Significance
To aid in prioritizing the development of tuberculosis (TB) vaccines most likely to reach the 2050 TB elimination goal, we estimated the impact and cost-effectiveness of a range of vaccine profiles in low- and middle-income countries. Using mathematical modeling, we show that vaccines targeted at adolescents/adults could have a much greater impact on the TB burden over a 2024–2050 time horizon than those vaccines targeted at infants. Such vaccines could also be cost-effective, even with relatively high vaccine prices. Our results suggest that to achieve the 2050 elimination goals, future TB vaccine development should focus on vaccines targeted at adolescents/adults, even if only relatively low efficacies and short durations of protection are technically feasible.
Keywords: mathematical modeling, epidemiology, threshold analysis
Abstract
To help reach the target of tuberculosis (TB) disease elimination by 2050, vaccine development needs to occur now. We estimated the impact and cost-effectiveness of potential TB vaccines in low- and middle-income countries using an age-structured transmission model. New vaccines were assumed to be available in 2024, to prevent active TB in all individuals, to have a 5-y to lifetime duration of protection, to have 40–80% efficacy, and to be targeted at “infants” or “adolescents/adults.” Vaccine prices were tiered by income group (US $1.50–$10 per dose), and cost-effectiveness was assessed using incremental cost per disability adjusted life year (DALY) averted compared against gross national income per capita. Our results suggest that over 2024–2050, a vaccine targeted to adolescents/adults could have a greater impact than one targeted at infants. In low-income countries, a vaccine with a 10-y duration and 60% efficacy targeted at adolescents/adults could prevent 17 (95% range: 11–24) million TB cases by 2050 and could be considered cost-effective at $149 (cost saving to $387) per DALY averted. If targeted at infants, 0.89 (0.42–1.58) million TB cases could be prevented at $1,692 ($634–$4,603) per DALY averted. This profile targeted at adolescents/adults could be cost-effective at $4, $9, and $20 per dose in low-, lower-middle–, and upper-middle–income countries, respectively. Increased investments in adult-targeted TB vaccines may be warranted, even if only short duration and low efficacy vaccines are likely to be feasible, and trials among adults should be powered to detect low efficacies.
The bacterium Mycobacterium tuberculosis was responsible for ∼8.6 million cases of tuberculosis (TB) disease and ∼1.3 million deaths in 2012 (1), of which over 80% were in low-income countries (LICs) and middle-income countries. This burden remains despite the widespread use of the infant TB vaccine, bacille Calmette–Guérin (bacillus Calmette–Guérin) (2). Dramatic levels of control are required to reach the World Health Organization (WHO) targets of TB elimination as a public health problem by 2050 (3). Previous mathematical modeling has suggested elimination can only be achieved through the use of new vaccines (4–7).
In 2013, there were more than a dozen new TB vaccines in clinical trials, using a large range of antigens and adjuvants (8). A variety of modes of action and target populations are being researched (9, 10). The most recent TB vaccine tested in a large-scale phase II trial reported a nonsignificant impact on TB disease of 17.3% [95% confidence interval (CI): −31.9 to 48.2] (11). However, such an undertaking highlights the progress that has been made in TB vaccine clinical trials, as well as the need for a reevaluation of the potential impact and need for increased investment in new TB vaccines (12).
Estimates of the likely impact and cost-effectiveness of new products before and during development are useful for informing target product profiles and guiding product prioritization. Such analyses of future vaccines have been undertaken for several other diseases (13–17) but have been limited to exploring the cost-effectiveness of infant vaccination in Asia and Sub-Saharan Africa for TB (18–20). To inform the products and targeting that will be most useful for achieving the 2050 elimination target (3), there is a need to systematically estimate the impact in a wide range of settings of a variety of potential TB vaccine profiles defined by efficacy, duration of protection, and potential target groups (e.g., by age). The aim of this study was to estimate this potential impact and cost-effectiveness for prospective TB vaccine profiles in LICs and middle-income countries over the time horizon 2024–2050.
We used an age-structured M. tuberculosis transmission model. To be conservative in vaccine impact, we model an aggressive scale-up of existing technologies before the introduction of the new vaccine, in line with the recently approved post-2015 WHO global TB strategy (21). New TB vaccines were assumed to be available in 2024, to prevent active TB in infected and uninfected individuals, to have a 5-y to lifetime duration of protection and 40–80% efficacy, and to be targeted at “infants” or “adolescents/adults.” The former involved vaccination at birth, and the latter involved vaccination at the age of 10 y in schools supplemented with mass campaigns directed to those individuals aged 11 y and older at a frequency corresponding to the duration of protection or every 10 y (whichever was longer). These age groups were chosen to reflect the feasibility of vaccine delivery. Infant vaccination would be alongside the routine schedule, and 10-y-olds could be reached in schools, where immunization is becoming increasingly common. Mass campaigns were included as a scenario in which all people at risk from TB could be targeted. Vaccine prices were tiered by country income group, as measured in US dollars ($1.50–$10 per dose), and cost-effectiveness was defined as a cost per disability adjusted life year (DALY) averted of less than the gross national income (GNI) per capita.
We carried out two main analyses. The first estimated the impact and cost-effectiveness of a broad range of prespecified potential vaccine characteristics when targeted at infants or adolescents/adults. The second analysis estimated the maximum price per vaccine dose at which the vaccine could still be deemed cost-effective. Full details are provided in Materials and Methods and SI Appendix.
Results
Model Calibration (“No New Vaccine Scale-Up” Scenario).
