Abstract
Aim:
To study the influence of β-receptor activation on sodium channel current and the physiological significance of increased sodium current with regard to the increased cardiac output caused by sympathetic excitation.
Methods:
Multiple experimental approaches, including ECG, action potential recording with conventional microelectrodes, whole-cell current measurements, single-channel recordings, and pumping-force measurements, were applied to guinea pig hearts and isolated ventricular myocytes.
Results:
Isoprenaline was found to dose-dependently shorten QRS waves, increase the amplitude and the Vmax of action potentials, augment the fast sodium current, and increase the occurrence frequencies and open time constants of the long-open and burst modes of the sodium channel. Increased levels of membrane-permeable cAMP have similar effects. In the presence of a calcium channel blocker, TTX reversed the increased pumping force produced by isoprenaline.
Conclusion:
Beta-adrenergic modulation increases the inward sodium current and accelerates the conduction velocity within the ventricles by changing the sodium channel modes, which might both be conducive to the synchronous contraction of the heart and enhance its pumping function.
Keywords: sodium-channel current, isoprenaline, cAMP, single channel recording, action potential, ECG, pumping strength
Introduction
In the heart, most ion channels, including calcium channels and potassium channels, are regulated by isoprenaline. However, the effects of isoprenaline on sodium-channel currents have not been resolved completely. Some studies have shown that sodium channels can be modulated via multiple mechanisms, including both direct and indirect pathways1, 2, 3, 4, 5. The direct mechanism of regulation, which is protein kinase A (PKA)-independent, increases sodium current without alternation of the single-channel characteristics and without shifting the activation of the voltage-dependent current. This effect of beta stimulation on sodium channels is performed by changing the number of functional sodium channels in the cell membrane6. The indirect mechanism, which is mediated by the cAMP-dependent PKA pathway, modulates sodium channels by phosphorylation within the interdomain I–II of the channel protein5, 7, 8. This may result in activation or inhibition of the sodium current, depending on the protocols used2, 3, 9, 10, 11, 12. A shift in the inactivation membrane-voltage relationship to more negative potentials2 is observed in multi-cellular preparations and in isolated myocytes. In sum, the influence of adrenergic stimulation on the cardiac sodium-channel current is uncertain and needs to be further explored.
In our previous work13, 14, we confirmed the work done by Yuri et al15, who showed that the sodium-channel currents can be separated into two components: the early fast sodium current and the late sodium current. The early sodium current occurs immediately after depolarization, which produces phase 0 of an action potential; the current appearing 20 ms later, after the step depolarization, is arbitrarily defined as the late sodium current here. According to our experiments13, late sodium currents can be further classified into four modes: (1) an isolated brief opening with an open time as short as that of the early sodium-channel current; (2) scattered openings, which occur more frequently than the first mode; (3) a long open mode lasting more than 10 ms; and (4) a burst mode that lasts for a very long period, which is sometimes sustained from the beginning to the end of the depolarized clamping13. It is not known if the modes can be regulated by the autonomic nervous system.
The hypotheses of this study are based on our preliminary experiments. We found that isoprenaline could increase the amplitude of the action potential (APA) of the ventricular muscle, even if the preparation was treated with the calcium channel blocker verapamil. As APAs mainly depend on the inward Na+ current, if sympathetic activation does increase the inward Na+ current, there should be changes visible on ECG, in the whole cell Na+ current and in single Na+ channel dynamics. The increased Na+ current would cause faster impulse conduction within the atria and ventricles, which could influence the synchronous pumping action of the heart.
The purpose of this study is to determine 1) whether the activation of β-receptors can influence the sodium current via four experimental approaches (ECG, recording of the action potentials of cardiac myocytes, patch clamp recording of whole cell currents, and recording of single sodium-channel currents), 2) whether cAMP mediates the regulation, and 3) whether there is any physiological significance for the increased sodium current hearts excited by β-receptor activation.
Materials and methods
All animal experiments were approved by the Animal Care and Use Committee at Shanghai Jiaotong University. The investigation conformed to the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (NIH Publication No 85–23, revised 1996).
Recording of ECG in vitro
Guinea pigs of either sex (weighing approximately 350 g) were anesthetized by intraperitoneal injection of urethane (20%, 5 mL/kg). The heart was removed and perfused through the aorta in a Langendorff set-up with Tyrode's solution [in mmol/L: NaCl, 137; NaHCO3, 23; MgCl2, 0.5; KCl, 5.4; CaCl2, 1.8; NaH2PO4, 0.4; glucose, 10 (pH 7.4±0.05, 37±0.5 °C, with 95% O2 and 5% CO2)] at a pressure of 76 cmH2O. Three silver electrodes were placed on the surface of the epicardium to record the epicardial ECG. The positive, negative and ground electrodes were positioned on the apex, the right atrium, and the root of the aorta, respectively. In observing the effects of isoprenaline on the ECG, isoprenaline at different concentrations (from 1×10−7 mol/L to 1×10−5 mol/L) was consecutively added into the perfusion solution. The purpose of this protocol was to see whether isoprenaline could increase the conduction velocity within the ventricles as measured by a shortened QRS wave. The signals were monitored and stored in a computer through a bioamplifier and interface (PowerLab ML135, ML 845, ADInstruments, Australia NSW). The sample rate was 10 kHz, with high- and low-pass filtrations set at 1 kHz and 0.01 Hz. The data were measured by the software suite Chart 5 for Windows (ADInstruments).
Recording of heart pumping force
Isolated hearts were prepared in the same manner as for in vitro ECG. The left ventricular apex was gently pinched by a tiny metal clip that was connected by a thread to a force transducer (PowerLab MLT050/D, ADInstruments), and the heart contraction force was measured in grams. The stimulation electrodes were placed on the surface of the right atrium as a pacing point. The heart was driven to a rate of 300 beats/min (5 Hz) with a stimulator (SEN-7103, NIHON KOHDEN, Japan). The contraction force was monitored and stored in a computer through the force transducer interface.
