Abstract
Background
Accumulated evidence has demonstrated that both oxidative stress and abnormal cytokine production, especially tumor necrosis factor-α (TNF), play important etiological roles in the pathogenesis of alcoholic liver disease (ALD). Agents that have both antioxidant and anti-inflammation properties, particularly anti-TNF production, represent promising therapeutic interventions for ALD. We investigated the effects and the possible mechanism(s) of silymarin on liver injury induced by acute ethanol (EtOH) administration.
Methods
Nine-week-old mice were divided into 4 groups, control, silymarin treatment, EtOH treatment, and silymarin/EtOH treatment, with 6 mice in each group. Because control and silymarin values were virtually identical, only control treatment is shown for ease of viewing. Ethanol-treated mice received EtOH [5 g/kg body weight (BW)] by gavage every 12 hours for a total of 3 doses. Control mice received an isocalorical maltose solution. In the silymarin/EtOH group, silymarin was dissolved in the EtOH and gavaged simultaneously with EtOH at a dose of 200 mg/kg BW. At 4 hours after the last dosing, the mice were anesthetized and subsequent serum alanine aminotransferase (ALT) level, hepatic lipid peroxidation, enzymatic activity of hepatic cytochrome P450 2E1, hepatic TNF-α, and glutathione (GSH) levels were measured. Histopathological change was assessed by hematoxylin and eosin staining.
Results
Acute EtOH administration caused prominent hepatic microvesicular steatosis with mild necrosis and an elevation of serum ALT activity, induced a significant decrease in hepatic GSH in conjunction with enhanced lipid peroxidation, and increased hepatic TNF production. Supplementation with a standardized silymarin attenuated these adverse changes induced by acute EtOH administration.
Conclusions
Silymarin protects against the liver injury caused by acute EtOH administration. In view of its nontoxic nature, it may be developed as an effective therapeutic agent for alcohol-induced liver disease by its antioxidative stress and anti-inflammatory features.
Keywords: Silymarin, Ethanol, Oxidative Stress, Liver Injury, Glutathione, CYP2E1, TNF
Although much progress has been made in understanding the pathogenesis of alcoholic liver disease (ALD), there remains no effective therapy for this disease. Accumulated evidence has demonstrated that both oxidative stress and abnormal cytokine production, especially tumor necrosis factor (TNF), play important etiological roles in the pathogenesis of ALD. Therefore, agents that have both antioxidant and anti-inflammatory properties, particularly anti-TNF production, represent promising therapeutic interventions for ALD.
There is increasing evidence that oxidative stress plays an important etiologic role in the development of ALD. Alcohol administration causes accumulation of reactive oxygen species (ROS), including superoxide, hydroxyl radical, and hydrogen peroxide (Nordmann et al., 1992). Reactive oxygen species, in turn, cause lipid peroxidation of cellular membranes and protein and DNA oxidation, which result in hepatocyte injury (Kurose et al., 1997; Navasumrit et al., 2000; Rouach et al., 1997). Reduced glutathione (GSH) is one of the most prominent defense molecules in the liver. Besides serving as a substrate for GSH-related enzymes, GSH acts as a free radical scavenger and a regenerator of α-tocopherol and plays an important role in the maintenance of protein sulfhydryl groups (Fernandez-Checa et al., 1998; Kurose et al., 1996). Several studies have reported that acute ethanol (EtOH) administration decreases hepatic GSH content (Masini et al., 1994; Shaw et al., 1983; Song et al., 2003). The decrease in GSH has been related to an enhanced oxidation of GSH to oxidized glutathione (GSSG) as a consequence of increased generation of ROS (Videla and Valenzuela, 1982). Restoration of GSH has been shown to inhibit EtOH-induced liver injury (Fermandez-Checa et al., 1995; Iimuro et al., 2000). It is conveivable that agents with a hepatic antioxidant property would be a potential therapy for ALD.
Abnormal cytokine metabolism, especially TNF, is another major feature of ALD (McClain and Cohen, 1989). Previous investigations showed that rats chronically fed alcohol had much higher lipopolysaccharide (LPS)-stimulated plasma levels of TNF than control rats, and liver injury could be attenuated by agents such as prostaglandin analog that down-regulated TNF production (Honchel et al., 1992). Moreover, acute EtOH administration to mice has been found to induce hepatic TNF-α production and mRNA expression and down-regulated TNF production by antioxidants was paralleled by attenuated hepatic lipid peroxidation, steatosis, and liver injury (Zhou et al., 2003).
Silymarin is a flavonoid extracted from the milk thistle Silibum marianum. It has been reported to prevent liver injuries induced by various chemicals or toxins including EtOH (Farghali et al., 2000; Halim et al., 1997; Lieber et al., 2003). Although the hepatoprotective properties of silymarin have been reported both from in vitro and in vivo studies, its mechanism of action still has not been established. Two major mechanisms that have been proposed include functioning as an antioxidative scavenger of free radicals (mainly active toward HO and HOCl and less so for H2O2 or ) and as a regulator of immune functions by modulating cytokine production (Ferenci et al., 1989; Luper, 1998).
The current study was undertaken to determine whether silymarin supplementation could attenuate acute EtOH-induced hepatotoxicity and to explore the possible mechanism (s) involved in this protection.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The mice were housed in the animal quarters at the University of Louisville Research Resources Center. They were maintained at 22 °C on a 12:12-hour light–dark cycle, and they had free access to rodent chow and tap water. The experimental procedures were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association of Accreditation of Laboratory Animal Care.
