Abstract
Background
Ikaros, the product of IKZF1, is a regulator of lymphoid development and polymorphisms in the gene have been associated with the childhood acute lymphoblastic leukemia (ALL). Additionally, IKZF1 deletions and mutations identify high risk biological subsets of ALL [1, 2].
Procedure
To discover the underlying pathways modulated by Ikaros we performed gene expression and gene ontology analysis in IKZF1 deleted primary B-ALL pediatric patient samples. To validate downstream targets we performed qPCR on individual patient samples. We also created IKZF1 knockdown B-ALL cell lines with over 50% reduction of Ikaros, mimicking haplosufficient Ikaros deletions, and again performed qPCR to investigate the downstream targets. Finally, to understand the association of Ikaros deletion with a poor prognosis we challenged our IKZF1 knockdown cell lines with chemotherapy and compared responses to IKZF1 wild-type controls.
Results
We report a specific gene expression signature of 735 up-regulated and 473 down-regulated genes in IKZF1 deleted primary B-ALL pediatric patient samples. Gene ontology studies revealed an up-regulation of genes associated with cell adhesion, cytoskeletal regulation, and motility in IKZF deleted patient samples. Validated up-regulated target genes in IKZF1 deleted patient samples included CTNND1 and PVRL2 (p=0.0003 and p=0.001), and RAB3IP and SPIB (p=0.005 and p=0.032) were down-regulated. In further studies in IKZF1 knockdown cell lines, apoptosis assays showed no significant chemoresistance.
Conclusion
IKZF1 knockdown alone does not impart intrinsic chemotherapy resistance suggesting that the association with a poor prognosis may be due to additional lesions, microenvironmental interactions with the bone marrow niche, or other factors.
Keywords: acute lymphoblastic leukemia, Ikaros, molecular biology of ALL, molecular genetics, adhesion
Introduction
Breakthroughs in the treatment of childhood acute lymphoblastic leukemia (ALL) have led to cure rates approaching 90%, through development of better risk stratification, more effective chemotherapy regimens and enhanced supportive care [3]. In spite of these advances, 10 to 20% of patients will suffer a relapse and their prognosis is poor. While the percentage of treatment failures is low, the prevalence of ALL still makes it the second most common cause of cancer-related mortality in children [4].
Ikaros is a zinc finger transcription factor, encoded by the gene IKZF1, responsible for the regulation of the development of B and T lymphoid lineages as well as immunoglobulin rearrangement [5-7]. Without normally functioning Ikaros, precursor-B lymphocytes arrest in a self-proliferative, adherent state that predisposes them to malignant transformation [8]. While predominantly T-lineage leukemia is seen in mice with IKZF1 deletions, in humans deletions and mutations are most commonly associated with B-cell leukemia, especially with BCR-ABL1+ B lymphoblastic ALL (B-ALL) [9-12]. Initial reports estimated an 84% incidence of IKZF1 deletion in pediatric BCR-ABL1+ patients and 29% in high risk BCR-ABL1− pediatric patients [2, 12]. Overall, the incidence of IKZF1 deletion has been reported to be 16-27% in all childhood BALL [13-15]. Deletion of IKZF1 has been reported to be associated with higher minimal residual disease (MRD) at the end of induction, higher rates of relapse, and in some reports, an independent predictor of survival in childhood ALL [16, 17]. IKZF1 mutations or deletions are often reported to be associated with other poor prognostic factors such as BCR-ABL1 translocation, BCR-ABL1-like gene expression signatures, JAK mutations, and CRLF2 rearrangements [14, 18, 19]. While the majority of Ikaros deletions are present at diagnosis, IKZF1 deletions can also be acquired at relapse in patients without alterations at diagnosis [17, 20]. IKZF1 may be deleted by three different mechanisms: complete loss of Ikaros expression through biallelic deletions (12%), null mutations on one allele resulting in haplosufficiency (55%), or intragenic deletions effecting exons 4 to 7 producing dominant negative isoforms (33%) [14]. It is unclear if different mutations produce different molecular signatures, but different forms of deletion may influence clinical outcomes as van der Veer et al. reported only haplosufficient IKZF1 deletions were prognostically significant in childhood B-ALL [21].
To explore the possible role of Ikaros in transformation and chemosensitivity we sought to provide an in-depth analysis of transcriptional alterations present specifically in pediatric BCRABL1− IKZF1 deleted B-ALL and to discover biological pathways unique to patients who harbor IKZF1 deletions. As many patients with IKZF1 deletions have other accompanying mutations, we evaluated the specific impact of Ikaros levels on drug sensitivity in a panel of B-lineage acute leukemia cell lines. We discovered a specific gene expression signature associated with IKZF1 deletions; however these deletions were not associated with resistance to conventional chemotherapeutic agents in vitro. Our results indicate that IKZF1 deletions impact the transcriptional program of B-ALL and that the adverse prognostic impact of IKZF1 deletions may be due to associated genetic lesions or alterations in microenvironmental interactions.