Preparation of cardiac papillary muscle of guinea pig and the recording of transmembrane action potentials
Guinea pigs of either sex (weighing approximately 250−300 g) were sacrificed by venesection under deep anesthesia with injection of urethane (20%, 5 mL/kg). The hearts were rapidly removed into a dissection chamber submerged in Tyrode's solution. The right ventricular papillary muscles were excised and transferred to a recording chamber perfused with Tyrode's solution at a constant rate of 3 mL/min. The preparation was pinned to the bottom of the chamber. The chamber solution was maintained at a temperature of 36±0.5 °C and gassed with 95% O2 and 5% CO2. Bipolar platinum electrodes were applied to drive the preparations with rectangular current pulses at a frequency of 1 Hz. The duration of each pulse was 0.1 ms with an amplitude about 1.5 times the threshold current. After a 30 min perfusion for recovery, transmembrane action potentials were recorded with conventional glass microelectrodes filled with 3 mol/L KCl and with a tip resistance of 15 to 20 MΩ. The signals were sampled at a rate of 100 kHz and stored in the computer through the amplifier (MEZ8201, Nihon Kohden, Japan, with a high frequency filter of 20 kHz) and PowerLab interface (PowerLab ML845, ADInstruments). The parameters of the action potential that were measured were the resting potential (RP), the action potential amplitude (APA), the maximum upstroke velocity of phase 0 (dV/dtmax, Vmax), and the action potential durations at 50% (APD50) and 90% (APD90) of repolarization. Verapamil (4×10−7 mol/L) was used to block the calcium channels in the action potential recording. Isoprenaline or cAMP was later added into the perfusion system according to the experimental protocols.
Enzymatic isolation of ventricular myocytes and patch-clamp recording of Na+ channel current
The hearts of anesthetized guinea pigs (weighing 250–300 g) were taken out and perfused consecutively with calcium-free and collagenase-containing Tyrode's solution at a constant hydrostatic pressure of 76 cmH2O for about 10 min (Langendorff perfusion)13. The Tyrode's solution contained (in mmol/L): NaCl, 138; NaOH, 2.1; MgCl2, 1; KCl, 4.6; CaCl2, 1.8; glucose, 10; and HEPES, 5; pH 7.4±0.05; saturated with 100% oxygen. After that, the heart was moved to and kept in a Ca2+-free solution for 2 h for slow digestion of the connective tissue of the heart. The ventricles were then cut into pieces and shaken in K-B solution (in mmol/L: KCl, 85; MgSO4, 5; creatine, 5; K2HPO4, 30; sodium pyruvate, 5; taurine, 20; HEPES, 5; Na2-ATP, 2; EGTA, 0.5; glucose, 20; pH 7.4±0.05; saturated with 100% oxygen). The calcium-tolerant ventricular myocytes were thus obtained.
A patch clamp was used to record the whole-cell and single-channel currents. For whole-cell Na+ current recording, the pipette solution contained (in mmol/L): KCl, 120; CaCl2, 0.1; MgCl2, 2; EGTA, 1.1; and HEPES, 10; and the bathing solution was the Tyrode's solution. The single Na+ channel current was recorded in the cell-attached configuration. The pipette was filled with solution containing (in mmol/L): NaCl, 180; KCl, 1.3; MgCl2, 0.5; CaCl2, 1.5; glucose, 5; Hepes, 5; CoCl2, 3; TEA, 10; 4-AP, 10; and CsCl, 10. Since potassium and calcium channels are blocked by the TEA, 4-AP, CoCl2 and CsCl in the pipette solution16, the only channel that allowed an inward current to pass through it was the Na+ channel. The perfusion solution contained (in mmol/L): KCl, 120; MgCl2, 2; CaCl2, 0.1; HEPES, 10; and EGTA, 0.1; pH 7.4. Different concentrations of isoprenaline (SIGMA, USA) or membrane-permeable adenosine 3′,5′-cyclic monophosphate (8-bromo-cAMP, Sigma) were added into the perfusion solution according to the protocols. An Oscilloscope (DSS6521, Kikusui, Japan) and Axopatch-1-D amplifier (Axon Instruments, Foster City, CA, USA) were used to monitor and record the clamped voltage and channel currents. The data were sampled with the Clampex 9.0 software suite (Axon Instruments) and stored in the computer through the interface (Digidata 1320, Axon Instruments). The sample rate was 100 kHz with a filtering frequency of 10 kHz.
Data analysis
Chart 5 (ADInstruments) software, Clampex 9.0, and Origin 6 (Microcal Software, Inc, USA) were used for measurement and statistical analysis, including the open time constants of the sodium channel17. For measuring the open time constant of the sodium channel, all of the openings were grouped into histograms of different open durations by Clampex9. The first-order exponential decay equation was fit to the histogram, and the open time constants of the sodium channel were obtained. All of the results are expressed as mean±SEM. Statistical significance was determined by using the Student's t-test for paired data. P<0.05 was considered statistically significant.
