Abstract
Obesity is a multi-factorial disorder, which is often associated with many other significant diseases such as diabetes, hypertension and other cardiovascular diseases, osteoarthritis and certain cancers. The management of obesity will therefore require a comprehensive range of strategies focussing on those with existing weight problems and also on those at high risk of developing obesity. Hence, prevention of obesity during childhood should be considered a priority, as there is a risk of persistence to adulthood. This article highlights various preventive aspects and treatment procedures of obesity with special emphasis on the latest research manifolds.
Introduction
Obesity can be described as the "New World Syndrome". Its prevalence is on continuous rise in all age groups of many of the developed countries in the world. Statistical data reveals that the problem of obesity has increased from 12–20% in men and from 16–25% in women over the last ten years [1]. Recent studies suggest that nearly 15–20% of the middle aged European population are obese [2] and that in USA alone it is responsible for as many as 3,00,000 premature deaths each year [3]. Obese patients have been associated with increased risk of morbidity and mortality relative to those with ideal body weight [4]. Even modest weight reduction in the range of 5–10% of the initial body weight is associated with significant improvements in a wide range of co-morbid conditions [5-9]. Obesity, which was once viewed as the result of lack of will power, or a lifestyle "choice" – the choice to overeat and under exercise, is now being considered more appropriately by the modern world as a chronic disease, which requires effective strategies for its management.
Obesity, in simple terms, may be defined as a state of imbalance between calories ingested versus calories expended which would lead to excessive or abnormal fat accumulation. Body Mass Index (BMI) is a measure of weight corrected for height and which reflects the total body fat and has been the most accepted parameter for defining over weight [10].
![]()
Optimal BMI increases with age. WHO also classified over weight according to BMI [11]. There is a very good correlation between BMI and the percentage of body fat in large populations.
Percent Body fat = 1.2 (BMI) + 0.23 (age) - 10.8 (gender) - 5.4
Where gender = '1' for men and '0' for women.
It follows from this equation that for a given height and weight, the percentage of body fat is about 10% higher in women compared to men. The reason for this could be that in women, the excess body fat is usually distributed as subcutaneous fat and is mainly peripheral (thighs, buttocks, breasts) where as in men there is a relative excess of body fat stored in abdominal cavity as abdominal subcutaneous fat.
New classifications of over weight may be based on cut-off points for simple anthropometric measures such as waist hip ratio, total adiposity and intra-abdominal fatness. There exists a correlation between increased BMI, mortality due to allied risks which is depicted in Fig. 1
Figure 1.
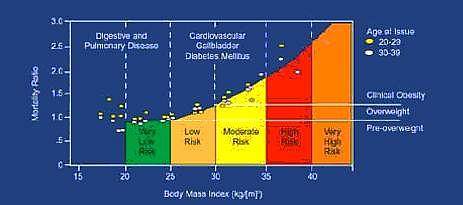
Correlation between increased BMI and risk of mortality
Aetiology of obesity
Obesity is not a single disorder but a heterogeneous group of conditions with multiple causes each of which is ultimately expressed as obese phenotype. Obesity involves complex aetiological links between the genetic, metabolic and neural frameworks on one hand and behavior, food habits, physical activity and socio-cultural factors on the other (Table 1).
Table 1.
Some important causes and precautionary measures of obesity.
| Obesity | |
| Causes | Management |
| Sedentary life style | Physical activity |
| Food availability | Diet control |
| High fat diet | Behavioural therapy |
| Hereditary | Medication |
| Drug induced weight gain | Surgery |
Genetic considerations
Although obesity had a genetic component, it is not a simple genetic disorder. There is an underlying genetic predisposition to obesity on to which environmental factors are layered. The discovery of 'ob' gene, which was mapped to chromosome 7, has led to a renewed interest in understanding the patho-biological basis of genetic predisposition in obesity. The 'ob' gene codes a hormone called leptin, a 167 amino acid protein and was supposed to be produced in white and brown adipose tissue and placenta [12]. The leptin receptors are concentrated in hypothalamus and belong to the same class of IL-2 and growth hormone receptors [13]. Any mutation of 'ob' gene leads to improper coding of leptin, which further results in obesity [14]. The effects of the 'ob' gene are mediated through effects on both energy intake and energy expenditure. Obesity can also be considered as a "complex trait" as many other genes coding proteins like apolipoprotein B, D, E, β3-adrenergic receptor [15], dopamine D2-receptor, tumor necrosis factor (TNF), glucocorticoid receptor etc. are associated with it. So far, 200 genes, gene markers and chromosomal regions have been associated with human obesity [16].
