Abstract
Background
The Rio Grande River is the natural boundary between U.S. and Mexico from El Paso, TX to Brownsville, TX. and is one of the major water resources of the area. Agriculture, farming, maquiladora industry, domestic activities, as well as differences in disposal regulations and enforcement increase the contamination potential of water supplies along the border region. Therefore, continuous and accurate assessment of the quality of water supplies is of paramount importance. The objectives of this study were to monitor water quality of the Rio Grande and to determine if any correlations exist between fecal coliforms, E. coli, chemical toxicity as determined by Botsford's assay, H. pylori presence, and environmental parameters. Seven sites along a 112-Km segment of the Rio Grande from Sunland Park, NM to Fort Hancock, TX were sampled on a monthly basis between January 2000 and December 2002.
Results
The results showed great variability in the number of fecal coliforms, and E. coli on a month-to-month basis. Fecal coliforms ranged between 0–106 CFU/100 ml while E. coli ranged between 6 to > 2419 MPN. H. pylori showed positive detection for all the sites at different times. Toxicity ranged between 0 to 94% of inhibition capacity (IC). Since values above 50% are considered to be toxic, most of the sites displayed significant chemical toxicity at different times of the year. No significant correlations were observed between microbial indicators and chemical toxicity.
Conclusion
The results of the present study indicate that the 112-Km segment of the Rio Grande river from Sunland Park, NM to Fort Hancock, TX exceeds the standards for contact recreation water on a continuous basis. In addition, the presence of chemical toxicity in most sites along the 112-Km segment indicates that water quality is an area of concern for the bi-national region. The presence of H. pylori adds to the potential health hazards of the Rio Grande. Since no significant correlation was observed between the presence of H. pylori antigens and the two indicators of fecal contamination, we can conclude that fecal indicators cannot be used to detect the presence of H. pylori reliably in surface water.
Background
The Rio Grande river is the natural boundary between U.S. and Mexico from El Paso, TX. to Brownsville, TX, and it is one of the major water resources of the area. El Paso, TX. and Ciudad Juarez, Mexico comprise the largest metropolitan area of the bi-national region with a semi-arid environment receiving an average of 17.7 cm of rain per year. The Rio Grande/Rio Bravo is the major watershed of this bi-national region. The major groundwater reservoirs of the area are the Hueco Bolson and the Mesilla Bolson. The river serves as an important natural resource for industry, agriculture, domestic water supply, recreation, and wildlife habitat for both countries [1]. Unfortunately, the Rio Grande is also a reservoir for infectious micro-organisms and toxic pollutants [2]. A variety of activities contributing to the chemical and microbial contamination of water supplies have been identified and include improperly installed and maintained septic systems, landfills, injection wells, land application of waste, irrigation, runoff, animal feed lots, etc [3]. It is estimated that at the present rate of consumption groundwater supplies will be depleted in approximately twenty years.
The presence of "colonias" (unincorporated and economically disadvantaged communities) with inadequate wastewater disposal methods, the application of untreated or improperly treated sewage for disposal or irrigation purposes, the numerous maquiladoras [international industry in Mexico], and differences in disposal regulations between U.S. and Mexico result in a high probability of anthropogenic activities being responsible for the contamination of Rio Grande water supplies. Although groundwater has traditionally been considered a safe source of drinking water, more than half of the reported waterborne disease outbreaks have been linked to contaminated groundwater [4].
Infectious diseases including cholera, amoebiasis, hepatitis A, salmonellosis, shigellosis, giardiasis, ascariasis and other intestinal infections are not uncommon in the border region. The Texas Department of Health showed that hepatitis A, salmonellosis, dysentery, cholera, and other diseases occur at much higher rates in colonias than in Texas as a whole [2]. The occurrence of infectious diseases is associated with conditions prevalent in border counties, i.e., potentially contaminated water from shallow wells in colonias, poor hygiene, and low socioeconomic status. Results from previous toxic chemicals studies by the Texas Commission on Environmental Quality (TCEQ), the International Boundary and Water Commission (IBWC) and the United States Environmental Protection Agency (USEPA) ranked several Rio Grande sites as areas of concern [1,5]. The main pollutants found in water were arsenic, copper, nickel, chloride, unionized ammonia, and phenolic compounds. The USEPA has recently included Helicobacter pylori on the Contaminant Candidate List (CCL) (62FR 52193). The CCL identifies contaminants which are not currently regulated but known or anticipated to occur in public water systems. Little is known about the mode of transmission of H. pylori. A waterborne transmission route has been proposed since this microorganism has been found in surface water, groundwater and drinking water [6-9]. The occurrence and persistence of H. pylori in border water supplies has not been established. However, H. pylori antibodies were detected in 21% of children between the ages of 4–7 in a study of 365 primary school children conducted in an area of El Paso where half of the population do not drink piped water and 86% use septic tanks [10]. In order to fully understand the risk factors that promote H. pylori infections, it is extremely important to determine if this bacterium is present in border water supplies. Several studies have indicated that microorganisms can be found in the environment in a "viable, non culturable state." While these microorganisms cannot be cultured in regular culture media, their genomes remain viable, and given the right conditions, they can become infectious [11,12]. Viruses have been reported to be in a similar state and to be activated and become infectious under certain environmental conditions [13]. It has been suggested that H. pylori can be found in a viable, non-culturable, metabolically active state in water supplies [8,12]. Information on the occurrence of H. pylori in border water supplies will provide valuable information on the route of transmission and survival of this infectious agent.
Agrochemicals, pesticides, heavy metals, arsenic, and PCBs (presumably from illegal dumping, agriculture and maquiladora activities) have been detected in the river and may be associated with fish deformities, leukaemia, and congenital malformations in humans. Fecal coliforms were also identified as an area of concern in three of the 19 segments of the Rio Grande Basin [2]. Two of these sites were located in the El Paso/Ciudad Juarez region. In order to determine the extent of chemical contamination of the river, it is important to develop and utilize assays that are appropriate for field work. Botsford's toxicity assay is inexpensive, rapid, and can be conducted with minimal training [14]. It is a novel assay that provides values comparable to the fat head minnow (Pimephales promelas) assay, and two commercial assays (Microtex and Polytox) that use bacteria as indicator organisms. Botsford's assay has been used to detect toxicity due to the presence of several inorganic and organic chemicals. It uses the ability of Rhizobium meliloti to reduce MTT (3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyltetrazolium bromide) under non toxic conditions [14].
