Significance
Receptor-interacting kinase-3 (RIP3) and its substrate mixed lineage kinase domain-like protein (MLKL) are now recognized as the core cellular regulators of programmed necrosis. Programmed necrosis is thought be involved in host defense against pathogens, but the mechanistic studies on the topic are still scarce. We discover that human herpes simplex virus 1 (HSV-1) triggers RIP3/MLKL-dependent necrosis in host cells. HSV-1 ribonucleotide reductase large subunit (ICP6) is found to be a direct activator of RIP3 and sufficient for triggering programmed necrosis. Mice lacking RIP3 exhibited severely impaired control of HSV-1 replication and pathogenesis. This study provides a clear case how host cells use programmed necrosis to mount an antiviral response.
Keywords: programmed necrosis, HSV-1, ICP6, RIP3, MLKL
Abstract
The receptor-interacting kinase-3 (RIP3) and its downstream substrate mixed lineage kinase domain-like protein (MLKL) have emerged as the key cellular components in programmed necrotic cell death. Receptors for the cytokines of tumor necrosis factor (TNF) family and Toll-like receptors (TLR) 3 and 4 are able to activate RIP3 through receptor-interacting kinase-1 and Toll/IL-1 receptor domain-containing adapter inducing IFN-β, respectively. This form of cell death has been implicated in the host-defense system. However, the molecular mechanisms that drive the activation of RIP3 by a variety of pathogens, other than the above-mentioned receptors, are largely unknown. Here, we report that human herpes simplex virus 1 (HSV-1) infection triggers RIP3-dependent necrosis. This process requires MLKL but is independent of TNF receptor, TLR3, cylindromatosis, and host RIP homotypic interaction motif-containing protein DNA-dependent activator of IFN regulatory factor. After HSV-1 infection, the viral ribonucleotide reductase large subunit (ICP6) interacts with RIP3. The formation of the ICP6–RIP3 complex requires the RHIM domains of both proteins. An HSV-1 ICP6 deletion mutant failed to cause effective necrosis of HSV-1–infected cells. Furthermore, ectopic expression of ICP6, but not RHIM mutant ICP6, directly activated RIP3/MLKL-mediated necrosis. Mice lacking RIP3 exhibited severely impaired control of HSV-1 replication and pathogenesis. Therefore, this study reveals a previously uncharacterized host antipathogen mechanism.
Cell death triggered by pathogens is a crucial component of mammalian host-defense system. Apoptosis, a predominant programmed cell death in mammals, functions as an effective host-defense mechanism for preventing pathogen replication. Apoptosis is initiated by either mitochondria or cell-death receptors, and it is executed by a group of cysteine proteases called caspases (1). The apoptotic pathway can be subverted by pathogen-encoded apoptotic suppressors such as caspase inhibitors (2). Recent studies have revealed that caspase inhibition can lead to alternative activation of necrosis, releasing the damage-associated molecular patterns (DAMPs) signal to trigger the activation of the host immune system (3, 4).
Cytokines of the TNF family are classical inducers of programmed necrosis that are morphologically characterized by the swelling of intracellular organelles and disrupted plasma membranes. Programmed necrosis triggered by death cytokines such as TNF, also known as necroptosis (5–7), is tightly regulated by receptor-interacting kinase-1 (RIP1) (8), its deubiquitin enzyme cylindromatosis (CYLD) (9), and receptor-interacting kinase-3 (RIP3) (10–12). The RIP homotypic interaction motif (RHIM) domains of RIP1 and RIP3 are required for the formation of the RIP1–RIP3 complex that is called a necrosome (13). Recently, mixed lineage kinase domain-like (MLKL) protein has been identified as a functional substrate of RIP3 kinase (14, 15). Upon phosphorylation, MLKL forms oligomers, and these oligomers translocate to both the plasma and the intracellular membranes to elicit necrosis (16–18).
Additionally, Toll-like receptor (TLR) ligands activate programmed necrosis through the RHIM-dependent formation of the Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF/TICAM-1)–RIP3 complex (19). A recent study demonstrated that RIP1 is dispensable for TLR3-induced necrosis in fibroblasts (20). Furthermore, the RHIM-containing protein DAI forms a complex with RIP3 (DAI–RIP3 complex) to mediate programmed necrosis that is induced by the mutant murine cytomegalovirus (MCMV), a process that is independent of RIP1 (21, 22). Therefore, RIP3 and MLKL constitute the core biochemical executioners in necrotic cell death, which is activated by the specific recognition of a particular RHIM-containing protein.
RIP3-dependent necrosis has been implicated in host defenses against invading pathogens. Vaccinia virus (VV), which encodes caspase inhibitor B13R (23, 24), has been shown to sensitize mouse embryonic fibroblasts (MEFs) to TNF-α–induced necroptosis (11). Upon VV infection, both RIP3 and TNF receptor knockout mice showed reduced inflammation and necrosis, suggesting the involvement of necroptosis in the host defenses against VV (11). In the case of MCMV infection, the virus encodes a potent RHIM-containing protein, M45/vIRA, which is capable of forming an interaction with RIP3, thus preventing RIP3 from receiving a necrotic signal mediated by a host protein named DNA-dependent activator of IFN regulatory factor (DAI) (21, 22). Although these observations suggest that programmed necrosis can function as part of host defense, the molecular mechanisms that drive the activation of RIP3-dependent necrosis by pathogens remain largely unknown.
Herpes simplex virus 1 (HSV-1) is a common human pathogen that infects around 80% of adults. HSV-1 is able to establish a latent infection in sensory neurons that lasts for the entire life of the host (25). The virus uses multiple receptors for successful infection in a variety of cell types, including MEFs. Studies in animal models and in humans have shown that HSV-1 infection is not only the major cause of severe herpes infections on the mouth and lips, but also an important cause of fatal sporadic encephalitis (25, 26). In the current study, we report that HSV-1 infection triggers RIP3/MLKL-dependent necrosis via an RHIM-containing viral ribonucleotide reductase large subunit (ICP6). Ectopic expression of ICP6 directly activates RIP3/MLKL-mediated necrosis in a RHIM-dependent manner. Importantly, RIP3−/− mice showed defective control of viral replication and pathogenesis. Thus, our work provides, to our knowledge, the first evidence that the direct activation of RIP3-dependent necrosis through a pathogen-encoded RHIM-containing protein acts as a host-defensive mechanism.