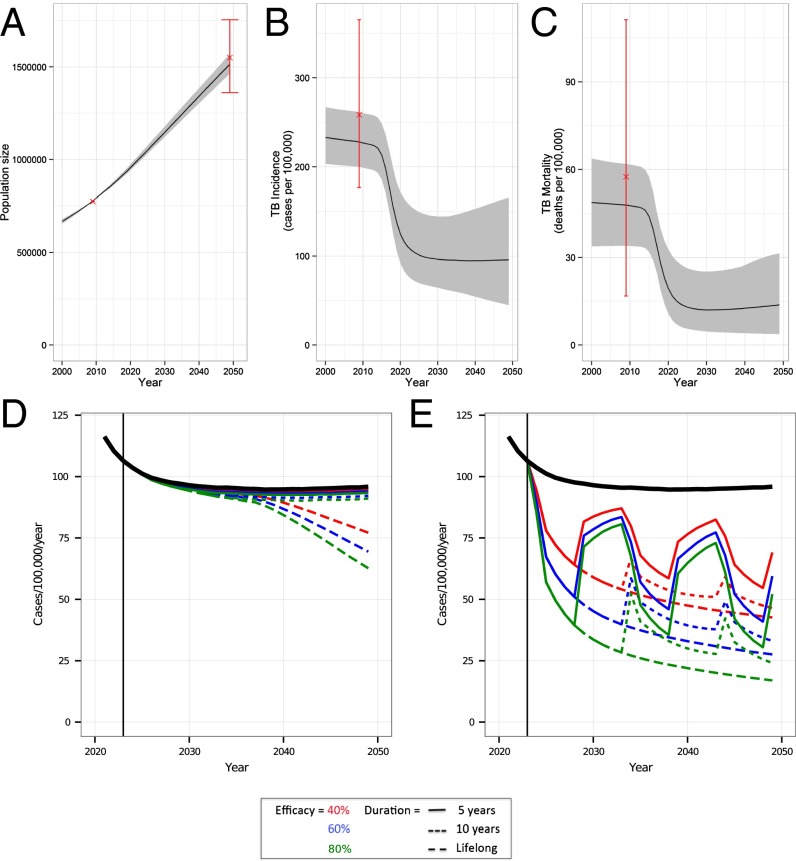
The results from the calibration of the model to 91 countries are shown in Fig. 1 A–C for LICs and in SI Appendix, Fig. S4 for lower middle-income countries (LMICs) and upper middle-income countries (UMICs). TB incidence and mortality fall rapidly between 2012 and 2020 as a result of our assumed aggressive scale-up of existing technologies to be conservative about potential vaccine benefit. Our fitting method incorporated uncertainty in TB/HIV natural history parameters and WHO and United Nations (UN) estimates of burden, which is shown as the gray areas on the graphs (Fig. 1 A–C). A summary of the natural history parameters used to generate the calibration (posterior parameter distributions) is shown in SI Appendix, Fig. S5.
Fig. 1.
Model calibration (A–C) and vaccine impact (D and E) in LICs. In A–C, the median (solid black line) and 95% range (gray cloud) of model fits to data (red, cross and range) are shown. (A) Human population size (in 1,000s) in LICs for the years 2000–2050. (B) TB incidence (cases per 100,000 per year) for the years 2000–2050. (C) TB mortality (deaths per 100,000 per year) for the years 2000–2050. TB incidence (cases per 100,000 per year) for the years 2000–2050 with the median model output (black line) and vaccine profile impact. Characteristics of efficacy (color) and duration of protection (line type) are shown for vaccines targeted at infants (D) or adolescent/adults (E). A vaccine targeted at infants (D) has a smaller impact on TB disease incidence than one targeted at adolescents/adults (E). In E, “waves” within the adolescent/adult incidence are due to mass campaigns.
The fitted model predicted the highest TB incidence to be in LICs (Fig. 1 B and C). With our optimistic scale-up of TB control (Fig. 1 B and C), TB incidence in 2050 is in line with the level that could be achieved assuming the current global rate of decline of 2% a year is continued (SI Appendix, Fig. S1), but it is higher than the newly proposed post-2015 WHO targets for 2035 (21). The proportion of the TB burden in children (aged <15 y) predicted by the model was 2–7%, compared with global estimates of 5% in 2011 (22).
Epidemiological Impact of New TB Vaccine Introduction.
When targeting infants, a new TB vaccine was predicted to avert less than 12% of the TB burden in LICs across the 2024–2050 time horizon (Fig. 1D and Tables 1 and 2). This prediction is primarily due to the fact that vaccinated cohorts do not reach the age of high TB risk before 2050. Compared with LICs, a smaller percentage of decrease in cases and deaths is predicted for LMICs and UMICs due to the greater decrease in incidence prior to vaccine introduction in the latter two groups (Table 2). For example, a vaccine with a 10-y duration of protection and 40% efficacy could avert 1.8% (95% range: 1.1–2.9%) of cases in LICs but only 1.1% (95% range: 0.5–2.4%) and 0.5% (95% range: 0.2–1.3%) of cases in LMICs and UMICs, respectively.
Table 1.
Predicted total TB cases and deaths across 2024–2050 by income group without new vaccine [median (95% range)]
| LIC | LMIC | UMIC | |
| Total cases, millions | 32 (23–44) | 46 (30–87) | 19 (11–32) |
| Total deaths, millions | 4.2 (2.2–7.1) | 5.0 (2.0–13.5) | 0.9 (0.3–3.5) |
Table 2.