Water quality of the river is one of the most important concerns facing communities that are dependent on the river for drinking water, agriculture, and watershed. Microbial and chemical contamination must be monitored continuously to determine the condition of the water in order to contain the spread of diseases and to eliminate non-point sources of contamination.
River flow
The demands on the water of the Rio Grande river have changed in the last two years (2001–2003) due to the drought conditions that the area is experiencing. Agriculture as well as residential "drought-condition" limits have been in place for the last several years. To conserve water and to control pollution, most of the water is now being diverted into concrete-lined canals leaving part of the river sites between El Paso, TX and Fort Hancock, TX with low or no flowing water. The concrete lined-canals are used for irrigation purposes. The decreased flow in some of the sample sites has resulted in observable changes in the microbial population and chemical composition of the water. The seven sites extend over 112-Km distances that vary in terms of the potential sources of contamination. Table 1 and Figure 1 describe the seven surface water sites with the potential sources of contamination. The least impacted in terms of flow are Sites 1 and 2 since they receive water from New Mexico and are before the diversion dams that distribute water between Mexico and the USA. Two secondary-treatment Waste Water Treatment Plants (WWTPs) in New Mexico discharge their effluent into the river approximately 3 Km upstream from Site 1. The standards for fecal coliforms in WWTP's effluent is 1000 cfu/100 ml (monthly average) for New Mexico, while in Texas the standard for fecal coliform is 200 cfu/100 ml. The Sunland Park Horse Race Track in New Mexico is almost adjacent to Site 1 and wild avian species frequent this area in large quantities. Conditions between Site 1 and Site 2 are influenced by street runoff, the Montoya agricultural drain, and other industrial discharge. Water treated for drinking purposes in the Canal Treatment Plant is taken from a concrete-lined canal approximately 5 Km downstream from Site 2. Sites 3 and 5 are located around the most populated areas of the city and are likely to be affected by anthropogenic activity. These sites are in a concrete-lined section of the river and have only a trickle of flow due to the diversion of the river above Site 3. Two international vehicle and pedestrian bridges are located close to this concrete-lined section of the river. The flow in Site 3 consists mostly of leakage from the International Dam. Street runoff and municipal streams also contribute to the flow in Site 3. The Haskell Waste Water Treatment Plant (which is a tertiary treatment plant) returned most of its treated effluent into the river between Sites 3 and 5 before the Americas canal was completed. Site 5 has same flow as Site 3, except when the Americas Canal cannot handle effluent from Haskell WWTP. Since the completion of this canal, most of this treated wastewater is returned to the Americas canal, which then empties into the Riverside Canal. Water that is treated in the Jonathan Rogers Water Treatment Plant for municipal use is taken from the Riverside canal at this point. Both of the drinking water plants are closed during non-irrigational seasons. Site 6 is located close to this point and some of the overflow, which the Riverside Canal cannot handle, is diverted back into the river. The majority of the year this site is stagnant or has insignificant flow. Since the water in the Americas canal comes from a mixture of river water and treated water, Site 6 shows differences from Site 5 in microbial contamination and chemical toxicity. For most of this study, the 17.6-Km natural segment between Site 5 and Site 6 has been completely dry. The conditions at Site 7 are unique since this area is far from populated areas and water taken from the river at American and International diversion dams merges from both sides of the border. Most of the flow at Site 7 is due to a return gate from the Mexican side, which includes partially treated wastewater (primary treatment only) along with some gates from irrigation canals in the U.S. side of the river.
Table 1.
Sample site descriptions
| Site 1. Rio Grande at Sunland Park bridge near Texas/ New Mexico state line | A horse race track is located upstream and El Paso Electric is downstream [influenced by urban and agricultural runoff] |
| Site 2. Rio Grande at Courchesne Bridge in El Paso | This site is influenced by flows coming from Elephant Butte Dam. The use of water for irrigation upstream contributes large volumes of irrigation return flow and agricultural runoff. It also receives urban runoff and wastewater discharges from Anthony, Canutillo, and El Paso |
| Site 3. Rio Grande 2.4 Km upstream of Haskell street [WWTP] | Vehicle traffic is heavy and the area is also affected by urban runoff |
| Site 4. El Paso Haskell Street WWTP outfall | Wastewater is discharged to the concrete lined/ channel part of the river |
| Site 5. Rio Grande 1.3 Km downstream from Haskell WWTP | Vehicle traffic is heavy and the area is affected by urban runoff |
| Site 6. Rio Grande at Riverside Diversion Dam | Influenced by urban runoff, wastewater discharges from the Haskell Street WWTP and runoff from industrial facilities on both sides of the border |
| Site 7. Rio Grande at Alamo Grade Control structure 9.7 Km upstream of Fort Hancock Port of Entry | Receives large amounts of wastewater from domestic and industrial sources |
Figure 1.
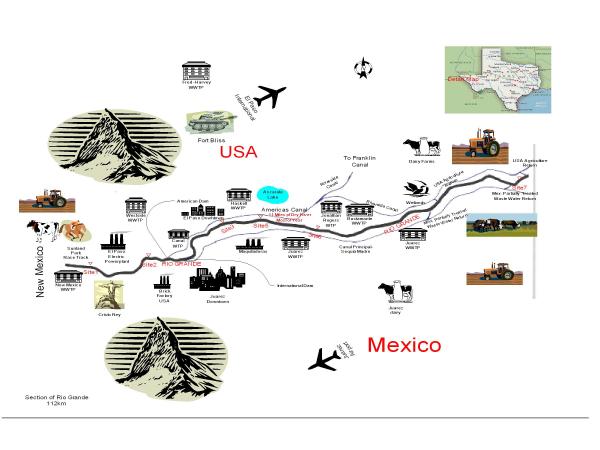
Sampling Locations in the Rio Grande basin.
