Significance
Spontaneous low-frequency oscillations (LFOs) of blood-oxygen-level-dependent (BOLD) signals in brain constitute the basis for mapping resting functional connectivity with functional MRI (fMRI). However the origin of these LFOs is not well understood. Using optical imaging we provide evidence that (i) LFOs in calcium (marker of cellular oscillations) show frequencies similar to those of deoxyhemoglobin (main contributor to the BOLD signal) and precede them by 5–6 s; (ii) hemodynamic slow oscillations (including LFOs in deoxyhemoglobin) also correlate with spontaneous neuronal firing activity (as assessed with slow local field potentials); and (iii) LFOs of deoxyhemoglobin (HbR) are observed in arteries, veins, and capillaries. These findings therefore corroborate the cellular basis underlying resting-state fMRI and indicate that oscillating HbR signals are detectable across the vascular tree.
Keywords: spontaneous low-frequency brain oscillations, resting-state functional connectivity, neuronal calcium, cerebral hemodynamic, neuroimaging
Abstract
Spontaneous low-frequency oscillations (LFOs) of blood-oxygen-level-dependent (BOLD) signals are used to map brain functional connectivity with functional MRI, but their source is not well understood. Here we used optical imaging to assess whether LFOs from vascular signals covary with oscillatory intracellular calcium (Ca2+i) and with local field potentials in the rat’s somatosensory cortex. We observed that the frequency of Ca2+i oscillations in tissue (∼0.07 Hz) was similar to the LFOs of deoxyhemoglobin (HbR) and oxyhemoglobin (HbO2) in both large blood vessels and capillaries. The HbR and HbO2 fluctuations within tissue correlated with Ca2+i oscillations with a lag time of ∼5–6 s. The Ca2+i and hemoglobin oscillations were insensitive to hypercapnia. In contrast, cerebral-blood-flow velocity (CBFv) in arteries and veins fluctuated at a higher frequency (∼0.12 Hz) and was sensitive to hypercapnia. However, in parenchymal tissue, CBFv oscillated with peaks at both ∼0.06 Hz and ∼0.12 Hz. Although the higher-frequency CBFv oscillation (∼0.12 Hz) was decreased by hypercapnia, its lower-frequency component (∼0.06 Hz) was not. The sensitivity of the higher CBFV oscillations to hypercapnia, which triggers blood vessel vasodilation, suggests its dependence on vascular effects that are distinct from the LFOs detected in HbR, HbO2, Ca2+i, and the lower-frequency tissue CBFv, which were insensitive to hypercapnia. Hemodynamic LFOs correlated both with Ca2+i and neuronal firing (local field potentials), indicating that they directly reflect neuronal activity (perhaps also glial). These findings show that HbR fluctuations (basis of BOLD oscillations) are linked to oscillatory cellular activity and detectable throughout the vascular tree (arteries, capillaries, and veins).
Measures of resting-state functional connectivity with functional MRI (fMRI) are based on spontaneous low-frequency blood-oxygen-level-dependent (BOLD) oscillations that occur throughout the brain with the assumption that regions with correlated oscillations are functionally connected (1, 2). The networks that emerge from resting-state functional connectivity correspond roughly with neuroanatomical connectivity (3, 4) and are modified by brain diseases (5–7). BOLD signals in fMRI reflect the interplay between hemodynamics (including blood volume and velocity of blood flowing in the vessels) and cellular (neuronal and glial) metabolism, which affect the amount of deoxygenated hemoglobin (HbR) in brain tissue that leads to changes in BOLD fMRI (8, 9). Human studies using near-infrared spectroscopy (NIRS) (10) have reported low-frequency oscillations (LFOs) of ∼0.04–0.1 Hz for oxygenated hemoglobin (HbO2) and HbR in the brain consistent with those measured by BOLD (11). However, there is still no quantitative understanding of the relative direct contribution of spontaneous oscillations in cellular activity (neuronal and glial) vs. oscillations that reflect hemodynamic coupling (velocity and vessel diameter) (12) to the resting-state signal. It is also unclear how fluctuations in HbR progress through the vascular tree (13); whereas BOLD signals are believed to predominantly reflect postcapillary and venous compartments, recent evidence suggests that capillaries and arteries also contribute (14).
Here we test the hypothesis that slow BOLD oscillations reflect neuronal oscillatory activity that drives the hemodynamic changes detected with fMRI. For this purpose we use a multimodal optical imaging platform whose high spatiotemporal resolution allowed us to measure spontaneous LFOs in cerebral blood flow velocity (CBFv), HbO2, and HbR in different vascular compartments (veins vs. arteries) and in parenchymal tissue in the rat’s somatosensory cortex both under normocapnia (baseline) and hypercapnia (5% CO2). In parallel we measured spontaneous LFOs in intracellular calcium fluorescence (Ca2+i) using the fluorescent indicator Rhod2-AM (Molecular Probes), which serves as a marker of cellular activity (15). In addition, local field potentials (LFPs) from neurons were measured to assess their correlations with the hemodynamic LFOs. Hypercapnia dilates cerebral blood vessels, increasing blood flow, but has minimal effects on neuronal activity (16, 17) and neurovascular coupling (18–20). Thus, we used hypercapnia as a strategy to differentiate oscillatory components that are due to neurovascular coupling as opposed to other mechanisms that affect vascular tone. Fluorescence histochemistry experiments of Rhod2-Ca2+i revealed that the Ca2+i signal reflected cellular activity (neuronal and perhaps also glial activity). The results indicate that HbR fluctuations occur throughout the vascular tree (arteries, veins, and capillaries) and that Ca2+i oscillations are strongly correlated but occur before HbR fluctuations. Interestingly, we uncovered that CBFv fluctuations have two distinct components, one at the frequency of HbR that was insensitive to hypercapnia and one at a higher frequency that was reduced by hypercapnia, suggesting its dependence on vascular effects. Parallel studies of LFP signals of neurons showed oscillations correlated with those of CBFv, HbR, and HbO2 that indicate they directly reflect neuronal oscillatory activity. Thus, the HbR fluctuations that are the basis of resting-state fMRI are linked to cellular oscillations and observed in arteries, veins, and capillaries.
Results
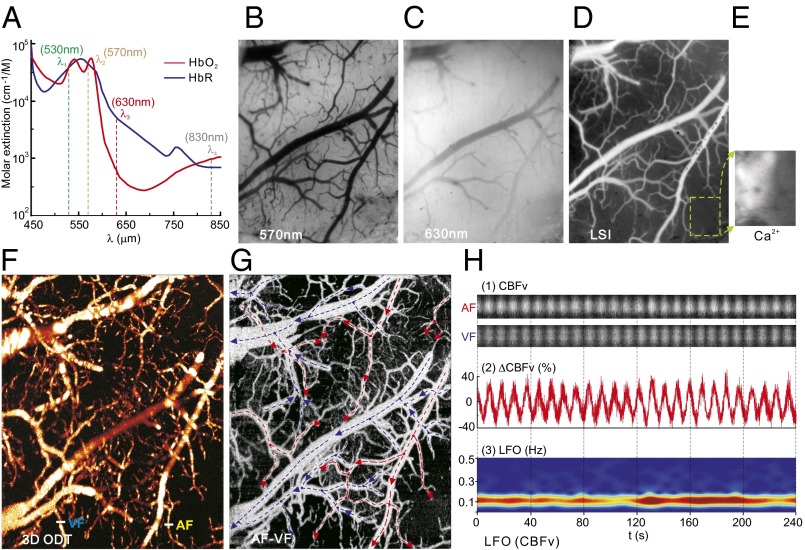
Rats were anesthetized with α-chloralose to minimize anesthesia-induced depression of neuronal activity (21–23), after which a cranial window was created over the somatosensory cortex for in vivo assessment using an optical/fluorescence imaging (OFI) platform (SI Appendix, Fig. S1). Simultaneous dynamic images of CBFv, HbO2, and HbR from relatively large cerebrovascular vessels (>ϕ30 μm) and from parenchymal cortical tissue (including vessels <ϕ30 μm), and of Ca2+i fluorescence fluctuations in the resting state were acquired with OFI at ∼30-μm spatial and 16-Hz temporal resolution over a 5 × 6 mm2 field of view. OFI (Fig. 1A) integrates laser speckle imaging with four-wavelength spectral imaging (SI Appendix, Fig. S1) to permit concurrent dynamic measures of CBFv (λ4 = 830 nm, Fig. 1D); total hemoglobin (tHb) concentration from λ2 = 570 nm, which is an isosbestic point at which the absorption of HbO2 and HbR is equal (Fig. 1B); and HbR from λ3 = 630 nm at which the absorption of HbR predominates (Fig. 1C), allowing separation of HbR from HbO2 (24). The spontaneous LFOs were retrieved by time-frequency analysis of the acquired image sequences (Fig. 1H, Top and SI Appendix, Fig. S2). To empirically analyze the relationship of the hemodynamic LFOs with the oscillatory cellular activity (neurons and glia), a fluorescence imaging technique was applied to measure intracellular calcium concentration ([Ca2+]i) using Rhod2-AM with excitation at λ1 = 530 nm and emission at λem = 590 ± 10 nm (Fig. 1E). The integration of OFI with optical coherence Doppler tomography (ODT) allowed us to obtain 3D CBFv images of the neurovascular network (Fig. 1 F and G) and to separately assess the spontaneous LFOs of CBFv in arteries from those in veins (AF and VF in Fig. 1H). SI Appendix, Fig. S2 illustrates the retrieval of spontaneous LFOs in CBFv from CBF images obtained from OFI and ODT. A similar approach was used to extract the LFOs in HbO2, HbR, and Ca2+i pixel by pixel from the time-lapse images of OFI and averaged over the region of interest (ROI) (e.g., within a vessel or within parenchymal tissue with vessels <ϕ30 μm) to enhance signal-to-noise ratio. For example, Fig. 1H, Middle shows a vascular LFO in CBFv (unit: %), and Fig. 1H, Bottom shows its spectrogram obtained by a short-time Fourier transform (STFT), which illustrates that the LFO frequency of CBFv peaked at ∼0.12 Hz.