Neurobiology
Two neurotransmitters neuropeptide Y (NPY) and serotonin (5-HT) are found to play a major role in body weight regulation. NPY is a 36 amino acid peptide, which is concentrated mainly in the hypothalamus; a region crucial to regulation of appetite [17] has emerged as a possible key neurotransmitter candidate for the regulation of energy homeostasis. Increased NPY activity has been found in the hypothalamus of obese rodents [18]. NPY increases food in-take through its interaction with a unique Y5 subtype of NPY receptor and hence Y5 receptor antagonists could be effective in the treatment of obesity [19].
The inhibitory actions of 5-HT on food in-take have been localized to the hypothalamic para ventricular nucleus (PVN), the site at which NPY is most active in inducing feeding behavior [20]. 5-HT induced reduction in food in-take is mediated by post-synaptic 5-HTIB receptors. The hypophagic actions of 5-HT may be mediated at least partly through the NPY pathway. For example, 5-HT antagonist which stimulates feeding increases NPY concentrations in the arcuate and para ventricular nuclei of the hypothalamus [21]. Similarly, a 5-HT agonist, which reduces food intake significantly, reduces NPY concentrations in the hypothalamic para ventricular nucleus. Corticotrophin releasing factor (CRF) which also causes weight loss by reducing appetite and act in opposing to NPY on the regulation of energy balances. Cholecystokinin (CCK), a neurotransmitter present in the brain plays a physiological role as a meal termination (satiety) signal between the two receptors such as CCKA and CCKB, CCK acted at CCKA receptors [22]. Hence, CCKA agonist could also be useful in the treatment of obesity.
Environmental factors
These factors play a critical role in the development of obesity by unmasking genetic or metabolic susceptibilities. Environmental influences act via an increase in energy intake or a decrease in energy expenditure with little physical activity and hence there is increased likelihood of becoming obese. Sedentary behaviors, notably television watching, car ownership also contributes to the risk of obesity. The role of passive over consumption [23], eating disorders, and preference for high carbohydrate diet also play an important role in increasing the risk of obesity. Other food habits like smoking and alcohol consumption lowers body weight and results in higher BMI respectively.
Psycho-social impact
A number of individual characteristics may place individuals at increased risk of obesity. Restrained eating also plays a role in aetiology of obesity. Restrained eaters report more food carvings and binge eating [24]. One of the characteristic features of dietary restraints is the tendency towards disinhibited eating in particular circumstances. Restrained eaters may be more susceptible to the availability of highly palatable foods, which act as a stimulus for excess food consumption.
Obesity-associated diseases and risk factors
Cardiovascular diseases (CVD)
Hypertension
Coronary heart disease
Cerebrovascular disease
Varicose veins
Deep venous thrombosis
The increased risk of CVD is 2-fold in women of BMI 25–28.9 kg/m2 and 3.6 fold for BMI in 29 kg/m2 or more. In males a 10% increase in body weight increases risk of CVD by 38%, where as 20% weight risk corresponds with 86% increased risk. Blood pressure is increased by 6 mm systole and 4 mm diastole for a 10% gain in body fat. Hyper tension is prevalent in obese adults at a rate of 2.9 fold than non-obese population and weight reduction reduces risk of developing hyper tension [25].
Respiratory diseases
Breathless
Sleep apnoea
Hypoventilation syndrome
There are a number of ways in which obesity affects lung function [26]. An increased amount of fat in the chest wall and abdomen limits respiratory excursion reducing lung volume. As the obesity worsens, so do the apnoeic episodes resulting in frequent awakening and the resultant sleep deprivation produces daytime somnolence.
Metabolic disorders
Hyperlipidemia
Diabetes mellitus
Insulin resistance
Menstrual irregularities
There is a consistent graded relationship between increased BMI and prevalence of NIDDM and insulin resistance [27]. Over 10 to 15 million Americans with type 2 diabetes are obese [28]. A mean weight loss of 7% weight reduces risk of developing type 2 diabetes by more than 55% [29]. BMI above 35 kg/m2 increases the risk by 93 fold in women and by 42 fold in men. Obesity is associated with lipid disorders in which elevated levels of cholesterol, triglycerides, LDL-cholesterol and low levels of HDL-cholesterol are observed. For every 1 kg of weight loss, there is a corresponding reduction by about 1% in HDL and reduction by 3% of triglycerides. It has been observed that modest weight loss reduces lipid abnormalities [30] and diabetes mellitus [31].
Gastrointestinal disorders
Fatty liver and cirrhosis
Haemorrhoids
Hernia
Colorectal cancer
Gallstones
Gall bladder disease is the most common gastrointestinal disorder in obese individuals. Obese women have a 2.7 fold increase in the prevalence of gall bladder disease. There is an increased risk of gallstones in individuals having BMI of 20 kg/m2 or more. The mortality rates of cancer of the stomach and pancreas were higher in obese individuals.