Results
Microbial
Fecal coliform counts for Sites 1–3 (Fig. 2) showed values ranging between 0 and 1.9 × 105 CFU/100 ml for Site 1, from 1.3 × 102 to 2.9 × 105 for Site 2, and from 0 to 3.7 × 105 for Site 3. Site 4 counts were from 0 to 27 CFU/100 mL, and most counts were below 10 CFU/100 mL (these results were not plotted). The Site 4 sample is from an effluent sampling faucet located inside Haskell WWTP and was not taken directly from the river. Fecal coliforms results for Sites 5–7 (Fig. 3) ranged between 0 and 1.9 × 105 CFU/100 ml for Site 5, between 0 and 1 × 105 for Site 6 and between 0 and 3 × 106 for Site 7. E. coli most probable number (MPN) determined with the IDDEX Colilert system for all sites are shown in (Fig. 4). Values ranged between 6 and 2419, which is the upper detection limit. A high percentage of the samples were at the upper detection limit. Site 4 was not analysed for MPN of E. coli. Table 2 shows the results for H. pylori determinations using the HpSA Antigen test. Out of 31 months, Site1 tested positive for H. pylori 17 times, Site 2 (13 times), Sites 3 and 5 (17 times), Site 4 (15 out of 24 samples) data not shown, Site 6 (14 times), and Site 7 (16 times).
Figure 2.
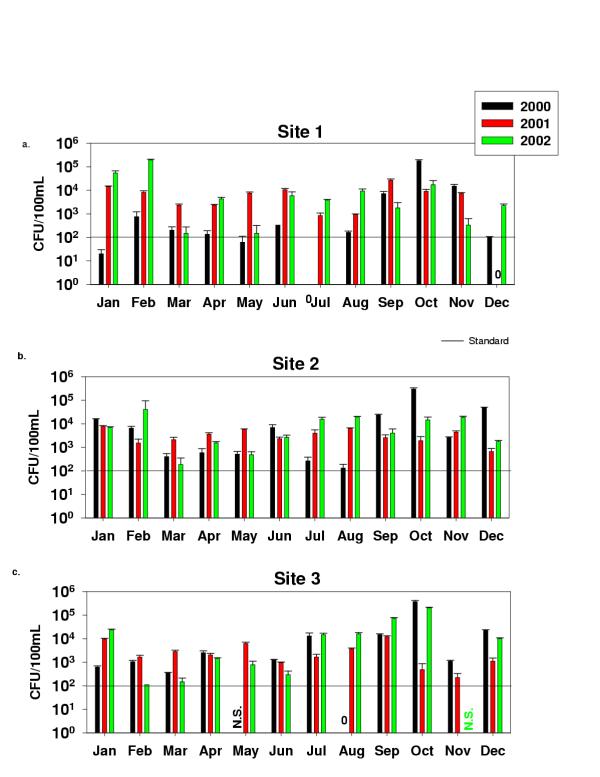
Fecal coliform bacteria on M-FC agar media for Sites 1–3
Figure 3.
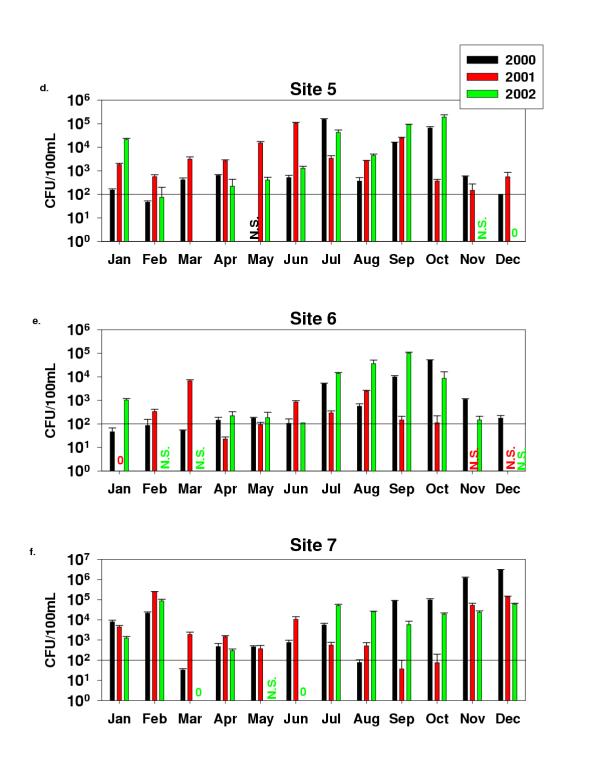
Fecal coliform bacteria on M-FC agar media for Sites 5–7
Figure 4.
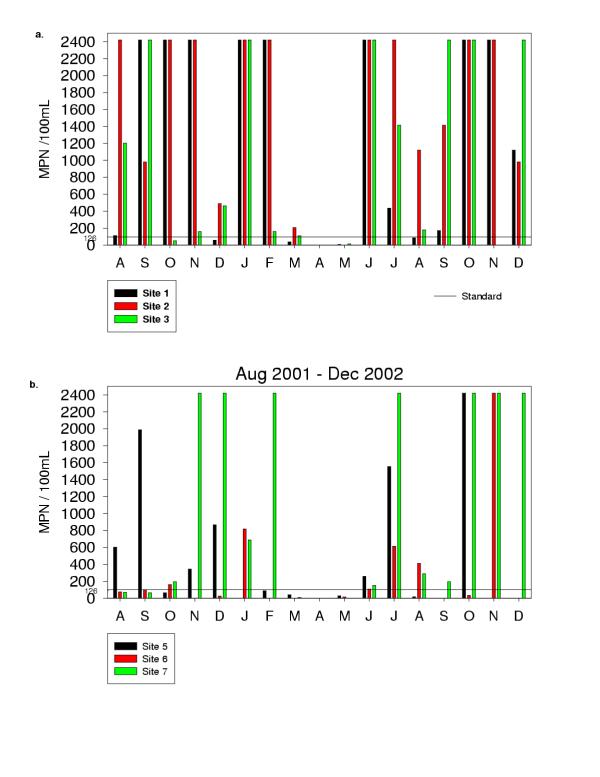
E. coli most probable number (MPN) using the IDEXX Colilert assay for August 2001 – December 2002
Table 2.