Silymarin Supplementation and EtOH Administration
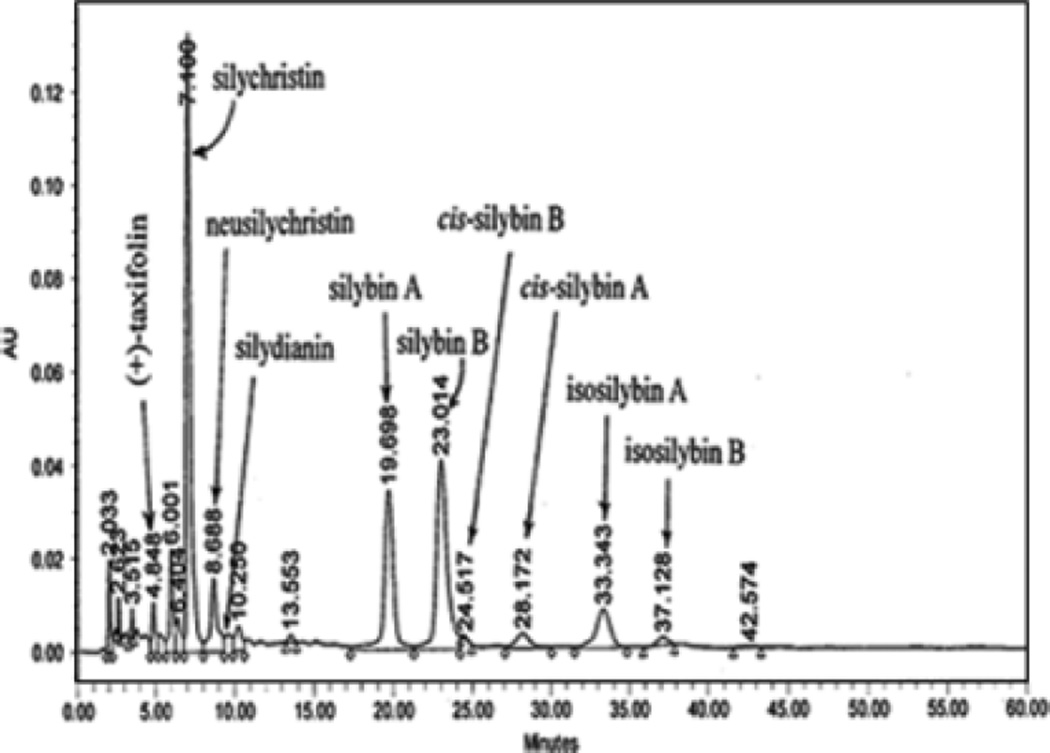
Silymarin, a mixture of flavonolignans, is extracted from the seed of S. marianum. However, there are more than a dozen silymarin products on the United States market with no quality controls of qualitative and quantitative information on the active ingredients. The silymarin used in our study was provided by the Natural Pharmacia Interntinal Inc (Belmont, MA) and is standardized (Lee et al., 2005). This standardized silymarin contains a mixture of flavonolignans that were fully characterized and marked in the high-performance liquid chromatography (HPLC) fingerprint as shown in Fig. 1. It is important to use a standardized silymarin to generate reliable and reproducible results.
Fig. 1.
High-performance liquid chromatography fingerprint of standarized silymarin.
The binge drinking mouse model developed by Carson and Pruett (1996) was utilized for EtOH challenge. This model was designed to achieve blood alcohol levels, behavioral effects, and physiological changes comparable with human binge drinking. As we did not detect any adverse effects with only silymarin supplementation, 9-week-old mice were divided into 3 groups, control, EtOH treatment, and EtOH/silymarin treatment, with 6 mice in each group. Mice received EtOH [5 g/kg body weight (BW)] by gavage every 12 hours for a total of 3 doses. Control mice received an isocalorical maltose solution. In the EtOH/silymarin group, silymarin was dissolved in EtOH and gavaged simultaneously with EtOH at a dose of 200 mg/kg BW. At 4 hours after the last dosing, the mice were anesthetized and blood samples were taken for measurement of plasma ALT levels. Liver samples were taken at the same time for biochemical assays.
Serum Enzymes Assay
Serum alanine aminotransferase (ALT, EC 2.6.1.2.) activity was measured colorimetrically using a diagnostic kit (Procedure No. 505, Sigma Chemical Co., St Louis, MO) according to the instructions provided.
Histopathological Examination
Liver tissues were cut into ~3-mm-thick slices and fixed with 10% neutral formalin. The tissue slices were embedded in paraplast. Tissue sections of 5 µm were stained with hematoxylin and eosin (H&E), and were observed with a Nikon Eclipse E400 light microscope.
Determination of Hepatic GSH
Hepatic GSH concentrations were determined using a GSH assay kit (Category No. 354,102, Calbiochem, San Diego, CA) according to the instructions provided and the result was confirmed by HPLC. For HPLC assay, 20-µL tissue homogenates were injected onto a reversed-phase C18 column (Val-U-Pak HP, fully endcapped ODS, 5 µm, 250 × 4.6 mm; ChromTech Inc). The mobile phase, which consisted of a solution of 0.1 M monochloroacetic acid, 2 mM heptanesulfonic acid at pH 2.8 (98%), and acetonitrile (2%), was delivered at a flow rate of 1 mL/min. The compounds were detected in the eluant with a Bioanalytical Systems dual LC4B amperometric detector, using 2 Au/Hg electrodes in series with potentials of −1.2 and +0.15 V for the upstream and downstream electrodes, respectively. Standard curves for the analytes were plotted as peak area versus concentration of the analyte. The GSH content was expressed as nanomoles per milligram of protein.
Hepatic Triglyceride Content Determination
For determination of total triglyceride content, 40 to 100 mg of liver was homogenized in 4 mL of chloroform:methanol (2:1). A total of 0.8 mL of 50 mM NaCl was added to each sample. Samples were then centrifuged and the organic layer was removed and dried. The resulting pellet was dissolved in phosphate-buffered saline containing 1% Triton X-100 and the triglyceride contents were determined by using an enzymatic reagent (Sigma Chemical Co.).
Lipid Peroxidation Assay
Liver tissue was homogenized in 9 volumes of 50 mM Tris-HCl buffer (pH 7.4) containing 180 mM KCl, 10 mM ethylenediamine-tetraacetic acid, and 0.02% butylated hydroxytoluene. To 0.2 mL of the tissue homogenate, 0.2 mL of 8.1% sodium dodecyl sulfate, 1.5 mL of 20% acetic acid, 1.5 mL of 0.9% thiobarbituric acid, and 0.6 mL of distilled water were added and vortexed. The reaction mixture was placed in a water bath at 95 °C for 1 hour. After cooling on ice, 1.0 mL of distilled water and 5.0 mL of butanol:pyridine mixture (15:1 v/v) were added and vortexed. After centrifugation at 10,000×g for 10 minutes, the resulting lower phase was determined at 532 nm. The thiobarbituric acid–reactive substances’ (TBARS) concentration was calculated using 1,1,5,5-tetraethoxypropane as a standard.