Fig. 1.
OFI for imaging of LFOs in CBFv, HbO2, and HbR from cerebrovascular vessels and cortical tissue. (A) Absorption spectra of HbO2 and HbR to illustrate the principle of OFI. (B and C) Spectral images at λ2 and λ3. (D) LSI at λ4 to image relative CBFv. (E) Ca2+ fluorescence with excitation at λ1. (F and G) Quantitative 3D ODT of CBFv and separation of AF (red arrows) and VF (blue arrows). (H) Extraction of LFOs in an AF and a VF highlighted in F. (Top) time-lapse ODT images to show CBFv LFOs. (Middle) LFO with DC removed. (Bottom) Time-varying LFO by STFT.
The Global Neurovascular Network Fluctuates at Low Frequency, and CBFv Oscillates Faster Than HbO2 and HbR and Dominates the CBFv Signal Within Blood Vessels.
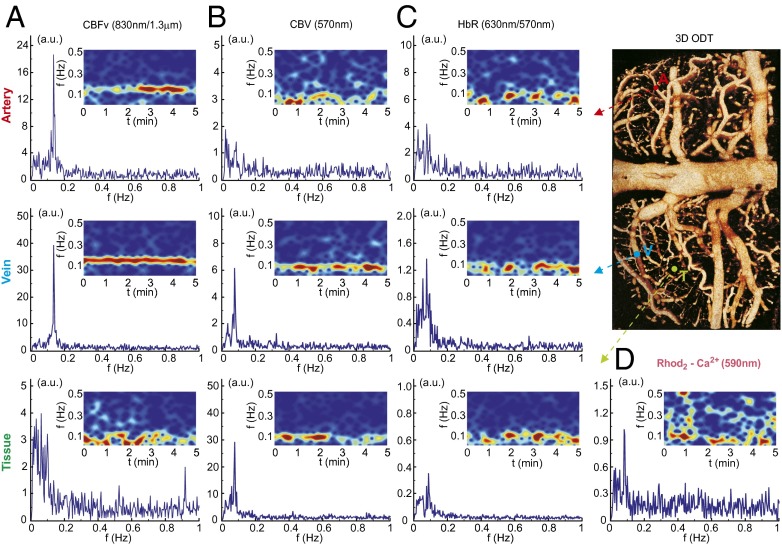
Fig. 2 represents the low-frequency fluctuations in vessels (artery and vein) and in parenchymal tissue observed from the different OFI channels and ODT, including CBFv (by λ4 and λODT), tHb (i.e., cerebral blood volume by λ2), and raw HbR (by λ3) and Rhod2 fluorescence (excited at λ1, emitted at λem) before calculation or correction. Whereas both OFI and ODT provided LFO signals of CBFv in vessels, OFI additionally detects CBFv fluctuations in tissue, which included signals from very small vessels (<ϕ30 μm) and from perfused blood flow in the cerebral parenchyma (SI Appendix, Fig. S2). This indicates that the neurovascular network fluctuates at low frequency.
Fig. 2.
LFO signals observed from multichannels of OFI and ODT modalities. Power spectra and spectrograms of cortical artery, vein, and parenchymal tissue of (A) CBFv (observed by ODT, λ = 1,300 nm or LSI, λ4 = 830 nm). (B) Total hemoglobin (tHb) or cerebral blood volume (CBV) (observed by OFI, λ2 = 570 nm). (C) Raw HbR by λ3 = 630 nm. With λ2 it can be used to separate HbO2 from HbR. (D) Rhod2-Ca2+i fluorescence (emitted at 590 nm while excited at λ1 = 530 nm). Location of the vessels is demonstrated in 3D ODT.
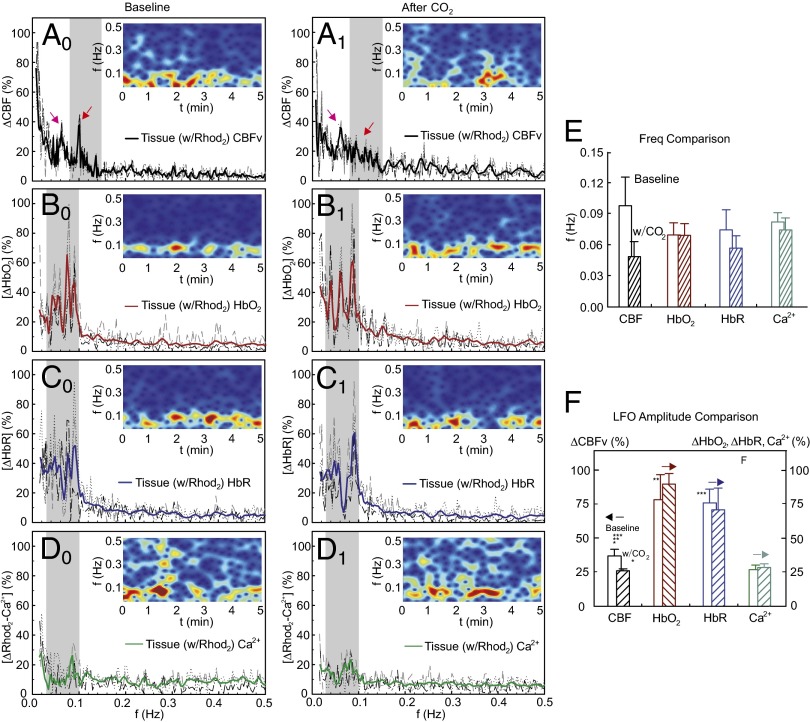
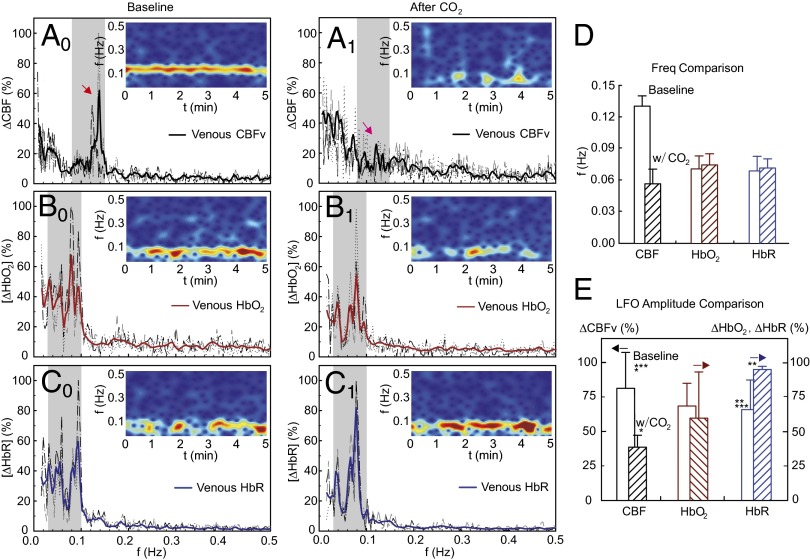
Figs. 3–5 illustrate LFOs of CBFv, HbO2, and HbR in arteries (Fig. 3), veins (Fig. 4), and parenchymal tissue (includes vessels <ϕ30 μm) (Fig. 5) during normocapnia (Left) and hypercapnia (Center). In each panel, a spectrogram is inserted to show the frequency-time characteristics. During normocapnia, LFOs oscillating at <0.15 Hz were observed for CBFv, HbO2, and HbR in arteries (Fig. 3, A0–C0) and veins (Fig. 4, A0–C0), and the full half bandwidth of the oscillation spectrum was defined as its oscillation frequency band as illustrated in Fig. 7, Inset, which was in the range of {0.08 Hz, 0.15 Hz} for CBFv and of {0.03 Hz, 0.1 Hz} for HbO2 and HbR and Ca2+fluoresence, as illustrated in Figs. 3–5 (gray strips). The CBFv in arteries and veins oscillated at higher frequencies (arteries: 0.125 ± 0.013 Hz; veins: 0.13 ± 0.01 Hz, P < 0.006) than those of HbO2 (arteries: 0.06 ± 0.016 Hz; veins: 0.07 ± 0.012 Hz) and HbR (arteries: 0.063 ± 0.017 Hz; veins: 0.069 ± 0.014 Hz) (Table 1, n = 10). Specifically, CBFv oscillated at approximately twice the frequency of the LFOs of HbO2 and HbR in arteries (Fig. 3D) and veins (Fig. 4D). However, in tissue, CBFv oscillated with two frequency bands (a lower band at CBFv-L = 0.061 ± 0.009 Hz and a higher band at CBFv-H = 0.113 ± 0.016 Hz). CBFv-L resembles the frequency of HbO2 (0.069 ± 0.012 Hz) and HbR (0.074 ± 0.019 Hz), whereas CBFv-H in tissue resembles that of CBFv in arteries (0.125 ± 0.013 Hz) and veins (0.13 ± 0.01 Hz).
Fig. 3.