H. pylori HpSa ELISA test results
| Site 1 | Site 2 | Site 3 | Site 5 | Site 6 | Site 7 | |
| Jun-00 | + | + | ||||
| Jul-00 | ||||||
| Aug-00 | + | + | + | + | ||
| Sep-00 | + | + | + | + | + | + |
| Oct-00 | + | |||||
| Nov-00 | ||||||
| Dec-00 | + | + | + | + | ||
| Jan-01 | + | + | + | + | + | + |
| Feb-01 | ||||||
| Mar-01 | ||||||
| Apr-01 | + | + | ||||
| May-01 | + | + | + | + | + | + |
| Jun-01 | ||||||
| Jul-01 | ||||||
| Aug-01 | + | + | + | + | + | + |
| Sep-01 | + | + | + | + | + | + |
| Oct-01 | + | + | + | + | + | + |
| Nov-01 | N/D | N/D | N/D | N/D | N/D | N/D |
| Dec-01 | + | |||||
| Jan-02 | + | + | + | + | + | |
| Feb-02 | + | + | + | + | N/D | + |
| Mar-02 | + | + | + | + | + | + |
| Apr-02 | + | N/D | + | + | + | + |
| May-02 | + | + | + | + | + | + |
| Jun-02 | + | + | + | |||
| Jul-02 | + | + | + | + | + | |
| Aug-02 | ||||||
| Sep-02 | + | + | + | + | + | + |
| Oct-02 | + | + | ||||
| Nov-02 | ||||||
| Dec-02 | + | |||||
Note: Squares with + indicate detection of H. pylori antigen.
Chemical toxicity
Results of Chemical toxicity for Sites 1–7 are shown in Figure 5. Values above 50% are considered toxic. Chemical toxicity ranged between 0 and 94% for Site 1, between 0 and 91% for Site 2, and between 0 and 92% for Site 3. Values for Site 4, out of the 24 samples, the highest chemical toxicity registered was 87% on three occasions, these values were not plotted. Chemical toxicity ranged between 0 and 94% for Sites 5 and 6, and between 0 and 90% for Site 7.
Figure 5.
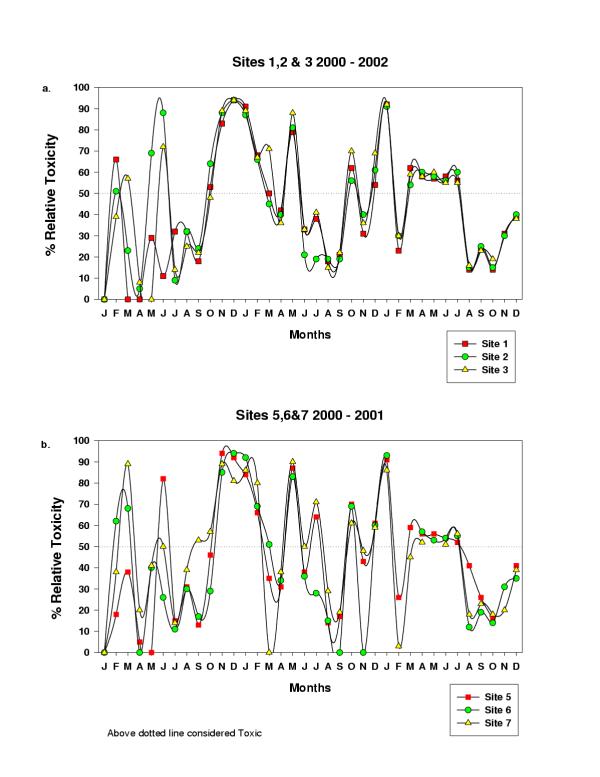
Relative chemical toxicity utilizing the Botsford assay
Discussion
Microbial
The Texas Commission on Environmental Quality (TCEQ)'s standard for fecal coliforms on contact recreation areas is 200 CFUs/100 ml (Chapter 307 Texas Surface Water Quality Standards, Appendix A). Although four of the seven tested sites do not support contact recreation activity because of the low flow most of the year, this standard was used for comparative purposes. During the three-year evaluation period, Site 1 exceeded the limit 75% of the time, Site 2 94.4% of the time, and Site 3 88.6% of the time. Sunland Park and Santa Teresa Waste Water Treatment plants located between New Mexico and Texas discharge their effluent before Site 1 and are potential contributors of contaminants to Sites 1 and 2. The Montoya agricultural drain is likely to impact Sites 2 and 3. The flow in Site 3 consists mostly of leakage from the International Dam and municipal streams. Site 5 exceeded 77.1% of the time, Site 6 exceeded only 42.9% of the time, and Site 7 exceeded the limit 80.6% of the time. Sites 1, 2, and 3 had fecal coliform counts around 103 CFU/100 ml most of the time. Site 5 had counts between 102 and 104 most of the time. In Site 6, the counts were between 102 and 103 CFU/100 most of the time. It was noted at Site 6, throughout the course of this study, that the counts remain close or below the standard. As mentioned before, Site 6 receives the overflow from the Riverside canal, which contains a chlorinated effluent from the Haskell WWTP and industrial discharges. This site has been stagnant the majority of the time. This may be the reason for the lower bacterial counts observed. Site 7 had fecal coliform counts of 104 the majority of the time. It was at this site that we observed the highest counts reaching 106 CFU/100 ml in November-December 2000. Extremely high fecal coliform counts were only observed in the first year of this study. During 2001, two wastewater treatment plants became operational in Mexico. The Mexican wastewater treatment plants only provide primary treatment. However, drought conditions have forced most crops to be irrigated with waters that would normally be returned to the river. The lower fecal coliform counts observed at Site 7 during the last two years may be the results of the WWTPs reducing the microbial contaminants combined with drought-condition irrigation practices.