Determination of Cytochrome P450 2E1 (CYP2E1) Activity
Hydroxylation of p-nitrophenol to 4-nitrocatechol, a reaction catalyzed specifically by CYP2E1, was determined colorimetrically. Liver tissue was homogenized in 0.15 KCl and was spun at 10,000×g for 30 minutes. Microsomes were isolated by further centrifugation at 105,000×g for 60 minutes. For the assay, 300 µL of microsomal protein was incubated for 5 minutes at 37 °C, and absorbance at 535 nm was measured with 4-nitrocatechol as a standard. The CYP2E1-catalyzed p-nitrophenol hydroxylation was expressed as nanomoles of product formed per minute per milligram of microsomal protein.
Hepatic TNF-α Determination
Liver samples were disintegrated in 5 volumes of ice-cold RIPA buffer. After incubation on ice for 30 minutes, samples were centrifuged twice at 20,000×g for 15 minutes at 4 °C. The resulting supernatants were used for assay. The TNF levels were detected by enzyme-linked immunosorbent assay (ELISA) using a murine kit (BioSource International Inc., Camarillo, CA) and expressed as picograms per gram of liver.
Statistics
All data were expressed as mean ± SD (n = 6). Statistical analysis was performed using Student’s t test and ANOVA where appropriate. Differences between groups were considered to be statistically significant at p < 0.05.
RESULTS
Effects of Silymarin Supplementation on Serum Enzyme Levels and Histopathological Changes
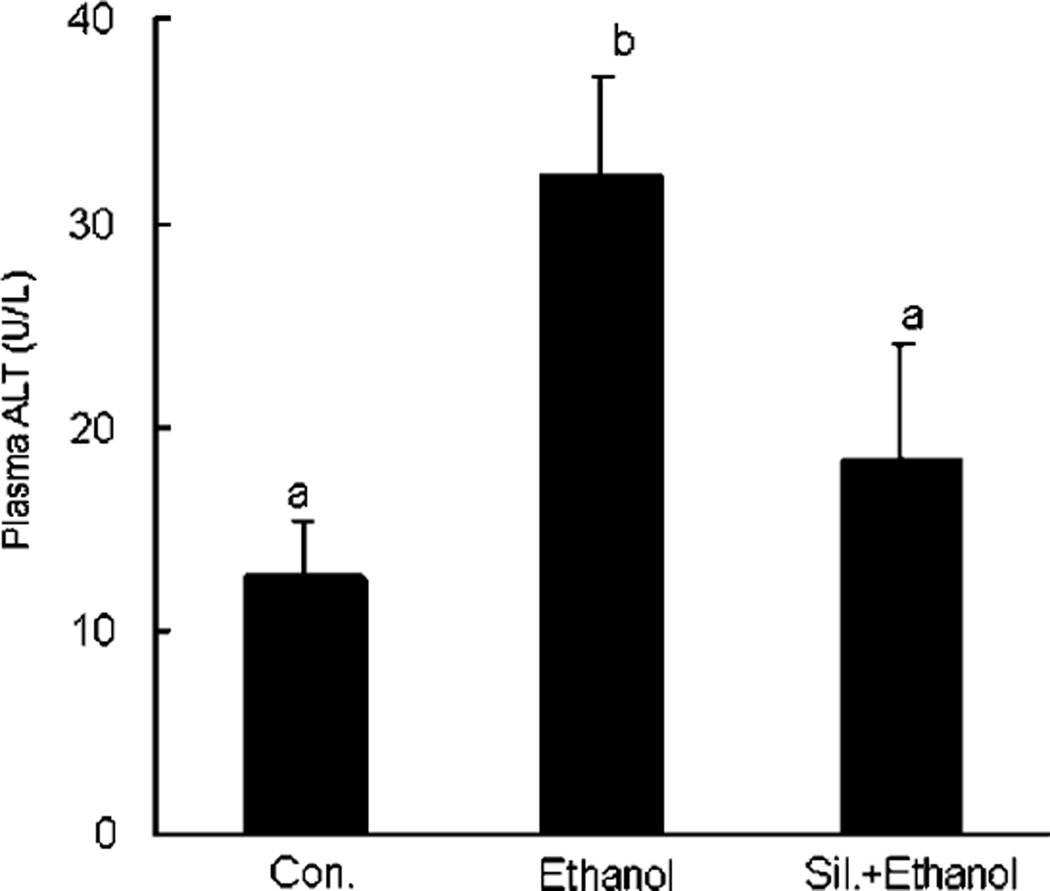
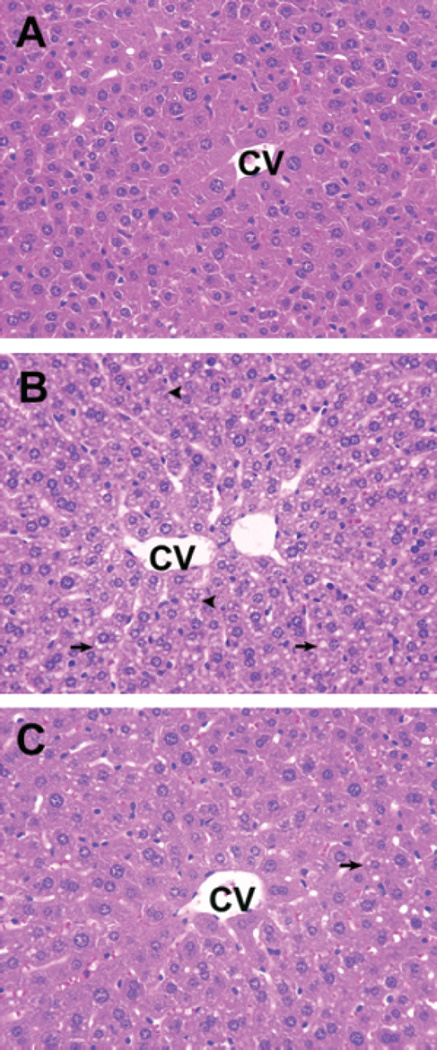
Because control and silymarin-treated animals yielded virtually identical results in all values monitored, only control animals were shown for ease of presentation. Acute EtOH administration caused an elevation of plasma ALT activity. However, silymarin supplementation attenuated the EtOH-induced increases in ALT activity (Fig. 2). Liver sections from mice treated with EtOH showed prominent microvesicular steatosis (arrows) along with necrosis (arrowheads) (Fig. 3). The necrotic hepatocytes were characterized by cell enlargement and nuclear dissolution. In the silymarin supplementation group, only microvesicular steatosis, which was less extensive than that in livers from mice receiving EtOH alone, was observed (Fig. 3).