Results
HSV-1 Infection Activates RIP3-Dependent Necrosis.
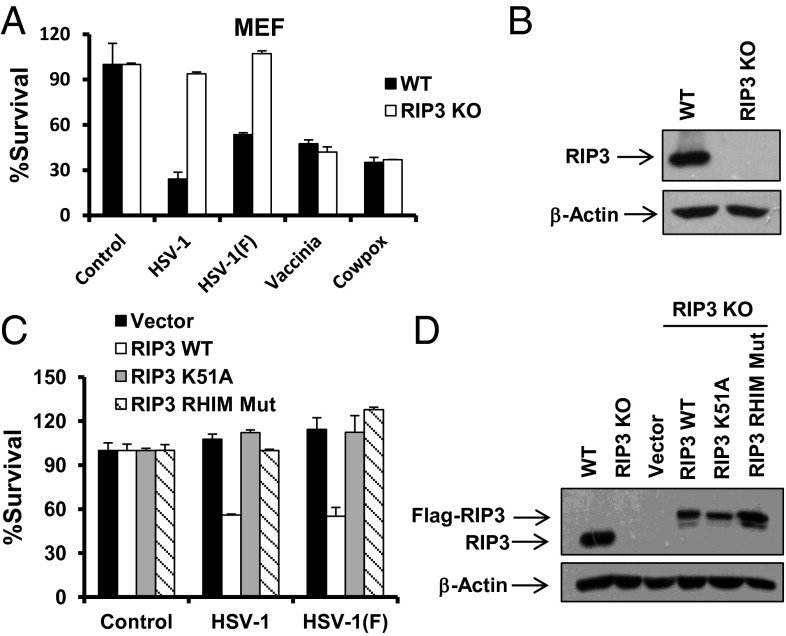
Host cells usually undergo cell death in response to infection of a number of viruses such as cowpox, vaccinia virus, and HSV-1. To address the role of RIP3 in the death of virus infected cells, we examined the sensitivity of WT and RIP3 knockout (KO) MEFs to these viruses. As shown in Fig. 1 A and B, RIP3 deficiency had no significant effect on cell death induced by VV or cowpox. Similar to VV, cowpox encodes a well-known caspase-8 inhibitor named CrmA (27). Interestingly, we found that the massive death of MEFs caused by infection of HSV-1 KOS (referred to henceforth as HSV-1) or HSV-1 F strain was attenuated in RIP3 KO MEFs (Fig. 1 A and B). Consistently, knockdown of RIP3 in mouse L929 cells also greatly diminished cell death induced by HSV-1 or HSV-1 F strain (Fig. S1A). Reintroducing a WT, but not a kinase dead mutant or a RHIM mutant form of RIP3 cDNA into the RIP3 KO MEFs, restored sensitivity to HSV-1–induced cell death (Fig. 1 C and D). We did not observe detectable activation of caspase-3 or caspase-8 in MEFs exposed to HSV-1 (Fig. S1B). Furthermore, we noted that the addition of zVAD-fmk (z-VAD), a pan-caspase inhibitor, did not affect the death of infected cells (Fig. S1C). The dying cells showed propidium iodide (PI)-positive nuclei, indicating disrupted cellular membranes (Fig. S1D). Taken together, these results demonstrate that HSV-1 infection activates RIP3-dependent necrosis.
Fig. 1.
HSV-1 infection activates RIP3-dependent necrosis. (A) WT and RIP3 KO MEFs were infected with the HSV-1 or HSV-1 F strain at a multiplicity of infection (MOI) of 5 for around 15 h, or infected with vaccinia or cowpox virus for around 28 h. Identical MOI was used in MEF in later experiments unless otherwise stated. The cell-survival rate was determined by measuring ATP levels. (B) Cell lysates collected from WT or RIP3 KO MEFs were subjected to Western-blot analysis. (C) RIP3 KO MEFs expressing WT RIP3 (RIP3 WT) or RIP3 kinase dead mutant (RIP3 K51A) or RHIM domain mutant form of RIP3 (RIP3 RHIM Mut) were infected with HSV-1 or F strain for 16–18 h. The cell-survival rate was determined by measuring ATP levels. (D) Cell lysates collected from RIP3 KO MEFs expressing RIP3 WT or RIP3 K51A or RIP3 RHIM Mut were subjected to Western-blot analysis.
RIP3 Is Dispensable for HSV-1 Entry, Replication, and Virus-Induced NF-κB Activation.