E. coli is the most specific test for fecal contamination. As the numbers of E. coli increase, there is a statistically greater risk that people using the river will experience gastro-intestinal illness. The TCEQ standard for this organism is 126 CFU/100 ml for multiple samples (Chapter 307-Texas Surface Water Quality Standards). E. coli counts during the last 17 months of assessment (Figure 4) revealed consistent trends. Site 1 exceeded the limit 62.5% of the time, Site 2 exceeded 87.5% of the time, Site 3 exceeded 68.8% of the time, Site 5 exceeded 43.8% of the time, Site 6 exceeded 31.2% of the time, and Site 7 exceeded the limit 68.8% of the time. These values agree with the results for fecal coliform counts, i.e., Site 2 exceeds the limit with the highest percentage, and Site 6 has the lowest percentage of time where the limit is exceeded. When river flow is diverted for irrigation beginning in middle of February, all river sites reveal lower E. coli counts. From August 2001 through October 2001 lower counts (around the standard or lower) were recorded in Site 6 and 7. Site 1, 2, and 3 reached the upper limit of the assay at least once on this period while Sites 5,6,7 remained very low. This could be the result of treated effluent entering the Rio Grande between Sites 3 and 5, which happen as a result of the Canal of the Americas not been able to handle all the effluent from Haskell WWTP. Precipitation at the El Paso International Airport for August 2001 was 4.3 cm, which supports the reasoning for this variation. However, E. coli counts appear to be influenced by rainfall, runoff and chemical discharges into the river including those used in the waste water treatment process.
Chemical toxicity
Botsford's Chemical Toxicity assay was standardized using a variety of toxic chemicals as previously reported [14]. According to this method, any samples showing greater than 50% inhibition are considered toxic (IC50) (Figure 5). Using this standard, Site 1 displayed significant toxicity 45.7% of the time, Site 2 was toxic 48.6% of the time, Site 3 was toxic 45.7% of the time, Site 5 was toxic 44.1% of the time, Site 6 was toxic 48.5% of the time, and Site 7 was toxic 50% of the time. Botsford's assay provides an initial assessment of the relative toxicity of a sample but does not identify the toxic chemicals present. It is not clear at this point if it is the synergistic effect of several chemical compounds that contribute to the toxicity displayed using Botsford's assay. Further detailed analyses of samples will be needed in order to fully establish the value of using Botsford's assay for surface water testing.
Statistical analyses
Analyses were performed to quantify relationships between biological variables and chemical toxicity, and to measure the strength of relationships between H. pylori and fecal contamination indicators such as fecal coliforms and E. coli. The assumption was that toxic chemicals present in the river may have an adverse effect on the bacterial population including fecal coliforms, E. coli and H. pylori. The All-Possible regression procedures using r2 and Stepwise were first used to find any significant relationship between independent variables such as chemical toxicity and H. pylori versus biological variables including fecal coliforms and E. coli. By-site and overall analyses were performed and no relationships of significant levels were observed (data not shown). Further analyses were performed using the same procedures between the same independent variables and environmental parameters such as pH, precipitation, specific conductivity, and DO. Chemical toxicity showed a significant relationship between temperature of sample with an r2 = 0.1615, p < 0.001 (data not shown).
To analyze any relationships between data from all parameters that were measured in the study, the Pearson-Moment correlation procedure was used. Tables 3, 4, 5, 6 were generated with the results from the analyses. First, an overall analysis was performed using data from all sites. As expected, there was a highly significant relationship between fecal coliforms and E. coli (shown in Table 3). Chemical toxicity shows a significant relationship with specific conductivity and a negative relationship with temperature of sample (shown in Table 3). Non-point contamination events and the decreased river flow in the winter months could be contributing to these results. The same analysis was performed by-site between chemical toxicity, H. pylori, Fecal coliforms, and physiochemical parameters. The same strong relationship was observed between E. coli and fecal coliforms, except for Site 6 which did not show a strong relationship between E. coli and fecal coliforms (shown in Table 4). As mentioned earlier, this site has unique hydrologic characteristics, which could temporarily affect the microbial population. No significant relationships were observed between the presence of H. pylori antigen and indicators of fecal contamination including fecal coliforms and E. coli. Differences in survival rates between H. pylori and fecal coliforms may explain the lack of correlation. Therefore, the traditional indicators of fecal contamination cannot be used to detect the presence of H. pylori.
Table 3.
Overall Pearson Product-Moment coefficients for correlation analysis between biological and environmental parameters.
| Fecal Coliform | Total Coliform | E. coli | H. pylori | Specific Conductivity | pH | Temperature | Dissolved Oxygen | |
| Chemical Toxicity | 0.0601 | 0.1637 | -0.1001 | -0.0454 | 0.2076 | -0.0599 | -0.4046 | 0.0634 |
| 0.3915 | 0.0208** | 0.3769 | 0.581 | 0.0093** | 0.4576 | 0.0001** | 0.4197 | |
| 205 | 208 | 80 | 150 | 156 | 156 | 167 | 164 | |
| Fecal Coliform | 0.53 | 0.6631 | -0.0782 | 0.0247 | 0.0271 | 0.0558 | -0.1368 | |
| 0.0001** | 0.0001** | 0.341 | 0.7595 | 0.7364 | 0.4734 | 0.0806 | ||
| 205 | 80 | 150 | 156 | 156 | 167 | 164 | ||
| Total Coliform | 0.7502 | 0.0686 | 0.0653 | 0.0273 | -0.2156 | 0.0168 | ||
| 0.0001** | 0.4039 | 0.4179 | 0.7352 | 0.0051** | 0.8304 | |||
| 80 | 150 | 156 | 156 | 167 | 164 | |||
| E. coli | -0.0863 | 0.2314 | -0.3289 | -0.01625 | -0.3455 | |||
| 0.4707 | 0.1134 | 0.0224** | 0.9054 | 0.0079** | ||||
| 72 | 48 | 48 | 56 | 58 | ||||
| H. pylori | 0.0269 | 0.1627 | 0.0291 | -0.0802 | ||||
| 0.7769 | 0.085 | 0.7456 | 0.3775 | |||||
| 113 | 113 | 126 | 123 | |||||
| Specific Conductivity | -0.3106 | -0.4432 | 0.1106 | |||||
| 0.0001** | 0.0001** | 0.1822 | ||||||
| 157 | 146 | 147 | ||||||
| pH | 0.1713 | 0.221 | ||||||
| 0.0387** | 0.0071** | |||||||
| 146 | 147 | |||||||
| Temperature | -0.4047 | |||||||
| 0.0001** | ||||||||
| 162 | ||||||||
Values are Pearson Correlation Coefficients/Probability |R| under Ho: Rho = O/Number Observations. ** Probability of 95% of greater level of significance.
Table 4.