Fig. 2.
Serum alanine aminotransferase (ALT) activity in different groups. N = 6 for each group. Values are means ± SD. Values in bars that do not share a letter differ (p < 0.05).
Fig. 3.
Histopathological changes in the liver of different groups. (A) Control mice. (B) Ethanol (EtOH) treatment induced prominent microvesicular steatosis (arrows) along with necrosis (arrowheads) in the liver. The necrotic hepatocytes are characterized by cell enlargement and nuclear dissolution. (C) Livers from silymarin supplemental group showed only microvesicular steatosis, which was less extensive than in livers from mice receiving EtOH alone. CV, central vein. ×40.
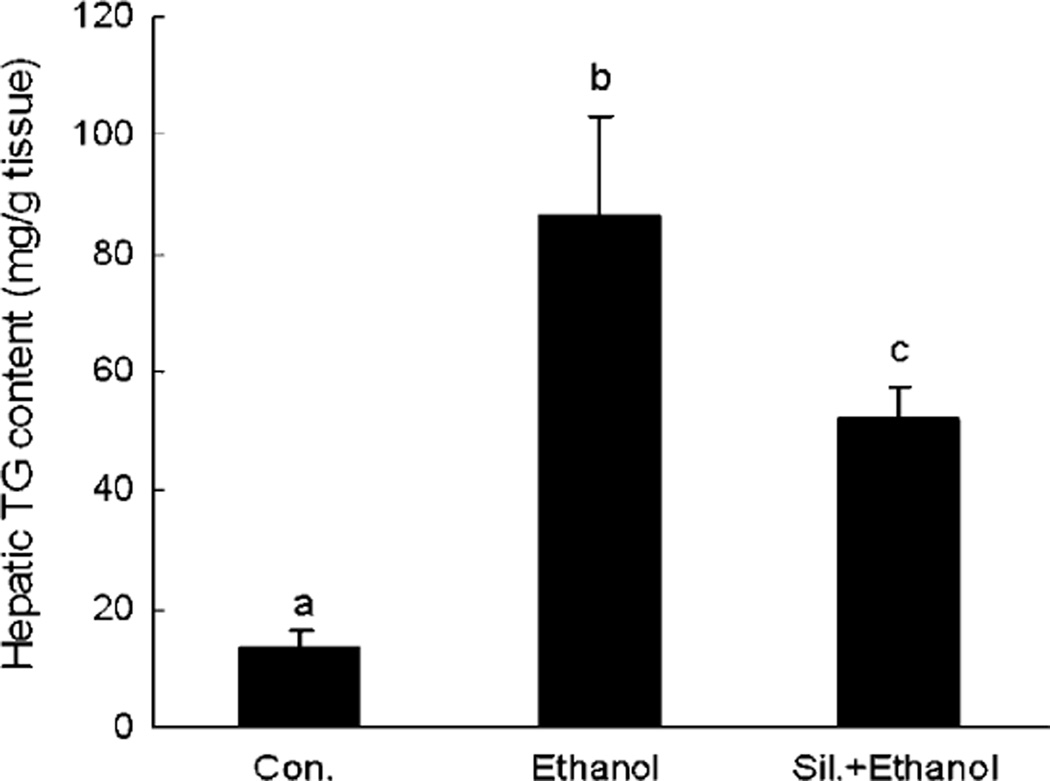
Silymarin Supplementation Attenuated EtOH-Induced Hepatic Triglyceride Accumulation
Ethanol administration induced a significant accumulation of triglyceride in the liver; however, this accumulation was attenuated by silymarin supplementation (Fig. 4).
Fig. 4.
Hepatic triglyceride content in different groups. N = 6 for each group. Values are means ± SD. Values in bars that do not share a letter differ (p < 0.05).
Silymarin Supplementation Attenuates EtOH-Induced Lipid Peroxidation
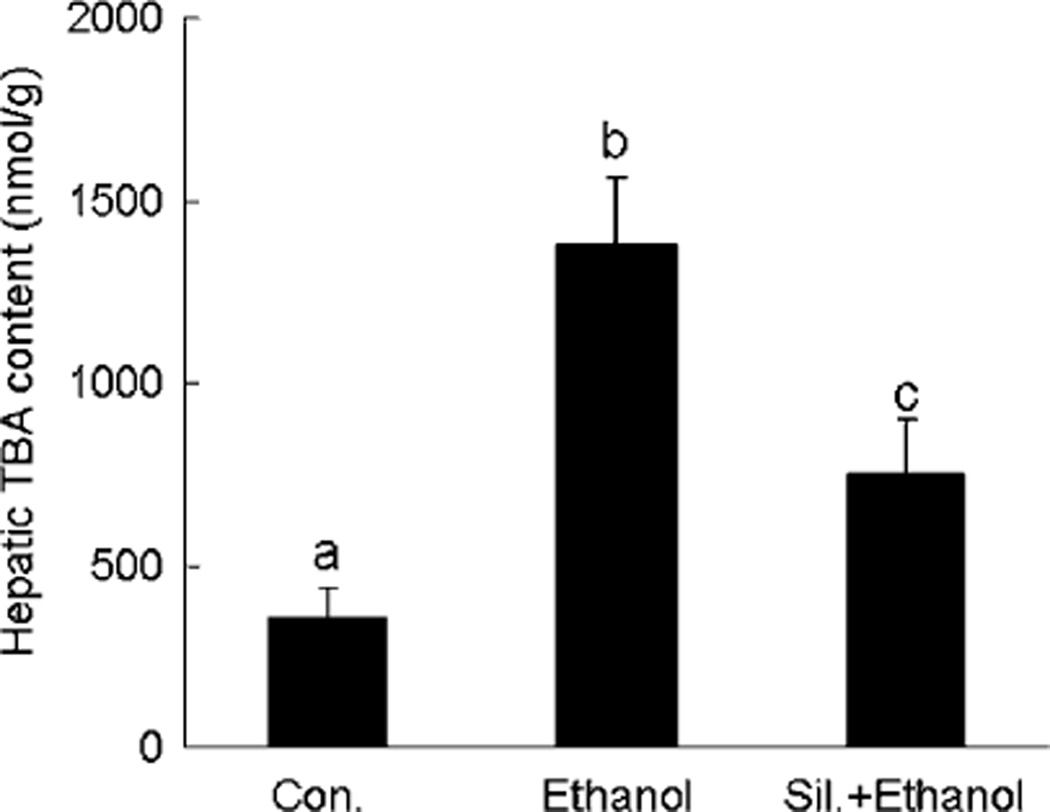
Ethanol-induced lipid peroxidation was assessed by measuring the extent of TBARS. In comparison with controls, EtOH administration caused a significant increase in the TBARS content of the liver. With silymarin supplementation, the EtOH-induced elevation of TBARS was significantly attenuated (Fig. 5).