Results
Isoprenaline reduces the duration of the QRS wave
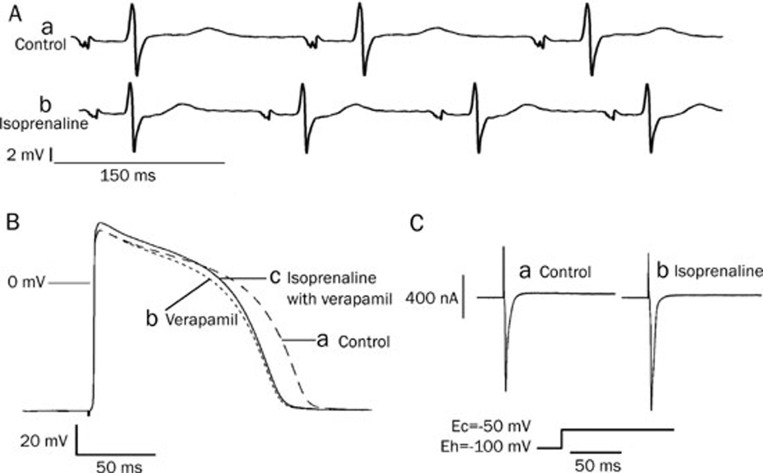
In order to avoid interference from neurohumoral regulation, ECGs were recorded in Langendorff-perfused hearts of guinea pigs. Figure 1A shows that isoprenaline not only increased the heart rate, but also shortened the duration of the QRS wave. The increased heart rate and the reduced values of the QRS wave are listed in Table 1 along with the different concentrations of isoprenaline. Isoprenaline dose-dependently decreased the durations of the QRS wave. At a concentration of isoprenaline of 1×10−6 mol/L, the duration of the QRS wave was shortened by 7.6% in eight experiments. The shortening of the QRS wave suggests it may be caused by an increased sodium current, which would accelerate the conduction velocity within the ventricles.
Figure 1.
The effect of isoprenaline on ECG, action potentials and the sodium current. (A) Isoprenaline increased the heart rate and shortened the QRS wave: a) Control ECG; b) isoprenaline (1×10−6 mol/L) shortened QRS waves. (B) Isoprenaline increased the amplitude of the action potentials after the calcium channels had been blocked by verapamil in guinea pig papillary muscle: a) Control; b) under verapamil (4×10−7 mol/L) treatment the action potential duration (APD) was shortened significantly; c) Isoprenaline (1×10−7 mol/L) increased the amplitude of the action potential after the cardiac myocyte was perfused with isoprenaline plus verapamil (4×10−7 mol/L). (C) Isoprenaline increased the early sodium current: a) before isoprenaline. b) isoprenaline (1×10−7 mol/L).
Table 1. Isoprenaline (ISO) shortens the duration of QRS wave of ECG in vitro. n=8. Values are given as mean±SEM. bP<0.05, cP<0.01 vs Control.
| Heart rate (per min) | Duration of QRS complex waves (ms) | |
|---|---|---|
| Control | 313±3 | 34.3±0.9 |
| ISO 1×10−7 mol/L | 320±4 | 33.6±0.6 |
| ISO 1×10−6 mol/L | 336±6c | 31.7±0.7b |
| ISO 1×10−5 mol/L | 350±4c | 30.1±0.3b |
Isoprenaline accelerates the depolarization rate of the action potential in ventricular myocytes
Stable action potentials were recorded from ventricular myocytes, and 30 min later, verapamil (4×10−7 mol/L) was added into the perfusion Tyrode's solution. As verapamil blocked the Ca2+ channels, it shortened the APD50 and APD90 (Figure 1Bb). Next, isoprenaline (1×10−7 mol/L) was added into the perfusion solution. Though there were no substantial changes in the resting potential (RP), the action potential duration was lengthened and the amplitude of the action potentials (APA) as well as the maximum upstroke velocity of phase 0 (Vmax) were increased significantly (Figure 1Bc). Table 2 lists the parameters that were measured from the action potentials when the ventricular myocytes were consecutively exposed to Tyrode's solution, verapamil, verapamil plus isoprenaline, and then a verapamil solution used to wash out the isoprenaline.
Table 2. The electrophysiological effects of isoprenaline (ISO) on the cardiac action potential. n=6. Values are given as mean±SEM. bP<0.05 vs control. eP<0.05 vs verapamil (Vera).
| APA (mV) | Vmax (V/s) | RP (mV) | APD50 (ms) | APD90 (ms) | |
|---|---|---|---|---|---|
| Control | 121.3±2.9 | 219.0±4.5 | 91.8±1.5 | 104.3±3.5 | 125.5±3.7 |
| Vera 4×10−7 mol/L | 120.2±2.4 | 218.6±5.7 | 91.2±1.6 | 89.6±3.5b | 108.7±3.0b |
| Vera 4×10−7 mol/L+ISO 1×10−7 mol/L | 125.6±0.8be | 233.1±5.4be | 92.2±1.6 | 99.0±3.1b | 119.1±2.9b |
| Wash out ISO with Vera 4×10−7 mol/L | 121.7±3.1 | 225.6±5.7 | 92.1±1.3 | 94.8±2.1 | 111.8±3.5 |
The effects of isoprenaline on sodium-channel current
Whole-cell current recording
In a whole-cell recording configuration, the isolated ventricular myocyte was perfused with verapamil (4×10−7 mol/L)-containing Tyrode's solution. The cell was depolarized from the holding potential (-100 mV) to a command potential (-50 mV) for 100 ms before the clamping voltage was decreased to the holding potential. A large inward current was immediately evoked by the depolarization (Figure 1Ca). The calcium channels were blocked by verapamil and the inward current could be blocked by TTX (1×10−5 mol/L) (data not shown); this large inward current was likely the fast sodium current. After the preparation was exposed to isoprenaline (1×10−7 mol/L), the amplitude of the inward current was increased substantially (Figure 1Cb). This inward current was considered to be the early sodium current. In all three experiments, isoprenaline (1×10−7 mol/L) increased the fast inward sodium current by about 7.1%.