Materials and Methods
Cells, Chemotherapy, and Transfections
B-lineage leukemia cell lines, Reh, RS4;11, and UOCB1 cells were grown in RPMI1640 medium supplemented with 10% fetal bovine serum, 10 mM HEPES buffer, 1% Penicillin/Streptomycin under 5% CO2 at 37 °C. The 293T cell line was grown in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin under 5% CO2 at 37°C. All cell lines were purchased from American Type Culture Collection (ATCC) and were authenticated according to their protocols (http://www.atcc.org except for UOCB1 cells which was kindly gifted by Dr. Terzah Horton at Texas Children's Cancer Center/Baylor College of Medicine). Stock solution of etoposide was prepared in dimethyl sulfoxide, 6-thioguanine (6-TG) was prepared in NaOH, and prednisolone was suspended in 0.9% NaCl (Sigma-Aldrich). Drugs were serially diluted in RPMI and added to the culture media at indicated concentrations. Cells were incubated with chemotherapy for 24-48 hours.
Reh and UOCB1 cells were infected with lentiviral vectors with either an IKZF1 shRNA plasmid (MISSION® pLKO Ikaros shRNA Plasmid DNA, Sigma-Aldrich) or a control (MISSION® pLKO.1-puro Empty Vector Control Plasmid DNA, Sigma-Aldrich). 293T cells were transfected using the calcium phosphate method. Forty-eight hours later virus was collected, pressure-filtered, and added to 3 million/ml previously described cell lines in six-well plates with polybrene (8 μg/ml). Infected cells were selected in puromycin (10 μg/mL). Knockdown of IKZF1 was confirmed by Western Blot and quantitative PCR (qPCR).
Patient Samples and Gene Expression Analysis
RNA was isolated from 46 diagnostic and relapse bone marrow samples, as previously described [22]. To obtain a list of differentially expressed genes distinguishing 14 patients harboring IKZF1 deletions from 32 wild-type patients lacking deletions, an unpaired T-Test was applied by utilizing the Broad Institute's GenePattern suite, using default parameters and a fold change cutoff of 1.2-fold (p-value ≤ 0.05). Heatmap representation of relative expression levels of responsive genes (samples as columns and gene probesets as rows) was carried out by utilizing GENE-E (http://www.broadinstitute.org/cancer/software/GENE-E/). Color scheme representative of relative high and low expression levels was applied by using minimum and maximum row values. The samples in each of the groups (‘IK-deletions’ and ‘Normal’) were further clustered by applying city-block distance metric, with single linkage as linkage method. Gene ontology analysis performed using Expression Analysis Systematic Explorer (EASE) software [23].
RNA Isolation and Quantitative reverse transcription PCR analysis
RNA was extracted from patient samples and the infected leukemia cell lines using the RNEasy Mini Kit (Qiagen, Valencia, CA), and reverse transcription PCR was performed using the I-Script II complementary DNA Synthesis kit (Biorad, Hercules, CA) and the PerfeCTA SYBR Green FastMix (Quanta Biosciences, Gaithersburg, MD). Synthesis of PCR products was monitored by the Stratagene MX3005P and normalized to β2 microglobulin levels. Data were plotted relative to mRNA levels in control samples using the ΔΔCt method. PCR primers used were all Qiagen Quantitect Primer Assays and included Hs_B2M_1_SG (QT00088935), Hs_IKZF1_1_SG (QT00061607), Hs_ARID1B_2_SG (QT01667617), Hs_CTNND1_1_SG (QT00033831), Hs_RAB3IP_1_SG (QT00044842), Hs_SPIB_1_SG (QT00223286). All experiments were done in triplicate.
Protein Isolation and Western blotting
Protein was extracted using RIPA buffer and measured against BSA standards on a Beckman Coulter DU-530 UV Vis Spectrophotometer. 9% SDS-Tris acrylamide gel was used and 30 μg of protein were loaded into each lane. PDVF membranes were blocked in 4% milk and incubated in primary antibody overnight using the following antibodies: Actin (Abcam, #ab6276) 1:1000 and IKZF1 (Abcam, #ab26083) 1:250. Membranes were then washed in PBST (0.1% Tween) and placed in secondary antibody using either ECL™ Anti-mouse IgG, Horseradish Peroxide linked (from sheep) whole antibody (GE Healthcare UK Limited) or ECL™ Anti-rabbit IgG, Horseradish Peroxide linked (from donkey) whole antibody (GE Healthcare UK Limited, Buckinghamshire, UK). Detection was performed using ECL Western Blotting Detection Kit (GE Healthcare Life Sciences, Piscataway, NJ, USA).
Apoptosis Assays
Cells were plated at a density of 0.4 million cells/mL in 48 well plates. Drugs were serially diluted to produce a range of concentrations that yielded optimal cell kill and then cells were treated in triplicate for 24-48 hours. Cells were stained using a PE Annexin-V Apoptosis Detection Kit (BD Pharmingen); flow cytometry analysis was done using a FACScan (BD), and analysis was conducted using FlowJo (Tree Star, Inc.).