Characteristics of arteriolar LFOs in rat cortex. (Left) LFO power spectra of CBFv (A0), HbO2 (B0) and HbR (C0) and their spectrograms (Insets) under normocapnia. (Center) LFO power spectra of CBFv (A1), HbO2 (B1), and HbR (C1) and their spectrograms (Insets) under hypercapnia. Dashed curves are traces from selected ROIs (m = 6–8) for each rat. Bold curves are averaged traces from the rat. (D and E) Comparison of mean LFO peak frequency and mean amplitude within its oscillation band between normocapnia and hypercapnia across the animals (n = 10). Asterisks indicate statistical significance (P < 0.05).
Fig. 5.
Characteristics of tissue LFOs in rat cortex. (Left) LFO power spectra of CBFv (A0), HbO2 (B0), HbR (C0), and Ca2+i (D0) and their spectrograms (Insets) under normocapnia. Mid (Center) LFO power spectra of CBFv (A1), HbO2 (B1), HbR (C1), and Ca2+i (D1) and their spectrograms (Insets) under hypercapnia. Dashed curves are traces from selected ROIs (m = 6–8) for each rat. Bold curves are averaged traces from the rat. (E and F) Comparison of mean LFO peak frequency and mean amplitude within its oscillation band between normocapnia and hypercapnia averaged across animals (n = 10). Asterisks indicate statistical significance (P < 0.05).
Fig. 4.
Characteristics of venular LFOs in rat cortex. (Left) LFO power spectra of CBFv (A0), HbO2 (B0), and HbR (C0) and their spectrograms (Insets) under normocapnia. (Center) LFO power spectra of CBFv (A1), HbO2 (B1), and HbR (C1) and their spectrograms (Insets) under hypercapnia. Dashed curves are traces from selected ROIs (m = 6–8) for each rat. Bold curves are averaged traces from the rat. (D and E) Comparison of mean LFO peak frequency and mean amplitude within its oscillation band between normocapnia and hypercapnia averaged across animals (n = 10). Asterisks indicate statistical significance (P < 0.05).
Fig. 7.
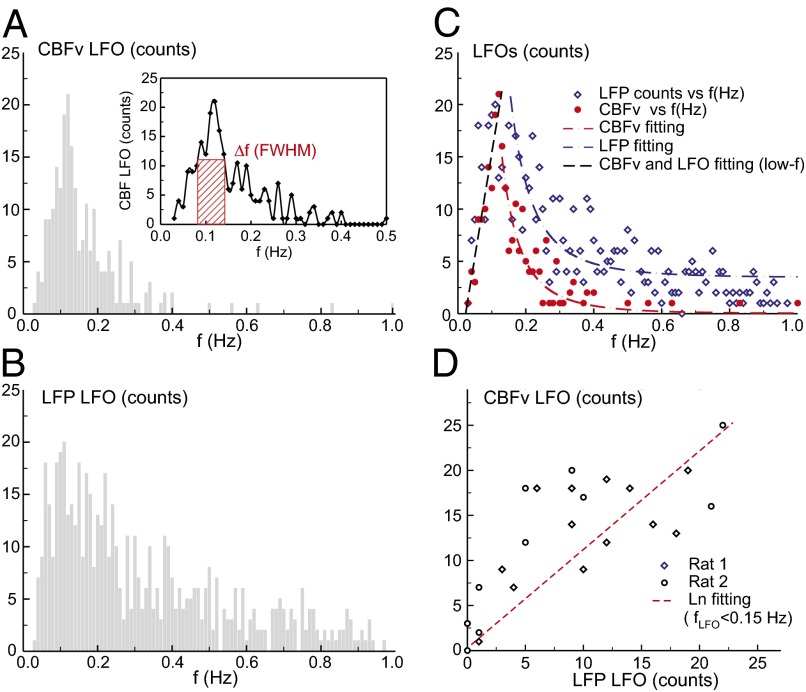
(A) Frequency distribution of CBFv LFO mostly oscillated at ∼0.1 Hz. (Inset) LFO profile and its FWHM bandwidth of 0.08–0.15 Hz (red box). (B) Distribution of LFP spontaneous firing frequency, indicative of neuronal firing rate also around 0.1 Hz. (C) Least-squares fitting of CBFv and LFP activity curves, indicating a corresponding linear increase (r = 0.93) in the low frequency range (<0.15 Hz) followed by rapidly decays with different offsets. (D) Correlation of LFP firing rates with CBFv LFOs (r = 0.83), implying that the spontaneous hemodynamic fluctuations are of neuronal origin.
Table 1.
Mean peak frequencies of LFOs in cortical CBFv, HbO2, and HbR of animals under normocapnia and hypercapnia (n = 10)
| State | Artery, Hz | Vein, Hz | Tissue, Hz |
| Normocapnia | |||
| fb(CBFv) | 0.125 ± 0.013 | 0.130 ± 0.010 | fb(CBFv-H): 0.113 ± 0.016 fb(CBFv-L): 0.061 ± 0.009 |
| fb(HbO2) | 0.060 ± 0.016 | 0.07 ± 0.012 | 0.069 ± 0.012 |
| fb(HbR) | 0.063 ± 0.017 | 0.069 ± 0.014 | 0.074 ± 0.019 |
| fb(Ca) | — | — | 0.082 ± 0.009 |
| Hypercapnia | |||
| fh(CBFv) | 0.05 ± 0.018 | 0.057 ± 0.014 | fh(CBFv-H): 0.047 ± 0.016 fh(CBFv-L): 0.058 ± 0.012 |
| fh(HbO2) | 0.065 ± 0.019 | 0.074 ± 0.011 | 0.069 ± 0.0117 |
| fh(HbR) | 0.06 ± 0.014 | 0.072 ± 0.009 | 0.057 ± 0.012 |
| fh(Ca) | — | — | 0.075 ± 0.012 |
Table 2 summarizes the computed LFO amplitudes of CBFv and HbO2 and HbR, defined as the mean value of the power spectrum within the frequency band (i.e., {0.08 Hz, 0.15 Hz} for CBFv and {0.03 Hz, 0.1 Hz} for HbO2 and HbR) against their background mean values. Results show that in the arteries the amplitude of CBFv LFO (75 ± 22%) is higher than that of HbO2 (41.2 ± 4.4%, P = 0.037) or HbR (38.4 ± 10%, P = 0.04), indicating that in the arteries under normocapnia CBFv dominates the signal intensity from LFOs (Fig. 3E). In veins (Fig. 4E), there were no differences in LFO amplitudes between CBFv (81.5 ± 26.9%) and HbO2 (68.8 ± 16.7%, P = 0.34) or HbR (66.2 ± 21.2%, P = 0.32). In contrast, in the parenchymal tissue, the LFO amplitudes of HbO2 (78.8 ± 18.3%, P = 0.03) and HbR (76.1 ± 10.3%, P = 0.02) were significantly higher than those of CBFv (37.1 ± 5.0%), indicating that in parenchymal tissue HbO2 and HbR dominate the signal intensity from LFOs.
Table 2.
Mean power spectral amplitudes of LFOs in CBFv, HbO2, and HbR oscillation band under normocapnia and hypercapnia (n = 10)
| State | Artery, % | Vein, % | Tissue, % |
| Normocapnia | |||
| Ab(CBFv) | 75.0 ± 22.0 | 81.5 ± 26.9 | 37.1 ± 5.0 |
| Ab(HbO2) | 41.2 ± 4.4 | 68.8 ± 16.7 | 78.8 ± 18.3 |
| Ab(HbR) | 38.4 ± 10.0 | 66.2 ± 21.2 | 76.1 ± 10.3 |
| Ab(Ca) | — | — | 28.2 ± 4.0 |
| Hypercapnia | |||
| Ah(CBFv) | 34.0 ± 23.0 | 38.9 ± 8.7 | 26.2 ± 1.5 |
| Ah(HbO2) | 30.9 ± 10.0 | 59.9 ± 33.4 | 89.9 ± 7.79 |
| Ah(HbR) | 79.7 ± 24.0 | 95.0 ± 2.16 | 71.1 ± 15.5 |
| Ah(Ca) | — | — | 29.8 ± 5.0 |
To rule out a potential aliasing effect of physiological artifacts related to the cardiac and respiratory cycles on the measured LFO signals, a high sampling acquisition of 12–16 Hz was applied during the experiments. As expected, the power spectrum exhibits oscillations at the respiratory (1.0 Hz, ventilated) and cardiac (4.3 Hz) cycles in CBFv and HbO2 in arteries (SI Appendix, Fig. S3 B, 1 and 2) but not in veins (SI Appendix, Fig. S3 D, 1 and 2). However, the LFOs in CBFv, HbO2, and HbR are clearly observed in both vessels (SI Appendix, Fig. S3, C1–C3 and E1–E3), indicating that the LFO signals were not due to respiration or heart rate.
CO2 Reduces the Higher-Frequency Oscillations in CBFv in Arteries and Veins But Does Not Affect the LFOs in HbO2, HbR, and Lower-Frequency CBFv in Tissue.
To investigate the contribution of vasomotion to the LFOs in CBFv, HbO2, and HbR, we measured the effects of hypercapnia (5% CO2 added to the respiratory gases), which dilates vessels (25), thus reducing vasomotion (26). The values for arterial blood gases, mean arterial blood pressure (MABP) (obtained from a femoral artery), and heart rate (HR) corresponded to PCO2 = 35.7 ± 0.5 mmHg, MABP = 98.7 ± 7.0 mmHg, and HR = 284.0 ± 3.5 beats per minute for normocapnia and PCO2 = 60.0 ± 2.9 mmHg, MABP = 94.6 ± 6.9 mmHg, and HR = 289.3 ± 4.3 beats per minute for hypercapnia.