Malignancies
Breast cancer
Endometrial Cancer
Prostrate Cancer
Cervical Cancer
Obese women have higher incidence of endometrial, ovarian, cervical and postmenopausal breast cancer, while obese men have incidents of prostrate cancer.
However, it remains to be confirmed whether these malignancies occur as a result of hormonal changes associated with obesity or due to specific dietary pattern.
Miscellaneous
Pregnancy
Stress
Arthritis and bone mass
Stress is associated with the consumption of high fat foods and leads to weight gain. Obesity is also associated with osteoarthritis of hip and knee although in some cases, mechanical stress associated with obesity leads to osteoarthritis [32]. Obese women have a higher risk of obstetric complication and have increased risk of caesarean delivery due to variety of foetal size. Recently, an increased risk of neural tube defects especially spinabifida has been reported in women with BMI greater than 29 kg/m2.
Prevention of obesity
Obesity is a serious, chronic medical condition, which is associated with a wide range of debilitating and life threatening conditions. The fact that obesity prevalence continues to increase at an alarming rate in almost all regions of the world is of major concern. Hence, an effective control of obesity requires the development of coherent strategies that tackle the main issues related to preventing:
i) The development of over weight in normal weight individuals
ii) The progression of over weight to obesity in those who are already over weight
iii) Weight regain in those who have been over weight or obese in the past but who have since lost weight and
iv) Further worsening of a condition already established.
The prevention of obesity involves action at several levels i) Primary ii) Secondary iii) Tertiary [33]. Objective of primary prevention is to decrease the number of new cases, secondary prevention is to lower the rate of established cases in the community and tertiary prevention is to stabilize or reduce the amount of disability associated with the disorder. When the attention is focused on the multi-factorial condition such as coronary heart disease (CHD), primary prevention of this involves national programmes to control blood cholesterol levels and secondary prevention deals with reducing CHD risk in those with existing elevated blood cholesterol levels while tertiary action would be associated with preventing re-infarction in those who had a previous heart attack. However, this classification system for prevention of obesity results in a great deal of ambiguity and confusion. To avoid this, the US institute of medicine [34] has proposed alternative classification of system. The new system separates prevention efforts into 3 levels. Universal (or) public health measures (directed at every one in the population), selective (for a sub-group who may have an above average risk of developing obesity) and indicated (targeted at high risk individuals who may have a detectable amount of excess weight which fore-shadows obesity). However, preventive measures for any disorder may not be helpful in all cases hence, proper management strategies can be integrated along with prevention programmes.
Management of obesity
Management include both weight control or reducing excess body weight and maintaining that weight loss, as well as, initiating other measures to control associated risk factors. Periodic evaluation for obesity should be done by the measurement of BMI, measurement of waist circumference etc., to assess risk factors. Based on the evaluation, appropriate treatment can be suggested. Treatment may consist of modification of diet, increased physical activity, behavioral therapy, and in certain circumstances weight loss medication and surgery.
Dietary therapy
Restrictions of calories represent the first line therapy in all cases except in cases with pregnancy, lactation, terminal illness, anorexia nervosa, cholelithiasis and osteoporosis. Low calorie diets (LCD), which provide 100–1500 kcal/day, resulted in weight loss of 8% of baseline body weight over six months but on long run most of the lost weight is regained [35].
Very low calories diets (VLCD), which provide 300–800 kcal/day, can be useful in severely obese patients under strict medical supervision. They are found to produce 13% weight loss over six months, i.e. they produce greater initial weight loss than LCDs, however, the long-term (>1 year) weight loss by VLCD's is not found superior to that of the LCDs.
Meal replacement programmes and formula diets can be used as an effective tool in weight management [36]. Optifast, Medifast are available through physians or hospitals as part of packaged weight-reduction programmes. These products appear to be safe, but maintenance of weight loss over the long term is difficult.
Other over the counter (OTC) variations to formula diets includes Slimfast and Ultra slimfast. The consumer is instructed to drink the formulations and use it to replace one or two meals.
Fat substitutes like Olestra (Olean), which is a non-digestible, non-caloric fat, can be used in food preparations taken by obese patients.
It has been observed that calorie restriction alone has remarkable effects compared to exercise alone [37-39]. A loss of 5% initial weight achieved with diet and exercise is associated with significant improvement in glycylated haemoglobin AIC and that diet control can be useful to treat co morbidities of obesity such as diabetes [40].