Impact of introduction of a new TB vaccine across 2024–2050: Percentage reduction in TB cases, cost per DALY averted (av.), and cost-effective (CE) vaccine price, by income group, vaccine age target group, vaccine duration of protection, and vaccine efficacy [median (95% range)]
| LIC (GNI = $563) | LMIC (GNI = $2,250) | UMIC (GNI = $7,149) | ||||||||
| Dur. | Eff., % | Reduction in cases, % | Cost per DALY av.,* US $1,000s | CE vaccine price,* US $ | Reduction in cases, % | Cost per DALY av.,* US $1,000s | CE vaccine price,* US $ | Reduction in cases, % | Cost per DALY av.,* US $10,000s | CE vaccine price,* US $ |
| Infant | ||||||||||
| 5 y | 40 | 1.0 (0.6–1.6) | 4.72 (1.78–12.77) | NA | 0.6 (0.3–1.3) | 33.11 (9.94–91.32) | NA | 0.3 (0.1–0.7) | 54.42 (5.84–232.15) | NA |
| 60 | 1.5 (0.9–2.4) | 3.09 (1.17–8.39) | NA | 0.9 (0.4–2.0) | 21.55 (6.60–60.68) | 0.11 (NA–1.55) | 0.4 (0.1–1.1) | 34.87 (3.76–150.75) | 0.24 (NA–3.24) | |
| 80 | 1.9 (1.2–3.2) | 2.28 (0.87–6.24) | 0.07 (NA–0.86) | 1.2 (0.6–2.7) | 15.82 (4.63–45.06) | 0.42 (NA–2.40) | 0.5 (0.2–1.4) | 25.39 (2.58–110.06) | 0.66 (NA–4.71) | |
| 10 y | 40 | 1.8 (1.1–2.9) | 2.61 (0.98–7.11) | NA | 1.1 (0.5–2.4) | 18.24 (5.32–51.85) | 0.25 (NA–1.97) | 0.5 (0.2–1.3) | 28.63 (3.08–128.89) | 0.42 (NA–4.33) |
| 60 | 2.7 (1.6–4.3) | 1.69 (0.63–4.60) | 0.28 (NA–1.29) | 1.7 (0.8–3.6) | 11.62 (3.47–34.52) | 0.82 (NA–3.26) | 0.7 (0.3–1.9) | 18.11 (1.67–80.91) | 1.25 (NA–6.84) | |
| 80 | 3.5 (2.1–5.7) | 1.22 (0.44–3.50) | 0.54 (NA–1.97) | 2.3 (1.1–4.8) | 8.38 (2.52–25.45) | 1.36 (0.06–4.50) | 0.9 (0.4–2.6) | 12.34 (1.06–59.97) | 2.06 (NA–9.14) | |
| Lifelong | 40 | 6.2 (4.1–8.3) | 0.65 (0.26–1.87) | 1.26 (0.25–3.03) | 3.9 (2.1–6.2) | 5.12 (1.31–18.63) | 2.39 (0.37–7.76) | 1.6 (0.6–3.1) | 5.38 (0.42–32.15) | 3.53 (0.23–11.75) |
| 60 | 8.9 (5.9–11.9) | 0.40 (0.15–1.20) | 2.07 (0.61–4.47) | 5.7 (3.1–9.0) | 2.99 (0.68–11.45) | 3.97 (0.89–11.27) | 2.3 (0.9–4.6) | 2.75 (CS–20.33) | 5.81 (0.90–18.82) | |
| 80 | 11.5 (7.6–15.2) | 0.30 (0.08–0.83) | 2.81 (1.01–5.82) | 7.4 (4.0–11.7) | 2.03 (0.24–8.61) | 5.40 (1.49–15.06) | 3.1 (1.2–6) | 1.36 (CS–13.99) | 8.15 (1.48–23.58) | |
| Adult | ||||||||||
| 5 y | 40 | 23.9 (18.8–29.1) | 0.63 (0.30–1.38) | 1.30 (0.39–2.89) | 24.3 (18.3–32) | 3.50 (1.48–9.17) | 3.45 (1.13–7.1) | 17.5 (11.2–25.8) | 2.45 (CS–11.61) | 6.97 (2.39–15.87) |
| 60 | 33.1 (26.3–39.7) | 0.38 (0.15–0.88) | 2.18 (0.85–4.27) | 33.9 (26.0–43.4) | 2.05 (0.79–5.75) | 5.36 (2.29–9.94) | 25.0 (16.3–36.1) | 0.40 (CS–5.70) | 10.90 (4.47–22.22) | |
| 80 | 40.7 (32.8–48.2) | 0.26 (0.08–0.65) | 2.88 (1.30–5.37) | 41.9 (33.1–52.9) | 1.28 (0.27–3.95) | 7.08 (3.31–12.33) | 31.9 (21.1–44.9) | CS (CS–3.28) | 14.69 (6.10–28.89) | |
| 10 y | 40 | 39.6 (32.7–46.4) | 0.28 (0.09–0.69) | 2.73 (1.20–5.19) | 39.6 (31.6–49.4) | 1.53 (0.47–4.48) | 6.44 (3.01–11.58) | 30.4 (21.3–41.0) | CS (CS–3.37) | 13.18 (5.62–25.48) |
| 60 | 52.1 (44.1–59.5) | 0.15 (CS–0.39) | 3.98 (2.05–7.07) | 52.8 (44.0–63.4) | 0.72 (CS–2.54) | 9.04 (4.58–15.87) | 42.4 (30.8–54.9) | CS (CS–1.10) | 19.95 (9.25–37.04) | |
| 80 | 61.8 (53.5–69.0) | 0.07 (CS–0.24) | 5.03 (2.75–8.48) | 63.5 (54.9–73.5) | 0.26 (CS–1.59) | 11.52 (6.20–19.48) | 53.0 (39.7–66.2) | CS (CS–0.28) | 25.78 (12.14–47.90) | |
| Lifelong | 40 | 44.1 (38.4–49.9) | 0 (CS–0.13) | 7.39 (3.93–12.54) | 42.1 (34.6–50.9) | 0.14 (CS–1.49) | 13.25 (6.19–28.43) | 34.4 (28.6–43.2) | CS (CS–CS) | 30.38 (13.54–55.94) |
| 60 | 58.5 (52.6–64.3) | CS (CS–0.05) | 10.10 (5.67–16.47) | 56.6 (48.7–65.6) | CS (CS–0.48) | 18.31 (9.12–39.20) | 48.5 (41.5–57.8) | CS (CS–CS) | 41.95 (20.91–82.04) | |
| 80 | 70.0 (64.4–75.0) | CS (CS–CS) | 12.22 (7.00–19.82) | 68.3 (61.0–76.2) | CS (CS–0.05) | 22.56 (11.23–44.06) | 61.0 (53.5–69.7) | CS (CS–CS) | 54.49 (26.16–97.53) | |
CS, cost-saving (i.e., negative cost per DALY, the intervention was dominant); Dur., duration; Eff., efficacy; NA, negative vaccine price.
Discounting included.
If, instead, a vaccine was targeted at adolescents/adults, up to 70% of the TB burden could be averted (Fig. 1E and Table 2). For example, a vaccine with 10-y duration of protection and 40% efficacy targeted at adolescents/adults could avert 40% (95% range: 33–46%) of cases in LICs (Table 2). In LMICs, the percentage of decline could be similar, but a higher absolute number of cases and deaths could be averted (Table 2). The impact in UMICs is predicted to be smaller, because TB incidence is already low, but the percentage of decline could still be substantial: 30% (95% range: 21–41%) of cases and 28% (95% range: 19–38%) of deaths may be averted (Table 2).
The impact on TB incidence is highlighted in Fig. 1 D and E, where the wave patterns in TB incidence reflect the impact of mass campaigns (Fig. 1E). The majority of incidence cases are due to recent infections, although there is some variation by country. When the exact duration of vaccine protection has ended, there is a rapid increase in the number of latent and susceptible individuals in the population, who are vulnerable to (re)infection and subsequent disease, causing the rapid increase in new cases.
