Significance
Pyrrolysine, the 22nd amino acid, is found in few proteins. One, the trimethylamine methyltransferase MttB, forms a small portion of a large family of proteins. Most in this family lack pyrrolysine and have no known activity. We show that one such protein, MtgB, is a glycine betaine methyltransferase, providing functional context that may explain the relationship between family members with and without pyrrolysine. Close relatives of MtgB are encoded in many of the abundant bacteria in the oceans, as well as different microbes undertaking symbioses ranging from plants to humans. This finding implies that MtgB might partake in a widespread and underappreciated pathway of GB metabolism contributing significantly to global carbon and nitrogen cycling as well as human health.
Keywords: glycine betaine, trimethylamine methyltransferase, COG5598, l-pyrrolysine, Desulfitobacterium hafniense
Abstract
COG5598 comprises a large number of proteins related to MttB, the trimethylamine:corrinoid methyltransferase. MttB has a genetically encoded pyrrolysine residue proposed essential for catalysis. MttB is the only known trimethylamine methyltransferase, yet the great majority of members of COG5598 lack pyrrolysine, leaving the activity of these proteins an open question. Here, we describe the function of one of the nonpyrrolysine members of this large protein family. Three nonpyrrolysine MttB homologs are encoded in Desulfitobacterium hafniense, a Gram-positive strict anaerobe present in both the environment and human intestine. D. hafniense was found capable of growth on glycine betaine with electron acceptors such as nitrate or fumarate, producing dimethylglycine and CO2 as products. Examination of the genome revealed genes for tetrahydrofolate-linked oxidation of a methyl group originating from a methylated corrinoid protein, but no obvious means to carry out corrinoid methylation with glycine betaine. DSY3156, encoding one of the nonpyrrolysine MttB homologs, was up-regulated during growth on glycine betaine. The recombinant DSY3156 protein converts glycine betaine and cob(I)alamin to dimethylglycine and methylcobalamin. To our knowledge, DSY3156 is the first glycine betaine:corrinoid methyltransferase described, and a designation of MtgB is proposed. In addition, DSY3157, an adjacently encoded protein, was shown to be a methylcobalamin:tetrahydrofolate methyltransferase and is designated MtgA. Homologs of MtgB are widely distributed, especially in marine bacterioplankton and nitrogen-fixing plant symbionts. They are also found in multiple members of the human microbiome, and may play a beneficial role in trimethylamine homeostasis, which in recent years has been directly tied to human cardiovascular health.
Quaternary amines, such as glycine betaine (N,N,N-trimethylglycine or GB), carnitine, and choline are abundant in nature, playing wide-ranging roles in the ecology and physiology of microbes, marine organisms, plants, and animals. GB is an important compound in marine or brackish environments in which it acts as a common compatible solute for many prokaryotic and eukaryotic organisms (1–3). Carnitine can also function as a compatible solute but is especially abundant in animal tissues due to its role in mitochrondrial transport of fatty acids for energy metabolism (4, 5). Legume plants make various quaternary amines, some of which are found in the rhizosphere (6). In many environments, choline results from the breakdown of phosphatidylcholine, ensuring choline as an intermediate of phospholipid breakdown in environments as diverse as lake sediments and the human colon (7, 8).
GB can be produced as an intermediate during choline and carnitine degradation (9, 10). The route of catabolic bacterial degradation of GB differs markedly depending on the presence or absence of oxygen. Aerobically, various oxidases or dehydrogenases can oxidatively demethylate GB to dimethylglycine (DMG), and then to sarcosine (monomethylglycine) (11–16). Anaerobically, catabolic pathways containing GB reductase cleave GB to acetate and trimethylamine (TMA) as excreted products. GB reduction is often considered the sole fate of the compound under anaerobic conditions (17–19). However, there have been sporadic reports in the literature that GB reduction is not the only route of microbial GB catabolism under anoxic conditions. Some sulfidogenic bacteria have been documented to demethylate GB to DMG while oxidizing methyl groups to CO2 (1). Acetogenic bacteria have been documented to use GB as a source for methyl groups and reducing equivalents (20, 21). The pathway of GB catabolism by such organisms has not been elucidated.
Recent work has revealed a surprising connection between anaerobic degradation of quaternary amines and human health. Circulating trimethylamine N-oxide (TMAO) has been implicated in atherosclerosis leading to heart disease and stroke (22, 23). The TMAO has been shown to arise from TMA, which is generated by anoxic gut microbial activity from precursors such as choline, carnitine, and GB (22, 23).
If alternative means of quaternary amine degradation exist that do not generate TMA, the composition of gut microbiota between individuals could moderate the risk of heart disease. A putative oxygenase that demethylates GB has been identified (12), but, thus far, no enzymes that could be active under anaerobic conditions. We have now found a source of such an enzyme in Desulfitobacterium hafniense strain Y51, an anaerobically respiring Gram-positive bacterium. One strain of D. hafniense, DP7, was isolated from the human intestine (24) and lacks the ability to carry out organohalide respiration, a hallmark of the rest of this species (25).