Physical activity
All individuals can benefit from regular exercise [41]. Physical activity, which increases energy expenditure, has a positive role in reducing fat storage and adjusting energy balance in obese patients. Various exercises preceded and followed by short warm up and cool down sessions help to decrease abdominal fat, prevent loss of muscle mass. Studies revealed that patients who exercise regularly had increased cardio vascular fitness [42,43] along with betterment in their mental and emotional status. Hence a minimum of 30 minutes exercise is recommended for people of all ages [44] as part of comprehensive weight loss therapy.
Behaviour therapy
Behaviour therapy is a useful adjunct when incorporated into treatment for weight loss and weight maintenance. Patients need to be trained in gaining self-control of their eating habits. Behaviour modification programmes which seek to eliminate improper eating behaviours (eating while watching TV, eating too rapidly, eating when not hungry etc.,) include individual or group counseling of patients.
Self-help groups (weight watchers, Nutri-System) use a program of diet, education and self-monitoring like maintenance of logbook, keeping an account of food intake etc are beneficial.
Pharmacotherapy
Drug treatment is advised only for subjects with BMI > 27 and with associated risk factors or with a BMI > 30 [45] and thus at medical risk because of their obesity. It should not be used for "cosmetic" weight loss. Weight loss medications should be used only as an adjunct to dietary and exercise regimes coupled with a program of behavioural treatment and nutritional counseling.
Pharmacological approaches in obesity treatment
Most available weight loss medications are "appetite–suppressant" medications. The initial drugs used for appetite suppression were amphetamine [46], metamphetamine and phenmetrazine (Preludin) and are no longer used in treatment of obesity because of their high potential for abuse.
Inhibitors of 5-hyroxytryptamine (5-HT) reuptake, fenfluramine and dexfenfluramine were licensed for obesity but proved to cause pulmonary hyper tension and increased valvular heart disease [47] and have been withdrawn from the market. Drugs like phendimetrazine (Plegine), diethylpropion (Tenuate), phentermine (Lonamin) etc., are being marketed but have been classified as controlled substances and are recommended for short-term use only.
The newest agents available for weight loss are sibutramine (Meredia) and orlistat (Xenical). They are the only weight loss medications approved by the US Food and Drug Administration (FDA) for long-term use [48] in significantly obese patients, although their safety and effectiveness have not been established for use beyond one year.
Sibutramine is the serotonin and norepinephrine re-uptake inhibitor, which induces decreased food intake and increased thermogensis [49-52]. In clinical trials, sibutramine showed a statistical improvement in amount of weight lost versus placebo [53]. It limits decline of metabolic rate that typically accompanies weight loss [54]. However, this agent is contraindicated in-patient with known seizure disorders, high blood pressure, congestive heart failure (CHF) a history of myocardial infraction and arrhythmias.
Orlistat is a potent and irreversible inhibitor of gastric, pancreatic lipases. It blocks the digestion of approximately 30% of the ingested dietary triglycerides. Studies proved that it produces 5% more weight loss than in control groups [55]. It is now available on prescription as Xenical® (Orlistat-120 mg). The most commonly reported side effects include oily stools, soft stool [56], and increased defecation and decreased absorption of fat-soluble vitamins (A, D, E and K). Hence, patient may be recommended intake of fat-soluble vitamins [57] along with it. When used in conjugation with diet it was found to improve glycemic control and cardiovascular disorders [58,59].
In general, monotherapy in obese patients produced sub-optimal weight loss [60] but the use of more than one weight loss medication at a time (combined drug therapy) is not approved [61] and hence such an off-label use of combinations of drugs for weight loss is not recommended except as part of a research study.
Drugs under development
There has been a wide search for effective drugs for the treatment of obesity. Some of the promising drug development research areas are mentioned below.
Amylin is a peptide secreted with insulin in response to food intake that shares many other properties with established adiposity signals like insulin and leptin. Its circulating levels can be correlated with body fat. Preclinical studies have shown that amylin complements the effects of insulin in mealtime glucose regulation via several effects, which include a suppression of post meal glucagon secretion, a decrease in gastric emptying, and a decrease in food intake [62]. The drug pramlintide, a synthetic analogue of amylin is currently in phase III trials.
11β-hydroxysteroid dehydrogenase type-1 (11β-HSD-1) is an enzyme that increases cortisol levels in adipocytes. Studies on mice lacking gene for 11β-HSD-1 suggest that they are resistant to diet induced obesity [63]. An 11β-HSD-1 inhibitor being developed by Biovitrum is currently in clinical testing.
Stimulation of β3 adrenoreceptors (β3-ARs) by selective agonists improves insulin action and stimulates energy metabolism. In animals, chronic β3-AR agonist treatment causes body weight reduction, which is almost entirely due to decrease in body fat [64]. At least a dozen pharmaceutical companies are in the process of developing β3-AR drugs, some of which are already in human testing. AD9677 a β-adrenoceptor agonist is in phase II trails.