The LFOs in CBFv that oscillated at ∼0.12 Hz (red arrows) were greatly reduced by hypercapnia in arteries (Fig. 3A1), veins (Fig. 4A1), and parenchymal tissue (Fig. 5A1). Within the parenchymal tissue, CO2 reduced the higher-frequency LFOs of CBFv (i.e., CBFv-H at 0.113 ± 0.016 Hz) but did not affect the lower-frequency LFOs of CBFv (CBFv-L = 0.061 ± 0.009 Hz before CO2 vs. CBFv-L = 0.058 ± 0.012 Hz after CO2, P = 0.2) (Table 1). Also, CO2 did not change either the LFOs of HbO2 in arteries (e.g., 0.06 ± 0.016 Hz vs. 0.065 ± 0.019 Hz, P = 0.19) or those of HbR in veins (e.g., 0.069 ± 0.014 Hz vs. 0.072 ± 0.009 Hz, P = 0.22), thus indicating that LFOs of CBFv-L, HbO2, and HbR are distinct from LFOs from CBFv-H.
In the arteries, CO2 significantly reduced the LFO amplitudes in CBFv about 41% (from 75 ± 22% to 34 ± 23%, P = 0.04), presumably from arterial dilation, and increased LFO amplitudes in HbR (from 38.4 ± 10% to 79.7 ± 24%, P = 0.027) but did not change LFO amplitudes in HbO2 (from 41.2 ± 4.4% to 30.9 ± 10%, P = 0.09). In the veins, CO2 induced similar changes in LFO amplitudes: ∼42.6% decreases in CBFv (from 81.5 ± 26.9% to 38.9 ± 8.7%, P = 0.03), ∼43.5% increases in HbR (from 66.2 ± 21.2% to 95 ± 2.1%, P = 0.045), and no changes in HbO2 (68.8 ± 16.7% before vs. 59.9 ± 33.4% after CO2, P = 0.36).
In parenchymal tissue, CO2 reduced the LFO amplitudes in CBFv about 10.9% (37.1 ± 5% to 26.2 ± 1.5%, P = 0.01) but did not change those in HbO2 (78.8 ± 18.3% vs. 89.9 ± 7.8%, P = 0.21) or HbR (76.1 ± 10.3% vs. 71.1 ± 15.5%, P = 0.33), which indicates that in small vessels (<ϕ30 μm) CO2 influenced CBFv but not HbO2 or HbR. Thus, hypercapnia could conceivably be used to differentiate the oscillatory components in CBFv LFOs that are due to neurovascular coupling from other factors that affect vascular oscillations. This might be particularly helpful for studies that measure BOLD oscillations at rest with fMRI in regions with a relatively large contribution from blood vessels with diameters >30 μm.
To corroborate that hypercapnia resulted in blood vessel vasodilation we used angiographic optical coherence tomography (OCA) to image changes in blood vessel diameters induced by hypercapnia across an artery and a vein [SI Appendix, Fig. S4 A–C, time-lapse 2D OCA during normocapnia (PCO2 = 35.7 ± 0.5 mmHg) and hypercapnia (PCO2 = 60.0 ± 2.9 mmHg)], which showed that CO2 induced vasodilatation in both the artery and the vein (SI Appendix, Fig. S4D).
A potential concern for spontaneous LFOs is that their amplitudes may be too weak for reliable estimation. Thus, we compared the amplitudes of the spontaneous LFOs in somatosensory cortex with those of the activation signals under forepaw electrical stimulation. The signals we obtained with optical imaging showed that the magnitudes of the spontaneous oscillations were robust albeit weaker than the hemodynamic signals evoked by forepaw stimulation (18–25% vs. 40%; SI Appendix, Fig. S8).
Ca2+i Fluctuates Within a Frequency Band Similar to That of HbO2 and HbR in Parenchymal Tissue.
To assess cellular oscillations, we measured LFOs of Ca2+i fluorescence using the calcium indicator Rhod2-AM locally microinjected into the somatosensory cortex (27). The LFO power spectra of CBFv, HbO2, and HbR within the Rhod2-loaded tissue area were almost identical to those in surrounding areas without Rhod2 loading (SI Appendix, Fig. S5 B, 1–3), thus documenting no effect of Rhod2 loading on local hemodynamics. There were also no differences in CBFv, HbO2, and HbR responses to CO2 between Rhod2-loaded and non-Rhod2-loaded tissue (SI Appendix, Fig. S5 C, 1–3), indicating that Ca2+i labeling did not affect the physiological responses of local tissue to CO2.
Fig. 5D0 shows the power spectra of Ca2+i fluorescence and its spectrogram during the baseline period. The LFOs of Ca2+i florescence fluctuated at 0.082 ± 0.009 Hz (Table 1), similar to those in HbO2 and HbR (i.e., <0.1 Hz) but lower than the higher-frequency CBFv component (i.e., >0.1 Hz). Although CO2 slightly down-shifted the LFO frequency of cellular Ca2+i oscillations (from 0.082 ± 0.009 Hz to 0.074 ± 0.012 Hz, P = 0.04, Fig. 5E) it did not change their amplitude (i.e., from 28.2 ± 4% to 29.8 ± 7%, P = 0.39; Table 2).
To ensure that the Rhod2-Ca2+ oscillations were not the result of “cross-talk” from CBFv fluctuations due to speckle noise, we used another fluorescence indicator, Sulforhodamine 101 (SR101), as a control. SR101 (28) has excitation (λ1) and emission (λem) wavelengths similar to those of Rhod2 (27) but provides information on cell morphology instead of cellular activity, as is the case for the Ca2+i changes with Rhod2.
SI Appendix, Fig. S6(1) compares the fluorescence signals from SR101 and Rhod2 when loaded to the somatosensory cortex and measured in vivo and ex vivo (SI Appendix, Fig. S7). These results show (i) no evidence of changes in local CBFv after loading with either SR101 or Rhod2 [SI Appendix, Figs. S6(1) A and S7A], (ii) similar brightness of fluorescence [SI Appendix, Figs. S6(1) B and S7B] and mean intensity [SI Appendix, Fig. S6(1) D] with SR101 and Rhod2, and (iii) similar cortical penetration and cellular uptake with SR101 and Rhod2 [SI Appendix, Figs. S6(1) C and S7C]. SI Appendix, Fig. S6(2) shows the LFO power spectra of CBFv and fluorescence within SR101-loaded tissue under normocapnia [Fig. S6(2), B0 and C0] and hypercapnia [SI Appendix, Fig. S6(2), B1 and C1]. The CBFv oscillation appeared in the SR101-loaded tissue and also responded to hypercapnia. However, there were no oscillations in the SR101 fluorescence [SI Appendix, Fig. S6(2), C0 and C1], in contrast to the oscillations in the Rhod2 fluorescence (Fig. 5, D0 and D1). This indicates that LFOs observed with Rhod2-Ca2+i fluorescence are not due to cross-talk from CBFv or hemoglobin fluctuations.
To further evaluate the effects of hypercapnia on oscillatory neuronal activity, we also measured LFP, which predominantly reflects neuronal signals (29), in contrast to Ca2+i LFOs, which reflect both neuronal and glial activity. SI Appendix, Fig. S4E shows the density of LFP spikes as we progressively increased PCO2 from 32 mmHg to 66 mmHg. The average LFP spikes did not change (from 92.6 ± 4.3 spikes per minute to 92.3 ± 3.3 spikes per minute) with PCO2 (from 33 mmHg to 66 mmHg), indicating that neuronal LFPs are not sensitive to PCO2 changes. This is consistent with the interpretation that the minimal effects of hypercapnia on Ca2+i LFOs reflect the widely held view that CO2 in the range used here does not have large effects on neuronal activity or on neurovascular coupling (18–20).
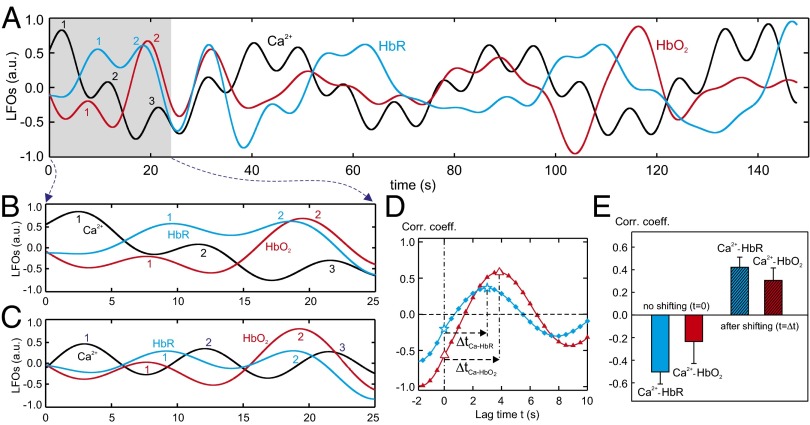
To analyze the temporal relationship of Ca2+i LFOs with those of HbO2 and HbR, their time traces were processed by low-pass filtering (fcutoff ≤0.1 Hz). Their typical time courses are plotted in Fig. 6A and the parameterized numbers of the oscillating waves in Rhod2-Ca2+i (1, black trace), HbR (2, blue trace) and HbO2 (3, red trace) signals within ∼25 s are shown in Fig. 6B. Fig. 6C shows the normalized oscillating waves from Fig. 6B, indicating that the Rhod2-Ca2+i waves tend to peak before HbR or HbO2 signals. Fig. 6D shows the temporal lags in spontaneous HbO2, HbR, and Rhod2-Ca2+i fluctuations in Fig. 6C obtained by cross-correlation and the positive lags of ΔtCa-HbR and ΔtCa-HbO present Rhod2-Ca2+i oscillation preceding HbO2 and HbR oscillations. Table 3 summarizes the time lags of HbR and HbO2 to Ca2+i signals, indicating that ΔtCa-HbR and ΔtCa-HbO are 5.59 ± 0.97 s and 5.7 ± 0.65 s, respectively.