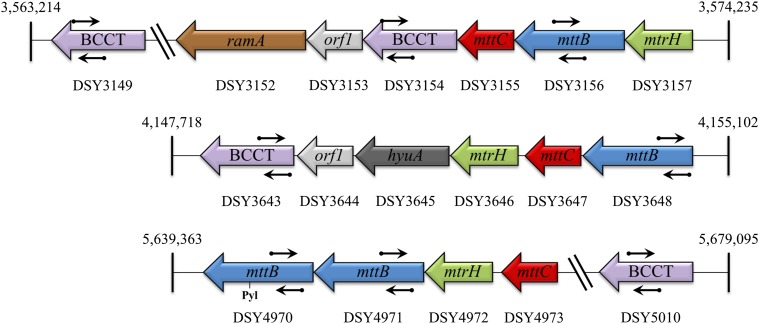
D. hafniense is one of few known microbes that carry the pyl genes to enable the biosynthesis and genetic encoding of pyrrolysine (Pyl), the 22nd amino acid (26, 27). Organisms with the pyl genes also carry examples of Pyl-containing methyltransferases (28). D. hafniense has an mttB gene with a pyrrolysine codon (pyl-mttB). Pyl-MttB proteins are the only known gene products to carry out TMA-dependent corrinoid methylation. Interestingly, the genome of D. hafniense Y51 encodes three homologs of mttB that lack pyl codons and are thus predicted to form non-Pyl MttB proteins (29). Each of these non-Pyl mttB homolog genes is found in close proximity in the chromosome to a gene encoding a member of the betaine/choline/carnitine transporter (BCCT) family as well as genes encoding predicted corrinoid-binding proteins and methylcorrinoid:pterin methyltransferases (Fig. 1).
Fig. 1.
The genomic context of mttB genes suggests a role in quaternary amine metabolism. The clustering of MttB family member genes (blue) with genes predicted to encode corrinoid-binding proteins (red), methylcorrinoid:pterin methyltransferases (green), and BCCT transporters (purple) suggests a role of non-Pyl MttB enzymes in the methylotrophic metabolism of quaternary amines. Gene names of nearest homologs are located within the indicated genes, with loci designations in the D. hafniense Y51 genome listed below. RamA functions in corrinoid protein activation whereas MtrH is a methylcobalamin:THF methyltransferase. HyuA is a predicted d-phenylhydantoinase. Pyl indicates the position of the amber codon encoding the pyrrolysine residue in DSY4970, a homolog of the bona fide TMA methyltransferase. The small arrows indicate the location of primers used for the qRT-PCR experiment.
This genomic proximity led us to hypothesize that non-Pyl MttB homologs function as corrinoid-dependent quaternary amine methyltransferases. We found that the DSY3156 gene encoding a non-Pyl MttB of D. hafniense is up-regulated during growth on GB, and the gene product is a GB:corrinoid methyltransferase, an enzymatic activity that has not been previously reported. These results reveal the function of a non-Pyl MttB for the first time, to our knowledge, and also a functional relationship that may underlie the evolutionary history of pyrrolysine and the large MttB superfamily. A large number of homologs of the GB methyltransferase are found encoded in available genomes and metagenomes and indicate a previously underappreciated pathway of quaternary amine oxidation to CO2, as also supported by the function of DSY3157 as a methylcorrinoid:tetrahydrofolate methyltransferase. This pathway is likely present in environments as diverse as the oceans, the rhizosphere, and the human colon.
Results
D. hafniense Uses GB as a Methylotrophic Growth Substrate.
D. hafniense has not previously been thought to use quaternary amines as substrates. However, GB supported growth when added to culture medium having an exogenous electron acceptor such as fumarate (Fig. S1A). We noticed a low, but significant growth on fumarate in the absence of GB and therefore also tested inorganic electron acceptors. We found that GB also supported growth with nitrate (Fig. S1B). Continued cultivation of GB cultures was dependent on the presence of both nitrate and GB. Yeast extract was stimulatory to growth but not essential and could not replace GB. However, CO2 could not replace fumarate or nitrate during growth on GB, suggesting that CO2 reduction for acetogenesis did not underlie growth on GB. Acetate was also not detected during growth.
The dependence on exogenous electron acceptors suggested that GB was serving as an electron donor; therefore, cultures were examined for possible degradation products of GB using TLC. In nitrate cultures at midlog, GB was demethylated to DMG (Fig. 2). We measured the ratio of DMG produced to GB consumed as 0.91 ± 0.18 (n = 5). In keeping with the absence of GB reductase in the genome, we could not detect trimethylamine using gas chromatography in growing cultures. Instead, we found that GB-metabolizing cultures also produced CO2 with a DMG:CO2 ratio of 1.1 ± 0.16 (n = 6). CO2 was not significantly produced when GB was not added to the medium. These results are consistent with a stoichiometry of ∼1 GB → 1 DMG + 1 CO2. We conclude that D. hafniense is capable of methylotrophic growth in which a methyl group of GB is oxidized to CO2 to provide reducing equivalents for anaerobic respiration. Additionally, we noted in some cultures given lower amounts of GB (<5 mM), a compound comigrating with monomethylglycine in TLC was also detectable in late log cultures, suggesting that under some circumstances GB could be further demethylated to form monomethylglycine.
Fig. 2.