The botanical P57 is an extract of steroidal glycosides derived from South African Cactus. The potent appetite suppression may occur via the melanocortin-4 (MCR-4) saponins from the Platycodi radix and Salacia reticulata have been shown to inhibit pancreatic lipase, producing weight loss and reduction of fatty liver in laboratory animals [65]. Currently, P57 is in Phase II testing and Table 2 summarizes some other important drugs union are under clinical trials for the treatment of obesity.
Table 2.
List of some important drugs under clinical trials for weight reduction.
| Drugs in phase II trials | Drugs in phase III trials |
| Bupropion (dopamine reuptake inhibitor) | Mazindol (adrenergic agonist) |
| Linitript (cholecystokinin A antagonist) | Sertraline (selective serotonin uptake inhibitor) |
| Pegylated leptin | Posatirelin (thyrotrphin-releasing hormone analogue) |
| Dipeptidyl peptidase IV inhibitors | Cannabinoid antagonists |
| Human growth hormone factor AOD9604 | Lipase inhibitor, ATL-962 |
| Phytostanol |
Surgery
Apart from drug treatment, surgery is also indicated when BMI is exceedingly high (>40 kg/m2 or >30 kg/m2 with obesity-related medical co-morbidities) and when other treatment modalities have failed [66]. The most popular surgical procedures used for treatment of severe obesities involve gastric portioning or gastroplasty and gastric by-pass. The gastroplasty procedures create a small gastric pouch, which is drained through a narrow calibrated stoma [67,68]. The intake of solids is therefore considerably limited. Gastric by-pass surgery creates a larger pouch emptied by an anastomosis directly into the jejunum, bypassing the duodenum. It is considered now as the most effective and safe surgery for morbid obesity [69,70]. This technique induces weight loss by combining restricted intake and a moderate degree of malabsorbtion [71]. Initial loss of weight is greater after this procedure than following gastroplasty [72].
Gastric and nutritional complications [73] may be serious implications of the surgery. Nutritional deficiencies and intractable vomiting are frequently associated with surgery. Surgical treatments for obesity resolve most co-morbidities of severe obesity such as hypertension [74,75], serum lipid levels [76] and diabetes mellitus [77,78].
Conclusion
Obesity is not a social condition but is a rampant disease. Obesity cannot be overviewed as just a matter of overeating and lack of will power but must be considered as a major genetic aetiology modified by environment and should be treated vigorously in the same manner that we now apply to other diseases. A better understanding of the aetiological determinants in individual subjects will provide a basis for more rational intervention to prevent this recalcitrant public health problem. With the increasing awareness and ongoing research in this area there is a considerable reason for optimism that the next coming years will bring better treatment for the obese.
Contributor Information
Srinivas Nammi, Email: nammi@rediffmail.com.
Saisudha Koka, Email: k_saisudha@yahoo.com.
Krishna M Chinnala, Email: chinnala_km@yahoo.com.
Krishna M Boini, Email: krishnamurthyboini@yahoo.com.
References
- Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Over weight and obesity in the United States: Prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Obesity. Lancet. 1997;350:423–426. doi: 10.1016/S0140-6736(97)04503-0. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Office of the Surgeon General The surgeon General's call to action to prevent and decrease overweight and obesity. Rockville MD: United States Department of Health and Human Services. 2001. [PubMed]
- Manson JE, Willett WC, Stamfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Eng J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- Blackburn GL. Effect of degree of weight loss on health benefits. Obes Res. 1995;3:211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization Obesity: Preventing and managing the global epidemic. World Health Organisation Geneva. 2000. [PubMed]
- Goldstein DJ. Beneficial health effects of a modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- Bosello O, Armellini F, Zamboni M, Fitchet M. The benefits of modest weight loss in type-II diabetes. Int J Obes Relat Metab Disord. 1997;21:S10–S13. [PubMed] [Google Scholar]
- Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long term effects of modest weight loss in type-II diabetic patients. Arch Intern Med. 1987;147:1749–1753. doi: 10.1001/archinte.147.10.1749. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Keil D, Gold EJ, Williams SM, Goulding A. Body mass index, waist girth and waist-to-hip ratio as indexes of total and regional adiposity in women: evaluation using receiver operating characteristic curves. Am J Clin Nutr. 1998;67:44–49. doi: 10.1093/ajcn/67.1.44. [DOI] [PubMed] [Google Scholar]
- Jacob CS, Katherine MH. Assessing obesity classification and epidemology. Br Med Bull. 1997;2:239. doi: 10.1093/oxfordjournals.bmb.a011611. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Proencea R, Maffei M, Barone M, Leopold L, Friedman JM. Positional clone of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Auwerx J, Stales B. Leptin. Lancet. 1998;351:732–742. doi: 10.1016/S0140-6736(97)06348-4. [DOI] [PubMed] [Google Scholar]