Early sodium-channel current recording
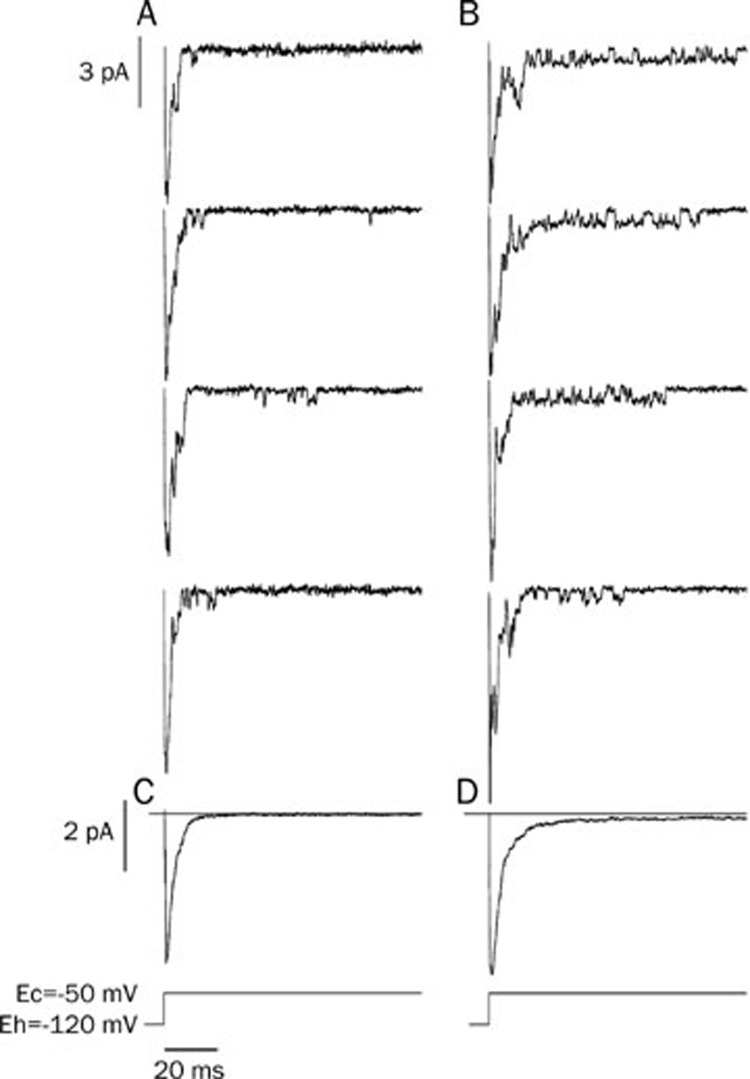
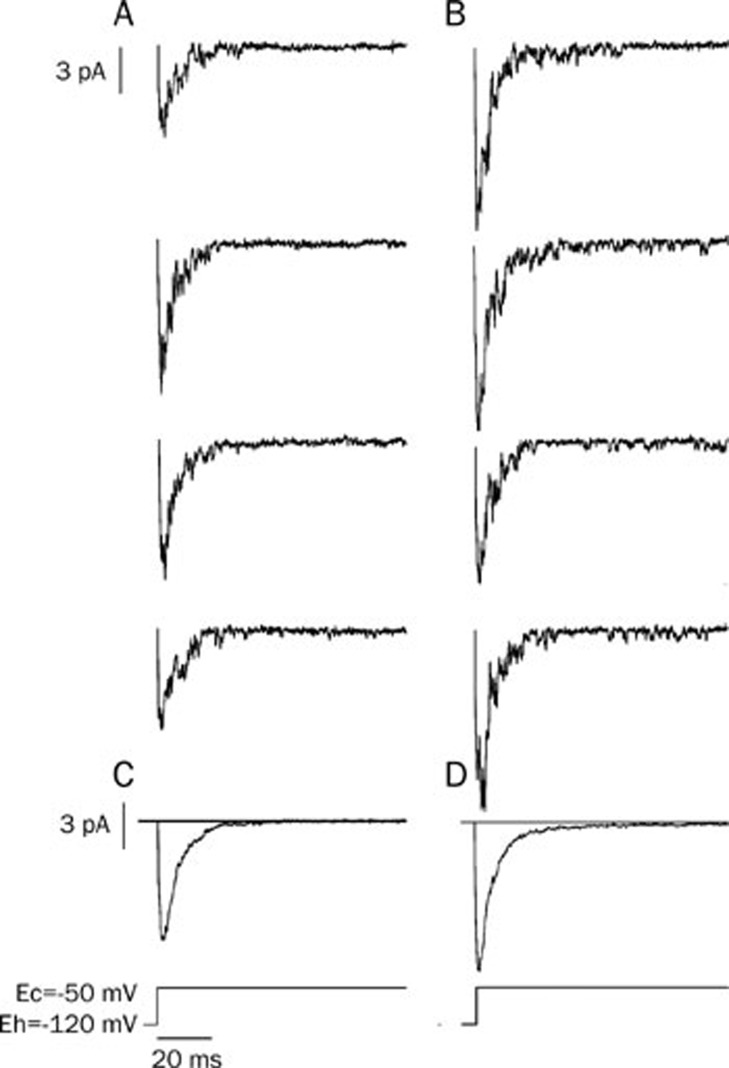
In order to record the early sodium-channel current, a cell-attached recording configuration was adopted. The membrane was first depolarized from -120 mV to -50 mV for 100 ms, and then was put back at the holding potential. The sodium-channel current was evoked by the depolarization and its conductance was measured as 19 pS (the conductance was measured by multiple depolarizations; data not shown). Most of the channels opened within the first 20 ms of depolarization, which was defined in this paper as the early sodium-channel current. After the preparation was exposed to isoprenaline (3×10−7 mol/L), the open time constant of the sodium channel was increased significantly compared with the control (Figure 2A, 2B and Table 3).
Figure 2.
Isoprenaline increased the sodium-channel current. Cell-attached recording was performed on isolated ventricular myocytes. The cells were held at -120 mV and depolarized to -50 mV to invoke the opening of Na+ channels. Isoprenaline increased the early sodium channel current. (A) Control; (B) Isoprenaline (3×10−7 mol/L); (C) The average of 49 sweeps from the control; (D) The average of 45 sweeps after isoprenaline.