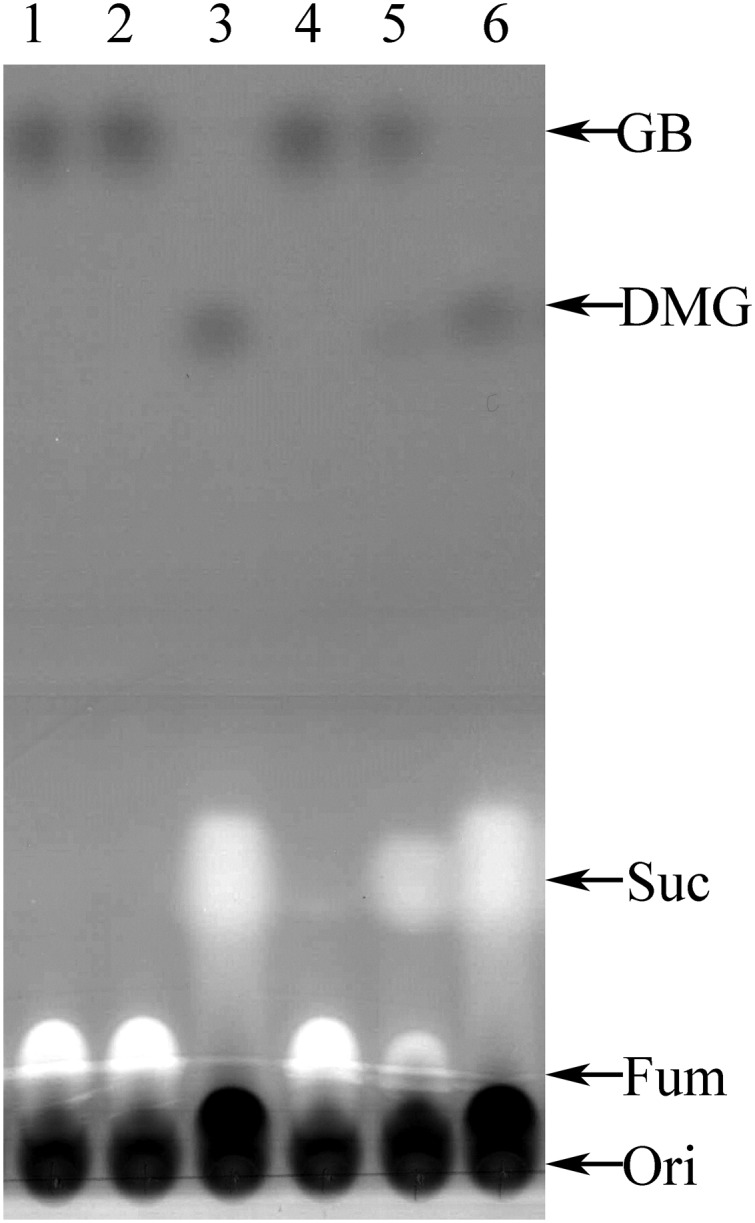
Thin-layer chromatographic analysis of D. hafniense culture supernatants. Lanes were spotted at the origin (Ori) with aliquots of supernatants from (lane 1) uninoculated tube at T0; (lane 2) uninoculated culture tube after 190 h incubation at 37 °C; (lane 3) stationary phase culture used to inoculate culture in lane 4; (lane 4) newly inoculated culture, O.D. = 0.0; (lane 5) culture after 83 h incubation at 37 °C, O.D. = 0.12; and (lane 6) culture after 190 h, O.D. = 0.19. Arrows indicate relative migration positions of authentic standards including succinate (Suc), fumarate (Fum), GB, and dimethylglycine (DMG).
qPCR Analysis of Non-Pyl mttB Genes.
Examination of the genome of D. hafniense Y51 revealed a possible pathway for conversion of a methyl group to CO2 on a tetrahydrofolate (THF) cofactor (Fig. S2) but supported no route from GB to methyl-THF. However, the genomic context of the genes encoding three non-Pyl MttBs in D. hafniense supported our hypothesis that these proteins may be quaternary amine:corrinoid methyltransferases. Each non-Pyl MttB is encoded in gene clusters that are potential transcriptional units and has, within the unit or nearby, a gene encoding a member of the BCCT family of proteins (2). Additionally, each gene cluster encodes homologs of a methylotrophic corrinoid protein (30) and a methylcorrinoid:THF methyltransferase (31, 32). Such proteins are part of the systems that initiate catabolism by Gram-positive bacteria such as D. hafniense during growth on methoxylated aromatic compounds (31, 32).
So as to determine whether one of the non-Pyl mttB genes might be involved in GB metabolism, transcript abundance was compared by qPCR in cells grown on either pyruvate or GB. Transcripts of the mttB genes (DSY3156, DSY3648, DSY4970, and DSY4971) as well as those encoding the putative BCCT genes (DSY3149, DSY3154, DSY3643, and DSY5010) were targeted at the locations indicated in Fig. 1. The melt curves indicated the formation of single amplified products for each gene, which was supported by gel electrophoresis. In pyruvate-adapted cultures, each of the transcripts of the mttB homologs was in lower abundance than the housekeeping gene rpoB. This trend is unchanged in GB-grown cells for most mttB transcripts, the exception being DSY3156, which became more abundant than rpoB. Analysis using the 2−ΔΔCT method (33) indicated that the DSY3156 transcript is 27- to 58-fold higher in GB-grown cultures relative to pyruvate, and this difference was significant (P ≤ 0.001). Similarly DSY3154, the predicted BCCT gene located near DSY3156, increased above rpoB levels in cells grown on GB relative to pyruvate; with DSY3154 23- to 42-fold higher in GB- versus pyruvate-grown cells, and this result also was significant (P ≤ 0.001).