- Andersson LB. Genes and obesity. Ann Med. 1996;28:5–7. doi: 10.3109/07853899608999066. [DOI] [PubMed] [Google Scholar]
- Arner P. The β3-adrenergic receptor – a cause & cure of obesity. N Engl J Med. 1995;333:382–383. doi: 10.1056/NEJM199508103330612. [DOI] [PubMed] [Google Scholar]
- Chagnon YC, Perusse L, Weisnagel SJ, Rankinen T, Bouchard C. The human obesity gene map: the 1999 update. Obes Res. 2000;8:89–117. doi: 10.1038/oby.2000.12. [DOI] [PubMed] [Google Scholar]
- Dryden S, Frankish H, Wang Q, Williams G. Neuropeptide Y and energy balance, one way ahead for the treatment of obesity? Eur J Clin Invest. 1994;24:293–308. doi: 10.1111/j.1365-2362.1994.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Flier JS, Flier EM. Obesity and the hypothalamus: Novel peptides for new pathways. Cell. 1998;92:437–440. doi: 10.1016/S0092-8674(00)80937-X. [DOI] [PubMed] [Google Scholar]
- Friedman JM. The alphabet of weight control. Nature. 1997;385:119–120. doi: 10.1038/385119a0. [DOI] [PubMed] [Google Scholar]
- Shor Posnar G, Grinker JA, Marinescu C. Hypothalamic serotonin in the control of meal patterns and macronutrient selection. Brain Res Bull. 1986;17:663–671. doi: 10.1016/0361-9230(86)90198-X. [DOI] [PubMed] [Google Scholar]
- Dryden S, Frankish H, Wang Q, Williams G. The serotonin antagonist methysergide increase NPY synthesis and secretion in the hypothalamus of rat. Brain Res. 1995;699:12–18. doi: 10.1016/0006-8993(95)00841-D. [DOI] [PubMed] [Google Scholar]
- Boosalis MG, Gemayel N, Lee A, Bray GA, Laine L, Cohen H. Cholecystokinin and satiety: effect of hypothalamic obesity and gastric bubble insertion. Am J Physiol. 1992;262:R241–244. doi: 10.1152/ajpregu.1992.262.2.R241. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Adipogenesis and obesity: round in out the big picture. Cell. 1996;87:377–389. doi: 10.1016/S0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- Wadley J. Dietary restraint and binge eating behaviour. Anal Modif. 1980;4:647–660. [Google Scholar]
- Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma rennin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- Kolarzyk E, Kiec E, Wiater M. Effect of obesity on the ventilatory capacity of the respiratory system. I. Relation between basic spirometric indicators: vital capacity (VC) and forced expiratory volume (FEV1) and obesity. Med Pr. 1985;36:87–95. [PubMed] [Google Scholar]
- Rahilly OS. Non insulin dependent diabetes mellitus: the gathering storm. Br Med J. 1997;314:955–960. doi: 10.1136/bmj.314.7085.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National health and Nutrition Examination survey. J Amer Med Assoc. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer_Davis E, Mooradian AD, Purnell JQ, Wheeler M. Evidence based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes care. 2002;25:148–198. doi: 10.2337/diacare.25.1.148. [DOI] [PubMed] [Google Scholar]
- Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- Anonymous UK Prospective study on maturity onset diabetes. I. Effect of diet and sulphonylurea, insulin or biguainide therapy on fasting plasma glucose and body weight over one year. Diabetologia. 1983;24:404–411. [PubMed] [Google Scholar]
- Davis MA, Neuhaus JM, Ettinger WH, Mueller WH. Body fat distributions and osteoarthritis. Am J Epidemiol. 1990;132:701–707. doi: 10.1093/oxfordjournals.aje.a115711. [DOI] [PubMed] [Google Scholar]
- Timothy PG. Key issues in the prevention of obesity. Br Med Bull. 1997;53:359–388. doi: 10.1093/oxfordjournals.bmb.a011618. [DOI] [PubMed] [Google Scholar]
- US Institute of medicine . Reducing risks of mental disorders. Frontiers for preventive intervention research. Washington, National Academy Press; 1994. [PubMed] [Google Scholar]
- Wadden TA. Treatment of obesity by moderate and severe caloric restriction results of clinical research tracts. Ann Intern Med. 1993;119:688–693. doi: 10.7326/0003-4819-119-7_part_2-199310011-00012. [DOI] [PubMed] [Google Scholar]
- Ashley JM, St Jeor ST, Schrage JP, Perumean_Chaney SE, Gilbertson MC, McCall NL, Bovee V. Weight control in the physician's office. Arch Intern Med. 2001;161:1599–1604. doi: 10.1001/archinte.161.13.1599. [DOI] [PubMed] [Google Scholar]
- Anderssen S, Holme I, Urdal P, Hjermann I. Diet and exercise intervention have favourable effects on blood pressure in hypertensives: The Oslo Diet and Exercise Study (ODES) Blood Press. 1995;4:343–349. doi: 10.3109/08037059509077619. [DOI] [PubMed] [Google Scholar]
- Bertram SR, Venter I, Stewart RI. Weight loss in obese women – exercise vs dietary education. S Afr Med J. 1990;78:15–18. [PubMed] [Google Scholar]
- Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, Superko HR, Fortman SP, Albers JJ, Vranizan KM, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight loss effects of a long term dietary intervention in obese patients. Am J Clin Nutr. 1999;69:198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human services Leading health indicators. Overweight and obesity. Healthy people 2010 (Conference ed. in two volumes) DC US Department of Health and Human Services, Washington. 2000. pp. 24–45.