Results
Patients with IKZF1 deletions form a distinct subset of pediatric B-ALL patients
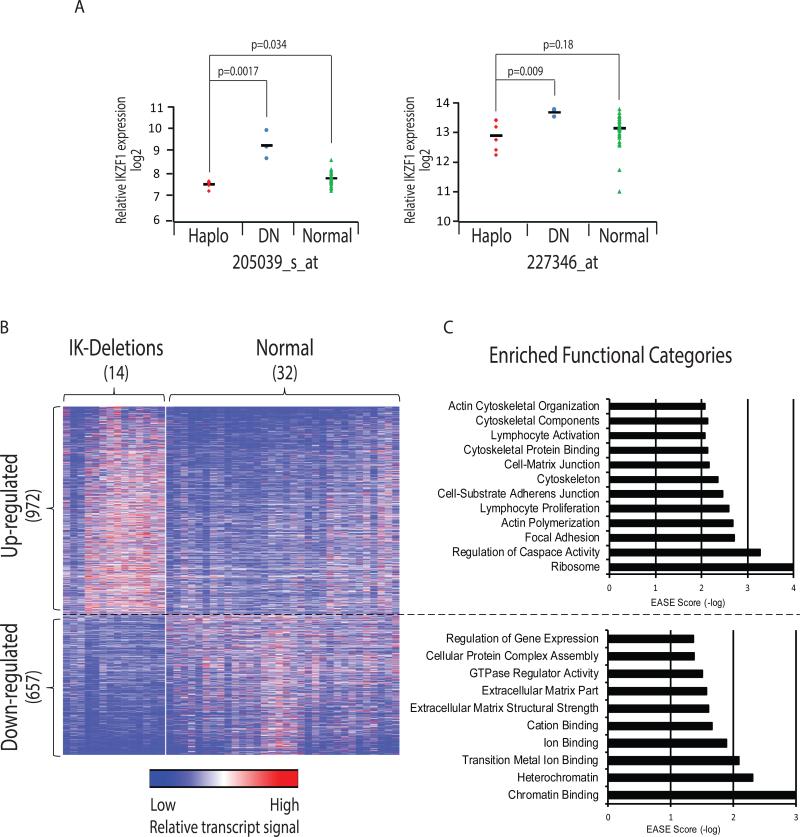
As described in Hogan et. al, frozen aspirates of pediatric patient's original diagnostic bone marrow sample were obtained from the Children's Oncology Group (COG) tissue bank. Total RNA and genomic DNA were extracted from a single aliquot of Ficoll-enriched blast cells. Gene expression profiling was completed on Affymetrix U133Plus2 array and copy number alterations were performed on Affymetrix SNP Version 6.0. for 46 primary pediatric B-ALL patient samples. Fourteen patients (30%) were found to harbor deletions in IKZF1 at diagnosis. Among these, three patients were classified as dominant negative IKZF1 isoforms and none of the patients had homozygous deletions. Two microarray probesets on Exon 8 of IKZF1 distinguished six IKZF1 haplosufficient patient samples and three patient samples with dominant negative (DN) Ikaros isoforms (Fig 1A). These two probesets, located at the 3’-end of IZKF1 were selected as they manifested the highest mRNA signals compared to other IKZF1 probesets, as expected due to oligo(dT) primers utilized in the reverse transcription step to obtain cDNA. Of the fourteen IKZF1 deleted samples, five had deletions that included the location of the two probesets used to determine transcript levels among IKZF1 subtypes. Therefore for specifically analyzing Ikaros transcript levels these five samples were omitted. Haplosufficient IKZF1 patient samples showed a decrease in mRNA expression compared to IKZF1 wild-type patients with significance in one probeset (205039_s_at) and a trend due to two outliers in the other (227346_at) (Fig 1A). Nevertheless, in this study all fourteen IKZF1 deleted patients were analyzed together as all would be expected to impact pathways modulated by Ikaros. In order to investigate transcriptional alterations that were associated with IKZF1 deletions, an unpaired T-Test (fold-change ≥ ±1.2; p-value ≤ 0.05) was applied and revealed 1,629 probesets whose expression differed between cohorts, of which 972 were up-regulated and 657 down-regulated (representing 735 and 473 genes, respectively) in the IKZF1 deleted cohort as compared to IKZF1 wild-type patients (Fig 1B). Gene ontology analysis performed by Expression Analysis Systematic Explorer (EASE) software [23] illuminated several interesting enriched functional categories, including down-regulation of genes associated with heterochromatin binding, suggesting broad genetic consequences of IKZF1 deletions (Fig 1C). The analysis also showed up-regulation of genes associated with caspase regulation. Expectedly we found up-regulation of genes associated with lymphocyte activation and B cell proliferation, but remarkably, there was also a significant up-regulation of genes involved in focal adhesions, cell-substrate adherens junction, cell-matrix junctions, cytoskeleton, and actin binding (EASE scores 0.001, 0.003, 0.004, 0.006, and 0.007, respectively). Within these categories we found several genes whose expression was significantly up-regulated in IKZF1 deleted patients, such as ARHGEF2, which was increased 1.4-fold in the Ikaros deleted subset (p<0.04) and is known to regulate cell migration and promote solid tumor metastases [24-26]. We also detected 1.5-fold increase of ITGA6 (p<0.02), previously shown to be a regulator of cell migration and adhesion [27].