Transcripts encoding the other non-Pyl MttB homologs and BCCT proteins were not elevated to this extent during growth on GB. For example, DSY3648 and DSY3643 were only slightly changed with GB relative to pyruvate, with the DSY3648 having a 0.77- to 2.3-fold difference and DSY3643 having a 1.1- to 2.8-fold increase in transcript abundance compared with pyruvate. The adjacent BCCT gene, DSY3149, showed a significant induction, with GB of 2.2- to 7.3-fold compared with pyruvate (P ≤ 0.001). In contrast, expression of other mttB and BCCT genes was actually lower on GB relative to pyruvate, with DSY4970 ∼0.07- to 0.41-fold lower and DSY4971 0.15- to 0.48-fold lower. This trend is also seen with DSY5010, the BCCT transporter gene nearest DSY4971, which showed a 0.34- to 1.34-fold difference in GB-grown cells relative to those grown on pyruvate. Overall, the expression patterns supported our hypothesis and indicated that non-Pyl mttB gene DSY3156 and BCCT gene DSY3154 could play important roles during growth on GB.
DSY3156 Is a Glycine Betaine:Cob(I)alamin Methyltransferase.
Proteins encoded adjacent to DSY3156 include a small corrinoid protein homologous to MttC, the cognate corrinoid protein of the pyrrolysine-containing MttB from Methanosarcina spp., suggesting that the DSY3156 protein might act to methylate corrinoid cofactors. If so, this activity would explain the up-regulation of the DSY3156 gene during growth on GB because this function would provide a path to methylate THF and thereby initiate a route to formation of CO2. Several different methyltransferases have been shown to methylate cob(I)alamin not bound to protein (34–36), and, therefore, we tested the ability of the recombinant DSY3156 protein (Fig. S3A) to methylate cob(I)alamin with various substrates. Quaternary amines such as carnitine and choline did not serve as substrates, nor did tertiary amines such as dimethylglycine or trimethylamine. Instead, DSY3156 protein carries out a robust methylation of cob(I)alamin in the presence of GB. Fig. 3A depicts the changes in the visible spectrum of cob(I)alamin as it is methylated in a reaction dependent on the presence of both GB and DSY3156. The presence of a clear isosbestic point at 578 nm indicates that cob(II)alamin was not generated by adventitious oxidation to a significant extent during the reaction, and that cob(I)alamin and methylCbl were the only detectable forms of cobalamin in the assay. Therefore, the rate of the MtgB-catalyzed reaction can be quantified by the increase in absorbance at 540 nm (Fig. 3B). No change in rate was detected when the reactions were performed under H2 or N2. DSY3156 carried out the methylation of cob(I)alamin with an apparent Km for GB of 1.96 ± 0.2 mM and a Vmax of 1.49 ± 0.04 µmol • min−1 • mg−1 (n = 3) (Fig. S4).
Fig. 3.
DSY3156 is a glycine betaine:cob(I)alamin methyltransferase. (A) UV-visible spectrum of 1.75 mM cobalamin reduced with Ti(III) citrate showing total change of absorbance vs. wavelength between 480 nm to 740 nm to demonstrate cob(I)alamin to methylcob(III)alamin (methylCbl) conversion in the presence of 5 mM GB and 50 μg of DSY3156. Spectra were gathered at 30-s intervals from 0 min to 9.5 min. Arrows indicate increasing or decreasing absorbance during course of reaction in different parts of spectrum. (B) Change in absorbance at 540 nm and 578 nm over time for an experiment in which the assay contained 50 mM GB, and at the arrow 50 μg of DSY3156 was added to the reaction mixture.
Stoichiometery of the Glycine Betaine:Cob(I)alamin Methyltransferase Reaction.
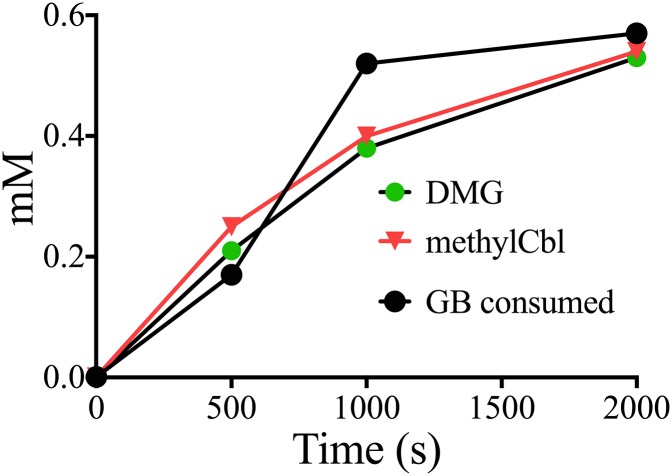
Our experiments with growing cultures revealed that GB was consumed with the concurrent production of dimethylglycine as a cellular product. The methylation of cob(I)almin with GB suggested that this DMG might be produced directly by DSY3156. Assays were first conducted in which the molar ratio of GB consumed to methylCbl produced was determined as 0.98. In separate experiments, the ratio of produced DMG to methylCbl was measured as 1.04, providing strong support for an overall reaction stoichiometry of 1 GB consumed to produce 1 DMG and 1 methylCbl. We confirmed this stoichiometry in an experiment in which GB and both products were measured simultaneously by removal of samples at specific time points for TLC from a spectrophotometrically monitored reaction (Fig. 4). Although GB was consumed, methylCbl and DMG were produced at approximately unit stoichiometry over the course of the reaction.
Fig. 4.
Stoichiometric demethylation of GB to produce DMG and methylCbl. The methylation of cob(I)alamin was monitored spectrophotometrically, and the conversion of GB to DMG was measured by TLC.
DSY3157 Is a Methylcorrinoid:Tetrahydrofolate Methyltransferase.