- Wyatt HR, Wing RR, Hill JO. The National weight control registry. In: Bessesen DH, Kushner RF, editor. Evaluation & Management of obesity. Philadelphia, Hanley & Belfus Inc; 2002. pp. 199–224. [Google Scholar]
- Schoeller DA, Shay K, Kushner RF. How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr. 1997;66:551–556. doi: 10.1093/ajcn/66.3.551. [DOI] [PubMed] [Google Scholar]
- Physical Activity and Health A Report of the surgeon General PA. US Department of Health and Human services. 1996.
- National Institutes of Health (NHLBI) Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults. The evidence report Washington DC National Institute of Health, Obese Res. 1998;6:51s–201s. [PubMed] [Google Scholar]
- Lessof MH, Myerson A. Benzedrine sulfate as an aid to be the treatment of obesity. N Engl J Med. 1938;218:119–205. [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensud DD, Edwards BS, Edwards WD. Valvular heart disease associated with fenfluramine – phentermine. N Engl J Med. 1997;337:783. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- James WP, Astrup A, Finer N, Hilsted J, Kopelman P, Rossner S, Saris WH, Van Gaal LF. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet. 2000;356:2119–2125. doi: 10.1016/S0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]
- Mun EC, Blackbur GL, Matthews JB. Current status of medical and surgical therapy for obesity. Gastroenterology. 2001;120:669–681. doi: 10.1053/gast.2001.22430. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Shide DJ, Thorwart ML, Ulbrecht JS. Sibutramine reduces food intake in non-dieting women with obesity. Obes Res. 1998;6:1–11. doi: 10.1002/j.1550-8528.1998.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Hansen DL, Toubro S, Stock MJ, Macdonald IA, Astrup A. Thermogenic effects of sibutramine in humans. Am J Clin Nutr. 1998;68 :1180–1186. doi: 10.1093/ajcn/68.6.1180. [DOI] [PubMed] [Google Scholar]
- Seagle HM, Bessesen DH, Hill JO. Effects of sibutramine on resting metabolic rate and weight loss in over weight women. Obes Res. 1998;6:115–121. doi: 10.1002/j.1550-8528.1998.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Astrup A, Toubro S. When, for whom and how to use sibutramine? Int J Obes Relat Metab Disord. 2001;25:52–57. doi: 10.1038/sj.ijo.0801930. Review. [DOI] [PubMed] [Google Scholar]
- Luque CA. Sibutramine: a serotonin-norepinephrine reuptake inhibitor for the treatment of obesity. Ann Pharmacother. 1999;33:968–978. doi: 10.1345/aph.18319. [DOI] [PubMed] [Google Scholar]
- Sjostrom L, Rissonen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, Krempf M. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight in obese patients. European Multicenter Orlistat study group. Lancet. 1998;352:167–172. doi: 10.1016/S0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- Hill JO, Hauptman J, Anderson JW, Fujioka K, O'Neil PM, Smith DK, Zavoral JH, Aronne LJ. Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: a 1-year study. Am J Clin Nutr. 1999;69:1108–1116. doi: 10.1093/ajcn/69.6.1108. [DOI] [PubMed] [Google Scholar]
- Mc Duffie JR, Calis KA, Booth SL, Uwaifo GI, Yanovski JA. Effects of orlistat on fat soluble vitamins in obese adolescents. Pharmacotherapy. 2002;22:814–822. doi: 10.1592/phco.22.11.814.33627. [DOI] [PubMed] [Google Scholar]
- Hollander PA, Elbein SC, Hirsch IB, Kelley D, McGill J, Taylor T, Weiss SR, Crockett SE, Kaplan RA, Comstock J, Lucas CP, Lodewick PA, Canovatchel W, Chung J, Hauptman J. Role of orlistat in the treatment of obese patients with type-2 diabetes. A 1-year randomized double blind study. Diabetes care. 1998;21:1288–1294. doi: 10.2337/diacare.21.8.1288. [DOI] [PubMed] [Google Scholar]
- Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, Heimburger DC, Lucas CP, Robbins DC, Chung J, Heymsfield SB. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. J Amer Med Assoc. 1999;281:235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Berkowitz RI, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: a randomized trial. Arch Intern Med. 2001;161:218–227. doi: 10.1001/archinte.161.2.218. [DOI] [PubMed] [Google Scholar]
- NHLBI Prescription medication for treatment of obesity http://www.niddk.nih.gov/health/nutrit/nutrit.htm
- Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC. Amylin: a novel action in the brain to reduce body weight. Endocrinology. 2000;141:850–853. doi: 10.1210/en.141.2.850. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Tomlinson JW. Cortisol, 11 beta-hydroxysteroid dehydrogenase type 1 and central obesity. Trends Endocrinol Metab. 2002;13:94–96. doi: 10.1016/S1043-2760(02)00566-0. [DOI] [PubMed] [Google Scholar]
- Clapham JC, Arch JRS, Tadayyon M. Anti-obesity drugs: a critical review of current therapies and future opportunities. Pharmacol Ther. 2001;89:81–121. doi: 10.1016/S0163-7258(00)00105-4. [DOI] [PubMed] [Google Scholar]
- Anonymous P 57 and food intake. obesity Meds and Research News. 2000.
- Anonymous NIH Conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–961. [PubMed] [Google Scholar]
- Karl JG. Overview of surgical techniques for treating obesity. Am J Clin Nutr. 1992;55:552s–555s. doi: 10.1093/ajcn/55.2.552s. [DOI] [PubMed] [Google Scholar]
- Ashley S, Bird DL, Sugden G, Royston CM. Vertical banded gastroplasty for the treatment of morbid obesity. Br J Surg. 1993;80:1421–1423. doi: 10.1002/bjs.1800801122. [DOI] [PubMed] [Google Scholar]
- Shikora SA, Benotti PN, Forre RA. Surgical Treatment of Obesity. In: Blackburn GL, Kanders BS, editor. Obesity, pathophysiology, psychology and treatment. New York Chapman & Hall; 1994. pp. 264–282. [Google Scholar]
- Sagar PM. Surgical treatment of morbid obesity. Br J Surg. 1995;82:732–739. doi: 10.1002/bjs.1800820606. [DOI] [PubMed] [Google Scholar]
- Lonroth H, Dalenback J, Haglind E, Josefsson K, Olbe L, Fagevik Olsen M, Lundell L. Vertical banded gastroplasty by laparoscopic technique in the treatment of morbid obesity. Surg Laparosc Endosc. 1996;6:102–107. doi: 10.1097/00019509-199604000-00004. [DOI] [PubMed] [Google Scholar]
- Salmon PA, McArdle MO. The rationale and results of gastroplasty/gastric by-pass. Obes Surg. 1992;2:61–68. doi: 10.1381/096089292765560565. [DOI] [PubMed] [Google Scholar]
- Seehra H, Macc Dermatt N, Lascelles RG, Taylor TV. Wernicke's encephalapathy after vertical banded gastroplasty for morbid obesity. Br Med J. 1996;312:434. doi: 10.1136/bmj.312.7028.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley EF, Benotti PN, Borlase BC, Hollingshead J, Blackburn GL. Impact of gastric restrictive surgery on hypertension in the morbidly obese. Am J Surg. 1992;163:294–297. doi: 10.1016/0002-9610(92)90005-C. [DOI] [PubMed] [Google Scholar]
- Carson JL, Ruddy ME, Duff AE, Holmes NJ, Cody RP, Brolin RE. The effect of gastric bypass surgery on hypertension in the morbidity obese patients. Arch Intern Med. 1994;154:193–200. doi: 10.1001/archinte.154.2.193. [DOI] [PubMed] [Google Scholar]
- Olsson SA, Petersson BG, Sorbris R, Nilsson-Ehle P. Effects of weight reduction after gastroplasty on glucose and lipid metabolism. Am J Clin Nutr. 1984;40:1273–1280. doi: 10.1093/ajcn/40.6.1273. [DOI] [PubMed] [Google Scholar]
- Herbst CA, Hughes TA, Gwynne JT, Buckwalter JA. Gastric bariatric operation in insulin treated adults. Surgery. 1984;95:209–214. [PubMed] [Google Scholar]
- Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]


