/j.neuropsychologia.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stebbins GT, Smith CA, Bartt RE, et al. HIV-associated alterations in normal-appearing white matter: a voxel-wise diffusion tensor imaging study. Journal of acquired immune deficiency syndromes. 2007 Dec 15;46(5):564–573. doi: 10.1097/qai.0b013e318159d807. [DOI] [PubMed] [Google Scholar]
- 80.Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. Journal of neurovirology. 2005 Jul;11(3):292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. AJNR. American journal of neuroradiology. 2001 Feb;22(2):277–283. [PMC free article] [PubMed] [Google Scholar]
- 82.Ragin AB, Storey P, Cohen BA, Edelman RR, Epstein LG. Disease burden in HIV-associated cognitive impairment: a study of whole-brain imaging measures. Neurology. 2004 Dec 28;63(12):2293–2297. doi: 10.1212/01.wnl.0000147477.44791.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ragin AB, Storey P, Cohen BA, Epstein LG, Edelman RR. Whole brain diffusion tensor imaging in HIV-associated cognitive impairment. AJNR. American journal of neuroradiology. 2004 Feb;25(2):195–200. [PMC free article] [PubMed] [Google Scholar]
- 84.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal fiber bundle compromise in HIV infection without dementia. Aids. 2009 Sep 24;23(15):1977–1985. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoare J, Westgarth-Taylor J, Fouche JP, et al. A diffusion tensor imaging and neuropsychological study of prospective memory impairment in South African HIV positive individuals. Metabolic brain disease. 2012 Sep;27(3):289–297. doi: 10.1007/s11011-012-9311-0. [DOI] [PubMed] [Google Scholar]
- 86.Stubbe-Drger B, Deppe M, Mohammadi S, et al. Early microstructural white matter changes in patients with HIV: a diffusion tensor imaging study. BMC neurology. 2012;12:23. doi: 10.1186/1471-2377-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Du H, Wu Y, Ochs R, et al. A comparative evaluation of quantitative neuroimaging measurements of brain status in HIV infection. Psychiatry research. 2012 Jul 30;203(1):95–99. doi: 10.1016/j.pscychresns.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu T, Zhong J, Hu R, et al. Patterns of white matter injury in HIV infection after partial immune reconstitution: a DTI tract-based spatial statistics study. Journal of neurovirology. 2013 Feb;19(1):10–23. doi: 10.1007/s13365-012-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wright PW, Heaps JM, Shimony JS, Thomas JB, Ances BM. The effects of HIV and combination antiretroviral therapy on white matter integrity. Aids. 2012 Jul 31;26(12):1501–1508. doi: 10.1097/QAD.0b013e3283550bec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, An H, Zhu H, et al. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. NeuroImage. 2009 Oct 1;47(4):1154–1162. doi: 10.1016/j.neuroimage.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rauch A, Rainer G, Logothetis NK. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2008 May 6;105(18):6759–6764. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nature reviews. Neurology. 2010 Jan;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 93.Ogawa S. Finding the BOLD effect in brain images. NeuroImage. 2012 Aug 15;62(2):608–609. doi: 10.1016/j.neuroimage.2012.01.091. [DOI] [PubMed] [Google Scholar]
- 94.Chang L, Speck O, Miller EN, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001 Sep 25;57(6):1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- 95.Ances B, Vaida F, Ellis R, Buxton R. Test-retest stability of calibrated BOLD-fMRI in HIV− and HIV+ subjects. NeuroImage. 2011 Feb 1;54(3):2156–2162. doi: 10.1016/j.neuroimage.2010.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ances BM, Roc AC, Korczykowski M, Wolf RL, Kolson DL. Combination antiretroviral therapy modulates the blood oxygen level-dependent amplitude in human immunodeficiency virus-seropositive patients. Journal of neurovirology. 2008 Oct;14(5):418–424. doi: 10.1080/13550280802298112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang L, Tomasi D, Yakupov R, et al. Adaptation of the attention network in human immunodeficiency virus brain injury. Annals of neurology. 2004 Aug;56(2):259–272. doi: 10.1002/ana.20190. [DOI] [PubMed] [Google Scholar]
- 98.Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002 Nov 12;59(9):1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- 99.Ernst T, Yakupov R, Nakama H, et al. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Annals of neurology. 2009 Mar;65(3):316–325. doi: 10.1002/ana.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Juengst SB, Aizenstein HJ, Figurski J, Lopez OL, Becker JT. Alterations in the hemodynamic response function in cognitively impaired HIV/AIDS subjects. Journal of neuroscience methods. 2007 Jul 30;163(2):208–212. doi: 10.1016/j.jneumeth.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 101.Maki PM, Cohen MH, Weber K, et al. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009 May 12;72(19):1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tracey I, Hamberg LM, Guimaraes AR, et al. Increased cerebral blood volume in HIV-positive patients detected by functional MRI. Neurology. 1998 Jun;50(6):1821–1826. doi: 10.1212/wnl.50.6.1821. [DOI] [PubMed] [Google Scholar]
- 103.Du Plessis S, Vink M, Joska J, Koutsilieri E, Stein DJ, Emsley R. HIV infection and the fronto–striatal system: a systematic review and meta-analysis of fMRI studies. JAIDS. 2014 doi: 10.1097/QAD.0000000000000151. in press. [DOI] [PubMed] [Google Scholar]
- 104.Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013 Mar 26;80(13):1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]