Treatment and Productivity Costs.
Our regression model for TB treatment costs predicted that mean cost per patient varied from $64 (95% CI: $27–$152) in the Democratic Republic of Congo to $3,913 (95% CI: $2,266–$6,759) in Chile (SI Appendix, Table S11). Mean multidrug-resistant (MDR) TB treatment costs in the same two countries were $196 (95% CI: $44–$872) and $14,894 (95% CI: $10,086–$21,995), respectively. Productivity costs per TB case were predicted to range from $54/$162 in the Democratic Republic of Congo to $2,034/$6,102 in Chile for a TB/MDR-TB case (SI Appendix, Table S14).
An infant vaccine with a 10-y duration of protection and 80% efficacy could lead to cumulated, discounted TB treatment cost savings of $185 million in LICs, $757 million in LMICs, and $1.6 billion in UMICs between 2024 and 2050. A similar profile adolescent/adult vaccine could reduce TB and MDR-TB treatment costs by $5.3 million in LICs, $35.6 billion in LMICs, and $133.4 billion in UMICs.
Cost-Effectiveness Estimates.
When targeted at infants, with our tiered pricing structure, only the lifelong duration of protection vaccine profiles with high efficacy in LICs and LMICs were considered cost-effective (Table 2). For example, a vaccine with a 10-y duration of protection and 40% efficacy is considered not to be cost-effective at an estimated cost per DALY averted of $2,605 (95% range: $984–$7,105) in LICs.
In contrast, vaccines targeted at adolescents/adults are potentially either cost-effective or cost-saving over the 2024–2050 time horizon (Table 2). For all income groups, vaccines with a duration of protection greater than 5 y are predicted to be cost-effective, and even cost-saving in UMICs. For example, in LICs, a profile with a 5-y duration of protection and 60% efficacy is cost-effective at an estimated cost per DALY averted of $378 (95% range: $150–$881).
With the inclusion of productivity costs (SI Appendix, Table S14), more profiles are predicted to be cost-saving (SI Appendix, Table S4).
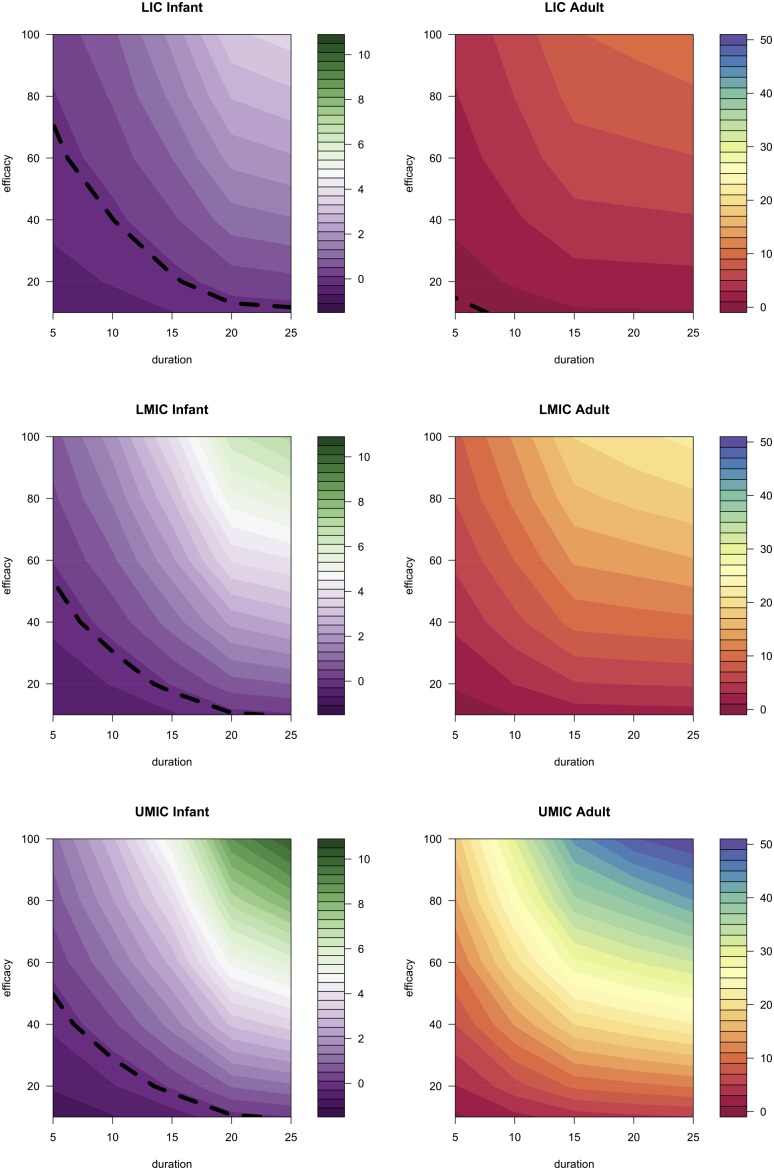
“Cost-Effective Vaccine Price” Analysis.
The second analysis estimated a lower bound on efficacies and duration for vaccines to be considered cost-effective (dashed lines in Fig. 2). In LICs, many of the vaccine profiles evaluated did not have a cost-effective vaccine price when targeted at infants (Fig. 2), implying that the price per dose could be zero and the vaccine would still not be cost-effective because of the resources used delivering it. Other profiles targeted at infants would also need to have a low price: the cost-effective vaccine price per dose of a vaccine profile with a 10-y duration of protection and 60% efficacy in LICs is predicted to be only $0.28 [95% range: negative vaccine price (NA) −$1.29] as is seen in Table 2 (Fig. 2). In LMICs, the same vaccine profile could have a cost-effective vaccine price of $0.82 (NA−$3.26), rising to $1.25 (NA−$6.84) in UMICs, reflecting the higher averted treatment costs and GNI.
Fig. 2.
Vaccine targeted at adolescents/adults can cost more per dose than one targeted at infants and still be cost-effective. The contour plots shown here are of the cost-effective vaccine price (US dollars) that results in the cost per DALY for a vaccine, of a certain duration and efficacy, equal to the mean GNI per capita. The color represents the price as indicated in the legend. The dashed black line represents the values below which no price would be cost-effective. For example, if given in LICs, an adolescent/adult vaccine with a 15-y duration of protection and 60% efficacy could be cost-effective when priced at ∼$7.