Figure 1. Gene expression microarrays and gene ontology in IKZF1 deleted B-cell acute lymphoblastic leukemia (ALL) patient samples.
(A) Gene expression values for IKZF1 probesets located in exon 8; 205039_s_at (left-hand side) and 227346_at (right-hand side). Color-coded samples are depicted (Red: IKZF1 haplosufficiency, Blue: dominant negative IKZF1 isoforms, Green: normal IKZF1). (B) Heatmap in which rows are individual genes and columns are patient samples, depicting distinct gene expression pattern in IKZF1 deleted ALL and wild-type ALL (see detailed analysis in Supplementary Table I). (C) Gene Ontology analysis of patient samples analyzed with EASE software showing significantly up-regulated and down-regulated gene sets in IKZF1 deleted patient samples (see detailed analysis in Supplementary Table II). Expression Analysis Systematic Explorer (EASE) score negative-log-transformed values are used, with higher values represent more significantly over-represented categories.
Validation of Differentially Expressed Target Genes in IKZF1 deleted Patient Samples
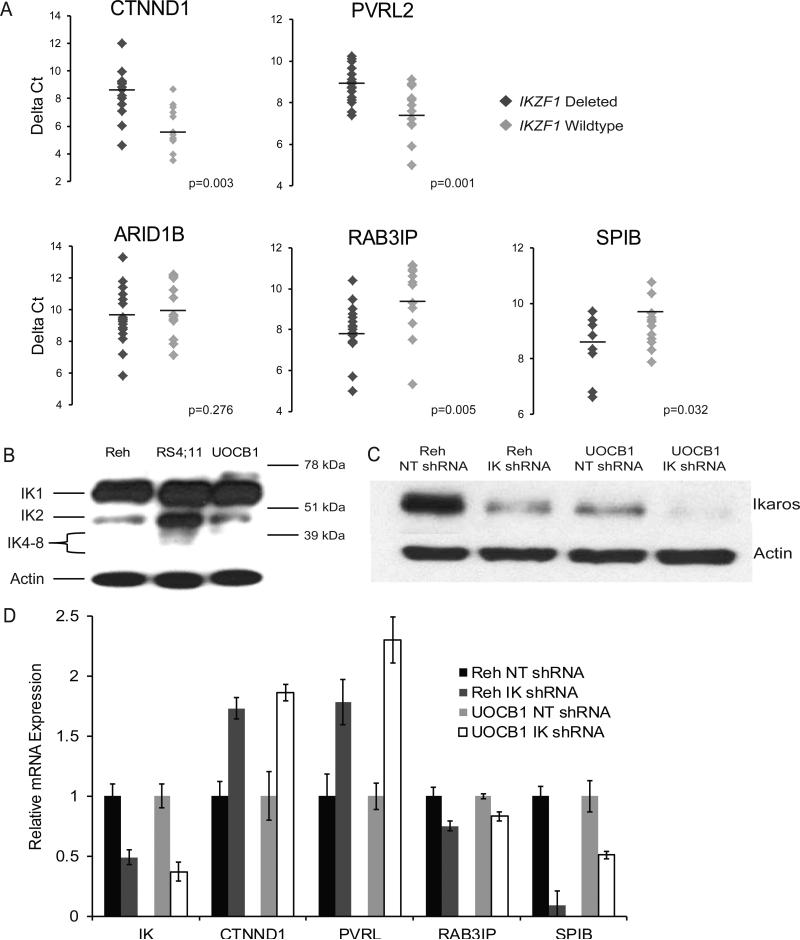
In order to validate our gene expression results, we chose 5 genes, including two of the most significantly up-regulated (CTNND1, PVRL2) and three of the most significantly down-regulated genes (ARID1B, RAB3IP, SPIB) that are known to have a role in cancer progression and we performed quantitative real-time PCR in triplicate on 29 patient samples (17 IKZF1 wild-type, 12 IKZF1 deleted) based on sample availability. This qPCR analysis confirmed the up-regulation of both candidate genes, CTNND1 and PVRL2, in the IKZF1 deleted patient samples compared to IKZF1 wild-type samples (1.41-fold, p<0.0003 and 1.18-fold, p<0.001, respectively) (Fig 2A). Our qPCR analysis additionally confirmed the reduced expression of two candidate genes, RAB3IP and SPIB (reduction of 17% with a p=0.005 and reduction of 10% with a p=0.032, respectively) (Fig 2A). Next, we compared our gene expression results with another independent gene expression analysis performed on a cohort of IKZF1 deleted BCR-ABL1+ patients [9]. Our comparison of the two IKZF1 deleted signatures identified 62 commonly up-regulated genes, including PVRL2 and 9 commonly down-regulated genes, further supporting a distinct gene signature associated with IKZF1 deletion that is not context dependent (Supplementary Table I).