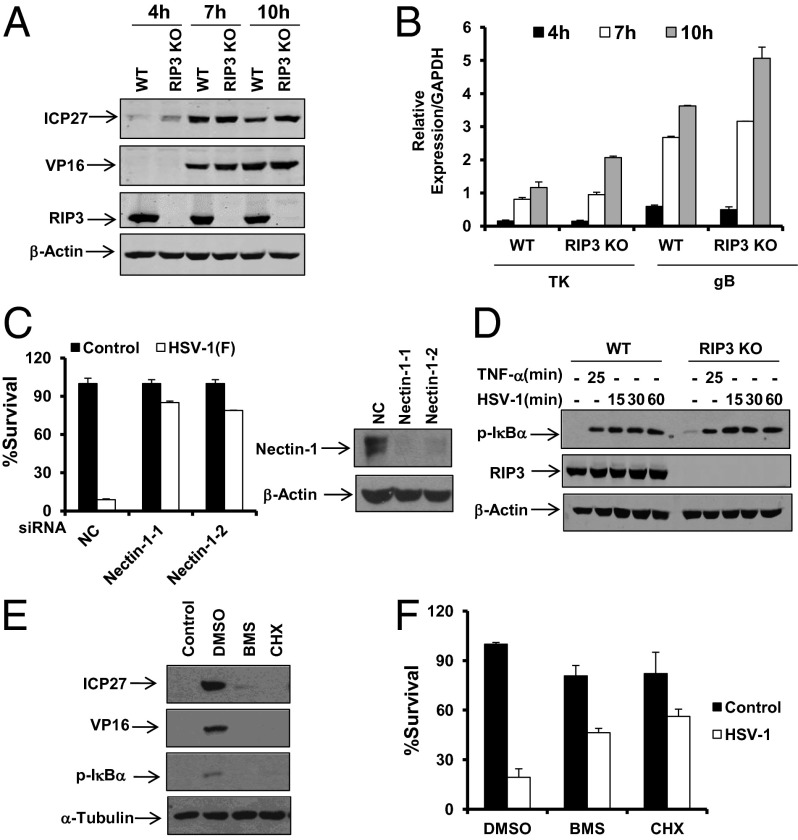
We further examined whether RIP3 deficiency affected HSV-1 entry and replication by measuring the expression levels of various HSV-1 proteins and genes between WT and RIP3 KO MEFs. No significant differences in the levels of the proteins and genes tested were observed in any of the cells exposed to HSV-1 at any time from between 4 h and 10 h postinfection (Fig. 2 A and B). Consistently, WT and RIP3 KO MEFs showed similar intensity of GFP signal after the infection of the GFP-labeled HSV-1 F strain (Fig. S2). In contrast, knockdown of the known HSV receptor nectin-1 reduced HSV-1–induced necrosis (Fig. 2C). These data suggest that RIP3 is dispensable for HSV-1 entry and replication. Because efficient replication of HSV-1 relies on the activation of the NF-κB pathway in host cells, we further tested the status of NF-κB activation in both WT and RIP3 KO MEFs. Compared with WT MEFs, the RIP3 KO cells exerted normal NF-κB activation in response to HSV-1 infection or TNF-α treatment (Fig. 2D). Addition of either the NF-κB inhibitor BMS-345541 (28) or protein synthesis inhibitor cycloheximide (CHX) abolished HSV-1–induced NF-κB activation and expressions of viral proteins (Fig. 2E). Because the addition of these inhibitors also resulted in reduced necrosis of infected cells (Fig. 2F), the NF-κB–mediated expression of HSV-1 proteins is likely required for the induction of programmed necrosis.
Fig. 2.
RIP3 is dispensable for HSV-1 entry, replication, and virus-induced NF-κB activation. (A and B) WT and RIP3 KO MEFs were infected with HSV-1 (MOI = 2) for the indicated time. Cell lysates were then collected and subjected to Western-blot analysis for the indicated proteins (A). Quantitative PCR (qPCR) was performed to measure the expression levels of thymidine kinase (TK) and gB (B). The error bars show the SD from duplicate qPCR reactions. (C) MEFs were transfected with negative control (NC) or nectin-1 siRNA oligos for 48 h. Cells were treated as indicated for an additional 16–18 h. The cell-survival rate was determined by measuring ATP levels. Cell lysates were collected 48 h posttransfection and subjected to Western-blot analysis. (D) WT and RIP3 KO MEFs were treated with TNF-α for 25 min or infected with HSV-1 (MOI = 2). At the indicated time points, cell lysates were collected and subjected to Western-blot analysis. (E) MEFs were treated with DMSO, BMS-345541 (BMS), or Cycloheximide (CHX) for 1 h before infection with HSV-1 (MOI = 2) for an additional 8 h. Cell lysates were then collected and subjected to Western-blot analysis. BMS, 20 µM; CHX, 5 µg/mL. (F) MEFs were treated with DMSO, BMS, or CHX for 1 h before infection with HSV-1 for an additional 16 h. The cell-survival rate was determined by measuring ATP levels.
HSV-1 Infection-Induced Necrosis Requires MLKL but Is Independent of TNFR, TLR3, CYLD, and DAI.
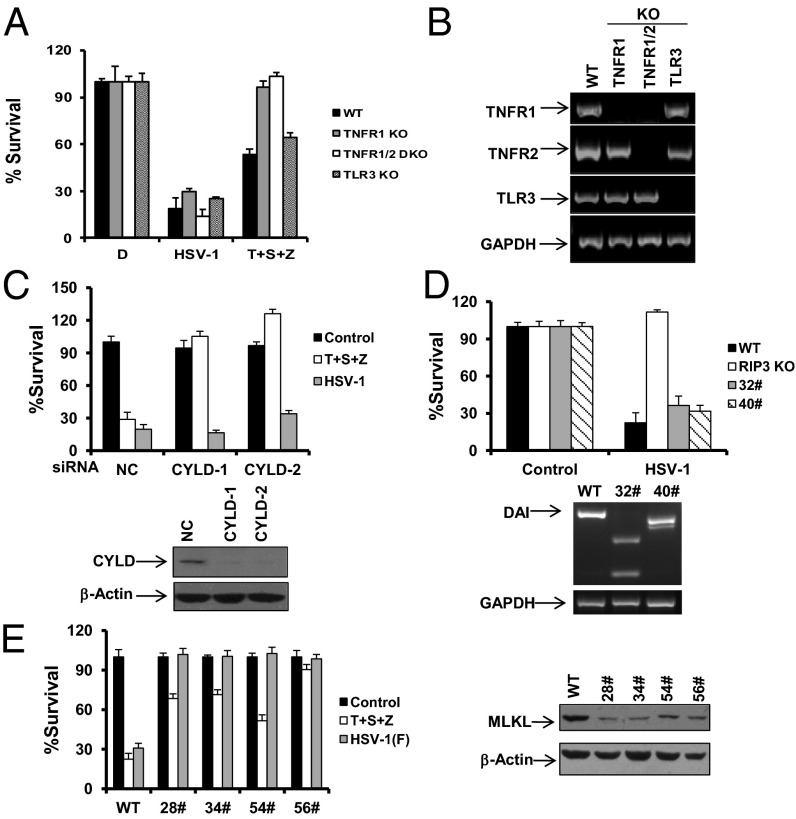
Having shown that HSV-1 triggered RIP3-dependent necrotic death, we sought to characterize the cellular requirements for this form of programmed necrosis. TNFR1 KO, TNFR1/TNFR2 double KO, and TLR3 KO MEFs had similar susceptibility to HSV-1 infection compared with WT cells even though TNFR1 KO cells completely lost sensitivity to TNF-α mediated necrosis, which is known to be induced by the treatment of TNF-α/Smac mimetic/z-VAD (10) (Fig. 3 A and B). It is therefore apparent that HSV-1 provokes the RIP3 signal independently of TNFR and TLR3. Furthermore, we found that reducing CYLD by RNAi failed to mitigate the necrosis induced by HSV-1 infection although it did greatly suppress TNF-α–induced necrosis (Fig. 3C and Fig. S3A). Despite the importance of DAI in MCMV M45 mutant-mediated necrosis, deletion of DAI by the CRISPR/Cas9 approach was unable to remit HSV-1–induced necrosis (Fig. 3D and Figs. S4 and S5). Notably, knockdown of MLKL provided effective protection against HSV-1 in both MEFs and L929 cells (Fig. 3E and Fig. S3B). These results indicate that HSV-1 activates RIP3/MLKL-dependent necrosis independently of TNFR, TLR3, and DAI.