Table 3. Isoprenaline and cAMP affect the occurrence frequency and open time constant of single sodium channel current. n=6. Data were shown as mean±SEM. bP<0.05 vs Ctrl.
| Early Na current | Late Na current | |||||
|---|---|---|---|---|---|---|
| Long | Burst | |||||
| |
T (ms) |
T (ms) |
OF (%) |
T (ms) |
OF (%) |
|
| Ctrl (n=7) | 0.3±0.1 | 4.0±0.6 | 0.47±0.11 | 1.4±0.3 | 0.04±0.02 | |
| ISO 3×10−7 mol/L | 1.0±0.2b | 6.8±0.5b | 0.71±0.17b | 2.0±0.4b | 0.12±0.05b | |
| Ctrl (n=7) | 0.3±0.2 | 4.1±0.4 | 0.37±0.13 | 1.3±0.2 | 0.06±0.02 | |
| cAMP 6×10−8 mol/L | 1.0±0.3b | 8.1±0.6b | 0.63±0.14b | 2.7±0.4b | 0.19±0.06b | |
T=Open time constant, OF=Occurrence frequency.
The late sodium-channel current recording
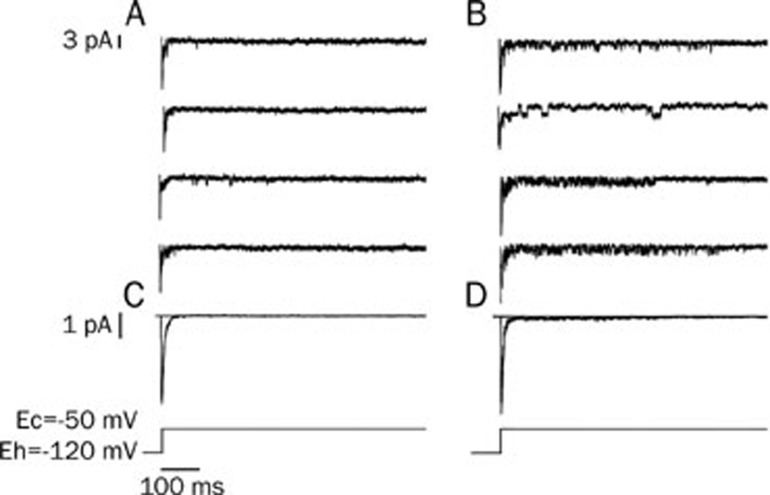
To investigate the influence of isoprenaline on open modes of the sodium channel, we prolonged the depolarizing duration from 100 ms to 700 ms when the cells were clamped from -120 mV to -50 mV. The perfusion solution, the pipette solution and the patch configuration were the same as described above. The inward channel current after the first 20 ms was designated as the late sodium-channel current. Different open modes of the late sodium-channel current such as a brief opening, scattered opening, long opening and burst opening were observed (Figure 3). Isoprenaline (3×10−7 mol/L) increased the occurrence frequencies of the burst and long-open modes (Figure 3B). The sweeps of each group (80 sweeps for control and 75 sweeps for isoprenaline group) were recorded and averaged, resulting in traces C and D (Figure 3C, 3D). Traces C and D were similar to the whole-cell sodium currents when the sodium channels were activated (Figure 1C). In trace D, isoprenaline not only increased the early sodium current, but also made it decay very slowly during the remaining long clamping time, which may indicate that isoprenaline augments the late sodium current.
Figure 3.
Isoprenaline can change the modes of the late sodium-channel current. The duration of depolarization was prolonged to 700 ms. (A) Control: brief opening of the late sodium-channel currents were observed once in a while in the late clamping time. (B) After adding isoprenaline (3×10−7 mol/L), the frequency of sodium channel opening was increased substantially. The open modes of the late sodium-channel current were identified as: brief opening, scattered opening, long opening and burst mode. The first three traces in group B show the burst mode of the sodium channel. (C and D) The averaged currents from group A and B. There was a small and slow-decaying inward current in trace D.
The time constant and occurrence frequency of the long-open and burst modes are influenced by isoprenaline
As each patch can contain different numbers of sodium channels, the following data were normalized for the calculation of single-channel properties. The occurrence frequencies and open time constants of the long-open and burst modes for a single sodium channel are presented in Table 3. The results show that in the control about 3000 depolarizations were needed before one burst mode was observed (0.04%±0.02%). Isoprenaline increased the occurrence frequency of burst modes by about three times (0.12%±0.05%). Isoprenaline also increased the occurrence frequency of long opening by 1.5 times (0.71%±0.17% vs 0.47%±0.11%) and prolonged the open time constants for the both modes (Table 3).
The sodium-channel current is regulated by cAMP
In the next set of experiments, we used membrane-permeable cAMP instead of isoprenaline and observed its effects on the action potential and the Na+ channel current.
cAMP accelerates the depolarization rate of the action potential in ventricular myocytes
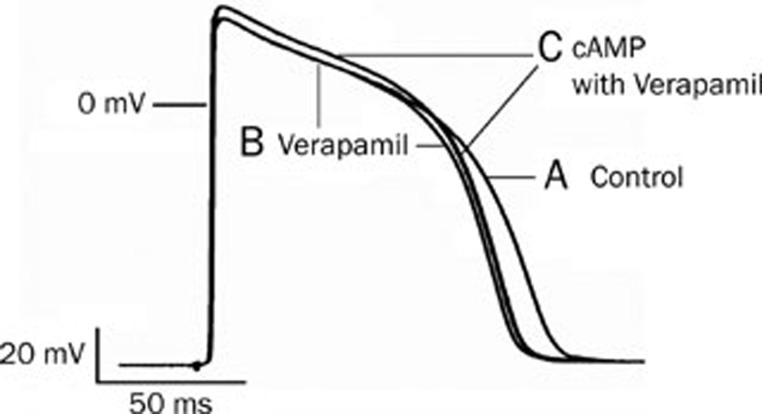
The methods and procedures for observing the effects of membrane-permeable cAMP on the cardiac action potentials were the same as in the experiments with isoprenaline. Verapamil (4×10−7 mol/L) and cAMP (6×10−8 mol/L) were consecutively added to the perfusion solution. It was found that after verapamil blocked the Ca2+ channels, cAMP, like isoprenaline, significantly increased the APA and the Vmax of the action potentials (Figure 4). In six experiments, cAMP (without substantially affecting the resting potential) prolonged the action potential duration (2.1%), increased the APA (from 119±3 to 127±2 mV) and increased the Vmax (from 211±4 to 226±5 V/s) by 6.7% and 7.2%, respectively.