Figure 2. qPCR validation of gene expression modulated by the deletion of IKZF1.
(A) qPCR of IKZF1 deleted ALL patient samples (dark grey diamonds) compared to wild-type samples (light grey diamonds). Lateral line in each patient set represents the mean. Y-axis is the mRNA expression relative to IKZF1 wild-type patient samples. (B) Western blot of Reh, RS4;11, and UOCB1 B-ALL cell lines for different Ikaros isoforms confirming no significant dominant negative Ikaros isoforms (IK4-8) in Reh or UOCB1 cell lines. (C) Western blot of Reh and UOCB1 B-cell ALL cell lines infected with a control non-targeting (NT) shRNA as a control or an IKZF1 (IK) shRNA. (D) qPCR of IK shRNA Reh and UOCB1 cell lines compared to NT shRNA Reh and UOCB1 cell lines validating all genes validated in patient samples. Y-axis is the mRNA expression relative to cell line-specific controls.
Validation of Differentially Expressed Target Genes in IKZF1 Knockdown B-ALL Cell Lines
We aimed to validate our findings further with an IKZF1 deleted leukemia cell line model. As we aimed to mimic the haplosufficient IKZF1 deletion most commonly seen in patients, we first evaluated the B-ALL cell lines Reh, RS4;11, and UOCB1 for baseline IKZF1 expression. Childhood leukemia with the t(4;11) translocation has been reported to express increased levels of IK6, a dominant negative isoform of Ikaros formed by deletion of exons 3-6 [28]. Western blot analysis of these leukemic cell lines showed similar expression of IK1 (57 kDa), but RS4;11 exhibited increased expression of both IK2 (47 kDa) and IK6 (38 kDa) compared to both the Reh and the UOCB1, therefore, to avoid conflicting results that could result from the endogenous expression of the DN form of Ikaros, we proceeded with the Reh and the UOCB1 cell lines (Fig 2B). Using a lentiviral vector system, IKZF1 shRNA cell lines were created with a greater than 50% reduction of IKZF1 expression. In parallel, these two cell lines were infected with a non-targeting plasmid to serve as control. Infected cells were sorted by flow cytometry for GFP expression and expanded for RT-PCR, western blots, and apoptotic cell death assays. With mRNA from these IKZF1 depleted cells we performed western blot and qPCR analysis to confirm IKZF1 knockdown (Figs 2C,D). In line with our gene expression signature of human patients (Supplementary Table I) and with its confirmatory qPCR results reflecting the concordant regulation of four candidate genes (Fig 2A), a qPCR analysis performed on samples of the two IKZF1 depleted cell lines clearly captured the same Ikaros-dependent transcriptional regulation manifested by significant up-regulation of CTNND1 and PVRL2 expression (increased 1.73-fold and 1.78-fold in the Reh IKZF1 knockdown cell line and 1.86-fold and 2.3-fold in UOCB1 IKZF1 knockdown cell line, respectively) and by down-regulation of RAB3IP and SPIB expression (reduction of 25% and 91% in the Reh IKZF1 knockdown cell line and 17% and 49% in UOCB1 IKZF1 knockdown cell line, respectively) (Fig 2D).
IKZF1 knockdown does not confer direct chemoresistance in B-cell leukemic cell lines
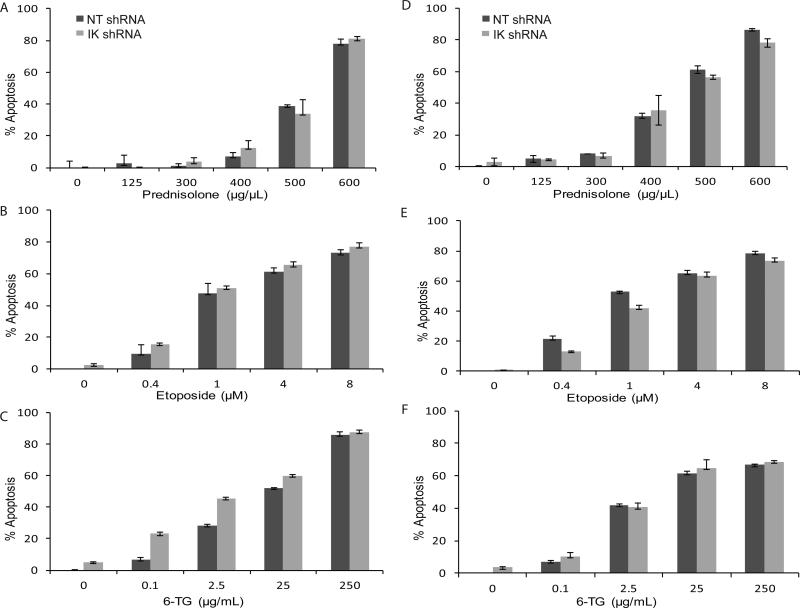
As IKZF1 deletion is associated with worse outcomes in high risk childhood B-ALL and our gene ontology analysis noted changes in caspase regulation (Fig 1C, Supplementary Table II), we sought to determine if IKZF1 down-regulation directly imparted chemoresistance. We challenged IKZF1 down-regulated Reh and UOCB1 cell lines with prednisolone, etoposide, or 6-thioguanine (6-TG), p53-independent and dependent cytotoxic chemotherapies used in the treatment of childhood acute leukemias. Apoptotic assays were only evaluated in cases where plated cells had a confirmed decrease of IKZF1 expression of at least 50% within 48 hours (as tested by qPCR). Our chemoresistance studies revealed a modest reduction in apoptosis only in the UOCB1 cell line treated with low etoposide dosing (Fig. 3E). However, generally in both cell lines with all three chemotherapeutic agents there was no major survival benefit detected in the IKZF1 deleted cells (Fig 3).