Fig. 6.
Cross-correlation between Ca2+i and HbR/HbO2 LFOs. (A) Time traces of Ca2+i (black), HbR (blue), and HbO2 (red) LFOs acquired from a rat. (B and C) A close view of LFOs in A and the normalized traces of B. (D) Cross-correlation between the time traces of Ca2+i-HbR (blue) and Ca2+i–HbO2 (red). (E) Statistical results (n = 10) of correlation coefficients of Ca2+i-HbR and Ca2+i-HbO2 LFOs without shifting (Δt = 0) and after shifting (time lag − Δt).
Table 3.
Time lags of HbR and HbO2 to Ca2+i signals from the experiments (n = 10)
| Experimental ROIs | |||||||||||
| Time lag, s | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean, s |
| ΔtCa-HbO | 8.6 | 7.8 | 7.7 | 5.4 | 5.9 | 6.9 | 4.7 | 2.5 | 4.3 | 3.1 | 5.70 ± 0.65 |
| ΔtCa-HbR | 8.8 | 8.7 | 8.6 | 5.4 | 6.9 | 7.8 | 4.3 | 0.3 | 2.8 | 2.3 | 5.59 ± 0.97 |
Fig. 6E shows the correlation of Ca2+i signaling with HbO2 and HbR within tissue statistically across the experiments. Without shifting of the oscillation waves (t = 0 s as demonstrated in Fig. 6D) there is a negative correlation between Ca2+i and HbR (k = −0.5 ± 0.11) or Ca2+i and HbO2 (k = −0.23 ± 0.19). However, after shifting a few seconds (i.e., ΔtCa-HbR and ΔtCa-HbO as specified in Table 3), positive correlations between Ca2+i and HbR (k = 0.42 ± 0.09) and HbO2 (k = 0.31 ± 0.12) were observed, thus confirming a 5- to 6-s time lag of HbR and HbO2 to Ca2+i slow fluctuations. This could be caused by the delays in the metabolic and vascular responses to the Ca2+ increase or the transient time of hemoglobin through the vasculature, which is on the order of a few seconds (30). Indeed, in rodent models peak BOLD response occurs 4–6 s after the onset of stimulation (18, 31).
To determine whether the Ca2+ signals measured with Rhod2-AM (Rhod2-[Ca2+]i) arise from neurons or glia, or both, we used immunohistochemistry to assess in which cell types the uptake of Rhod2 occurred. GFAP antibody was used to stain glial fibrillary acidic protein to identify astrocytes, NeuN antibody was used to identify neurons, and DAPI was used to label the nucleus of all cells. SI Appendix, Fig. S7 D–H show the confocal images of cortical tissue with fluorescent markers for nuclei (D), astrocytes (E), Rhod2 (F), neurons (G), and an overlay of all four channels (H), which indicates that Rhod2 uptake occurs in both astrocytes (in E, and light blue arrows in H) and neurons (in G, and yellow arrows in H). Because we used Ca2+ to assess cellular oscillations in the cortex, this indicates that our signals not only reflect oscillations in neurons but might also reflect oscillations in glia.
Spontaneous LFOs Correlate with Neuronal Activity.
The LFP is a direct measurement of neuronal activity in brain (32–36). To assess whether the slow hemodynamic oscillation was associated with neuronal activity, two additional studies were performed to simultaneously record CBFv and LFP using a laser Doppler probe and an LFP probe side by side on the somatosensory cortex. Because the laser Doppler probe was ∼ϕ1 mm it provides integrated information of CBFv signals from vessels and parenchymal tissue. CBFv fluctuation cycles and LFP firing frequency were analyzed during the resting state (>30 min, SI Appendix, Fig. S8).
Fig. 7 A and B shows the distribution of CBFv oscillation cycles and spontaneous neuronal firing frequency, respectively. It indicates that (i) CBFv mostly oscillated at ∼0.1 Hz with a FWHM bandwidth of {0.08 Hz, 0.15 Hz} and (ii) the peak LFP firing frequency of ∼0.1 Hz corresponded to that of CBFv oscillation. Fig. 7C illustrates the fitting process for the CBFv and LFP activity profiles. Interestingly, the CBFv oscillation followed a linear increase along with the LFP activity up to 0.15 Hz (r = 0.93), then both signals decreased rapidly as a function of activity frequency with the different offsets. This indicates that the spontaneous hemodynamic LFOs were associated with neuronal activity. A linear correlation across animals shown in Fig. 7D further confirms that the slow hemodynamic oscillations correlate with neuronal firing frequency.
Discussion
Spontaneous LFOs have been widely used in fMRI to map brain functional connectivity at resting state and are believed to reflect ongoing intrinsic activity of the brain (37). However, because BOLD reflects vascular and hemodynamic processes linked to neuronal and glial activity it is not possible to differentiate the relative contribution of these processes to the BOLD signals with fMRI (38). Here we used optical imaging, which allowed us to simultaneously measure both oxy- and deoxyhemoglobin and Ca2+i signals at high spatiotemporal resolutions to distinguish the contribution of hemodynamic and cellular oscillations to LFOs in vascular and tissue compartments.
In our study we show that, at the resting state, (i) LFOs in CBFv, HbO2, and HbR were observed both in large vessels and capillary networks; (ii) in arteries and veins CBFv oscillated at a higher frequency band (centered at ∼0.12 Hz) than HbO2 and HbR (centered at ∼0.06–0.07 Hz), whereas in the parenchymal tissue (composed of capillaries and small arterioles and venules <ϕ30 μm) CBFv oscillated at both the lower HbO2 and HbR frequency band and the higher vascular CBFv frequency band; (iii) Ca2+i signal oscillated at a frequency of 0.082 ± 0.009 Hz, closer to HbR (0.074 ± 0.019 Hz) and HbO2 (0.07 ± 0.012 Hz) than to CBFv-H (0.113 ± 0.016 Hz), and the Ca2+i oscillation preceded that of HbR and HbO2 and a shift of 5–6 s in the measured Ca2+i oscillations correlates with the HbR and HbO2 oscillations; (iv) hypercapnia reduced the CBFv-H LFOs in both vessels and tissue, whereas it had minimal effects on the LFOs of Ca2+i, HbO2, HbR, and CBFv-L and on LFPs; and (v) LFOs correlated with the neuronal spiking frequencies. Care was taken to remove the artifacts from respiratory and heart rate variations, which could be directly sampled at the high acquisition rate used.
The LFOs for HbO2 and HbR were preserved throughout the arterial and venous vessels and in parenchymal tissue (composed of capillaries and vessels <ϕ30 μm) regardless of whether the CBFv-H oscillations (centered at ∼0.12 Hz) were present (normocapnia) or attenuated (hypercapnia). In contrast, hypercapnia, which induces vasodilation, reduced the CBFv-H oscillations, thus indicating the contribution of factors that affect vascular tone distinct from those of neurovascular control. Hypercapnia did not affect LFPs (SI Appendix, Fig. S4), which reflect neuronal activity, and had minimal effects on Ca2+i oscillations, which most likely arise from neuronal and glial activity. This is consistent with the idea that hypercapnia at the level used here does not have large effects on neuronal function or neurovascular coupling (18–20).
Similar to hemoglobin and Ca2+i oscillations, the CBFv-L oscillations, which were only present in tissue, were also insensitive to hypercapnia, consistent with capillary oscillations reflecting direct influence by neuronal and glial oscillatory activity. Because capillaries are the blood vessels with closest interaction to neuronal and glial activity this could explain why in tissue, which comprised capillary vessels and small arterioles and venules, the CBFv components included not only the faster oscillations that predominated in the larger vessels (>ϕ30 μm) but also the slower oscillations that had frequencies correlated to those of HbR, HbO2, and Ca2+i oscillations.
The lack of an effect of hypercapnia on LFOs in HbR and CBFv-L is consistent with their being directly driven by oscillations in cellular activity. Moreover, the good correspondence between the frequencies of the oscillations in HbO2 and HbR and those in cellular Ca2+ provides evidence that the HbR oscillations are driven by neurovascular coupling. The minimal effects of hypercapnia on Ca2+i signals or on LFPs are consistent with studies showing no large changes in neuronal metabolism during hypercapnia in rodents (39) and in awake humans (40). Our results are also consistent with prior electrophysiological findings revealing a correlation between oscillations in neuronal activity as measured with LFP and resting BOLD signals (35, 41), further supporting the evidence that the resting BOLD oscillations are predominantly driven by neuronal activity. Our results extend these findings to show for the first time to our knowledge that Ca2+i signal also oscillates at a frequency similar to HbR/HbO2 and precedes HbR and HbO2 changes by 5–6 s. The 5- to 6-s time lag between HbR, HbO2, and Ca2+i signals is in agreement with that of BOLD to neuronal activity (35, 41), consistent with the model that HbR fluctuations (basis of spontaneous BOLD oscillations) are linked to oscillatory Ca2+i signals and thus indicating that they reflect oscillatory neuronal (perhaps also oscillatory glial) activity, which further clarifies the neuronal basis underling resting-state BOLD oscillations with fMRI.