Fig. 5.
Hepatic lipid peroxidation (TBARS content) in different groups. N = 6 for each group. Values are means ± SD. Values in bars that do not share a letter differ (p < 0.05).
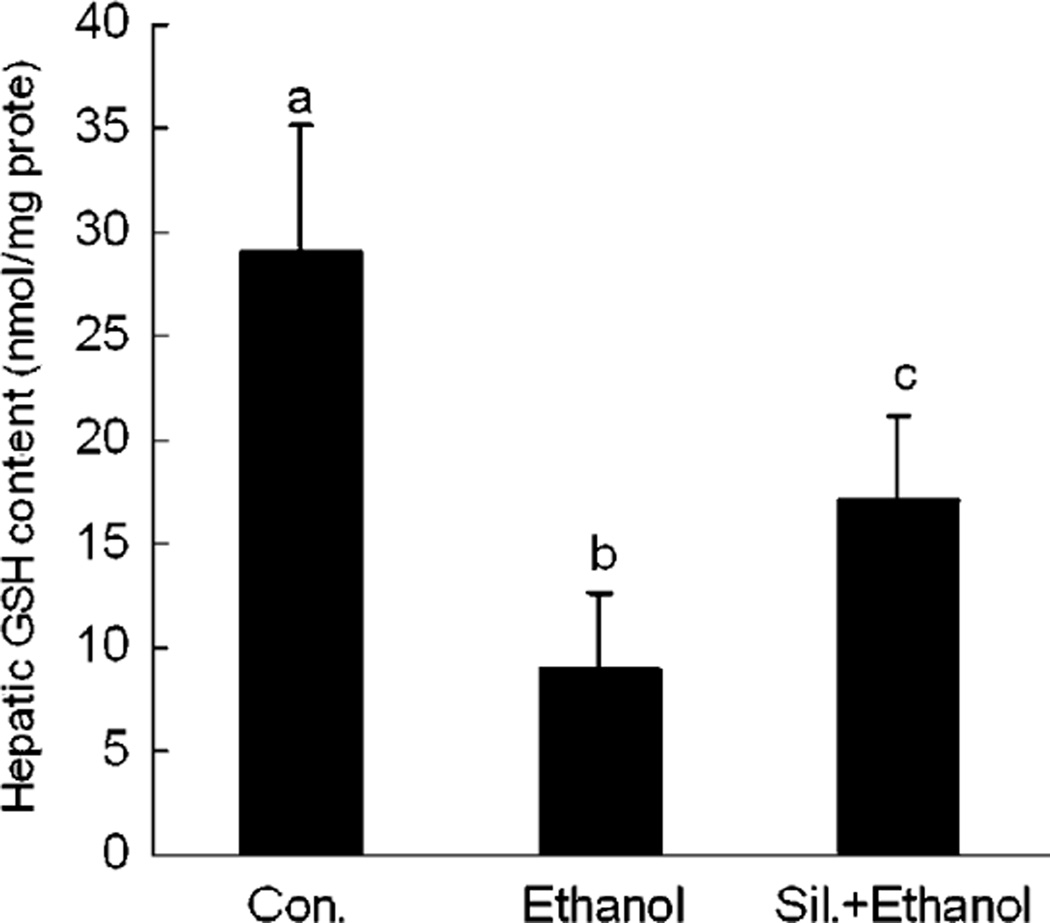
Effects of Silymarin on Hepatic GSH Concentration
Ethanol-treated mice showed a significantly decreased concentration of hepatic GSH in comparison with control mice. However, silymarin supplementation effectively attenuated the hepatic GSH decrease (Fig. 6).
Fig. 6.
Heptic glutathione (GSH) concentrations in different groups. N = 6 for each group. Values are means ± SD. Values in bars that do not share a letter differ (p < 0.05).