Figure 3. Deletion of Ikaros does not produce chemoresistance in vitro in Reh and UOCB1 cells.
Apoptosis assays using annexin-V and 7-AAD in control non-targeting (NT) shRNA and IKZF1 (IK) shRNA Reh cell lines treated with (A) prednisolone, (B) etoposide, and (C) 6-TG and in control non-targeting (NT) shRNA and IKZF1 (IK) shRNA UOCB1 cell lines treated with (D) prednisolone, (E) etoposide, and (F) 6-TG. Y-axis % apoptosis represents the percentage of cells that were annexin-V and 7-AAD positive.
Discussion
Ikaros, the product of IKZF1 on chromosome 7, contains two zinc finger regions which mediate DNA binding and interaction with other Ikaros family members. Ikaros has a profound transcriptional impact through epigenetic regulation, acting as a transcriptional activator or repressor depending on the type of cell and regulates stage-specific B cell development [29, 30]. In order to further define the prognostic role of Ikaros alterations on childhood ALL, we investigated BCR-ABL1− leukemia patient samples and describe an interesting set of genes whose transcriptional levels were altered as consequence of IKZF1 deletion without translocation of BCR-ABL1. Our results show up-regulation of a novel set of genes involved in cell communication and adhesion. CTNND1, a gene encoding a member of the Armadillo family of proteins that is involved in cell-cell adhesion and signal transduction, is over-expressed in IKZF1 deleted cells and was recently reported as up-regulated in mice lacking Ikaros zinc finger 4 [31]. We also show PVRL2, whose product functions as a component of adherens junctions, is an additional target gene that being inversely modulated by IKZF1 deletion. PVRL2 is also suggested to be involved in the formation of peripheral cutaneous T-cell lymphomas through a translocation involving BCL3 [32]. This significant up-regulation of CTNND1 and PVRL2, along with previous studies documenting their involvement in cell-cell interactions, mark these genes as exciting new downstream targets that may account for negative outcomes in subsets of patients carrying IKZF1 deletions [32, 33]. SPIB, validated to be down-regulated by IKZF1 depletion and is a known stage-specific transcription factor effecting pre-BCR signaling [34]. RAB3IP, validated to be down-regulated in IKZF1 deleted leukemia, was also found to be significantly downregulated (approximately 35%) in early T-cell precursor (ETP) ALL as captured by gene expression microarray analysis [35]. Interestingly, data from the same study indicates that ETP ALL also overexpresses both CTNND1 and PVRL2 by approximately 35% [35]. While ARID1B, a tumor suppressor gene, had decreased expression in the IKZF1 deleted cohort by gene expression array, it failed to validate by qPCR in individual IKZF1 deleted patient samples [36]. Despite trending toward significance, it did not meet significance possibly because of low power due to a small cohort or because of an outlying wild-type sample that we felt could not be excluded.
Despite our gene enrichment analysis showing dysregulation of the apoptotic pathway, we were unable to demonstrate that decreased levels of Ikaros protected cells from apoptosis by the chemotherapeutic agents we tested. As several agents are used in the treatment of childhood leukemias, the failure to see resistance may only be a consequence of the specific agents that were chosen for this study. The negative prognostic impact of IKZF1 deletions may be associated with the additional high risk genetic features commonly seen in these patients. Thus the lack of chemoresistance we saw in vitro may simply reflect the complex signature of IKZF1 deletion, whose prognostic impact may be in part related to other associated genetic abnormalities. These findings may be in keeping with recent clinical findings demonstrating that IKZF1 deletions were not an independent prognostic variable in BCR-ABL1− patients or only significant in patients with haplosufficient forms of IKZF1 deletions where large deletions may interrupt others neighboring genes [21, 37].