The genomic context of DSY3156 suggested that it was the first methyltransferase in a pathway to generate methyl-THF from GB and that the DSY3157 gene may encode the predicted pterin methyltransferase needed to catalyze the second half reaction of this pathway. BLASTp alignment revealed that DSY3157 was homologous to the soluble MtrH subunit of the membrane-bound methyltetrahydromethanopterin:cobalamin methyltransferase complex of methanogens (37). We therefore cloned the DSY3157 gene using the polymerase incomplete primer extension (PIPE) cloning technique (38) and heterologously expressed DSY3157 in Escherichia coli BL21 (DE3) to test this hypothesis. The enzyme had an N-terminal hexahistidine tag and was purified to apparent homogeneity by nickel-affinity chromatography (Fig. S3B). The DSY3157 enzyme catalyzed the transfer of methyl groups from methylcobalamin to THF at an initial rate of 0.64 ± 0.03 µmol • min−1 • mg−1 as measured by the change in UV-visible spectrum (Fig. S5A). The conversion of methylCbl to cob(II)alamin was followed by monitoring the decrease in absorbance at 525 nm (Fig. S5B). This reaction was dependent on the presence of both THF and recombinant DSY3157.
Aliquots from spectrally monitored reactions run to apparent completion (45 min) were analyzed by reverse-phase HPLC. Reactions lacking enzyme had only THF eluting as the major peak (Fig. S5C); however, in complete reactions, an additional peak having the retention time noted for methyl-THF was observed (Fig. S5D). In three separate reactions, 0.172 ± 0.003 mM methylcobalamin (calculated by ΔA525) was consumed whereas 0.19 ± 0.01 mM methyl-THF was produced. No methyl-THF peak was detectable during HPLC analysis of aliquots from assays lacking enzyme, methylCbl, or THF.
Discussion
The genes encoding members of the MttB protein superfamily are often annotated in genomes and metagenomes as probable TMA:corrinoid methyltransferases. However, only genes encoding one small clade within this large family of proteins (Fig. 5) have the pyrrolysine codon that is a hallmark of bona fide TMA methyltransferases (30, 39). This ambiguity has left the function of the non-Pyl MttB proteins an open question. Here, we show that a non-Pyl MttB is indeed a corrinoid-dependent methyltransferase, but with specificity for GB. To our knowledge, DSY3156 is the first known glycine betaine:corrinoid methyltransferase, and we propose that its gene be designated mtgB. We have also shown that the DSY3157 enzyme encoded adjacently to MtgB functions as a methylCbl:THF methyltransferase, and we propose that its gene be designated mtgA.
Fig. 5.
Phylogenetic tree of the COG5598 Superfamily. The evolutionary relations between the COG5598 members, or trimethylamine (TMA) methyltransferases (MttBs), are inferred with a maximum likelihood approach using the LG (81) substitution model with a discrete gamma distribution. Green coloring (light green, bacterial; dark green, archaeal) is used to highlight those proteins that are predicted to be l-pyrrolysine containing TMA methyltransferases. DSY3156 (MtgB) is found in the part of the tree colored in red.
D. hafniense Y51 is able to grow anaerobically at the expense of GB. Neither TMA nor acetate is produced as a product, consistent with the absence of genes specific for known GB reductases in the genome. This finding suggests that, unlike for many Gram-positive bacteria, GB does not serve as an electron acceptor. Rather, the requirement for an external electron acceptor for growth, the demethylation of GB, and production of stoichiometric CO2 indicates that GB provides a source of electrons for anaerobic respiration. Thus, we propose that D. hafniense grows via anaerobic methylotrophy: i.e., the oxidation of the methyl group provides reducing power for anaerobic respiration. D. hafniense now joins a small group of species known to carry out denitrification at the expense of reduced C1 compounds (40, 41).
The discoveries of MtgB-catalyzed corrinoid methylation with GB as well as the MtgA-catalyzed methylation of THF from methylCbl provide strong support for a hypothetical pathway of GB:THF methyl transfer, which can lead to oxidation of the methyl group to CO2 via THF intermediates (Fig. S2). We recently obtained preliminary proteomic data from cells grown on GB or pyruvate and found that the enzymes of the oxidative methyl-THF pathway, as well as MtgB, MtgA, and the accompanying corrinoid protein (DSY3155) (Fig. S2), are increased in abundance in GB-grown cells. These findings further support the qPCR data indicating that GB increases the transcript abundance of mtgB (DSY3156) and the putative GB transporter gene (DSY3154).
The chemistry of methyl-group transfer between GB and cob(I)alamin is not surprising, given that the quaternary amine is essentially already an activated methyl donor. However, the discovery that a member of the MttB superfamily carries out a GB-dependent methylation of corrinoid reveals a functional rationale that might underlie the evolutionary relationship between Pyl-MttB and its non-Pyl MttB homologs. MtgB lacks pyrrolysine and yet uses GB, a quaternary amine, whereas the pyrrolysine containing MttB uses a tertiary amine, trimethylamine. However, if the TMA methyltransferase MttB functions as has been proposed for other Pyl methylamine methyltransferases (26, 30, 42), TMA would form an adduct with pyrrolysine before methyl transfer. In other words, pyrrolysine would serve to convert TMA into a quaternary amine, such as used by other MttB superfamily members such as MtgB (Fig. S6). This hypothesis mandates further functional analysis of the superfamily because it remains to be seen whether those MttB family members closest to Pyl-MttBs have substrate specificity for quaternary amines. It is possible that the family has diversified to include quaternary amines, such as choline or carnitine, or tertiary amines such as dimethylethanolamine. The function of these non-Pyl MttBs will provide interesting context for evolutionary biologists who seek to understand the driving forces behind entrance of pyl into the MttB family of proteins.