For an adolescent/adult-targeted vaccine, the price per vaccine dose can be higher and the vaccine profile still be considered cost-effective because it has a larger epidemiological impact, and hence averts a greater treatment cost burden (Figs. 1, D and E, and 2). A profile with a duration of protection of 10 y and 60% efficacy could be cost-effective at a vaccine price per dose of $3.98 (95% range: $2.05–$7.07), $9.04 (95% range: $4.58–$15.87), and $19.95 (95% range: $9.25–$37.04) in LICs, LMICs, and UMICs, respectively (Table 2).
Crucially, when targeted at adolescents/adults, all vaccine profiles considered had a price per dose at which the vaccine could be considered cost-effective, whereas those vaccine profiles targeted at infants did not (Fig. 2). For example, a vaccine profile with an efficacy of 20% and a duration of protection of 10 y could be cost-effective at vaccine prices per dose of $1.19 (95% range: $0.28–$2.66), $3.04 (95% range: $1.05–$6.35), and $6.17 (95% range: $2.21–$13.8) in LICs, LMICs, and UMICs, respectively.
Sensitivity and Uncertainty.
A partial rank correlation coefficient (PRCC) analysis revealed that the most influential parameters on the number of TB cases averted by a vaccine were those parameters governing case detection rates and progression to disease.
In our scenario analyses, a less optimistic TB control scale-up scenario predicted a larger future burden of TB; hence, the impact of the same vaccine profiles with the same coverage would be greater (SI Appendix, Fig. S9). Overall cost-effectiveness results were similar (SI Appendix, Table S5). A second scenario analysis with decreasing HIV incidence found little effect of this decrease on the cost-effectiveness of the vaccine profiles (SI Appendix, Table S6). A small number of vaccine profiles were no longer cost-effective when HIV incidence was decreased. This finding reflects the lower TB burden with decreased HIV incidence, and therefore a lower impact of a vaccine.
Discussion
In this study, we have used a calibrated transmission model of TB to estimate the disease burden and costs of TB that could be averted by different vaccine profiles in LICs and middle-income countries before 2050. Our primary finding is that the number of cases and deaths averted by a TB vaccine targeted at infants is predicted to be substantially lower than one targeted at adolescents/adults. This finding is highlighted in the associated cost-effectiveness analysis, which estimates that vaccines targeted at infants would not be cost-effective before 2050 with our assumed vaccine prices unless the duration of protection were lifelong and the efficacy high. Meanwhile, vaccines targeted at adolescents/adults could be cost-effective (under different pricing assumptions), or even cost-saving before 2050, if the duration of protection were 10 y or longer or the efficacy were equal to or greater than 20%.
A novel TB vaccine targeted at infants has a smaller immediate impact than one targeted at adolescents/adults due to children having lower rates of TB notifications; lower proportions of smear-positive pulmonary TB; and, as a consequence, a smaller contribution to TB transmission (23). Thus, a vaccine targeted at infants has both a smaller direct impact (by preventing relatively smaller numbers of active cases) and a smaller indirect impact (due to fewer secondary cases being prevented). Conversely, a vaccine targeted at adolescents/adults has a high direct impact on the very population with the greatest burden of active TB (i.e., the targeted population of 10-y-olds vaccinated in schools and those individuals reached in mass campaigns). A vaccine targeted at adolescents/adults will also have a large indirect impact and is likely to prevent, before 2050, more infant cases of TB than a vaccine targeted at infants due to the reduction in transmission. Similarly, despite a lower efficacy in those individuals with HIV, as assumed here, a vaccine could significantly decrease overall TB transmission and avert the burden in both HIV-negative and HIV-positive individuals.
As shown in other modeling studies, this work predicts a large impact of new TB vaccines (4–7). Our approach differs in that we explored a range of vaccine profiles, instead of comparing different mechanisms or different targeting strategies. A strength of our modeling is that we calibrated data for the majority of LICs and middle-income countries to the individual country level. The limitation of this approach is that we only calibrated to a single time point. We also incorporated uncertainty in vaccine pricing by performing an analysis to combine impact and cost analysis. Further uncertainty was included in our scenario analysis around TB control scale-up and HIV incidence.
Our estimates were associated with a large level of uncertainty (Fig. 1 and SI Appendix, Fig. S4). We believe that this uncertainty is an appropriate reflection of the state of knowledge of TB natural history, country level burden, and cost variation, as well as uncertainty associated with predicting into the future. Due to this uncertainty, we grouped our analysis by income group to allow for broad conclusions to be made. Similarly, our comparisons between infant and adolescent/adult impact should be interpreted as magnitudes of difference and not precise estimates. Another limitation is that we assumed a homogeneous population, which does not capture the heterogeneity in TB risk or likelihood of being vaccinated. Thus, our cost-effectiveness estimates may be an overestimation, but the relative impact of infant vs. adolescent/adult vaccination is likely to remain the same. Further modeling could explore these issues.
Because the aim of this work was to explore a range of vaccine profiles with different vaccine characteristics and target populations, we decided to explore only one vaccine mode of action (to prevent active disease and not infection). This decision is in line with vaccine candidates in active research (24), whereas vaccines that prevent infection are considered less likely (4). With further data on candidates and an increased understanding of their true immunological effect, different assumptions could be more appropriate and could be explored.
Although vaccine coverage of infant vaccines can be predicted with good confidence due to relatively robust routine data, it is difficult to determine the likely coverage of the adolescent/adult campaigns. However, for future TB vaccines, the mass delivery option is considered the most feasible (25). We assumed that vaccine coverage of around 75% could be achieved in the mass campaigns, which is less than was achieved in most rubella vaccine campaigns targeting women of child-bearing age and in recent meningococcal A vaccine campaigns in West Africa targeting all 1- to 29-y-olds (26). If higher coverage can be obtained, we may have underestimated the impact, or we may have overestimated the impact if these levels cannot be reached in mass campaigns. The delivery costs of mass campaigns also remain highly uncertain, along with the costs of productivity loss; thus, the uncertainty in our cost-related estimates is a further limitation. We did not attempt to address the debate about the feasibility of frequent mass campaigns but chose to set 10 y as a minimum frequency based on expert opinion. With more frequent mass campaigns, the impact of the adolescent/adult vaccine could be greater but the incremental costs would be higher. For vaccines with a short duration of protection (<10 y), the mass campaign frequency is crucial for preserving their initial impact; there is a gradual increase in incidence following short duration of protection vaccination to the levels seen in the base case (Fig. 1E).