The vascular oscillations show a slow CBFv-L oscillation that follows the HbR and Ca2+i LFOs and also a faster CBFv-H oscillation that was sensitive to hypercapnia and thus probably reflects factors that affect vascular tone differently from neurovascular coupling mechanisms. However, because neuronal activity might also influence vascular tone (42) one has to reconcile the possibility that these CBFv-H frequencies might also be linked with neuronal activity and the processes that mediate hemodynamic changes. This contrasts with the CBFv-L oscillations in the tissue, which predominantly reflect oscillations in capillary networks where signals are proximal to the cellular metabolic changes that influence the content of HbR and HbO2 in capillaries, whereas in the larger vessels oscillations reflect also the regulatory mechanisms that modulate vasodilation of blood vessels to perfuse larger tissue areas. Indeed, increases in flow with activation occur in areas that are larger than the activated region, which results in the positive BOLD signals detected by fMRI.
There is no evidence that increases in CBF and oxygenation owing to mild hypercapnia influence spontaneous neuronal oscillatory activity and because oscillations in HbR, CBFv-L, and Ca2+i reflect neuronal activity this explains the lack of an effect with hypercapnia. Whereas hypercapnia did not affect the frequencies of HbR, HbO2, and CBFv-L LFOs and had a minimal effect on Ca2+i LFOs, it significantly influenced the amplitudes of CBFv, HbR, and HbO2 in the vessels. The relative strength of the hemodynamic signals might be expected to change with increase in blood vessel diameter triggered by hypercapnia, which we observed both for CBFv and HbR in arterioles (CBFv: from 75 to 34%, HbR: from 38 to 79%) and venules (CBFv: from 81 to 38%, HbR: from 66 to 95%) and to a lesser extent in tissue (CBFv: from 37 to 26%), which would also explain why BOLD activation signals are influenced by hypercapnia (19). Here we make the distinction between observing an effect of hypercapnia in oscillatory frequencies (Table 1) vs. an effect of hypercapnia in amplitudes (Table 2). Nevertheless, a linear correlation of LFOs in CBFv with LFP firing rates in the low-frequency range (<0.15 Hz) further corroborates that spontaneous hemodynamic LFOs are neuronal in origin.
There is growing interest in using optical imaging to study resting-state fluctuations in human (38, 43) and rodent cortex (44–47). For example, a recent study (10) demonstrated LFOs in HbO2, HbR, and cytochrome oxidase in human visual cortex using NIRS. The LFOs of HbO2 and HbR were centered between 0.04 s−1 (i.e., 0.04 Hz) and 0.1 s−1 (i.e., 0.1 Hz) and the HbR changes preceded HbO2 oscillations, which is in agreement with our findings. However, different from our report, attenuation was observed in HbO2 and HbR oscillations with hypercapnia (5% CO2 in 21% O2, 74% N2). This discrepancy might be due to differences in human vs. rodents or methodologies (e.g., anesthetics and spatial resolution differences, which for NIRS required that they average signals from vessels of various diameters). As shown by our results, CO2 reduced the amplitude of the fluctuation in CBFv across the vascular network (i.e., artery, vein and capillary within the tissue, Table 2) and thus attenuations observed with NIRS in HbO2 and HbR could result from the corresponding decrease in CBFv.
In this study we show that the LFO amplitudes of CBFv in arteries and veins did not differ significantly (P = 0.23). A study with multiphoton microscopy (46) also observed spontaneous oscillations in arterioles and venules but at a higher frequency (0.2–1 Hz) and weaker in venules than arterioles. The discrepancy of findings could reflect the different methodologies used, including different parameters measured [e.g., changes in CBF velocity in current study vs. changes in vessel diameters by multiphoton microscopy (46, 48)] and different dimensions of the vessels measured [e.g., >ϕ30 μm here vs. <ϕ20 μm (46)].
A limitation for this study was that we required the use of anesthesia, which affects neuronal responses. To minimize this confound and make it relevant to fMRI studies we chose α-chloralose, which has been widely used for rodent fMRI studies because (i) it preserves metabolic coupling for somatosensory stimulation (21), (ii) it provides a normal CBF baseline close to that measured in the awake state compared with other anesthetic agents such as isoflurane (22), and (iii) it preserves cerebrovascular reactivity (23). In addition, because of the slow Ca2+i acquisition rate used, we cannot rule out the possibility that the “slow” Ca2+i oscillations might be due to folding over from higher-frequency Ca2+i signals (e.g., Ca2+ transients) or from slow variations in higher-frequency Ca2+i oscillations. Thus, we cannot precisely determine the neuronal and/or glial frequency oscillations that are contributing to the slow Ca2+i oscillations that precede the slow HbR oscillations.
The detection of CBFv at a higher frequency (∼0.12 Hz) than HbR and HbO2 (∼0.06–0.07 Hz) is interesting. It is not clear why a fluctuation in CBFv is not reflected in a corresponding HbR and HbO2 frequency. Because HbR and HbO2 reflect effects of both oxygenation and velocity as well as metabolic factors owing to oxygen consumption, this result indicates that metabolic demands of the tissue determine HbR and HbO2 oscillators in the resting state. The measure of CBFv was based on the Doppler shift of particles in the vessel or speckle variation that reflects RBC velocity (49). It may be that changes in RBC velocity are not reflecting changes in total blood flow through the capillary bed. The changes in CBFv oscillations in the veins follow the CBFv changes in arteries, suggesting the possibility that the origin is in the arterial compartment. A direct measure of bulk CBF would help distinguish whether flow is changing independent of RBC velocity and whether this is occurring throughout the vascular tree. Recent work on MRI has demonstrated CBF fluctuations at frequencies similar to those of BOLD fMRI oscillations (50, 51), but it is unclear that the higher frequencies reported in this study would have been detected owing to technical issues. It will be interesting to determine whether there is a higher-frequency cerebral blood flow (CBF) oscillation that correlates with the higher-frequency red cell velocity (CBFv) measured here.
No consensus has yet arisen about the detailed molecular mechanisms of neurovascular coupling (37, 40). A mechanistic understanding that links specific neuromodulators to hemodynamic effects still needs to be developed. Release of most neurovascular modulators requires an increase in Ca2+i, and here we show that slow Ca2+i oscillates at a frequency similar to HbR, HbO2.
In summary, we provide evidence of spontaneous slow oscillations in cellular activity in cortical brain tissue that match and precede the oscillations of HbR and HbO2 and the CBFv-L oscillations in neuronal tissue. Thus, BOLD oscillations most likely reflect neuronal oscillations. This adds to the growing evidence that BOLD oscillations reflect oscillations in neuronal activity.
Methods
Animal Preparation.
All experiments were carried out according to National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee. A total of 13 Sprague–Dawley rats (250–300 g each) were used in this study: 10 for OFI/ODT imaging to retrieve the LFOs in CBFv, HbO2, HbR and Ca2+i fluorescence, 2 for simultaneous CBFv and LFP measurements, and 1 for microinfusion of SR101 fluorescence indicator to compare with the studies obtained with Rhod2-loaded animals. Each rat was anesthetized and ventilated with ∼2% isoflurane mixed in oxygen/air during the surgical procedures and its femoral artery was catheterized for continuous arterial blood pressure monitoring. An ∼6-mm cranial window was created on side of the parietal bone to expose the somatosensory motor cortex area. The dura was carefully removed and the exposed brain was immediately covered with 2% (vol/vol) agarose gel and affixed with a glass coverslip using biocompatible cyanocrylic glue to avoid changes in cranial pressure. After the surgery, the anesthesia was switched to α-chloralose using an initial bolus of 50 mg/kg followed by continuous infusion of 25 mg⋅kg−1⋅h−1 through a femoral vein. After the measurements under normocapnia, mild hypercapnia was induced by switching the respiratory gas from O2/air mixture to one that contained 5% CO2 to repeat the measurements after 15 min. For in vivo detection of Ca2+i fluorescence shown in Fig. 1E and Fig. 5, 6 of the 10 rats received microinfusion of the Ca2+i indicator Rhod2-AM (100μM, 3 μL/min; Molecular Probes) locally in the cortex (27) before the dura was removed, whereas other the 4 rats were used to assess effect of PCO2 on vascular dilation and to measure local field potentials (LFP) for assessing changes in neuronal activity from normocapnia to hypercapnia. Hemodynamic parameters were compared between an ROI near the Rhod2 injection spot (red circle and red curves) and a distant ROI (light blue circle and light blue curves) as shown in SI Appendix, Fig. S5 to ensure that the injection did not affect the parameters measured.
Multimodal OFI.