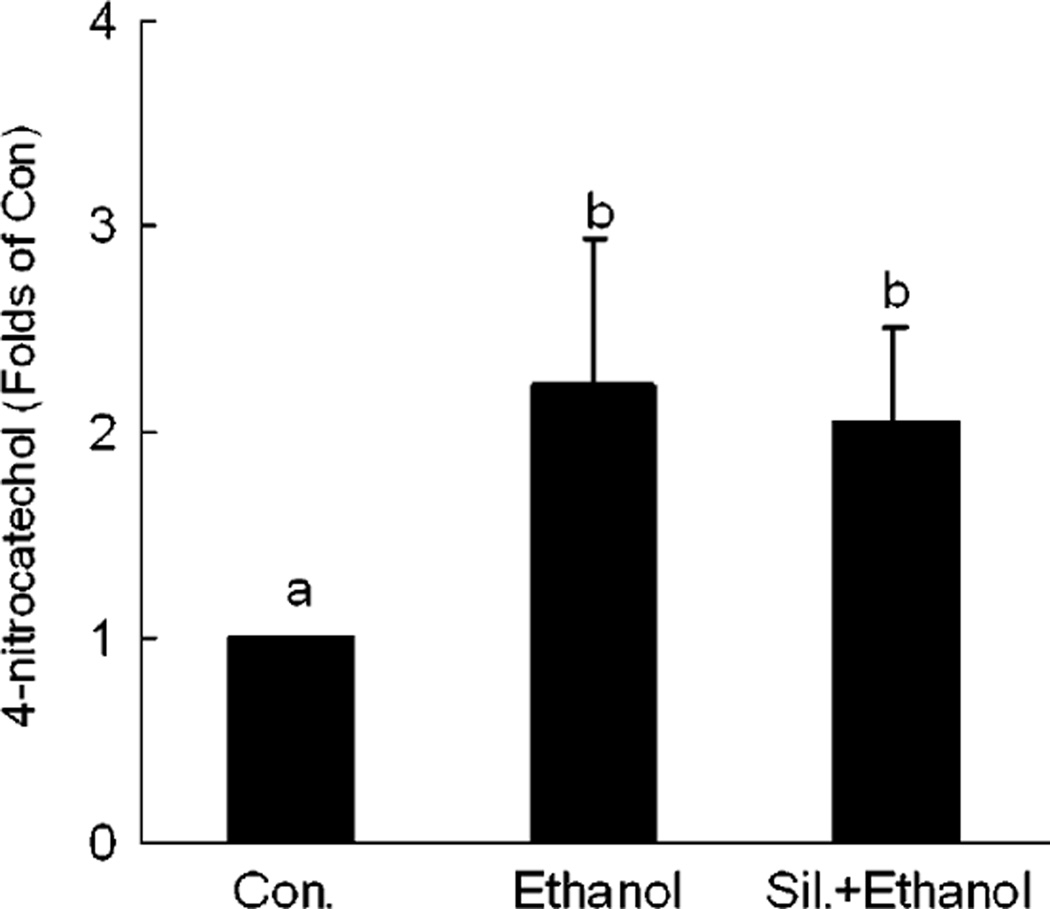
Changes in CYP2E1 Activity
To investigate the possible effects of silymarin on the activity of the major hepatic EtOH metabolizing enzyme, CYP2E1, the activity of this enzyme was determined. Our results showed that there was about a 2-fold increase in hepatic microsomal CYP2E1 activities in both EtOH alone and silymarin plus EtOH groups in comparison with controls. This suggests that the observed beneficial effect of silymarin was not via modulation of enzymatic activity of hepatic CYP2E1 (Fig. 7).
Fig. 7.
Changes of cytochrome P450 2E1 (CYP2E1) enzymatic activity in different groups. N = 6 for each group. The CYP2E1 activity in control is 1.643 ± 0.476 µmol · min/mg protein. Values are means ± SD. Values in bars that do not share a letter differ (p < 0.05).
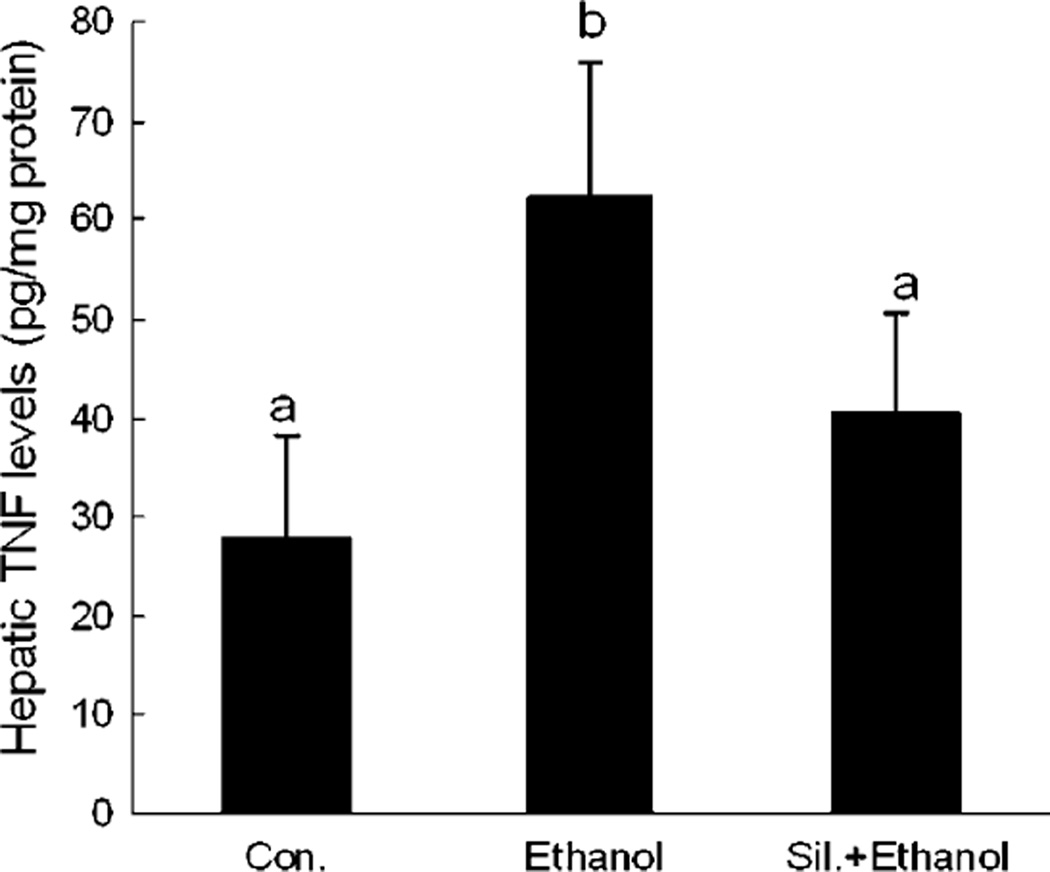
The Effect of Silymarin on Hepatic TNF Production
To determine the role of silymarin in hepatic TNF production under acute EtOH exposure, hepatic TNF productions from different groups were measured. As shown in Fig. 8, acute EtOH administration significantly increased hepatic TNF production, and this increase was attenuated by silymarin supplementation.