To be conservative in vaccine impact, in our main analysis, we modelled an aggressive scale-up of existing technologies in line with the recently approved WHO global TB strategy (21). A less aggressive scale-up was explored in our scenario analysis, showing that with lower TB control before vaccination, a new TB vaccine would have a bigger impact, and hence be more cost-effective. With future data, the actual improvements in health care could be included in subsequent models before vaccine implementation. Future models could also include increasing levels of MDR-TB. With a future higher burden of MDR-TB, we would expect any new TB vaccine to have a bigger impact, and hence be more cost-effective. Further limitations of our modeling lie in our HIV assumptions, with only late-stage HIV being captured and antiretroviral coverage taken as a weighted average.
A key focus of this analysis was the time horizon (2024–2050). We decided to evaluate impact up to 2050 because this date is the WHO elimination target date (21), and it is already far beyond the time horizon of most policy making. However, this time frame meant that we did not measure the full lifetime impact of a vaccine on all individuals vaccinated, and our results should be seen as being specific to 2024–2050. An infant-targeted vaccine would be more cost-effective if the time horizon were extended, but these benefits would not help achieve the elimination goal. If a vaccine were introduced later than 2024 or if the end date were earlier (e.g., 2040 or 2045), our key results would remain the same. The chance of a vaccine being cost-effective would decrease with later vaccine introductions or earlier end dates.
The cost per DALY averted declines rapidly for higher income countries due to the increasing treatment and productivity costs averted. Thus, a vaccine is more likely to become cost-saving with increasing duration of protection and efficacy in UMICs than in LICs, despite our tiered vaccine pricing assumption. This pattern is reflected in the higher vaccine prices that can be cost-effective in UMICs.
Our regression analysis method for approximating treatment and productivity costs in all study countries has been used in previous studies (27, 28), and we consider these results to be relatively robust estimates despite heterogeneity between studies. Less evidence is available about vaccine delivery costs, particularly for mass campaigns, and we had to make relatively crude assumptions about this parameter. Further analysis could investigate the costs associated with the logistical challenges of carrying out the mass campaigns modelled here.
To address the uncertainty in vaccine price, we performed a threshold analysis, reversing the question to find the vaccine price that would be cost-effective by vaccine profile. This analysis revealed that the cost-effective vaccine price for an infant-targeted vaccine would be in a similar range to those prices charged for other vaccines (<$10) (28, 29). Adolescent/adult-targeted vaccine could be priced at a higher level, but still less than the price of human papillomavirus vaccines (<$50) (30).
TB vaccines have the potential to tackle both the large latent TB burden and the increasing levels of MDR-TB. This potential appears largest if the vaccine is targeted at adolescents/adults, providing further evidence, along with the report on increased efficacy against pulmonary TB in screened adults (31), for a recently suggested strategy (32) of giving bacillus Calmette–Guérin to screened adults. However, for those individuals already infected with TB or other environmental Mycobacteria (i.e., who fail the screen), there is still a need, and a large market, for a new TB vaccine.
The broader implications of our study are that increased investments in the development of TB vaccines targeted at adolescents/adults may be rewarded with a rapid impact on TB disease. Specifically, such may be the case, even if only vaccines of short duration or low efficacy are likely to be technically feasible, suggesting that future TB clinical trials among adults should be powered to detect low efficacies.
Materials and Methods
Data.
Demographic data were extracted from the UN population division 2010 revision (33). TB incidence and mortality by HIV status estimates were obtained from the WHO [full methods are available in Annex 1 of the 2013 global TB report (1)], as were case detection rates and treatment success levels (22). HIV incidence and antiretroviral coverage were extracted from the Joint United Nations Program on HIV/AIDS (34). World Bank income group classifications were used (35), and treatment costs were estimated from the literature (SI Appendix). Ninety-one LICs and middle-income countries were included in the final analysis (>96% of the TB burden) (SI Appendix, Table S2).
Model and Analysis.
We used an age- and HIV status-structured transmission model to estimate the TB burden in a base case and a range of alternative vaccination scenarios (SI Appendix, Fig. S3). The model was programmed in R (36). This model was calibrated to the TB burden in 2009 and population size in 2009 and 2050 in each of the 91 countries via Sobol sequence sampling and approximate Bayesian computation. Natural history parameter ranges were assumed to be the same for all countries, but TB and HIV control parameters varied by country (SI Appendix, Tables S1 and S3). We explicitly model only those people with late stage HIV infection (stages 3 and 4). Generating 1,000 calibrations for each country allowed for the inclusion of the uncertainty in both natural history parameters and data. Estimates of TB burden between 2009 and 2050 were calculated under optimistic assumptions of improved TB and HIV control in each country to be conservative about vaccine impact and to reflect ambitious WHO targets for improved TB control (21) (Fig. 1, decline pre-2024, and SI Appendix, Figs. S1 and S2). Country-level projections were combined to give an estimate (median and range) of the TB burden without the introduction of a new TB vaccine, but with bacillus Calmette–Guérin at current levels, by income group (Fig. 1). The impact due to the introduction in 2024 of several potential TB vaccine profiles, which differ by duration of protection and efficacy, to different target populations was then considered in terms of cases and deaths averted. After expert consultation, we investigated nine profiles with efficacies of 40%, 60%, or 80% and durations of protection of 5 y, 10 y, or lifelong. The vaccine was assumed to prevent active disease in both infected and noninfected individuals (24), and be 40% (10–70%) less effective in HIV-infected individuals (37, 38). Vaccination was modelled as “take” (i.e., all or nothing protection). “Efficacy” was then the proportion of all those individuals vaccinated in whom the vaccine was 100% effective. Duration of protection was exact. The two target populations were infants via routine vaccination at birth and at 6 mo or adolescents/adults. The latter involved vaccination of 10-y-olds in schools with two doses given 6 mo apart and additional mass campaigns in two rounds directed to all persons aged 11 y or older in 2024 and at a frequency corresponding to the duration of protection or 10 y (whichever was longer). For each country, 2011 vaccine coverage of the third dose of the diphtheria/tetanus/pertussis vaccine was used for the infant schedule (39) and school attendance levels were used for coverage of 10-y-olds (29). Coverage of mass campaigns was assumed to be 20% less than obtained in rubella vaccine campaigns targeting women of child-bearing age (SI Appendix).