We used a custom-built OFI platform in this study (SI Appendix, Fig. S1), which integrates spectral/fluorescence imaging, laser speckle contrast imaging (LSI), and 3D ODT. For spectral imaging, two high-brightness light-emitting diodes (LEDs, 150 mW each) at the wavelengths of λ2 = 570 nm (sensitive to total hemoglobin absorption, i.e., tHb in Fig. 1C) and λ3 = 630 nm (sensitive to deoxygenated hemoglobin absorption, i.e., HbR in Fig. 1D) were coupled into a ϕ3-mm fiber bundle (N.A. 0.25) for illumination of the cortex to image the dynamic characteristics of total blood volume ([tHb]) and hemoglobin oxygenation ([HbO2] = [tHb] − [HbR]). A third 100-mW LED at λ1 = 530 nm was coupled into the fiber bundle to excite Rhod2(AM) for intracellular Ca2+ fluorescence imaging. In parallel, a pigtailed diode laser (60 mW) at λ4 = 830 nm was delivered through a monomode fiber (N.A. 0.12) for LSI imaging. The fiber-guided illumination was incident on the cortical brain through a cranial window at an oblique angle to reduce surface specular reflection; all four-wavelength channels were pulse-modulated to sequentially illuminate cortical brain synchronized by a time-base (time-sharing) interfaced with a workstation to permit “simultaneous” imaging at 12–16 Hz. The back-reflected light from each channel was collected through microscope optics (2×/0.22 N.A., a modified Nikon AZ100 microscope), filtered by a long-pass barrier filter (λBP2 > 570 nm), and imaged by a 14-bit electron-multiplying CCD (iXon3 885; Andor). Changes in [HbO2] and [HbR] were calculated pixel by pixel directly through the time-lapse images at λ2 and λ3. Because hemoglobin absorption affects Ca2+i or SR101 fluorescence both at excitation and emission wavelengths between normocapnia and hypercapnia, spectral correction (SI Appendix, section 9-2) was implemented to minimize the artifacts using a frequency-domain approach to extract Ca2+i fluorescence images (Fig. 5, D0 and D1). For LSI flow image reconstruction the dynamic speckle contrast was calculated based on a 5 × 5 pixel moving window (spatial resolution of 5 × 6 ∼30 μm for LSI) across the whole field of view (FOV, e.g., 5 × 6 mm2) to convert flow index (proportional to flow rate) from the acquired raw photographic images (24, 51).
In parallel a fast 3D optical coherence tomography (OCT) system (illuminated by a λ = 1.3-μm broadband source with Δλ ≥90 nm spectral bandwidth) was integrated into the microscope via a custom dichroic mirror (DM1) reflecting λ >1 μm light for simultaneous 3D ODT (lower dashed box, SI Appendix, Fig. S1). Light exiting the sample arm of the fiberoptic Michelson interferometer was connected to a custom scan head (C1) mounted on the microscope objective, in which light a ϕ5-mm collimated beam was transversely scanned by a pair of servo mirrors (VM500; General Scanning), focused by an achromate (f40 mm/0.1 N.A.) and reflected by DM1 onto the cortex. The backscattered light from brain was recombined with the reference light and detected by a high-speed spectrograph (a 1,024-pixel linear InGaAs array sampling rate up to 47 kHz; Goodrich). By synchronizing with sequential x–axis scanning (e.g., 500 pixels), 2D OCT image (z–x cross-section) was acquired at up to 94 frames per second and 3D OCT image was acquired by additional y-axis scanning. The OCT dataset was transferred to a Raid hard disk array (300 MB/s, Raid 0 configured) on the workstation for parallel image processing and display. An axial resolution (defined by the coherence length Lc = 2(ln2)1/2/π⋅λ2/Δλ) of 8 μm and a transverse resolution of ∼ϕ12 μm (for the achromate f40 mm/0.1 N.A. used) were reached in brain tissue. A typical FOV of 5 × 6 × 2 mm3 on cortex was imaged to register and compare with LSI. Different from OCT for structural imaging, specific raster scanning schemes were implemented to optimize the flow detection sensitivity for 3D ODT, by which the camera was configured to operate at 20,000 A-lines per second with dense sampling (e.g., 0.05-μm pitch) along the x axis for fast flows and down-binned to 10,000 and 5,000 A-lines per second in postimage processing to enhance slow minute flows (52).
High Spatiotemporal Resolution Acquisition for LFO Analyses.
OFI provides uniquely high spatial resolution (e.g., ∼6 μm for λ1–λ3 channels, ∼30 μm for λ4 channel or LSI, and ∼10 μm for ODT) and large FOV (e.g., 5 × 6 mm2), which allows the simultaneous study of hemodynamic, metabolic, and cellular characteristics in various vascular compartments [e.g., arteriolar flow (AF) and venular flow (VF)] and in brain tissue.. For brain functional studies in rats (e.g., LFOs), two major sources of artifacts include heartbeats at fsys ∼4–4.5 Hz (may vary slightly from animal to animal and with its physiological conditions) and respiration rate at fres = 1 Hz (incubated, controllable). According to the Nyquist sampling theorem, a sampling rate of fs/2 ≥ max{fsys, fres}, i.e., fs >9 Hz, is needed to eliminate the aliasing artifacts resulting from the folding back of fsys and fres components to the LFOs in the low-frequency range (53–57). Because LSI is a full-field acquisition modality, simultaneous images in CBFv, HbO2, and HbR from cerebrovascular vessels and cortical tissue (including capillaries and vessels <ϕ30 μm) along with Ca2+i fluorescence fluctuations in the resting state can be acquired with OFI at ∼30 μm/16 Hz spatiotemporal resolutions and over FOV of 5 × 6 mm2. However, for ODT to study the CBFv oscillation in vessels, a small FOV of 3 × 0.2 × 2 mm3 was imaged to increase the acquisition rate to 12 frames per second to avoid aliasing artifact (SI Appendix, Fig. S3).
Image Processing and LFO Analysis.
We used a new image processing algorithm (52), phase intensity mapping, for quantifying 3D Doppler flow velocity (i.e., vascular CBFv). In addition to significantly enhanced flow detection (e.g., Fig. 1F), this method allowed for separation of AF and VF as highlighted in Fig. 1G.
For analysis, an ROI was selected (e.g., along a vein, an artery, and an avascular area for tissue perfusion) and its temporal dynamics was extracted from the time-lapse image traces. For instance, Fig. 1H, Top shows the time-lapse images of an AF and a VF highlighted in Fig. 1F (3D ODT). By applying fast Fourier transform (FFT) to the time traces (e.g., Fig. 1G), their frequency characteristics (e.g., LFO) were analyzed. Specifically, two FFT analysis methods were performed; one was to produce the power spectrum of each ROI (shown in Figs. 2–5) and the other was to generate the spectrogram (shown in the insets in Figs. 2–5) by STFT (SI Appendix, section 9-1). For the power spectral analysis of each ROI, ∼5 min of continuous data under a stable physiological state (normocapnia or hypercapnia) were used, and the FWHM bandwidth of the “main” spectral peak in CBFv, HbO2, HbR, or Ca2+i LFOs was calculated to define their oscillation frequency band (as shown by gray shadows in Figs. 3–5). The LFO amplitude was calculated from its mean value of the power against background within the frequency band. In each animal, six to eight ROIs (i.e., m = 6∼8, dished traces) for each vessel type (e.g., artery or vein) or tissue area were manually selected (SI Appendix, Fig. S2), and their spectra were averaged to produce its final power spectra (solid traces) of AF (Fig. 3), VF (Fig. 4), and tissue (Fig. 5). Further averaging across different animals (n = 10) was conducted to obtain the average peak frequency of CBFv, HbO2, HbR, and Ca2+i (Figs. 3, 4D, and 5E) and the mean amplitude of LFOs in its frequency band (Figs. 3, 4E, and 5F), as summarized in Tables 1 and 2.
LFP Acquisition and Laser Doppler Measurement.
LFP signal traces of the cortex were recorded (at 5 kHz sampling rate, 0.1–35 Hz band-pass-filtered) by a pair of referenced electrodes (ϕ300 μm) with the corresponding multichannel electroencephalogram amplifier (EL450/MP150/EEG100C; Biopac). The signal electrode was affixed on the thinned bone of the cortex of interest, the reference electrode was placed on the symmetric side of the cortex, and a third electrode was inserted under the neck skin for grounding reference. Continuous acquisition of LFP was performed throughout the entire experiment (e.g., along with PCO2 from 33 mmHg to 66 mmHg), and a custom peak detection algorithm counted the LFP spikes per minute as shown in SI Appendix, Fig. S4. For simultaneous measurement of LFP and laser Doppler flowmeter (Moor Instruments), the Doppler probe (ϕ1 mm) and LFP electrode were placed side by side on the rat somatosensory cortex. Continuous recording of both LFP and CBFv was conducted throughout the experimental period during which electrical forepaw stimulation (2 mA, 1 Hz, 10 s) was induced periodically (2–3 min). Both CBFv fluctuation cycles and LFP firing frequency were analyzed during the resting state.
All of the statistics were performed within a two-tail Student t test, unless otherwise indicated, and all of the results are reported as mean ± SD.
Supplementary Material
Acknowledgments
We thank Dr. H. Ren, W. Chen, and J. Li for partially assisting with data representation; K. Park for assisting with animal handling; Dr. Z. Luo for laser speckle contrast imaging; S. Sundaresh for assisting with in vitro studies; and Dr. J.R. Walters [National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH)] for helpful discussions. This research was supported in part by NIH Grants K25-DA021200 (to C.D.), 1RC1DA028534 (to C.D. and Y.P.), R21DA032228 (to Y.P. and C.D.), and R01DA029718 (to C.D. and Y.P.) and by the NIH intramural program (N.D.V. and A.P.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410800111/-/DCSupplemental.