Fig. 3.
HSV-1 infection-induced necrosis requires MLKL but is independent of TNFR, TLR3, CYLD, and DAI. (A) MEFs isolated from WT, TNFR1 KO, TNFR1/TNFR2 double KO, and TLR3 KO mice were treated as indicated for 16–18 h. The cell survival rate was determined by measuring ATP levels. T, TNF-α; S, Smac mimetic; Z, z-VAD. (B) Total mRNA was collected from the indicated MEFs. The mRNA expression levels of the indicated genes were measured. (C) MEFs were transfected with NC or CYLD siRNA oligos for 48 h. Cells were treated as indicated for an additional 16–18 h. The cell-survival rate was determined by measuring ATP levels. Cell lysates were collected 48 h posttransfection and subjected to Western-blot analysis. (D) WT MEFs, RIP3 KO MEFs, or two DAI KO clones (32# and 40#) were infected with HSV-1 for 16–18 h. The cell-survival rate was determined by measuring ATP levels. Genomic DNA was collected from DAI KO clone 32# or 40#, and alterations of DAI DNA sequence were analyzed by PCR. (E) MEFs or MLKL-shRNA stable cell lines as indicated were infected with the HSV-1 F strain for 16–18 h. Cell lysates were collected and subjected to Western-blot analysis. The cell-survival rate was determined by measuring ATP levels. MLKL-shRNA, MEFs stably expressing a shRNA targeting mouse MLKL.
Viral Protein ICP6 Interacts with RIP3 Through the RHIM Domains of both Proteins and Is Required for HSV-1–Induced Necrosis.
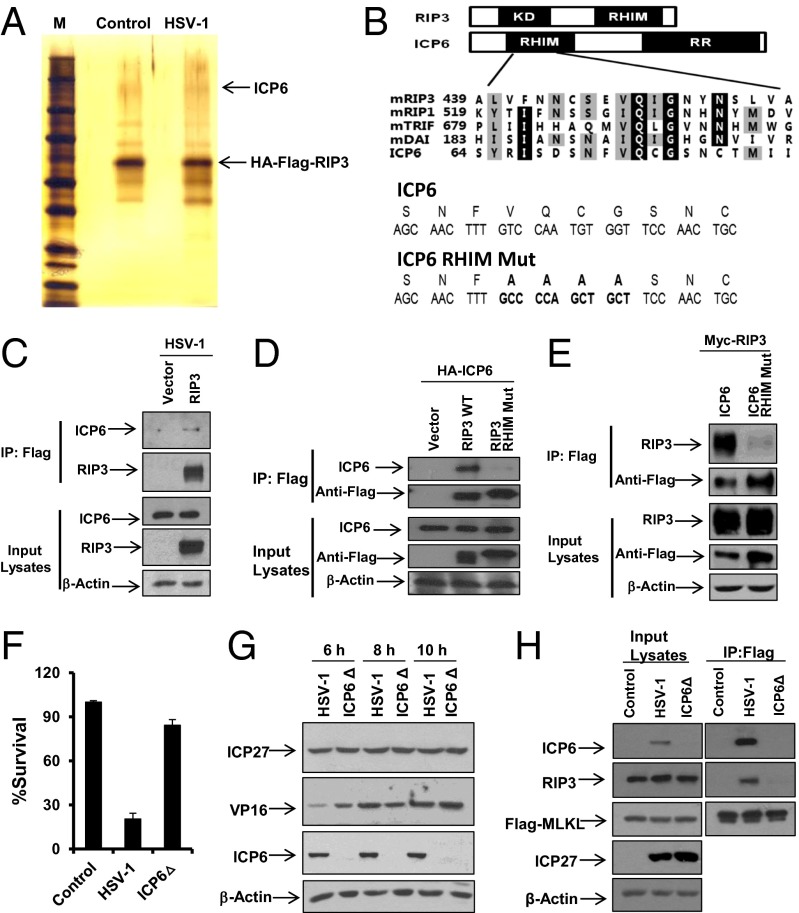
To explore how HSV-1 induces RIP3-dependent necrosis, we sought to identify the proteins that associate with RIP3 in cells after virus infection. As shown in Fig. 4A, a protein band at around 130 kDa was specifically observed with the RIP3 immunocomplex after HSV-1 infection. Protein mass spectrometry analysis revealed that this protein is an HSV-1 ribonucleotide reductase large (R1) subunit (called ICP6), encoded by the UL39 gene. Interestingly, ICP6 contains an N-terminal RHIM-like domain (29) in addition to its C-terminal reductase domain (Fig. 4B). To verify the specific interaction between ICP6 and RIP3, we infected RIP3 KO MEFs expressing empty vector control or Flag-tagged WT RIP3 with HSV-1 and then precipitated a Flag-tagged RIP3 immunocomplex, followed by Western blotting using an anti-ICP6 antibody. ICP6 was indeed specifically pulled down by Flag-tagged WT RIP3 (Fig. 4C). Furthermore, we found that Flag-tagged RHIM mutant RIP3 failed to interact with ICP6 (Fig. 4D). Additionally, the RHIM mutant form of ICP6 showed reduced ability to bind RIP3 compared with that of intact ICP6 (Fig. 4E) although it retained the capacity to form a complex with UL40, the ribonucleotide reductase small (R2) subunit (Fig. S6A). These results suggest that HSV-1 infection triggers the formation of an ICP6–RIP3 complex through the RHIM domains of both proteins. We then proceeded to investigate the contribution of ICP6 in HSV-1–induced necrosis.