TB Treatment, Productivity, and Vaccine Delivery Costs.
We undertook a systematic literature review on mean TB treatment cost per patient and used a regression analysis to extrapolate these data to all study countries (27, 28) (SI Appendix, Table S11). Productivity costs due to TB were estimated using the human capital method (40) by assuming a productivity loss of 2 mo or 6 mo for a case of TB or MDR-TB, respectively (41, 42) (SI Appendix, Table S14). All costs are expressed in 2012 US dollars.
Vaccines were assumed to be delivered from a single vial with 5% wastage (43), and with tiered pricing per dose of $1.5, $5, and $10 in LICs, LMICs, and UMICs, respectively (44). Vaccine delivery cost per dose also varied according to income group, ranging from $0.59–$1.72 (39) (SI Appendix, Table S7).
Cost-Effectiveness Analysis.
The cost-effectiveness of a new TB vaccine was compared with the current scenario with bacillus Calmette–Guérin only. The analysis was undertaken from a health sector and societal perspective, with the difference being that productivity costs were included in the latter. The primary outcome measure was DALYs, estimated using 5 mo of disability for TB and no age weighting (45). DALYs were calculated using the standard formula (46) (SI Appendix). We classified a vaccine as cost-effective a priori if the incremental cost per DALY averted, calculated as the average across all countries in an income group, was less than the GNI per capita in 2011, which was $563 for LICs, $2,250 for LMICs, and $7,149 for UMICs, in line with the WHO “very cost-effective” threshold (47). Future values were discounted at 3% a year.
Our secondary analysis determined the price at which the cost per DALY averted equalled the average GNI per capita across all countries in an income group (SI Appendix). A larger range of vaccine profiles was explored: 10–100% efficacy and 5- to 25-y duration of protection. The same infant and adolescent/adult targeting strategies were used.
Sensitivity and Uncertainty Analysis.
Uncertainty in our estimates was generated by sampling from natural history parameter values and by sampling the treatment cost value for each fit from a gamma distribution generated from the 95% uncertainty range. The proportion of MDR-TB successfully treated was also varied between 10% and 90% for each fit. To investigate the influence of individual parameters on model outcomes, we used PRCCs. We also assessed the sensitivity of our results in two scenarios: a less optimistic TB control scale-up and a halving of HIV incidence (SI Appendix).
Supplementary Material
Acknowledgments
The work was funded by Aeras Grant PHGHVK5610 (to G.M.K., U.K.G., Y.V.L., and R.G.W.). R.G.W. is also funded by the UK Medical Research Council (Grant MR/J005088/1), the Bill and Melinda Gates Foundation [TB Modelling and Analysis Consortium (Grant 21675/OPP1084276) and Consortium to Respond Effectively to the AIDS/TB Epidemic (Grant 19790.01)], and the Centers for Disease Control and Prevention/the US President's Emergency Plan for AIDS Relief via the Aurum Institute (Grant U2GPS0008111).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404386111/-/DCSupplemental.
References
- 1.World Health Organization . Global Tuberculosis Report. 2013. . Available at www.who.int/tb/publications/global_report/en/. Accessed July 2, 2014. [Google Scholar]
- 2.World Health Organization . BCG Vaccine. 2014. . Available at www.who.int/biologicals/areas/vaccines/bcg/en/. Accessed July 2, 2014. [Google Scholar]
- 3.World Health Organization . The Stop TB Strategy. 2014. . Available at www.who.int/tb/strategy/stop_tb_strategy/en/. Accessed July 2, 2014. [Google Scholar]
- 4.Abu-Raddad LJ, et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci USA. 2009;106(33):13980–13985. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271–286. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 6.Lietman T, Blower SM. Potential impact of tuberculosis vaccines as epidemic control agents. Clin Infect Dis. 2000;30(Suppl 3):S316–S322. doi: 10.1086/313881. [DOI] [PubMed] [Google Scholar]
- 7.Ziv E, Daley CL, Blower S. Potential public health impact of new tuberculosis vaccines. Emerg Infect Dis. 2004;10(9):1529–1535. doi: 10.3201/eid1009.030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoever K, Thole J. TB Vaccine Research and Development: A Business Case for Investment. Aeras, Rockville, MD and Tuberculosis Vaccine Initiative; Lelystad, The Netherlands: 2013. [Google Scholar]
- 9.Cayabyab MJ, Macovei L, Campos-Neto A. Current and novel approaches to vaccine development against tuberculosis. Front Cell Infect Microbiol. 2012;2:154. doi: 10.3389/fcimb.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan MJ, Thole J. Tuberculosis vaccines: A strategic blueprint for the next decade. Tuberculosis (Edinb) 2012;92(Suppl 1):S6–S13. doi: 10.1016/S1472-9792(12)70005-7. [DOI] [PubMed] [Google Scholar]
- 11.Tameris MD, et al. MVA85A 020 Trial Study Team Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dye C, Fine PE. A major event for new tuberculosis vaccines. Lancet. 2013;381(9871):972–974. doi: 10.1016/S0140-6736(13)60137-3. [DOI] [PubMed] [Google Scholar]
- 13.Tediosi F, Maire N, Penny M, Studer A, Smith TA. Simulation of the cost-effectiveness of malaria vaccines. Malar J. 2009;8:127. doi: 10.1186/1475-2875-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee BY, Bacon KM, Bailey R, Wiringa AE, Smith KJ. The potential economic value of a hookworm vaccine. Vaccine. 2011;29(6):1201–1210. doi: 10.1016/j.vaccine.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BY, et al. Economic value of dengue vaccine in Thailand. Am J Trop Med Hyg. 2011;84(5):764–772. doi: 10.4269/ajtmh.2011.10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bishai D, Lin MK, Kiyonga CW. Modeling the economic benefits of an AIDS vaccine. Vaccine. 2001;20(3-4):526–531. doi: 10.1016/s0264-410x(01)00335-8. [DOI] [PubMed] [Google Scholar]
- 17.International AIDS Vaccination Initiative 2006. The Impact of an AIDS Vaccine in Developing Countries: A New Model and Preliminary Results, Policy Research Working Paper 8 (International AIDS Vaccination Initiative, New York)
- 18.BIO Ventures for Global Health 2006. Tuberculosis Vaccines: The Case for Investment. Available at www.bvgh.org/News-and-Publications/Reports-and-Publications.aspx. Accessed July 2, 2014.