Fig. 8.
Hepatic tumor necrosis factor (TNF) levels in different groups. N = 6 for each group. Values are means ± SD. Values in bars that do not share a letter differ (p < 0.05).
DISCUSSION
The active constituents of milk thistle are flavonolignans including silybin, silydianin, and silychristine, collectively known as silymarin. Medicinal use of milk thistle as a liver-protecting herb dates back to the earliest Greek references to the plant. The mechanisms involved in silymarin’s hepatoprotective effects mainly include antioxidant, anti-inflammation, and antifibrotic activity (Ferenci et al., 1989; Luper, 1998). Hepatoprotective effects of silymarin on EtOH-induced liver damage have been reported previously in chronic alcohol consumption models including the study from Lieber’s group, which has demonstrated that silymarin retards the development of alcohol-induced hepatic fibrosis in baboons fed alcohol with or without silymarin for 3 years; however, the data on its effects on acute alcohol exposure model are very few. In addition, the usage of nonstandardized silymarin also made results from different group controversial and sometimes even contradictory. In the current experiments, we used standardized silymarin in an in vivo animal model of acute binge drinking and acute EtOH toxicity. Our results showed that supplementation of standardized silymarin attenuated acute EtOH-induced liver injury.
It has been well established that both oxidative stress and abnormal cytokine production, especially TNF, play important etiological roles in the pathogenesis of ALD. Oxidative stress is generally considered the result of an imbalance between prooxidants and antioxidants (Nordmann et al., 1992). Hepatic lipid peroxidation associated with acute EtOH administration has often been assessed in both animal models and human clinical trials as an indicator of oxidative stress (Meagher et al., 1999). A marked increase in lipid peroxidation was noted after acute EtOH administration in our study, and this increase was significantly attenuated by silymarin supplementation.
Although there are many potential sources of ROS in response to acute EtOH exposure, CYP2E1 is one of the major sites involved in ROS production in the liver responding to alcohol. It has been reported that long-term alcohol exposure increased CYP2E1 activities (Zhou et al., 2002; Lieber, 1997). Furthermore, investigations using CYP2E1 inhibitors, including diallyl sulfide or chlormethiazole, have shown that inhibition of CYP2E1 activity inhibits alcohol-induced liver injury, indicating the importance of CYP2E1 in alcohol-induced ROS accumulation and liver injury (Gouillon et al., 2000; McCarty, 2001). Similarly, genetic overexpression of CYP2E1 in the liver causes enhanced alcohol-induced liver injury in mice (Morgan et al., 2002). To investigate the possible mechanisms by which silymarin attenuated acute EtOH-induced liver injury, we first evaluated the effect of silymarin on CYP2E1 enzymatic activity in response to acute EtOH exposure. Our study indicated that EtOH increased hepatic CYP2E1 activity; however, this increase was not diminished by silymarin supplementation.
Reduced GSH is the main antioxidant found in cells that plays a critical protective role in the metabolism of a large number of toxic agents, including EtOH. Studies conducted to evaluate the status of hepatic GSH in response to EtOH exposure have shown that both acute and chronic exposure to EtOH cause time-dependent and dose-dependent decreases in the hepatic GSH content (Fernandez-Checa et al., 1998; Kurose et al., 1996). In addition, a decline in hepatic GSH has also been reported in patients chronically dependent on alcohol (Jewell et al., 1986). Furthermore, GSH decrease has been found to be associated with enhanced toxicity of EtOH, which may reflect the consumption of GSH by the overgeneration of ROS and subsequent oxidative stress caused by EtOH (Fermandez-Checa et al., 1995; Iimuro et al., 2000; Videla and Valenzuela, 1982). Results obtained from the present study confirmed previous observations by us and others that acute EtOH administration significantly decreased the hepatic GSH level (Song et al., 2003; Zhou et al., 2003). Moreover, our results showed that silymarin supplementation attenuated the hepatic GSH depletion induced by acute EtOH administration. In combination with our observation that silymarin supplementation had no effect on alcohol-induced increase in CYP2E1 activity, our results suggested that one of the major therapeutic effects of silymarin in acute EtOH-induced liver injury might result from either its direct free radical scavenging property, which shares the antioxidant properties of hepatic GSH, or an unknown mechanism by which silymarin could enhance hepatic GSH production in response to acute EtOH administration.
Abnormal metabolism of cytokine, especially TNF, is another major feature of ALD. It was initially observed that cultured monocytes from alcoholic hepatitis (AH) patients spontaneously produced TNF and produced significantly more TNF in response to an LPS stimulus than control monocytes (McClain and Cohen, 1989). Subsequently, Thurman’s group demonstrated that anti-TNF antibody prevented liver injury in alcohol-fed rats and mice lacking the TNF-type I receptor also did not develop alcoholic liver injury (Iimuro et al., 1997; Yin et al., 1999). Consistent with chronic alcohol effects, increased hepatic TNF production by acute EtOH exposure has recently been reported (Zhou et al., 2003). In vitro studies demonstrated that silymarin-inhibited Kupffer cell functions and TNF production in LPS-stimulated RAW264.7 cells (Cho et al., 2000; Dehmlow et al., 1996). Our results showed that acute EtOH administration enhanced hepatic TNF production, and in vivo silymarin supplementation attenuated this increased TNF production.
In conclusion, we have demonstrated that acute EtOH administration caused prominent microvesicular steatosis with mild necrosis and an elevation of serum ALT activity. Silymarin supplementation significantly attenuated the liver injury. In association with the hepatocyte injury, acute EtOH administration induced marked decreases in hepatic GSH levels along with enhanced lipid peroxidation. Silymarin supplementation attenuated hepatic GSH depletion following acute EtOH exposure and inhibited EtOH-associated lipid peroxidation. Furthermore, silymarin supplementation prevented acute EtOH-induced enhancement of hepatic TNF production. Taken together, our present study suggests that silymarin may prove to be an effective therapeutic agent in toxin-induced liver injuries, such as those induced by EtOH.