Fig. 4.
Virus protein ICP6 interacts with RIP3 through the RHIM domains of both proteins and is required for HSV-1–induced necrosis. (A) RIP3 KO MEFs expressing WT RIP3 (RIP3 was double tagged with Flag and HA) infected with HSV-1 (MOI = 2) for 5 h. Cell lysates were collected and immunoprecipitated with anti-Flag and anti-HA agarose beads as described in Materials and Methods. The eluted RIP3-associated complexes were subjected to SDS/PAGE and detected by silver staining. The indicated bands were excised and then digested and analyzed by mass spectrometry. (B) Domain structures of RIP3 and ICP6. KD, kinase domain; RR, ribonucleotide reductase. Compared with WT ICP6, residues from 73 to 76 are mutated to four alanine residues in ICP6 RHIM domain mutant (ICP6 RHIM Mut). Bold letters represent the changed sequences. (C) RIP3 KO MEFs expressing empty vector or WT RIP3 were infected with HSV-1 (MOI = 2) for 5 h. Cell lysates were collected and immunoprecipitated with anti-Flag agarose. The Flag-RIP3 immunocomplex was analyzed by Western-blot analysis. (D) The 293T cells were cotransfected with a DNA plasmid expressing HA-tagged ICP6 plus the plasmid expressing Flag-tagged WT RIP3 or Flag-tagged RIP3 RHIM Mut. Cell lysates were collected 48 h posttransfection and immunoprecipitated with anti-Flag agarose. The Flag-RIP3 immunocomplex was analyzed by Western-blot analysis. (E) The 293T cells were cotransfected with a DNA plasmid expressing Myc-tagged RIP3 plus the plasmid expressing Flag-tagged WT ICP6 or Flag-tagged RHIM domain mutant form of ICP6 (ICP6 RHIM Mut). Cell lysates were collected 48 h posttransfection and immunoprecipitated with anti-Flag agarose. The Flag-ICP6 immunocomplex was analyzed by Western-blot analysis. (F) MEFs were infected with HSV-1 or HSV-1 ICP6Δ for 16–18 h. The cell-survival rate was determined by measuring ATP levels. (G) MEFs were infected with HSV-1 or HSV-1 ICP6Δ for the indicated hours. Cell lysates were collected and subjected to Western-blot analysis. (H) MEFs stably expressing MLKL (MLKL was double tagged with Flag and HA) infected with HSV-1 or ICP6Δ (MOI = 2) for 5 h. Cell lysates were collected and immunoprecipitated with anti-Flag agarose. The Flag–MLKL immunocomplex was analyzed by Western-blot analysis of the indicated proteins.
We infected MEFs and L929 cells with WT or ICP6 deletion (ICP6Δ) HSV-1. These cells had severely reduced sensitivity to HSV-1 ICP6Δ compared with the WT virus even though these viruses replicated to similar levels (Fig. 4 F and G and Fig. S6 B and C). Moreover, we found that HSV-1 infection was able to trigger the RIP3/MLKL complex, which is known to be essential for RIP3-dependent necrosis. In contrast, HSV-1 ICP6Δ failed to induce the interaction of RIP3 with MLKL (Fig. 4H). Thus, these results demonstrate that ICP6 is required to activate RIP3/MLKL-mediated necrosis upon HSV-1 infection.
ICP6 Is Sufficient to Activate RIP3/MLKL-Mediated Necrosis.
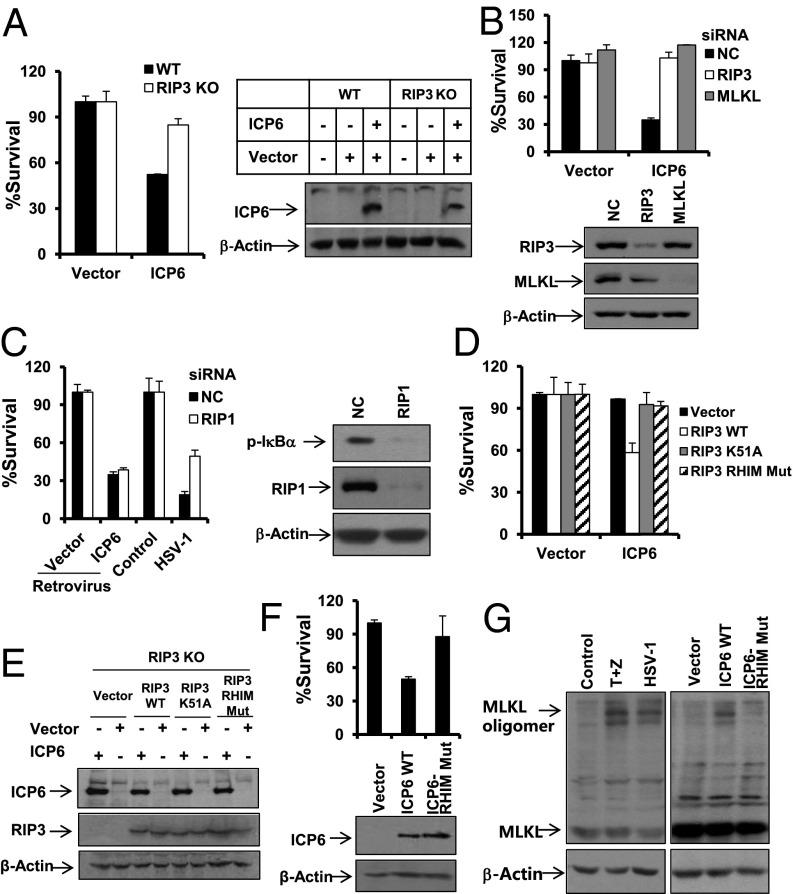
To address whether ICP6 directly activates RIP3 signaling, we applied retrovirus-mediated expression of ICP6. Interestingly, MEFs expressing ICP6, but not those expressing the empty vector control, underwent cell death whereas the observed cell death was attenuated in RIP3 KO cells (Fig. 5A). Knockdown of MLKL, as well as knockdown of RIP3, prevented ICP6-induced cell death dramatically (Fig. 5B and Fig. S7A). However, RIP1 RNAi had no impact on this cell death although it affected HSV-1 infection-induced NF-κB activation, as well as necrosis (Fig. 5C and Fig. S7A). As expected, RIP3 KO MEFs with ectopic expression of WT, but not the kinase-dead or RHIM-mutant forms of RIP3, restored the sensitivity to ICP6 (Fig. 5 D and E). The importance of the RHIM domain of ICP6 was verified by the fact that retrovirus-mediated expression of RHIM mutant ICP6 did not induce significant cell death of infected MEFs or L929 cells although expression of the mutant form was at a comparable level with that of WT ICP6 (Fig. 5F and Fig. S7B). Because activated MLKL molecules are known to form oligomers to mediate necrosis, we examined whether HSV-1 infection or ectopic expression of ICP6 could trigger MLKL to form oligomers. As shown in Fig. 5G, MLKL oligomers were induced upon HSV-1 infection, and the pattern of MLKL oligomerization was the same as that observed in TNF-α–induced necrosis (Fig. 5G, Left). Notably, ectopic expression of ICP6, but not RHIM mutant ICP6, was sufficient to drive MLKL into oligomers (Fig. 5G, Right). Thus, ICP6 is able to directly activate RIP3/MLKL-dependent necrosis.