- 19.Ditkowsky JB, Schwartzman K. Potential cost-effectiveness of a new infant tuberculosis vaccine in South Africa—Implications for clinical trials: A decision analysis. PLoS ONE. 2014;9(1):e83526. doi: 10.1371/journal.pone.0083526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng CL, Oxlade O, Menzies D, Aspler A, Schwartzman K. Cost-effectiveness of novel vaccines for tuberculosis control: A decision analysis study. BMC Public Health. 2011;11:55. doi: 10.1186/1471-2458-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Executive Board . Global Strategy and Targets for Tuberculosis Prevention, Care and Control after 2015 (EB134.R4) 2014. . Available at http://apps.who.int/gb/e/e_eb134.html. Accessed July 2, 2014. [Google Scholar]
- 22.World Health Organization . Global Tuberculosis Report. 2012. . Available at www.who.int/tb/publications/en/. Accessed July 2, 2014. [Google Scholar]
- 23.Styblo K. Epidemiology of Tuberculosis. Selected Papers. Royal Netherlands Tuberculosis Association; The Hague, The Netherlands: 1991. [Google Scholar]
- 24.Aagaard C, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011;17(2):189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 25.Aeras 2013. Planning for Adult Vaccination in Middle- and Low-Income Countries, HIV, TB, and Malaria Workshop, September 4–5, 2013. Available at www.aeras.org/blog/planning-for-adult-vaccination-in-middle-and-low-income-countries-hiv-tb-an. Accessed July 2, 2014.
- 26.Centers for Disease Control and Prevention Serogroup A meningococcal conjugate vaccine coverage after the first national mass immunization campaign-Burkina Faso, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(50):1022–1024. [PubMed] [Google Scholar]
- 27.Adam T, Evans DB, Murray CJ. Econometric estimation of country-specific hospital costs. Cost Eff Resourc Alloc. 2003;1(1):3. doi: 10.1186/1478-7547-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths UK, Clark A, Hajjeh R. Cost-effectiveness of Haemophilus influenzae type b conjugate vaccine in low- and middle-income countries: Regional analysis and assessment of major determinants. J Pediatr. 2013;163(1 Suppl):S50–S59.e59. doi: 10.1016/j.jpeds.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.UNICEF 2011. Childinfo (Monitoring the Situation of Children and Women). Available at http://data.unicef.org/. Accessed July 2, 2014.
- 30.Nguyen A, et al. 2011. Working towards affordable pricing for HPV vaccines for developing countries: The role of GAVI. GTF.CCC Working Paper and Background Note Series, No. 3 (Harvard Global Equity Initiative, Boston)
- 31.Mangtani P, et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin Infect Dis. 2014;58(4):470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 32.Dye C. Making wider use of the world’s most widely used vaccine: Bacille Calmette-Guerin revaccination reconsidered. J R Soc Interface. 2013;10(87):20130365. doi: 10.1098/rsif.2013.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Department of Economics and Social Affairs PD . World Population Prospects: The 2010 Revision. United Nations; New York: 2011. [Google Scholar]
- 34.UNAIDS 2013. Know Your Epidemic. Available at www.unaids.org/en/dataanalysis/knowyourepidemic/. Accessed July 2, 2014.
- 35.World Bank 2012. Country Classifications. Available at http://data.worldbank.org/news/newest-country-classifications-released. Accessed July 2, 2014.
- 36. R (2005) R: A Language and Environment (R Foundation for Statistical Computing, Vienna). Available at www.r-project.org/. Accessed July 2, 2014.
- 37.de Vries-Sluijs TE, et al. A randomized controlled study of accelerated versus standard hepatitis B vaccination in HIV-positive patients. J Infect Dis. 2011;203(7):984–991. doi: 10.1093/infdis/jiq137. [DOI] [PubMed] [Google Scholar]
- 38.Wilson CM, et al. Serologic response to hepatitis B vaccine in HIV infected and high-risk HIV uninfected adolescents in the REACH cohort. Reaching for Excellence in Adolescent Care and Health. J Adolesc Health. 2001;29(3 Suppl):123–129. doi: 10.1016/s1054-139x(01)00278-6. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization . Immunization Surveillance, Assessment and Monitoring. 2013. . Available at www.who.int/immunization/monitoring_surveillance/en/. Accessed July 2, 2014. [Google Scholar]
- 40.Krol M, Brouwer W, Rutten F. Productivity costs in economic evaluations: Past, present, future. Pharmacoeconomics. 2013;31(7):537–549. doi: 10.1007/s40273-013-0056-3. [DOI] [PubMed] [Google Scholar]
- 41.Sawert H, et al. Costs and benefits of improving tuberculosis control: The case of Thailand. Soc Sci Med. 1997;44(12):1805–1816. doi: 10.1016/s0277-9536(96)00289-4. [DOI] [PubMed] [Google Scholar]
- 42.Rouzier VA, Oxlade O, Verduga R, Gresely L, Menzies D. Patient and family costs associated with tuberculosis, including multidrug-resistant tuberculosis, in Ecuador. Int J Tuberc Lung Dis. 2010;14(10):1316–1322. [PubMed] [Google Scholar]
- 43.GAVI Alliance 2014. Supplementary guidelines for pneumococcal vaccine applications in 2014. New and Underused Vaccines Support. Available at www.gavi.org/support/apply/#NVS. Accessed July 2, 2014.
- 44.Wilson P. 2010. Giving Developing Countries the Best Shot: An Overview of Vaccine Access and R&D (Médecins Sans Frontières, Geneva)
- 45.Salomon JA, et al. Common values in assessing health outcomes from disease and injury: Disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray CJL, et al. GBD 2010: Design, definitions, and metrics. Lancet. 2012;380(9859):2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 47.Choosing Interventions that are Cost Effective (WHO-CHOICE) 2012. Cost-Effectiveness Thresholds. Available at www.who.int/choice/costs/CER_thresholds/en/. Accessed July 2, 2014. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.