Homologs of MtgB are found in a large number of species, with concentration in the Firmicutes and the alpha proteobacteria and with fewer examples in the gamma and delta proteobacteria. Homologs of MtgB are particularly well-represented in alpha proteobacteria. Prominent are members of the Rhizobiales family, including well-known organisms such as Sinorhizoium meliloti and Mesorhizobium loti. Rhizobiales are known users of glycine betaine, and a number of species have been shown to acquire it for osmotic control or degrade it as sole carbon and energy source (43, 44). Bacteroids are noted to uptake GB and then either maintain or consume it depending on external salt concentration (43). Choline is at mM concentrations in legume-root nodules, and bacteroids induce genes to convert it to GB within legumes (45). It has been shown that the pSym plasmids of S. meliloti carry the genes for degradation of a number of quaternary amines (46), and a homolog of mtgB is carried on one of the pSymA plasmids in this organism. One of these homologs is under quorum-sensing control within S. meliloti 1021 (47), supporting a potential role during some stage of plant symbiosis. The betaine:homocysteine methyltransferase was suggested as a first step in the demethylation of GB for Rhizobiales (15); however, it has now been shown by mutagenesis of S. meliloti that this methyltransferase is likely to be an anabolic enzyme (48), leaving the possibility that, at least under anaerobic conditions, MtgB homologs might serve this purpose in Rhizobiales.
GB is used as a compatible solute by cyanobacteria and other phototrophic bacteria, as well as by eukaryotic uni- and multicellular algae (49–51). Thus, many microbes in marine environments acquire exogenous GB to serve as an osmolyte or degrade it during changing osmotic conditions for use as a source of nitrogen, carbon, and/or energy (7, 52). In this regard, it is of interest that a large number of proteobacterial genera with genomes carrying homologs of mtgB inhabit marine environments. Of these homologs, the largest number of sequences can be attributed to those Rhodobacteriales within the Roseobacter clade. We identified homologs of mtgB that are found in isolated representatives of six subclades (53) of this ubiquitous major group of heterotrophs. Species have been isolated from both open-water columns or associated with eukaryotic unicellular or multicellular algae, where an enzyme having capability to use GB might be of advantage. Members of the Roseobacter clade, such as Ruggeria pomeroyi, are known to carry out one-carbon metabolism involving dimethylsulfoniopropionate (54) or TMAO (55), and the Roseobacter–algal symbiosis is thought to involve C1 metabolism with such compounds (56). A number of Roseobacter genera carry up to five different GB transporters (57) and have been shown to degrade GB in studies with enriched bacterioplankton from coastal regions (58). In contrast, members of the widespread SAR11 group such as Pelagibacter ubique, known to metabolize GB (59), do not possess homologs of non-Pyl MttBs (MtgB), with the exception of an mtgB homolog identified in the hypervariable region of one SAR11 subclade of P. ubique (60). In addition, a number of marine gamma proteobacterial genomes have been sequenced that possess mtgB homologs, including many from deep-ocean environments (61). Finally, GB was recently found to be demethylated by some marine archaea in the methanogenic genus Methanococcoides (62, 63). Only the genome of Methanococcoides burtonii has been sequenced and is publically available (64), but it is the only methanogen presently known to carry a non-Pyl MttB, and it will be interesting to see whether this enzyme plays a role in quaternary amine demethylation.
In the last few years, a surprising connection has been made between microbial metabolism of quaternary amines and human health. Gut microbiota produce trimethylamine when given precursors such as carnitine, choline, or GB commonly found in meats, fish, and other foodstuffs (8, 22). The TMA then leaves the intestine and is converted to TMAO by liver monooxygenases. Circulating TMAO correlates strongly with incidence of cardiovascular disease, which has been suggested to act as a trigger for deposition of lipids by foam macrophage, leading to atherosclerosis and subsequent risk for stroke and heart attack. Gnotobiotic mice do not produce TMA from the precursors mentioned above except when given a complement of gut microbiota, implicating microbial metabolism as essential to TMA production (8, 22). Presumably, the anoxic microbial route to TMA includes GB reductase (65) and choline-TMA lyase (66), as well as carnitine oxygenase (55) in the oxic portions of the digestive tract.
However, competing microbial metabolism has the potential to mitigate TMA production from quaternary amines within the intestine. For example, depletion of the GB pool by demethylation could lessen the total amount of TMA produced in the intestine. Dimethylglycine, the direct product of glycine betaine, has been identified in serum and urine and has been sometimes attributed to liver activities (67, 68). However, we identified, at the National Center for Biotechnology Information and the Joint Genome Institute-Integrated Microbial Genomes, multiple intestinal or fecal inhabitants having MtgB homologs encoded in their sequenced genomes. One of these organisms is D. hafniense DP7 (24), a fecal isolate that shares nearly identical mtgB-containing gene clusters with the D. hafniense Y51 used in this study. This finding suggests that microbially mediated demethylation of GB via MtgB is likely to occur in the gut.
Recently a novel family of methanogens closely related to the Thermoplasmatales was described whose genomes encode pyl genes and pyrrolysine-dependent TMA, DMA, and MMA methyltransferase for the complete demethylation of TMA (69). It has been suggested that these methanogens might serve to mediate levels of TMA in the intestine (70). With the results we described here for MtgB, it seems possible that a non-Pyl member of the TMA-dependent methyltransferase family found in intestinal bacteria might also affect TMA levels by removing a TMA precursor: that is, glycine betaine. If so, both Pyl and non-Pyl members of the MttB family could together be a means by which the deleterious effects of TMAO on human metabolism might be ameliorated.