Fig. 5.
ICP6 is sufficient to activate RIP3/MLKL-mediated necrosis. (A) WT or RIP3 KO MEFs were infected for 48 h with retrovirus expressing empty vector or ICP6. Cell lysates collected 24 h postinfection with retrovirus as indicated were subjected to Western-blot analysis. The cell-survival rate was determined by measuring ATP levels. (B) MEFs were transfected with the indicated siRNA oligos for 48 h. Cells were infected with retrovirus as indicated for an additional 48 h. The cell-survival rate was determined by measuring ATP levels. Cell lysates were collected 48 h posttransfection and subjected to Western-blot analysis. (C) MEFs were transfected with the indicated siRNA oligos for 48 h. Cells were infected with the indicated retrovirus for 48 h or with HSV-1 for 16 h, and then the cell-survival rate was determined by measuring ATP levels. Cells were infected with HSV-1 for 6 h, and cell lysates were collected and subjected to Western-blot analysis of p-IκBα, RIP1, and β-Actin. (D and E) RIP3 KO MEFs expressing RIP3 WT or RIP3 K51A or RIP3 RHIM Mut were infected for 48 h with retrovirus as indicated. The cell-survival rate was determined by measuring ATP levels. Cell lysates were collected 24 h postinfection with the indicated retrovirus and then subjected to Western-blot analysis for ICP6 and β-Actin levels. (F) MEFs were infected with retrovirus expressing ICP6 or ICP6 RHIM Mut for about 48 h. The cell-survival rate was determined by measuring ATP levels. Cell lysates collected from cells infected with retrovirus expressing empty vector or WT ICP6 or ICP6 RHIM Mut for 24 h were subjected to Western-blot analysis. (G) L929 cells were treated with TNF-α/z-VAD for 9 h, or infected with HSV-1 for 11h, or infected with retrovirus expressing the indicated gene for about 36 h. The cell lysates were resolved on nonreducing gel and analyzed by Western blot of MLKL and β-Actin.
RIP3 Is Critical for Host Defense Against HSV-1 Replication and Pathogenesis.
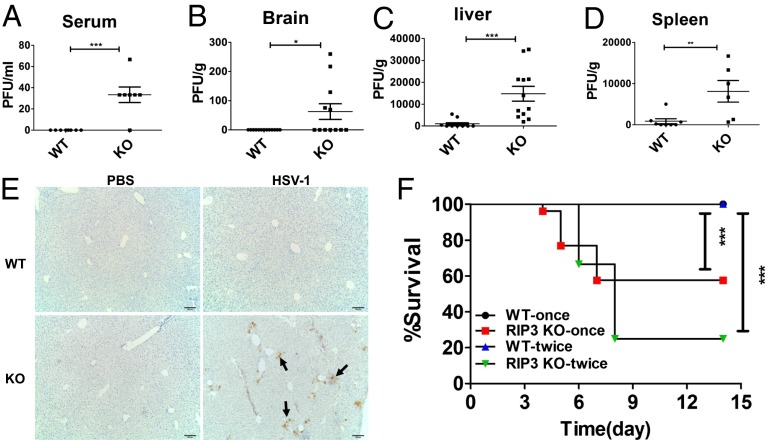
WT MEFs died after infection of GFP-labeled HSV-1 F strain for 16 h, leading to reduced GFP signal intensity compared with that observed in the RIP3 KO cells (Fig. S8A). Consistently, analysis of viral titers showed that WT MEFs produce less HSV-1 compared with RIP3 KO MEFs (Fig. S8B). These data support the hypothesis that RIP3-mediated necrosis contributes to the clearance of HSV-1. To further assess the role of RIP3 in the control of HSV-1 in vivo, WT and RIP3 KO mice were inoculated with the same dose of HSV-1 via i.p. injection, and the viral titers were measured 2 d postinfection. As shown in Fig. 6 A–D, viral titers in serum, brain, liver, and spleen were significantly elevated in RIP3 KO mice compared with WT mice. Immunohistochemistry analysis also revealed increased expression of HSV-1 glycoprotein gB in the liver of RIP3 KO mice compared with that of WT mice (Fig. 6E). Importantly, RIP3 KO mice showed increased lethality to the infection in a dose-dependent manner whereas all WT mice survived after the same infection (Fig. 6F). Taken together, these results establish an essential role for RIP3 in the host defense of HSV-1 pathogenesis.
Fig. 6.