Our results are in contrast to a recent report describes chemoresistance to glucocorticoids in a IKZF1 shRNA infected Nalm6 cell line designed to exhibit 50% Ikaros production, however Nalm6 cells harbor a dominant-negative IK6 isoform which may affect drug resistance differently than the engineered cell lines we analyzed [38, 39]. Alternatively, our findings are consistent with a model where IKZF1 deletions and the aberrant transcriptional program might alter micro-environmental interactions. This hypothesis is consistent with a recent report by Joshi et al. demonstrating that increased Ikaros expression in pre-B cells results in a transition from a proliferative stroma-adherent phase to a differentiated non-adherent phase [8]. Furthermore, Joshi et. al also found increased expression of genes involved in cellular adhesion pathways, including CTNN and genes coding for adhesion integrins (ITGA1, ITGA5, and ITGB1) [8]. As we found up-regulation of ITGA6, a regulator of cell adhesion and migration, the role of integrins may provide clinically targetable options, as a related integrin 4alpha blocking agent, Natalizumab, has already been shown to sensitize resistant cells to chemotherapy [40]. Previous work supports these findings as BCR-ABL1+ leukemia up-regulates several genes involved in signal transduction and cell adhesion [40, 41].
In summary, our work has discovered and validated a unique set of genes in childhood B-cell ALL that appear to be regulated directly or indirectly by IKZF1 and may be attractive candidates for further evaluation. In addition the poor prognostic impact of IKZF1 deletion may be related to genetic events commonly associated with this subtype or to interactions with the bone marrow niche facilitated by the altered transcriptional program.
Supplementary Material
Acknowledgments
We would like to thank all the members of the Carroll laboratory for their valuable contribution and discussions. This work was supported by NIH R01 CA140729, R21 CA152838-02, and CCSG P30CA016087 grants to W.L. Carroll. NV is generously supported by the American Society of Hematology; WZ was generously supported by the St. Baldrick's Foundation; JAM is supported by NIH T32 CA009161; TB is supported by the Ira Sohn Conference Foundation. We gratefully acknowledge the Children's Oncology Group for patient specimens and the NYU Cytometry Core for their expertise and assistance.
Footnotes
Authorship
NV, WZ, JM, TB, CJ, ER and DM designed research, performed research, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript. JW and RB analyzed data, performed statistical analysis, and wrote the manuscript. WC designed and directed the research, analyzed and interpreted data, and wrote the manuscript.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Avitahl N, Winandy S, Friedrich C, et al. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10(3):333–43. doi: 10.1016/s1074-7613(00)80033-3. [DOI] [PubMed] [Google Scholar]
- 2.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–80. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol. 2012;30(14):1663–9. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 5.Georgopoulos K, Bigby M, Wang JH, et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79(1):143–56. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 6.Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258(5083):808–12. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 7.Medina KL, Pongubala JM, Reddy KL, et al. Assembling a gene regulatory network for specification of the B cell fate. Dev Cell. 2004;7(4):607–17. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Joshi I, Yoshida T, Jena N, et al. Loss of Ikaros DNA-binding function confers integrin-dependent survival on pre-B cells and progression to acute lymphoblastic leukemia. Nat Immunol. 2014;15(3):294–304. doi: 10.1038/ni.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iacobucci I, Storlazzi CT, Cilloni D, et al. Identification and molecular characterization of recurrent genomic deletions on 7p12 in the IKZF1 gene in a large cohort of BCR-ABL1-positive acute lymphoblastic leukemia patients: on behalf of Gruppo Italiano Malattie Ematologiche dell'Adulto Acute Leukemia Working Party (GIMEMA AL WP). Blood. 2009;114(10):2159–67. doi: 10.1182/blood-2008-08-173963. [DOI] [PubMed] [Google Scholar]
- 10.Kuiper RP, Schoenmakers EF, van Reijmersdal SV, et al. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia. 2007;21(6):1258–66. doi: 10.1038/sj.leu.2404691. [DOI] [PubMed] [Google Scholar]
- 11.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758–64. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 12.Mullighan CG, Miller CB, Radtke I, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–4. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 13.Caye A, Beldjord K, Mass-Malo K, et al. Breakpoint-specific multiplex polymerase chain reaction allows the detection of IKZF1 intragenic deletions and minimal residual disease monitoring in B-cell precursor acute lymphoblastic leukemia. Haematologica. 2013;98(4):597–601. doi: 10.3324/haematol.2012.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupuis A, Gaub MP, Legrain M, et al. Biclonal and biallelic deletions occur in 20% of B-ALL cases with IKZF1 mutations. Leukemia. 2013;27(2):503–7. doi: 10.1038/leu.2012.204. [DOI] [PubMed] [Google Scholar]
- 15.Mullighan CG. Molecular genetics of B-precursor acute lymphoblastic leukemia. J Clin Invest. 2012;122(10):3407–15. doi: 10.1172/JCI61203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiper RP, Waanders E, van der Velden VH, et al. IKZF1 deletions predict relapse in uniformly treated pediatric precursor B-ALL. Leukemia. 2010;24(7):1258–64. doi: 10.1038/leu.2010.87. [DOI] [PubMed] [Google Scholar]