Materials and Methods
Bacterium Strain and Growth Conditions.
D. hafniense Y51 was a kind gift from Taiki Futagami (Department of Bioscience and Biotechnology, Kyushu University, Fukuoka, Japan) and was routinely cultured with a modified DSMZ 720 medium specified by the Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (www.dsmz.de/home.html). The modified DSMZ 720 medium designated as MM1 uses a nonchelated SL-10 trace elements solution and lacks NaHCO3. The pH was adjusted to 7.2, and ultra high purity nitrogen was the sole gas phase. Potassium phosphate, pH 7.2, was added to the medium at a final concentration of 22 mM after autoclaving. D. hafniense Y51 was cultivated anaerobically in the presence of either 25 mM filter-sterilized sodium pyruvate or 25 mM filter-sterilized glycine betaine monohydrate to act as both an electron donor and carbon source. Cultures were also supplemented with 50 mM sodium fumarate or 50 mM sodium nitrate to act as an external electron acceptor. Complete MM1 was inoculated with 0.5–1.0% (vol/vol) stationary phase cultures and grown statically, in the dark, at 37 °C. All O.D. values were measured at 600 nm. All chemical reagents were obtained from either Fisher Scientific or Sigma-Aldrich with a purity ≥ 98%.
Product Stoichiometry of GB Degradation by D. hafniense.
Liquid and gas phase samples were taken shortly after inoculation into a total of 11 mL of MM1 medium in Balch anaerobe tubes (27 mL total volume). MM1 medium lacked sodium sulfide and was supplemented with 3 mM methionine, 3 mM cysteine, and 2 mM DTT. The tubes were also amended with 10 mM GB and 50 mM sodium nitrate. Samples were again taken when the optical density reached ∼0.1 units. Liquid samples were analyzed for GB, DMG, and MMG by TLC as described in Analysis of GB and Demethylation Products. The culture was then acidified by addition of 1 mL of 1 M HCl. Total culture carbon dioxide was then determined from gas-phase measurements using a thermal conductivity detector-equipped model 8A gas chromatograph (Shimadzu Scientific) with a Carbosieve S-II (Sigma-Aldrich) column eluted with a flow rate of 45 mL/min He with injector, detector, and oven temperatures at 120 °C. Cultures incubated without GB did not produce significant amounts of CO2 above background (determined by total CO2, the amount present in T0 acidified controls). TMA and acetate were analyzed by gas chromatography as described previously (71, 72).
Analysis of GB and Demethylation Products.
The GB, DMG, or monomethylglycine present in samples was determined by TLC (73). A total of 15 µL of standard or unknown sample was spotted onto Silica Gel 60 plates (Merck-Millipore). Plates were then developed in 80% (vol/vol) phenol in H20, dried, and stained with 0.04% (wt/vol) bromocresol green (titrated to dark green with a 0.1-M NaOH solution). The plates were scanned, and then the entire image was converted to monochrome before densitometry. Standard curves were linear between 25 nmol and 125 nmol of analyte, and the volumes of unknown samples were adjusted to fit the analyte concentration within this range.
RNA Isolation and Quantitative Real-Time PCR.
D. hafniense Y51 cultures were harvested at the middle of exponential phase (OD600 circa 0.450 for pyruvate and 0.250 for betaine cultures) and centrifuged aerobically at 7,500 × g at 4 °C for 15 min. RNA was extracted using a modified total RNA isolation protocol for RNAzolRT (Molecular Research Center, Inc.) involving lysing cells with RNAzolRT heated to 95 °C with repeated vortexing for 5 min followed by cooling samples on ice for 5 min. RNA samples were treated for genomic DNA contamination with TURBO DNA-free DNase (Ambion). The RNA quality was evaluated and quantitated with a BioAnalyzer 2100 (Agilent Technologies) using an Agilent RNA 6000 Pico Kit according to the manufacturer’s instructions. Isolated RNA samples had RNA integrity numbers (RINs) in the range of 7.9–9.1.
Gene-specific primers (Table S1) were generated using Primer3 (74). Each primer was used as a query against the D. hafniense Y51 genome in a BLASTn search to ensure that only a single region of significant sequence identity was detected (75). Each qRT-PCR (10 µL) was performed using isolated total RNA from either pyruvate or GB-grown cultures with the iScript One-Step RT-PCR Kit with SYBR Green (Bio-Rad Laboratories, Inc.). The complete master mix for each PCR containing iScript reverse transcriptase and SYBR Green RT-PCR mix was used as per the manufacturer’s recommendation (Bio-Rad Laboratories, Inc.). Reactions contained 200 nM forward and reverse gene-specific primers and 10 ng of total RNA. Negative control reactions were performed by omitting either RNA or gene-specific primers. Three independent sets of samples were done in triplicate for each gene of interest and for the negative controls. The reactions were run on a CFX Connect 5200 (Bio-Rad Laboratories, Inc.) thermocycler in Hard-Shell 96-well plates (Bio-Rad Laboratories, Inc.) using the following conditions: 50 °C for 10 min., 95 °C for 5 min., followed by 34 cycles of 95 °C for 10 s, 55 °C for 30 s, and a plate-reading step before each cycle. A melt-curve analysis was also performed under the following conditions: 95 °C for 10 s, and 65 °C to 95 °C ramp with a step increase of 0.5 °C/5 s followed by a plate read before each incremental step.