References
- 1.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 2.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 3.Cordes D, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21(9):1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent JL, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 5.Zeng LL, et al. Identifying major depression using whole-brain functional connectivity: A multivariate pattern analysis. Brain. 2012;135(Pt 5):1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- 6.Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32(11):1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53(2):593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10(4–5):165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Elwell CE, Springett R, Hillman E, Delpy DT. Oscillations in cerebral haemodynamics. Implications for functional activation studies. Adv Exp Med Biol. 1999;471:57–65. [PubMed] [Google Scholar]
- 10.Obrig H, et al. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage. 2000;12(6):623–639. doi: 10.1006/nimg.2000.0657. [DOI] [PubMed] [Google Scholar]
- 11.Tong Y, Frederick BD. Time lag dependent multimodal processing of concurrent fMRI and near-infrared spectroscopy (NIRS) data suggests a global circulatory origin for low-frequency oscillation signals in human brain. Neuroimage. 2010;53(2):553–564. doi: 10.1016/j.neuroimage.2010.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razavi M, et al. Source of low-frequency fluctuations in functional MRI signal. J Magn Reson Imaging. 2008;27(4):891–897. doi: 10.1002/jmri.21283. [DOI] [PubMed] [Google Scholar]
- 13.Boas DA, Jones SR, Devor A, Huppert TJ, Dale AM. A vascular anatomical network model of the spatio-temporal response to brain activation. Neuroimage. 2008;40(3):1116–1129. doi: 10.1016/j.neuroimage.2007.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, et al. A three-compartment model of the hemodynamic response and oxygen delivery to brain. Neuroimage. 2005;28(4):925–939. doi: 10.1016/j.neuroimage.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 15.Helmchen F, Waters J. Ca2+ imaging in the mammalian brain in vivo. Eur J Pharmacol. 2002;447(2-3):119–129. doi: 10.1016/s0014-2999(02)01836-8. [DOI] [PubMed] [Google Scholar]
- 16.Bandettini PA, Wong EC. Magnetic resonance imaging of human brain function. Principles, practicalities, and possibilities. Neurosurg Clin N Am. 1997;8(3):345–371. [PubMed] [Google Scholar]
- 17.Hoge RD, et al. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA. 1999;96(16):9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennerley AJ, et al. Early and late stimulus-evoked cortical hemodynamic responses provide insight into the neurogenic nature of neurovascular coupling. J Cereb Blood Flow Metab. 2012;32(3):468–480. doi: 10.1038/jcbfm.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieger SN, et al. Using carbogen for calibrated fMRI at 7Tesla: Comparison of direct and modelled estimation of the M parameter. Neuroimage. 2014;84:605–614. doi: 10.1016/j.neuroimage.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Desjardins M, Berti R, Pouliot P, Dubeau S, Lesage F. Multimodal study of the hemodynamic response to hypercapnia in anesthetized aged rats. Neurosci Lett. 2014;563:33–37. doi: 10.1016/j.neulet.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Ueki M, Mies G, Hossmann KA. Effect of alpha-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand. 1992;36(4):318–322. doi: 10.1111/j.1399-6576.1992.tb03474.x. [DOI] [PubMed] [Google Scholar]
- 22.Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17(4):942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- 23.Bonvento G, et al. Is alpha-chloralose plus halothane induction a suitable anesthetic regimen for cerebrovascular research? Brain Res. 1994;665(2):213–221. doi: 10.1016/0006-8993(94)91340-4. [DOI] [PubMed] [Google Scholar]
- 24.Yuan Z, Luo Z, Volkow ND, Pan Y, Du C. Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. Neuroimage. 2011;54(2):1130–1139. doi: 10.1016/j.neuroimage.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grubb RL, Jr, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5(5):630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- 26.Morita-Tsuzuki Y, Bouskela E, Hardebo JE. Vasomotion in the rat cerebral microcirculation recorded by laser-Doppler flowmetry. Acta Physiol Scand. 1992;146(4):431–439. doi: 10.1111/j.1748-1716.1992.tb09444.x. [DOI] [PubMed] [Google Scholar]
- 27.Du C, et al. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J Neurosci. 2006;26(45):11522–11531. doi: 10.1523/JNEUROSCI.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masamoto K, et al. Repeated longitudinal in vivo imaging of neuro-glio-vascular unit at the peripheral boundary of ischemia in mouse cerebral cortex. Neuroscience. 2012;212(212):190–200. doi: 10.1016/j.neuroscience.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchinson EB, Stefanovic B, Koretsky AP, Silva AC. Spatial flow-volume dissociation of the cerebral microcirculatory response to mild hypercapnia. Neuroimage. 2006;32(2):520–530. doi: 10.1016/j.neuroimage.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 31.Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci USA. 2002;99(23):15182–15187. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolelis MA, Baccala LA, Lin RC, Chapin JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science. 1995;268(5215):1353–1358. doi: 10.1126/science.7761855. [DOI] [PubMed] [Google Scholar]
- 33.von Stein A, Chiang C, König P. Top-down processing mediated by interareal synchronization. Proc Natl Acad Sci USA. 2000;97(26):14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kara P, Reinagel P, Reid RC. Low response variability in simultaneously recorded retinal, thalamic, and cortical neurons. Neuron. 2000;27(3):635–646. doi: 10.1016/s0896-6273(00)00072-6. [DOI] [PubMed] [Google Scholar]
- 35.Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29(7):751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14(4):180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 38.White BR, et al. Resting-state functional connectivity in the human brain revealed with diffuse optical tomography. Neuroimage. 2009;47(1):148–156. doi: 10.1016/j.neuroimage.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barzilay Z, Britten AG, Koehler RC, Dean JM, Traystman RJ. Interaction of CO2 and ammonia on cerebral blood flow and O2 consumption in dogs. Am J Physiol. 1985;248(4 Pt 2):H500–H507. doi: 10.1152/ajpheart.1985.248.4.H500. [DOI] [PubMed] [Google Scholar]
- 40.Jain V, et al. Rapid magnetic resonance measurement of global cerebral metabolic rate of oxygen consumption in humans during rest and hypercapnia. J Cereb Blood Flow Metab. 2011;31(7):1504–1512. doi: 10.1038/jcbfm.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schölvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci USA. 2010;107(22):10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urban A, Rancillac A, Martinez L, Rossier J. Deciphering the neuronal circuitry controlling local blood flow in the cerebral cortex with optogenetics in PV:Cre transgenic mice. Front Pharmacol. 2012;3:105. doi: 10.3389/fphar.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mesquita RC, Franceschini MA, Boas DA. Resting state functional connectivity of the whole head with near-infrared spectroscopy. Biomed Opt Express. 2010;1(1):324–336. doi: 10.1364/BOE.1.000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biswal BB, Hudetz AG. Synchronous oscillations in cerebrocortical capillary red blood cell velocity after nitric oxide synthase inhibition. Microvasc Res. 1996;52(1):1–12. doi: 10.1006/mvre.1996.0039. [DOI] [PubMed] [Google Scholar]
- 45.Saka M, Berwick J, Jones M. Linear superposition of sensory-evoked and ongoing cortical hemodynamics. Front Neuroenergetics. 2012;2:23. doi: 10.3389/fnene.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drew PJ, Shih AY, Kleinfeld D. Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc Natl Acad Sci USA. 2011;108(20):8473–8478. doi: 10.1073/pnas.1100428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein EW, Maslov K, Wang LV. Noninvasive, in vivo imaging of blood-oxygenation dynamics within the mouse brain using photoacoustic microscopy. J Biomed Opt. 2009;14(2):020502. doi: 10.1117/1.3095799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blinder P, et al. The cortical angiome: An interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16(7):889–897. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo Z, Wang Z, Yuan Z, Du C, Pan Y. Optical coherence Doppler tomography quantifies laser speckle contrast imaging for blood flow imaging in the rat cerebral cortex. Opt Lett. 2008;33(10):1156–1158. doi: 10.1364/ol.33.001156. [DOI] [PubMed] [Google Scholar]
- 50.Chuang KH, et al. Mapping resting-state functional connectivity using perfusion MRI. Neuroimage. 2008;40(4):1595–1605. doi: 10.1016/j.neuroimage.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukunaga M, et al. Metabolic origin of BOLD signal fluctuations in the absence of stimuli. J Cereb Blood Flow Metab. 2008;28(7):1377–1387. doi: 10.1038/jcbfm.2008.25. [DOI] [PubMed] [Google Scholar]
- 52.Luo Z, Yuan Z, Tully M, Pan Y, Du C. Quantification of cocaine-induced cortical blood flow changes using laser speckle contrast imaging and Doppler optical coherence tomography. Appl Opt. 2009;48(10):D247–D255. doi: 10.1364/ao.48.00d247. [DOI] [PubMed] [Google Scholar]
- 53.Ren H, et al. Cocaine-induced cortical microischemia in the rodent brain: Clinical implications. Mol Psychiatry. 2012;17(10):1017–1025. doi: 10.1038/mp.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magn Reson Imaging. 2008;26(7):1055–1064. doi: 10.1016/j.mri.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Anderson JS. Origin of synchronized low-frequency blood oxygen level-dependent fluctuations in the primary visual cortex. AJNR Am J Neuroradiol. 2008;29(9):1722–1729. doi: 10.3174/ajnr.A1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magnuson M, Majeed W, Keilholz SD. Functional connectivity in blood oxygenation level-dependent and cerebral blood volume-weighted resting state functional magnetic resonance imaging in the rat brain. J Magn Reson Imaging. 2010;32(3):584–592. doi: 10.1002/jmri.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hutchison RM, Mirsattari SM, Jones CK, Gati JS, Leung LS. Functional networks in the anesthetized rat brain revealed by independent component analysis of resting-state FMRI. J Neurophysiol. 2010;103(6):3398–3406. doi: 10.1152/jn.00141.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.