Figure 4.
The effect of cAMP on the action potential in guinea pig ventricular myocytes. (A) Control; (B) Verapamil (4×10−7 mol/L) inhibited the calcium channel and shortened the APD; (C) cAMP (6×10−8 mol/L)+verapamil (4×10−7 mol/L) increased the APA significantly.
cAMP increased the early averaged sodium current
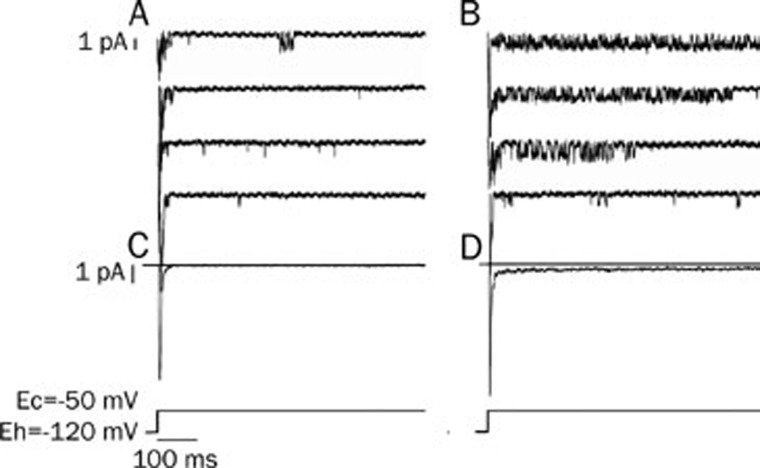
With the same protocol as in Figure 2, the isolated ventricular myocytes were perfused with a cAMP (6×10−8 mol/L)-containing solution in patch clamp experiments. The inward sodium-channel currents were increased substantially 16 min after the isolated cells were perfused with cAMP (Figure 5D).
Figure 5.
cAMP modulated the early sodium-channel current. With the same protocol as Figure 2, the isolated ventricular myocytes were perfused with cAMP at a concentration of 6×10−8 mol/L. cAMP increased the early sodium currents significantly. (A) control; (B) cAMP (6×10−8 mol/L) increased the inward currents. (C and D) The averaged currents from 42 (control) and 56 sweeps (cAMP).
cAMP can change the sodium channel modes
The occurrence frequencies of the long-open and burst modes of the sodium channel were increased from 3.7%±1.3% and 0.6%±0.2% to 6.3%±1.4% and 1.9%±0.6%, respectively by cAMP (Figure 6 and Table 3). Similarly to the experiments with isoprenaline, the open time constant for the long-open mode of the sodium channel was prolonged from 4.1±0.4 ms to 8.1±0.6 ms when perfused with cAMP. For the burst mode, cAMP changed the open time constant from 1.3±0.2 ms to 2.7±0.4 ms (Table 3). Therefore, the averaged current curve of the sodium channel after the addition of cAMP was different from that of the control, especially during the late clamping time (Figure 6C and 6D).
Figure 6.
cAMP modulated the opening of the late sodium-channel current. (A) Control; (B) cAMP (6×10−8 mol/L) increased the occurrence frequency of open modes of the sodium channel. The first sweep shows the scattered open mode; the second, long openings; the other 2, burst modes. C and D: the averaged currents from 57 traces (control) and 62 traces (cAMP).
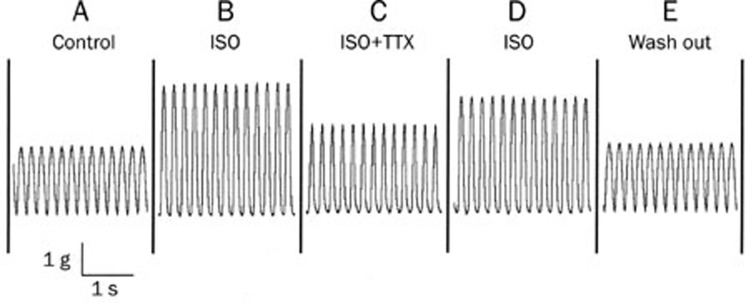
TTX decreased the isoprenaline-enhanced contraction force of the heart
Isolated guinea pig hearts were first perfused with Tyrode's solution in the Langendorff set-up and then electrically paced by a stimulator at a rate of 5 Hz. The contraction force was recorded and measured through a transducer as shown in Figure 7A. After the heart was perfused with isoprenaline-containing (1×10−7 mol/L) Tyrode's solution, the contraction force was increased dramatically (Figure 7B). Then TTX (1×10−6 mol/L) was added to the above isoprenaline-containing solution, and the contraction strength was diminished (Figure 7C). This effect of TTX was reversed after the TTX was washed out by isoprenaline-containing solution (Figure 7D). Finally, the isoprenaline was washed out of the perfused heart with control Tyrode's solution (Figure 7E). Table 4 shows the results from five experiments.
Figure 7.
TTX decreased the isoprenaline-strengthened contraction force of the heart. An isolated guinea pig heart was electrically stimulated at the right atrium at a rate of 5 Hz. Rhythmic contraction traces of the heart in various perfusion solutions are presented. (A) Control in the Tyrode's solution; (B) in the presence of isoprenaline (ISO) 1×10−7 mol/L; (C) ISO 1×10−7 mol/L+TTX 1×10−6 mol/L; (D) ISO 1×10−7 mol/L without TTX; (E) washout with the control Tyrode's solution.