RIP3 is essential for host defense against HSV-1 infection in vivo. (A–D) Replication levels in serum, liver, spleen, and brain of mice infected with HSV-1 of 2 × 107 pfus per mouse via i.p. injection (i.p.) for 2 d. Viral titers were determined by plaque assay. (E) Immunohistochemistry of the liver tissue from WT and RIP3 KO mice infected (i.p.) with HSV-1 of 2 × 107 pfus per mouse for 2 d. Arrows indicate the expression of viral protein gB. The results shown here are representative of five mice. (F) WT and RIP3 KO mice were infected (i.p.) with HSV-1 of 2 × 107pfus once per mouse or 1.8 × 107pfus twice per mouse. The second injection was done 3 d after the first injection. Survival rate of mice was monitored for 14 d. WT-once (n = 24), RIP3 KO-once (n = 26); WT-twice (n = 12), RIP3 KO-twice (n = 12). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Although necrosis has been traditionally considered as accidental cell death, we now know that some forms of necrotic cell death are programmed and that these processes are commonly mediated by RIP3 kinase and its substrate MLKL. The concept of programmed necrosis is emerging as an alternative cell death to apoptosis that may function critically for host defenses against microbes. However, the mechanisms underlying pathogen-initiated programmed necrosis are largely unknown. Our present work reveals a mechanism of HSV-1 infection-triggered programmed necrosis. This form of necrosis is directly induced by the viral protein ICP6, which forms an interaction with RIP3 through the RHIM domains of both proteins. Such an interaction activates RIP3, leading to the activation of MLKL and subsequent necrosis. To our knowledge, this study provides the first evidence that pathogen-encoded ICP6-mediated direct activation of RIP3/MLKL-dependent necrosis triggers an effective host-defense mechanism against HSV-1.
Multiple necrosis-initiating signaling pathways converge on RIP3. The RHIM domain of RIP3 is critical in the characterized programmed necrotic pathways induced by death ligands, TLR ligands, or M45/vIRA mutant MCMV. Through its RHIM domain, RIP3 forms complexes with RIP1, TRIF, or DAI to mediate the respective necrotic pathways. Therefore, RHIM–RHIM interaction seems to be a common mechanism of RIP3 activation. In this study, we found that HSV-1 infection naturally triggers RIP3-dependent necrosis. Deletion of DAI is unable to block HSV-1–induced necrosis. Additionally, TLR3 knockout MEFs responded normally to HSV-1 infection, indicating that the downstream adaptor of TLR3, TRIF, is not involved in this process. Although RIP1 RNAi affected HSV-1–induced cell death, it is hard to determine the precise role of RIP1 due to its effect on virus-induced NF-κB activation (Fig. 5C). Our study demonstrates that the HSV-1–encoded protein ICP6 interacts with RIP3 through the RHIM domains of both proteins during virus infection and that the RHIM-dependent interaction between ICP6 and RIP3 is essential for virus-induced necrosis. Therefore, ICP6 functions as a RIP3 partner mediating HSV-1 infection-induced necrosis, supporting RHIM–RHIM interaction-mediated activation of RIP3. Furthermore, we found that ectopic expression of ICP6 is sufficient to activate RIP3-dependent necrosis. Disruption of RHIM in either ICP6 or RIP3 abolishes this cell death process. Additionally, MLKL RNAi blocks ICP6-induced necrosis, and MLKL oligomers are induced by the expression of ICP6. Notably, knockdown of RIP1 failed to block ICP6-induced necrosis, indicating a dispensable role of RIP1 in ICP6-triggered activation of RIP3. Therefore, these data suggest that ICP6 is able to directly activate RIP3. This activation mechanism is distinct from VV-induced necrosis, which is indeed TNF-dependent necroptosis (11). Although MCMV-encoded M45/vIRA and ICP6 share a common RHIM domain, they exhibit opposite effects on RIP3. M45/vIRA interacts with RIP3, but its function is to block RIP3 interaction with its activator proteins (21). As such, to our knowledge, ICP6 is the first known pathogen-encoded activator of RIP3. Interestingly, some other viral proteins such as the R45 and E45 proteins encoded by two strains of rat CMV, as well as ICP10 encoded by HSV-2, also contain a RHIM domain (29). Therefore, viral encoded RHIM domain-containing proteins may function as general activators of RIP3-mediated necrosis. Further studies will be required to address the roles of these viruses in programmed necrosis of host cells and to explore how pathogen–host interaction determines the regulation of programmed necrosis.
As a ribonucleotide reductase R1 subunit, ICP6 forms a holoenzyme with R2 subunit to regulate viral DNA regulation in quiescent cells. We observed that HSV-1 ICP6Δ replicates in both immobilized MEFs and L929 cells (Fig. 4G and Fig. S6D), consistent with the previous observation that ICP6 is dispensable for virus multiplication in dividing cells (30). It has been demonstrated that ICP6 constitutively interacts with caspase-8 and RIP1 through its reductase domain and functions in an antiapoptotic role in death triggered by TNF-α, FasL, or TLR3 (31, 32). Although the N-terminal RHIM sequence of ICP6 was shown to be dispensable for the interaction with RIP1 and caspase-8, we have found that it is essential for the interaction with RIP3 (Fig. 4E). In the HSV-1–infected MEFs, we did not observe any detectable activation of caspase-8 and caspase-3 (Fig. S1B), suggesting that cells are not committed to apoptosis. Apoptosis commonly suppresses programmed necrosis via active caspase-8, which is able to cleave RIP1 (33, 34), CYLD (35), and RIP3 (36). In many situations, programmed necrosis proceeds only when caspase-8 activation is compromised by chemical or viral inhibitors (3). Constitutive inhibition of caspase-8 tends to restrict apoptosis of HSV-1–infected cells through the extrinsic apoptotic pathway, but it may commit cells to undergoing programmed necrosis. Therefore, it is not surprising that ICP6 has the ability to activate programmed necrosis by performing dual roles in suppression of caspase-8 and activation of RIP3.