- 17.Mullighan CG, Phillips LA, Su X, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322(5906):1377–80. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125–34. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–21. doi: 10.1182/blood-2009-09-245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JJ, Bhojwani D, Yang W, et al. Genome-wide copy number profiling reveals molecular evolution from diagnosis to relapse in childhood acute lymphoblastic leukemia. Blood. 2008;112(10):4178–83. doi: 10.1182/blood-2008-06-165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Veer A, Waanders E, Pieters R, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122(15):2622–9. doi: 10.1182/blood-2012-10-462358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogan LE, Meyer JA, Yang J, et al. Integrated genomic analysis of relapsed childhood acute lymphoblastic leukemia reveals therapeutic strategies. Blood. 2011;118(19):5218–26. doi: 10.1182/blood-2011-04-345595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosack DA, Dennis G, Jr., Sherman BT, et al. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4(10):R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y, Ou Y, Wu K, et al. miR-143 inhibits the metastasis of pancreatic cancer and an associated signaling pathway. Tumour Biol. 2012;33(6):1863–70. doi: 10.1007/s13277-012-0446-8. [DOI] [PubMed] [Google Scholar]
- 25.Vega FM, Colomba A, Reymond N, et al. RhoB regulates cell migration through altered focal adhesion dynamics. Open Biol. 2012;2(5):120076. doi: 10.1098/rsob.120076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Thun A, Preisinger C, Rath O, et al. Extracellular signal-regulated kinase regulates RhoA activation and tumor cell plasticity by inhibiting guanine exchange factor H1 activity. Mol Cell Biol. 2013;33(22):4526–37. doi: 10.1128/MCB.00585-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golbert DC, Correa-de-Santana E, Ribeiro-Alves M, et al. ITGA6 gene silencing by RNA interference modulates the expression of a large number of cell migration-related genes in human thymic epithelial cells. BMC Genomics. 2013;14(Suppl 6):S3. doi: 10.1186/1471-2164-14-S6-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz A, Jiang J, Kempski H, et al. Overexpression of the Ikaros 6 isoform is restricted to t(4;11) acute lymphoblastic leukaemia in children and infants and has a role in B-cell survival. Br J Haematol. 2004;125(1):31–7. doi: 10.1111/j.1365-2141.2004.04854.x. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill DW, Schoetz SS, Lopez RA, et al. An ikaros-containing chromatin-remodeling complex in adult-type erythroid cells. Mol Cell Biol. 2000;20(20):7572–82. doi: 10.1128/mcb.20.20.7572-7582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson EC, Cobb BS, Sabbattini P, et al. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity. 2007;26(3):335–44. doi: 10.1016/j.immuni.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Schjerven H, McLaughlin J, Arenzana TL, et al. Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros. Nat Immunol. 2013;14(10):1073–83. doi: 10.1038/ni.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almire C, Bertrand P, Ruminy P, et al. PVRL2 is translocated to the TRA@ locus in t(14;19)(q11;q13)-positive peripheral T-cell lymphomas. Genes Chromosomes Cancer. 2007;46(11):1011–8. doi: 10.1002/gcc.20490. [DOI] [PubMed] [Google Scholar]
- 33.Juric D, Lacayo NJ, Ramsey MC, et al. Differential gene expression patterns and interaction networks in BCR-ABL-positive and -negative adult acute lymphoblastic leukemias. J Clin Oncol. 2007;25(11):1341–9. doi: 10.1200/JCO.2006.09.3534. [DOI] [PubMed] [Google Scholar]
- 34.Niebuhr B, Kriebitzsch N, Fischer M, et al. Runx1 is essential at two stages of early murine B-cell development. Blood. 2013;122(3):413–23. doi: 10.1182/blood-2013-01-480244. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Ding L, Holmfeldt L, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–63. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shain AH, Pollack JR. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS One. 2013;8(1):e55119. doi: 10.1371/journal.pone.0055119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmi C, Valsecchi MG, Longinotti G, et al. What is the relevance of Ikaros gene deletions as a prognostic marker in pediatric Philadelphia-negative B-cell precursor acute lymphoblastic leukemia? Haematologica. 2013;98(8):1226–31. doi: 10.3324/haematol.2012.075432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Crotty ML, Sensel M, et al. Expression of dominant-negative Ikaros isoforms in T-cell acute lymphoblastic leukemia. Clin Cancer Res. 1999;5(8):2112–20. [PubMed] [Google Scholar]
- 39.Havinga J, Y.L., Demkes M, et al. Loss of IKZF1 function mediates resistance towards gluococorticoid-induced apoptosis. Blood. 2013 ASH Abstract 612. [Google Scholar]
- 40.Hsieh YT, Gang EJ, Geng H, et al. Integrin alpha4 blockade sensitizes drug resistant pre-B acute lymphoblastic leukemia to chemotherapy. Blood. 2013;121(10):1814–8. doi: 10.1182/blood-2012-01-406272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hakansson P, Segal D, Lassen C, et al. Identification of genes differentially regulated by the P210 BCR/ABL1 fusion oncogene using cDNA microarrays. Exp Hematol. 2004;32(5):476–82. doi: 10.1016/j.exphem.2004.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.