Pearson Product-Moment coefficients for correlation analysis by site between fecal coliforms and biological and environmental parameters
| Site 1 | Site 2 | Site 3 | Site 5 | Site 6 | Site 7 | |
| Chemical Toxicity | 0.2158 | 0.1242 | 0.1972 | -0.0175 | -0.2365 | 0.0644 |
| 0.213 | 0.4772 | 0.2634 | 0.9217 | 0.1851 | 0.7174 | |
| 35 | 35 | 34 | 34 | 33 | 34 | |
| Total Coliform | 0.5844 | 0.3486 | 0.51221 | 0.393 | 0.5991 | 0.5132 |
| 0.0002** | 0.0401** | 0.002** | 0.0215** | 0.0002** | 0.0019** | |
| 35 | 35 | 34 | 34 | 33 | 34 | |
| E. coli | 0.6779 | 0.7753 | 0.61 | 0.6001 | 0.3386 | 0.8466 |
| 0.0055** | 0.0011** | 0.0205** | 0.0391** | 0.3083 | 0.0001** | |
| 15 | 14 | 14 | 12 | 11 | 14 | |
| H. pylori | -0.0065 | -0.3487 | -0.1881 | 0.00052 | 0.0623 | -0.1777 |
| 0.9752 | 0.0875 | 0.3679 | 0.998 | 0.7724 | 0.3952 | |
| 25 | 25 | 25 | 26 | 24 | 25 | |
| Specific Conductivity | 0.0943 | 0.2294 | 0.0607 | -0.4797 | -0.4706 | 0.03 |
| 0.6468 | 0.2497 | 0.7633 | 0.0113** | 0.0234** | 0.8843 | |
| 26 | 27 | 27 | 27 | 23 | 26 | |
| pH | 0.3704 | -0.1124 | -0.2436 | 0.1451 | 0.0527 | 0.0942 |
| 0.0624 | 0.5764 | 0.2206 | 0.47 | 0.811 | 0.6469 | |
| 26 | 27 | 27 | 27 | 23 | 26 | |
| Temperature | -0.1215 | -0.0054 | 0.0882 | 0.4589 | 0.4981 | -0.2452 |
| 0.5714 | 0.9771 | 0.6429 | 0.0107** | 0.0113** | 0.2083 | |
| 24 | 30 | 30 | 30 | 25 | 28 | |
Values are Pearson Correlation Coefficients/Probability |R| under Ho: Rho = O/Number of observations. ** Probability of 95% of greater level of significance
Table 5.
Pearson Product-Moment coefficients for correlation analysis by site between chemical toxicity and biological and environmental parameters
| Site 1 | Site 2 | Site 3 | Site 5 | Site 6 | Site 7 | |
| Fecal Coliform | 0.21584 | 0.1242 | 0.1972 | -0.0175 | -0.2365 | 0.0644 |
| 0.213 | 0.4772 | 0.2634 | 0.9217 | 0.1851 | 0.7174 | |
| 35 | 35 | 34 | 34 | 33 | 34 | |
| Total Coliform | 0.2666 | 0.0337 | 0.1474 | 0.039 | 0.2719 | 0.1182 |
| 0.12151 | 0.8473 | 0.4053 | 0.8263 | 0.1257 | 0.5053 | |
| 35 | 35 | 34 | 34 | 33 | 34 | |
| E. coli | -0.0747 | -0.0766 | -0.213 | -0.4612 | 0.1376 | -0.0093 |
| 0.7912 | 0.7945 | 0.4646 | 0.1312 | 0.6866 | 0.9748 | |
| 15 | 14 | 14 | 12 | 11 | 14 | |
| H. pylori | -0.1733 | -0.2405 | -0.2019 | -0.0709 | 0.2174 | 0.022 |
| 0.4074 | 0.2468 | 0.333 | 0.7307 | 0.3078 | 0.9167 | |
| 25 | 25 | 25 | 26 | 24 | 25 | |
| Specific conductivity | 0.2434 | 0.391 | 0.2393 | 0.1371 | 0.3778 | -0.0375 |
| 0.2307 | 0.0437** | 0.2293 | 0.4951 | 0.0758 | 0.8554 | |
| 26 | 27 | 27 | 27 | 23 | 26 | |
| pH | -0.0796 | -0.0303 | -0.07382 | 0.0665 | 0.0928 | -0.342 |
| 0.6988 | 0.8807 | 0.7144 | 0.7417 | 0.6734 | 0.0872 | |
| 26 | 27 | 27 | 27 | 23 | 26 | |
| Temperature | -0.1215 | -0.3753 | -0.4489 | -0.217 | -0.5488 | -0.1965 |
| 0.5714 | 0.0409** | 0.0128** | 0.2492 | 0.0045** | 0.3162 | |
| 24 | 30 | 30 | 30 | 25 | 28 | |
Values are Pearson Correlation Coefficients/Probability |R| under Ho: Rho = O/Number of observations. ** Probability of 95% of greater level of significance
Table 6.