REFERENCES
- Carson E, Pruett S. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res. 1996;20:132–138. doi: 10.1111/j.1530-0277.1996.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Cho JY, Kim PS, Park J, Yoo ES, Baik KU, Kim YK, Park MH. Inhibitor of tumor necrosis factor-alpha production in lipopolysaccharide-stimulated RAW264.7 cells from Amorpha fruticosa. J Ethnopharmacol. 2000;70:127–133. doi: 10.1016/s0378-8741(99)00154-3. [DOI] [PubMed] [Google Scholar]
- Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–754. doi: 10.1053/jhep.1996.v23.pm0008666328. [DOI] [PubMed] [Google Scholar]
- Farghali H, Kamenikova L, Hynie S, Kmonickova E. Silymarin effects on intracellular calcuim and cytotoxicity: a study in perfused rat hepatocytes after oxidative stress injury. Pharmacol Res. 2000;41:231–237. doi: 10.1006/phrs.1999.0575. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105–113. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- Fermandez-Checa JC, Morales A, Colell A, Bellesta A, Rodes J, Kaplowitz N. S-adenosyl-L-methionine prevents both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology. 1995;21:207–214. doi: 10.1002/hep.1840210133. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389–401. doi: 10.1055/s-2007-1007172. [DOI] [PubMed] [Google Scholar]
- Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, Fang C, Ingelman-Sundberg M, Donohue TM, Jr, French SW. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224:302–308. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- Halim AB, el-Ahmady O, Hassab-Allah S, Abdel-Galil F, Hafez Y, Darwish A. Biochemical effect of antioxidants on lipids and liver function in experimentally induced liver damage. Ann Clin Biochem. 1997;34:656–663. doi: 10.1177/000456329703400610. [DOI] [PubMed] [Google Scholar]
- Honchel R, Ray MB, Marsano L. Tumor necrosis factor in alcohol enhanced endotoxin liver injury. Alcohol Clin Exp Res. 1992;16:665–669. doi: 10.1111/j.1530-0277.1992.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Bradford BU, Yamashita S, Rusyn I, Nakagami M, Enomoto N, Kono H, Frey W, Forman D, Brenner D, Thurman RG. The glutathione precursor l-2-oxothiazolidine-4-carboxylic acid protects against liver injury due to chronic enteral ethanol exposure in the rat. Hepatology. 2000;31:391–398. doi: 10.1002/hep.510310219. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor-a attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- Jewell SA, Di Monte D, Gentile A, Guglielmi A, Altomare E, Albano O. Decreased hepatic glutathione in chronic alcoholic patients. J Hepatol. 1986;3:1–6. doi: 10.1016/s0168-8278(86)80139-8. [DOI] [PubMed] [Google Scholar]
- Kurose I, Higuchi H, Kato S, Miura S, Ishii H. Ethanol-induced oxidative stress in the liver. Alcohol Clin Exp Res. 1996;20:77A–85A. doi: 10.1111/j.1530-0277.1996.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Kurose I, Higuchi H, Kato S, Miura S, Watanabe N, Kamegaya Y, Tomita K, Takashi M, Horie Y, Fukuda M, Mizukami K, Ishii H. Oxidative stress on mitochondria and cell membrane of cultured rat hepatocytes and perfused liver exposed to ethanol. Gastroenterology. 1997;112:1331–1343. doi: 10.1016/s0016-5085(97)70147-1. [DOI] [PubMed] [Google Scholar]
- Lee YW, David Liu yanze, Fengyu Zhao. Development of a Standardized Silymarin Product. 2005 (in preparation) [Google Scholar]
- Lieber C. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37:336–339. doi: 10.1097/00004836-200310000-00013. [DOI] [PubMed] [Google Scholar]
- Luper S. A review of plants used in the treatment of liver disease: part 1. Alternative Med Rev. 1998;3:410–421. [PubMed] [Google Scholar]
- Masini A, Ceccarelli D, Galléis D, Giovannini D, Trenti Lipid hydroperoxide induced mitochondrial dysfunction following acute ethanol intoxication in rats. The critical role for mitochondrial reduced glutathione. Biochem Pharmacol. 1994;47:217–224. doi: 10.1016/0006-2952(94)90009-4. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Inhibition of CYP2E1 with natural agents may be a feasible strategy for minimizing the hepatotoxicity of ethanol. Med Hypotheses. 2001;56:8–11. doi: 10.1054/mehy.1999.1015. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- Meagher E, Barry O, Burke A, Lucey M, Lawson J, Rokach J, FitzGerald G. Alcohol-induced generation of lipid peroxidation products in humans. Clin Invest. 1999;104:805–813. doi: 10.1172/JCI5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- Navasumrit P, Ward TH, Dodd NJ, O’Connor PJ. Ethanol-induced free radicals and hepatic DNA strand breaks are prevented in vivo by antioxidants: effects of acute and chronic ethanol exposure. Carcinogenesis. 2000;21:93–99. doi: 10.1093/carcin/21.1.93. [DOI] [PubMed] [Google Scholar]
- Nordmann R, Ribiere C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Rad Biol Med. 1992;12:219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- Rouach H, Fataccioli V, Gentil M, French SW, Mirimoto M, Nordmann R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–355. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- Shaw S, Rubin KP, Lieber CS. Depressed hepatic glutathione and increased diene conjugates in alcoholic liver disease. Evidence of lipid peroxidation. Dig Dis Sci. 1983;28:585–589. doi: 10.1007/BF01299917. [DOI] [PubMed] [Google Scholar]
- Song Z, Zhou Z, Chen T, Hill D, Kang J, Barve S, McClain C. S-adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice small star, filled. J Nutr Biochem. 2003;14:591–597. doi: 10.1016/s0955-2863(03)00116-5. [DOI] [PubMed] [Google Scholar]
- Videla LA, Valenzuela A. Alcohol ingestion, liver glutathione and lipoperoxidation: metabolic interrelations and pathological implication. Life Sci. 1982;31:2395–2407. doi: 10.1016/0024-3205(82)90743-3. [DOI] [PubMed] [Google Scholar]
- Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luser MI, Thurman RG. Essential role of tumor necrosis factor a in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sun X, Kang Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med. 2002;227:214–222. doi: 10.1177/153537020222700310. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol. 2003;163:1137–1146. doi: 10.1016/s0002-9440(10)63473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]