Table 4. Effect of TTX on contraction force of isolated heart in the presence of isoprenaline. n=5. bP<0.05 vs control. eP<0.05 vs ISO. hP<0.05 vs ISO with TTX.
| Perfusion solution | Normalized value of contraction force |
|---|---|
| Control | 1±0 |
| TTX | 0.9±0.1 |
| ISO | 2.0±0.3b |
| ISO+TTX | 1.4±0.2be |
| ISO without TTX | 1.9±0.3bh |
| Wash out with Tyrode's solution | 0.9±0.1 |
ISO=isoprenaline 1×10−7 mol/L. TTX=Tetrodotoxin 1×10−6 mol/L.
Discussion
Changes in sodium channel opening modes may be responsible for the β-receptor activation increase in the sodium current
The data from ECG recordings showed that isoprenaline dose-dependently shortened the duration of the QRS wave. These results coincided with those of De Boer et al18. Namely, beta-adrenergic stimulation, and not alpha-adrenergic stimulation, enhances conduction velocity in cultures of neonatal cardiac myocytes. It is known that the QRS wave represents the time for the propagation of excitation across the cardiac ventricular muscles. The propagation velocity depends on the amplitude of the action potential (APA), the upstroke velocity of phase 0 (Vmax) and the dynamics of the sodium channels. Our results demonstrated that isoprenaline increased the APA and Vmax of the action potentials, which explains the shortening of the QRS wave on ECG. It should be acknowledged, however, that a shortened QRS duration may also involve increased cell-to-cell coupling, which has been shown to change with beta-adrenergic stimulation19, 20.
As the sodium-channel current is mainly responsible for the formation of the QRS wave and the action potentials, the shortening of QRS duration and the increases in APA and Vmax both suggested that isoprenaline might increase the sodium-channel current. The whole-cell recording shown in Figure 1C demonstrated that isoprenaline did increase the fast inward current, while in patch clamp experiments isoprenaline not only increased the averaged sodium-channel current but also augmented the late sodium-channel currents.
When a long-lasting depolarization pulse was applied to the sodium channels, four modes of late sodium-channel current could be identified (Figure 2B). The long-open and burst modes reflect the dynamic changes of sodium channel. In our experiments (Table 3), not only the occurrence frequencies but also the open time constants of both the long-open and burst modes were increased by isoprenaline. This causes changes in the average sodium current. Therefore, the increased and lengthened slow component of the sodium current (Figure 3D) could account for the prolongation of the APD produced by isoprenaline13. This result suggests that all of the changes produced by isoprenaline including the QRS wave of the ECG, and the amplitude and the Vmax of the action potentials, are to some extent due to the increased sodium inward current. As the single channel current is the basic component of the electrophysiological activity in the cell, one of the mechanisms for β-receptor-activation increases in the sodium current could be its changing of the sodium channel modes.
cAMP mediates the increase of sodium-channel current induced by isoprenaline
When cAMP was applied to the isolated ventricular myocytes, the amplitude and Vmax of the action potentials were increased by 6.7% and 7.2%, respectively. Like isoprenaline, cAMP also increased the averaged single sodium-channel current and positively influenced the occurrence frequencies and open time constants of the long-open and burst modes of the late sodium-channel current. In their experiment on adult rat cardiac myocytes, Palygin et al21 indicated that the stimulatory G protein subunit could directly modulate the cardiac sodium channel. This mechanism is independent of PKA, and leads to an increase in the number of functional channels. Their later study in 2008 demonstrated that Gs-alpha H41 was a critical residue in the regulation of the increase in I(Na) in ventricular myocytes. Our results do not exclude the direct effect of the Gs protein on the sodium current, but indicate that cAMP might also play a role in the increase in sodium-channel current when β-receptors are activated by isoprenaline.
The possible physiological effects of the increased sodium current evoked by sympathetic excitation
It is well-known that sympathetic stimulation has a positive inotropic effect on cardiac muscle by increasing the calcium current. In vivo sympathetic excitation not only increases the contraction strength, but also has a positive dromotropic effect on A–V conduction. This effect, through its increment of the amplitude of slow response action potentials in the A–V node, makes it easier for the action potential to excite each succeeding portion of the conducting fiber, thereby decreasing the conduction time from the atria to the ventricles. However, until now, there has been no report that sympathetic excitation can increase the fast sodium current. This current is responsible for the conduction of action potentials within the cardiac working muscle, especially the ventricles.
It is possible that the faster conduction of an impulse within the ventricles that is initiated by sympathetic excitation could cause ventricular muscle to contract more synchronously. It is known that the total time for the transmission of the cardiac impulse from the initial bundle branches to the last of the ventricular muscle fibers in the normal heart is about 30 ms in human beings. If sympathetic excitation does increase the sodium current, it would make the conduction of the action potential within the atria and ventricles faster, resulting in more-profitable synchronous contraction and enhancing the pumping function of the heart. Our results show that β-activation shortens the duration of the QRS wave, which means that the conduction velocity within the ventricles is increased. This faster conduction might be conducive to the synchronous contraction of the ventricles. Furthermore, the application of TTX to block the increased Na+ current in the isoprenaline-stimulated heart decreases the contraction force (Figure 7, Table 4). This result may help to uncover the physiological significance of the increased Na+ current in fulfilling the synchronous contraction of the heart during excitement by β-receptor activation.
Author contribution
Ci-zhen LI and Yuan-mou LIU designed research; Ci-zhen LI, Hong-wei WANG, and Yin ZHANG performed research; Zhi-fang YANG and Jian-min YANG contributed new analytical tools and reagents; Zhi-fang YANG, Hong-wei WANG analyzed data; Hong-wei WANG and Yuan-mou LIU wrote the paper.
Acknowledgments
This work was supported by the Science Foundation of the Shanghai Education Committee, No 05BZ12 and 2005JY01 and the Shanghai Science Committee Foundation No 06JC14045.