Pearson Product-Moment coefficients for correlation analysis by site between H. pylori, and biological and environmental parameters
| Site 1 | Site 2 | Site 3 | Site 5 | Site 6 | Site 7 | |
| Fecal Coliform | -0.0065 | -0.2405 | -0.1881 | 0.0005 | 0.0623 | 0.022 |
| 0.9752 | 0.2468 | 0.3679 | 0.998 | 0.7724 | 0.9167 | |
| 25 | 25 | 25 | 26 | 24 | 25 | |
| Total Coliform | 0.1202 | -0.2375 | 0.2339 | 0.203 | -0.2082 | 0.1187 |
| 0.567 | 0.2529 | 0.2604 | 0.3199 | 0.3289 | 0.572 | |
| 25 | 25 | 25 | 26 | 24 | 25 | |
| E. coli | -0.0094 | -0.0507 | 0.1142 | -0.4742 | 0.0259 | -0.0921 |
| 0.9755 | 0.8756 | 0.7102 | 0.1405 | 0.9432 | 0.7647 | |
| 13 | 12 | 13 | 11 | 10 | 13 | |
| Chemical Toxicity | -0.1733 | -0.2405 | -0.2019 | -0.0709 | 0.2174 | 0.022 |
| 0.4074 | 0.2468 | 0.333 | 0.7307 | 0.3073 | 0.9167 | |
| 25 | 25 | 25 | 26 | 24 | 25 | |
| Specific conductivity | -0.0242 | -0.2117 | -0.1239 | 0.2298 | -0.2479 | 0.0746 |
| 0.9239 | 0.3843 | 0.6131 | 0.3297 | 0.3374 | 0.7544 | |
| 18 | 19 | 19 | 20 | 17 | 20 | |
| pH | 0.2534 | 0.5685 | 0.3308 | 0.3344 | 0.1358 | 0.162 |
| 0.3102 | 0.0111** | 0.1665 | 0.1495 | 0.6032 | 0.4949 | |
| 18 | 19 | 19 | 20 | 17 | 20 | |
| Temperature | 0.0035 | 0.2599 | 0.1441 | 0.0722 | 0.32 | -0.2654 |
| 0.9884 | 0.2427 | 0.522 | 0.7433 | 0.1817 | 0.2449 | |
| 19 | 22 | 22 | 23 | 19 | 21 | |
Values are Pearson Correlation Coefficients/Probability |R| under Ho: Rho = O/Number of Observations. **Probability of 95% of greater level of significance
Correlation between chemical and microbial contamination, H. pylori, and biological indicators
Analyses were performed to quantify relationships between biological variables and chemical toxicity, and to measure the strength of relationships between H. pylori and fecal contamination indicators such as fecal coliforms and E. coli. The All Possible regression procedure using r2 and Stepwise was first used to find any significant relationship between independent variables chemical toxicity, and H. pylori and biological variables. By site and overall analyses were performed and no relationships of significant level were observed (data not shown). Further analyses were performed using the same procedure between same independent variables and physicochemical parameters. Chemical toxicity showed a relationship between temperatures of samples of r2 = 0.1615, p < 0.001 (data not shown).
The results of this comprehensive three-year river monitoring effort confirm what previous isolated studies have suggested, i.e., that the Rio Grande is heavily contaminated with bacteria of fecal origin. Since several areas of the Rio Grande within this segment are used for recreational purposes by individuals from both sides of the border, the public health implications need to be addressed. Studies have been initiated in our laboratory to determine the sources of fecal contaminants using Antibiotic Resistance Analyses (ARA), genotyping and ribotyping. Future studies will include more detailed chemical analyses of water samples showing a high degree of chemical toxicity.
Conclusions
The results of the present study indicate that the 112-Km segment of the Rio Grande river from Sunland Park, NM to Fort Hancock, TX exceeds fecal coliforms standards for contact recreation water on a continuous basis. In addition, the presence of chemical toxicity in most sites along the 112-Km segment as detected by Botsford's assay, indicate that water quality is an area of concern for the bi-national region. The presence of H. pylori adds to the potential health hazards of the Rio Grande. Since no significant correlation was observed between the presence of H. pylori antigens and the two indicators of fecal contamination, we can conclude that fecal indicators cannot be used to detect the presence of H. pylori antigens reliably in surface water. Also, no correlation was found between Botsford's chemical toxicity assay and fecal indicators indicating that the toxic chemicals present in the river are having a differential effect on the bacterial population. The river is a dynamic system where biological and chemical components may interact in a complex synergistic or antagonistic manner. The results of this study indicate that no simple conclusions can be drawn by studying a single indicator or parameter.
Methods
Seven Rio Grande sites described in Table 1 and Fig. 1 were sampled. Sampling was done on a monthly basis for a period of thirty six months. All samples were collected and processed according to methods described in the Study Methods section of the Second Phase Bi-national Study [1] and in Standard Methods for the Examination of Water and Wastewater [15]. Samples were collected midstream by submerging sterile 1-liter plastic cubitainers to a depth of 30 cm and opening the container underneath till full with sample then closing underwater. Environmental parameters were taken at each site using a Hydro Lab Quanta Multi Parameter Analyser. Samples were kept on ice until delivered to the laboratories.
Chemical toxicity
Botsford's assay was adapted and used to test for toxicity in water samples [14]. Rhizobium meliloti was grown in a chemically-defined medium (CDM) supplemented with 0.1% casamino acids with 1% mannitol as the carbon source. Bacterial cultures were incubated at 30°C overnight and centrifuged at 10,000 × g for 10 min. After washing the cells once with 0.01 M KH2PO4 buffer, pH 7.5, the cells were resuspended in 0.01 M phosphate buffer, pH 7.5 to an absorbance value between 0.31 to 0.38 at 550 nm. Assays were conducted by combining 0.2 ml of 0.1 M Tris-HCl buffer, pH 7.5 and 2.1 ml of test water sample. Nanopure-quality water was used as a negative control. Water samples were filtered using a 0.2-μm syringe filter to remove sediments and other organisms that could interfere with absorbance reading. The diluted bacterial suspension was then added to make a final volume of 3.3 ml and the initial absorbance reading was taken using a spectrophotometer at a fixed wavelength of 550 nm. 100 μl of a 3 mM solution of MTT (3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyltetrazolium bromide) was added and the mixture was incubated at 30°C for 20 min. To prevent the high concentrations of minerals in samples from interfering with the assay, 10 μl of EDTA 5 mM was added to the mixture before incubation. The difference in absorbance between the initial reading and the absorbance after 20 minute incubation (ΔA) was used in the calculation of MTT reduction. The difference between ΔA of river water samples and ΔA of pure-water controls determined the degree of inhibition of MTT reduction by potential toxic compounds in the water. Samples that show an inhibition capacity of 50% (IC50) or more indicate potential toxicity as determined by standardization using toxic chemicals [14].