Although the lysis of host cells is important for the release of mature viruses from cells, our in vitro and in vivo evidence shows that loss of RIP3 results in elevated HSV-1 viral titers (Fig. S8 and Fig. 6 A–D). Therefore, our data suggest that RIP3-dependent necrosis induced by HSV-1 infection causes premature death of host cells. It is noteworthy that the deficiency of RIP3 significantly enhanced the susceptibility of mice to HSV-1 infection, leading to the death of animals (Fig. 6F). Lethality was observed in the group of RIP3 KO mice whereas all WT mice recovered. These findings reveal a critical role for RIP3-dependent necrosis in the control of HSV-1 replication and pathogenesis. Our study provides strong mechanistic and in vivo evidence that one of the physiological functions of programmed necrosis is host defense against pathogens.
Materials and Methods
Reagents.
TNF-α recombinant protein and the Smac mimetic compound were generated as previously described (10). z-VAD was purchased from Bachem; Necrostatin-1 was from Alexis Biochemicals; BMS-345541 and cycloheximide were from Sigma; DMEM was from Thermo. The following antibodies were used for Western-blotting: CYLD (437700; Cell Signaling), RIPK1 (610459; BD Biosciences), mouse RIP3 (2283; Prosci), Caspase-8 (51-8125KC; BD Biosciences), Cl-Caspase-8 (8592; Cell Signaling), Caspase-3 (9662; Cell Signaling), Flag (A8592; Sigma), α-Tubulin (PM054-7; MBL), β-Actin (A2066; Sigma), gB (6506; Abcam), ICP27 (31631; Abcam), VP16 (110226; Abcam), and p-IκBα (9246; Cell Signaling). HSV-1 ICP6 polyclonal antibody was generated against a synthesized peptide corresponding to the N-terminal 15 amino acids.
Cell-Survival Assay.
Posttreatment cell survival was determined using a Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega). The cell-survival assay was performed by measuring ATP levels according to the manufacturer’s instructions.
Animal Model of HSV-1 Infection.
Male WT and RIP3 KO mice at 7–8 wk of age were infected with HSV-1 with 2 × 107 plaque forming units (pfus) once per mouse, or 1.8 × 107 pfus twice per mouse (3 d apart) by i.p. injection. Survival was monitored for 14 d. Brains, livers, and spleens of mice infected with HSV-1 for 48 h were excised and used to prepare homogenates, which were then titered by plaque assay. Sera were also extracted from mice infected with HSV-1 for 48 h and titered by plaque assay. For Western blotting, livers and spleens were ground and resuspended in lysis buffer with 0.1% SDS. All animal experiments were performed in accordance with protocols by the Institutional Animal Care and Use Committee at Soochow University.
Immunohistochemistry Analysis.
To identify HSV-1 infection, paraffin-embedded liver sections from WT and KO mice were stained with gB and visualized with DAB (Genetech).
Statistical Analyses.
Data of cell-survival rate are represented as the mean ± SD of duplicates. All experiments were repeated at least three times with similar results. Significance was evaluated using t tests (GraphPad Prism software).
Supplementary Material
Acknowledgments
We thank Dr. Xiaodong Wang [National Institute of Biological Sciences (NIBS)] for kindly providing RIP3 knockout mice, anti-MLKL antibody, and Smac mimetic, and for his critical reading of the manuscript. We also thank Dr. Sandra K. Weller (University of Connecticut Health Center) for the HSV-1 KOS strain and ICP6 deletion mutant (ICP6Δ), Dr. Chunfu Zheng (Soochow University) for HSV-1 F strain and GFP-labeled HSV-1 F strain, and Dr. Wenhui Li (NIBS) for insightful discussions. This work was supported by National Basic Research Program of China Grant 2013CB910102, National Natural Science Foundation of China Grants 31222036 and 31171330, a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Natural Science Foundation of Jiangsu Province Grants BK2012004 and BK2011287, NIH Grant1R01CA166413, and Undergraduate Training Programs for Innovation and Entrepreneurship Grant 5731554813.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412767111/-/DCSupplemental.
References
- 1.Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science. 1998;281(5381):1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 2.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8(1):44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Moriwaki K, Chan FK. RIP3: A molecular switch for necrosis and inflammation. Genes Dev. 2013;27(15):1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J, Zhong CQ, Zhang DW. Programmed necrosis: Backup to and competitor with apoptosis in the immune system. Nat Immunol. 2011;12(12):1143–1149. doi: 10.1038/ni.2159. [DOI] [PubMed] [Google Scholar]
- 5.Degterev A, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 6.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22(2):263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 8.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 9.Hitomi J, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135(7):1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 13.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138(2):229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1-2):213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA. 2012;109(14):5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54(1):133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Cai Z, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16(1):55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24(1):105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA. 2011;108(50):20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser WJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7(4):302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11(3):290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobbelstein M, Shenk T. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J Virol. 1996;70(9):6479–6485. doi: 10.1128/jvi.70.9.6479-6485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kettle S, et al. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J Gen Virol. 1997;78(Pt 3):677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 25.Egan KP, Wu S, Wigdahl B, Jennings SR. Immunological control of herpes simplex virus infections. J Neurovirol. 2013;19(4):328–345. doi: 10.1007/s13365-013-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steiner I, Benninger F. Update on herpes virus infections of the nervous system. Curr Neurol Neurosci Rep. 2013;13(12):414. doi: 10.1007/s11910-013-0414-8. [DOI] [PubMed] [Google Scholar]
- 27.Ray CA, et al. Viral inhibition of inflammation: Cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69(4):597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 28.Burke JR, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278(3):1450–1456. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- 29.Lembo D, Brune W. Tinkering with a viral ribonucleotide reductase. Trends Biochem Sci. 2009;34(1):25–32. doi: 10.1016/j.tibs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DJ, Weller SK. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: Characterization of an ICP6 deletion mutant. Virology. 1988;166(1):41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 31.Dufour F, et al. The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFalpha- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis. 2011;16(3):256–271. doi: 10.1007/s10495-010-0560-2. [DOI] [PubMed] [Google Scholar]
- 32.Dufour F, Bertrand L, Pearson A, Grandvaux N, Langelier Y. The ribonucleotide reductase R1 subunits of herpes simplex virus 1 and 2 protect cells against poly(I · C)-induced apoptosis. J Virol. 2011;85(17):8689–8701. doi: 10.1128/JVI.00362-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13(19):2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan FK, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278(51):51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 35.O’Donnell MA, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13(12):1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng S, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19(10):2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.