We would like to express our thanks to Professor Louise J DeFELICE (Dept of Pharmacology, Vanderbilt University, USA) for consultation.
References
- Matsuda JJ, Lee H, Shibata EF. Enhancement of rabbit cardiac sodium channels by β-adrenergic stimulation. Circ Res. 1992;70:199–207. doi: 10.1161/01.res.70.1.199. [DOI] [PubMed] [Google Scholar]
- Ono K, Fozzard HA, Hanck DA. Mechanism of cAMP-dependent modulation of cardiac sodium channel current kinetics. Circ Res. 1993;72:807–15. doi: 10.1161/01.res.72.4.807. [DOI] [PubMed] [Google Scholar]
- Schreibmayer W, Frohnwieser B, Dascal N, Platzer D, Spreitzer B, Zechner R, et al. β-Adrenergic modulation of currents produced by rat cardiac Na+ channels expressed in Xenopus laevis oocytes. Receptors Channels. 1994;2:339–50. [PubMed] [Google Scholar]
- Kirstein M, Eickhorn R, Kochsiek K, Langenfeld H. Dose-dependent alteration of rat cardiac sodium current by isoproterenol: results from direct measurements on multicellular preparations. Pflügers Arch. 1996;431:395–401. doi: 10.1007/BF02207277. [DOI] [PubMed] [Google Scholar]
- Murphy BJ, Rogers J, Perdichizzi AP, Colvin AA, Catterall WA. cAMP-dependent phosphorylation of two sites in the subunit of the cardiac sodium channel. J Biol Chem. 1996;271:28837–43. doi: 10.1074/jbc.271.46.28837. [DOI] [PubMed] [Google Scholar]
- Lu T, Lee HC, Kabat JA, Shibata EF. Modulation of rat cardiac sodium channel by the stimulatory G protein subunit. J Physiol. 1999;518:371–84. doi: 10.1111/j.1469-7793.1999.0371p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnweiser B, Chen LQ, Schreibmayer W, Kallen RG. Modulation of the human cardiac sodium channelα-subunit by cAMP-dependent protein kinase and the responsible sequence domain. J Physiol (Lond) 1997;498:309–18. doi: 10.1113/jphysiol.1997.sp021859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunami A, Fan Z, Nakamura F, Naka M, Tanaka T, Sawanobori T, et al. The catalytic subunit of cyclic AMP-dependent protein kinase directly inhibits sodium channel activities in guinea-pig ventricular myocytes. Pflügers Arch. 1991;419:415–7. doi: 10.1007/BF00371125. [DOI] [PubMed] [Google Scholar]
- Fozzard HA, Hanck DA. Structure and function of voltage-dependent sodium channels: comparison of brain II and cardiac isoforms. Physiol Rev. 1996;76:887–900. doi: 10.1152/physrev.1996.76.3.887. [DOI] [PubMed] [Google Scholar]
- Schubert B, VanDongen AMJ, Kirsch GE, Brown AM. Inhibition of cardiac Na+ currents by isoproterenol. Science. 1989;245:516–9. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Gintant GA, Liu DW. β-Adrenergic modulation of fast inward sodium current in canine myocardium: syncytial preparations versus isolated myocytes. Circ Res. 1992;42:844–50. doi: 10.1161/01.res.70.4.844. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yi J, Hu N, George AL, Murray KT. Activation of protein kinase A modulates trafficking of the human cardiac sodium channel in Xenopus oocytes. Circ Res. 2000;87:33–8. doi: 10.1161/01.res.87.1.33. [DOI] [PubMed] [Google Scholar]
- Li CZ, Huang HW, Liu YM. Four types of late Na channel current in isolated ventricular myocytes and their contribution to the lastingness of action potential plateau. Acta Physiol Sin. 1997;49:241–8. [PubMed] [Google Scholar]
- Liu YM, DeFelice LJ, Mazzanti M. Na channels that remain open throughout the cardiac action potential plateau. Biophys J. 1992;83:654–62. doi: 10.1016/S0006-3495(92)81635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter YuI, Starmer CF, Starobin J, Grant AO. Late Na channels in cardiac cells: the physiological role of background Na channels. Biophys J. 1994;67:153–60. doi: 10.1016/S0006-3495(94)80464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YM, DeFelice LJ. Na channels that remain open throughout the cardiac action potential plateau. Biophys J. 1992;63:654–62. doi: 10.1016/S0006-3495(92)81635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CZ, Wang HW, Liu JL, Liu K, Yang ZF, Liu YM. Effect of ATX II on opening modes of myocyte sodium channel, action potential and QT intervals of ECG. Acta Physiol Sin. 2001;53:111–6. [PubMed] [Google Scholar]
- De Boer TP, van Rijen HV, Van der Heyden MA, Kok B, Opthof T, Vos MA, et al. Beta-, not alpha-adrenergic stimulation enhances conduction velocity in cultures of neonatal cardiomyocytes. Circ J. 2007;71:973–81. doi: 10.1253/circj.71.973. [DOI] [PubMed] [Google Scholar]
- Lowenstein WR. Regulation of cell-to-cell communication by phosphorylation. Biochem Soc Symp. 1985;50:43–58. [PubMed] [Google Scholar]
- Arnar DO, Van Why KJ, Gleed K, Foreman B, Hopson JR, Lee HC, et al. Effect of beta-adrenergic stimulation on the QRS duration of the signal-averaged electrocardiogram. Am Heart J. 1997;134:395–8. doi: 10.1016/s0002-8703(97)70072-3. [DOI] [PubMed] [Google Scholar]
- Palygin OA, Pettus JM, Shibata EF. Regulation of caveolar cardiac sodium current by a single Gsalpha histidine residue. Am J Physiol Heart Circ Physiol. 2008;294:H1693–9. doi: 10.1152/ajpheart.01337.2007. [DOI] [PubMed] [Google Scholar]