Microbial
The procedure described in Standard Methods for the Examination of Water and Waste Water [15] and modifications described by Elmund et al. [16] was used. Fecal coliforms were detected using the MF technique, incubating the filters placed on sterile absorbent pads saturated with M-FC broth for fecal coliforms. Tests for H. pylori were done using the Premier Platinum HpSA (EIA) H. pylori assay, which detects H. pylori antigens (Meridian Diagnostics, Inc.). For increased sensitivity, 10 ml samples were concentrated 100-fold by ultrafiltration. The Premier Platinum HpSA test utilizes polyclonal antibodies absorbed to micro wells. Concentrated samples and a peroxidase conjugated polyclonal antibody were added to the wells and incubated for one hour at room temperature. A wash was performed to remove unbound material. Substrate was added and incubated for ten minutes at room temperature. Color developed in the presence of bound enzyme. Stop solution was added and the results were interpreted spectrophotometrically. The IDEXX®, using the Quanti-Tray®, assay was used to determine E. coli most probable number (MPN). This assay has an upper detection limit of 2419.2. IDEXX Colilert® medium was added to the samples, sealed in Quanti-Tray, incubated for 24 hours at 37°C, and then quantified under a 365-nm ultraviolet light. E. coli uses the enzyme called β-glucuronidase to metabolize β-D-glucoronide leaving 4-methyl-umbelliferyl (MUG) to glow under ultraviolet light. Data collection using this assay began in August 2001.
Statistical analyses
Statistical analyses of data were done in collaboration with Dr. Melchor Ortiz, Professor of Biometry at the U.T. Houston School of Public Health using SAS 6.12 analysis software. All-Possible regressions using Stepwise regression and r2 procedures and the Pearson Product-moment correlation procedures were used to analyze data on bacterial indicators, H. pylori, chemical toxicity and physical/chemical parameter. The Pearson Product-Moment correlation coefficient measures the strength of the linear relationship between two variables. For response variables X and Y, it is denoted as r and computed as shown to the right. If there is an exact linear relationship between two variables, the correlation is 1 or -1, depending on whether the variables are positively or negatively related. If there is no linear relationship, the correlation tends toward zero.
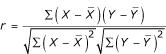
Authors' contributions
JM participated in the design of experiments and worked on sample collection, fecal coliforms, chemical toxicity assays, and statistical analyses.
JB developed and supervised chemical toxicity assays.
JH worked on H. pylori assay.
AM worked on sample collection, fecal coliforms, and chemical toxicity assays.
RS participated in the design of the study and worked on fecal coliforms.
AV worked on sample collection, fecal coliforms, and chemical toxicity assays.
AV participated in the design of the study and supervised sampling and fecal coliform enumeration.
MA conceived the idea for this study and participated in all aspects of its design, implementation and coordination.
All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This study was supported in part by the MBRS-RISE Grant (Number-R25 GM60424-01) to El Paso Community College, The Paso Del Norte Health Foundation's Center for Border Health Research, and The International Boundary and Water Commission's Clean Rivers Program-U.S. Section
Contributor Information
Jose Mendoza, Email: joseme@epcc.edu.
James Botsford, Email: jbotsfor@nmsu.edu.
Jose Hernandez, Email: jhernandez2@utep.edu.
Anna Montoya, Email: amonto33@cp.epcc.edu.
Roswitha Saenz, Email: roswitha@epcc.edu.
Adrian Valles, Email: adrian_valles@yahoo.com.
Alejandro Vazquez, Email: alejandv@epcc.edu.
Maria Alvarez, Email: mariaa@epcc.edu.
References
- International Boundary and Water Commission Second phase of the binational study regarding the presence of toxic substances in the Rio Grande/Rio Bravo and its tributaries along the boundary portion between the United States and Mexico Final Report, United States and Mexico. 1998.
- Texas Natural Resource Conservation Commission, Watershed Management Division Regional assessment of water quality in the Rio Grande Basin Austin, TX. 1994.
- Singh A. Detection methods for waterborne pathogens. In: Mitchell R, editor. In Environmental Microbiology. New York: A John Wiley & Sons, Inc; 1992. pp. 125–156. [Google Scholar]
- Craun GF, Berger PS, Calderon RL. Coliform bacteria and waterborne disease outbreaks. Journal of the American Water Works Association. 1997;89:96–104. [Google Scholar]
- International Boundary and Water Commission Second phase of the binational study regarding the presence of toxic substances in the Rio Grande/Rio Bravo and its tributaries along the boundary portion between the United States and Mexico Final Report United States and Mexico. 1997.
- Owen RJ. Bacteriology of Helicobacter pylori. Baillieres Clin Gastroenterol. 1995;9:415–46. doi: 10.1016/0950-3528(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Peura DA. The report of the international update conference on Helicobacter pylori. Digestive Disease Week Washington, DC. May 14, 1997.
- Megraud F. Transmission of Helicobacter pylori: fecal-oral versus oral-oral route. Aliment Pharmacol Ther. 1995;9:85–91. [PubMed] [Google Scholar]
- Hulten K, Han SW, Enroth H, Klein PD, Opejun AR, Gilman RH, Evans DG, Engstrand L, Graham DY, El-Zaatari FA. Helicobacter pylori in the drinking water in Peru. Gastroenterology. 1996;110:1031–5. doi: 10.1053/gast.1996.v110.pm8612990. [DOI] [PubMed] [Google Scholar]
- Redlinger T, O'Rourke K, Goodman KJ. Age distribution of Helicobacter pylori seroprevalence among young children in a United States/Mexico border community: evidence for transitory infection. Am J Epidemiol. 1999;150:225–230. doi: 10.1093/oxfordjournals.aje.a009991. [DOI] [PubMed] [Google Scholar]
- Rozak DB, Colwell RR. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahamat M, u Mai, Paszko-Kova C, Sessel M, Colwell RR. Use of autoradiography to assess viability of Helicobacter pylori in water. Appl Environ Microbiol. 1993;59:1231–1235. doi: 10.1128/aem.59.4.1231-1235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez ME, Aguilar M, Fountain A, Gonzalez N, Rascon O, Saenz D. Inactivation of MS2 phage and poliovirus in groundwater. Can J Microbiol. 2000;46:159–165. doi: 10.1139/cjm-46-2-159. [DOI] [PubMed] [Google Scholar]
- Botsford JL. A simple assay for toxic chemicals using a bacterial indicator. World Journal of Microbiology and Biotechnology. 1998;14:369–376. doi: 10.1023/A:1008813211422. [DOI] [Google Scholar]
- American Public Health Association . Standard Methods for the Examination of Water and Wastewater Washington DC. 19 1995. [Google Scholar]
- Elmund , Keith G, Allen MJ, Rice EW. Comparison of Escherichia coli, total coliform, and fecal coliform populations as indicators of wastewater treatment efficiency. Water Environmental Research. 1999;71:332–339. [Google Scholar]