The Bio-Rad CFX Manager 3.0 (Bio-Rad Laboratories, Inc.) was used to obtain and analyze the qRT-PCR run data to calculate quantification cycles (Cq) of each reaction. Cq values were averaged between triplicate reactions and then statistically analyzed between independent replicates to obtain 1σ and then normalized to rpoB using the 2−ΔΔCT method (33).
DSY3156 and DSY3157 Production and Purification.
The expression vector for DSY3156, pSpeedET_DSY3156, maintained in E. coli DH5α was obtained from the DNASU plasmid repository (dnasu.org/DNASU/). DSY3157 was cloned using the polymerase incomplete primer extension (PIPE) cloning technique (38) using the primers shown in Table S1. The clone was made in the EC100 strain of E. coli. Both plasmids were purified from cells grown in Luria–Bertani (LB) supplemented with 50 µg/mL kanamycin (Kan) using a Wizard Plus SV Miniprep kit as per the manufacturer’s directions (Promega). Chemically competent E. coli BL21(DE3) cells (Life Technologies) were transformed with pSpeedET_DSY3156 and pSpeedET_DSY3157, separately, and grown on LB agar supplemented with 50 µg/mL Kan. Five-milliliter starter cultures of E. coli containing each clone were grown overnight at 37 °C with shaking and used for inoculation of 1 L of LB broth supplemented with 50 µg/mL Kan and placed into an incubating shaker at 37 °C rotating at 250 rpm until they reached an OD600 of ∼0.5. The cultures were induced with l-arabinose at a final concentration of 0.1% (wt/vol) for 4 h at 37 °C shaking at 250 rpm. Cells were harvested by centrifugation at 7,500 × g at 4 °C for 15 min and then washed with Buffer A (50 mM sodium phosphate, 500 mM NaCl, and 40 mM imidazole at pH 7.2) and spun at 7,500 × g at 4 °C for 15 min. The wet-cell weight for each culture was ∼6.5 g and was suspended in 10 mL of Buffer A before lysing the cells three times via French Press at 20,000 psi. The cell lysate was then spun at 250,000 × g at 4 °C for 1.5 h, and the soluble fractions were isolated from the insoluble fractions via decanting.
The cell lysates were loaded on 1-mL HisTrap columns (GE Healthcare) equilibrated with Buffer A at 0.35 mL/min and then washed with 10 mL of Buffer A before the linear gradient. A 40-mL gradient was applied to the column from 0% to 100% Buffer B (50 mM sodium phosphate, 500 mM NaCl, and 500 mM imidazole at pH 7.2) at 0.75 mL/min. DSY3156 eluted in a single peak between 70 mM and 200 mM imidazole. DSY3157 eluted in a single peak between 160 mM and 260 mM imidazole. The eluted fractions were pooled and quantitated via Bradford reagent (76). The purity of recombinant DSY3156 and DSY3157 was assessed by SDS/PAGE (Fig. S3). SDS-polyacrylamide gel electrophoresis was performed in accordance with the method of Laemmli with a Minislab electrophoresis system (Idea Scientific) (77).
Spectrophotometric Assay of GB:Cob(I)alamin Methylation.
Reaction mixtures were assembled in anaerobic 0.2-cm quartz cuvettes under a 2-psi stream of either H2 (100%) or N2 (100%) gas with the following components: 50 µg of purified recombinant DSY3156 (1.6 μM), 50 mM glycine betaine, 16.25 mM Ti(III) citrate, 1.75 mM hydroxocobalamin in 50 mM Mops, pH 6.5, in a final volume of 0.6 mL. A mixture excluding both hydroxocobalamin and DSY3156 was used to blank the HP 8453 Diode-Array Biochemical Analysis Spectrophotometer (Hewlett-Packard Development Company). Hydroxocobalamin conversion to cob(I)alamin was monitored using ΔA540 and ΔA578 after the addition of hydroxocobalamin. When conversion to cob(I)alamin was complete, the assay was initiated with either recombinant DSY3156 or GB injection. The reactions were performed under dim red light at 37 °C for 10 min with UV-Vis spectra recorded every 30 s. The formation of methylCbl from cob(I)alamin was monitored using Δ540 with an extinction coefficient of 4.4 mM−1 • cm−1 (78). MethylCbl was not generated in the absence of recombinant DSY3156 or GB in these assays. Assays with alternative substrates included either 50 mM tetramethylammonium ion, trimethylamine, dimethylamine, monomethylamine, dimethylglycine, monomethylglycine, γ-butyrobetaine, choline, or carnitine as substrates using solutions first adjusted to pH 6.5.
The apparent kinetic parameters of MtgB were investigated by varying the GB concentration from 0.25 mM to 50 mM. Results were repeated in triplicate with three independently purified preparations of recombinant MtgB. The results were analyzed using Prism 6 (GraphPad) and fitted with a nonlinear Michaelis–Menten algorithm.
The stoichiometry of GB consumed to DMG and methylCbl produced was determined in assays as described above except containing 18 mM potassium phosphate, pH 7.0, 5 mM Ti(III) citrate, 1.8 mM GB, and 50 μg/mL MtmB in a total volume of 560 μL under an N2 gas phase. MethylCbl formation was followed spectrophotometrically, and the reaction was terminated at a fixed time point by removal of the cuvette contents and application to an aerobic C18 1-mL Sep-Pak (Waters Corp.) that had been previously eluted with 10 mL of 100% methanol and then equilibrated with 10 mL of deionized H2O. The flow-through was collected, then the column was further eluted with a total of 1.4 mL deionized H20. The fractions were concentrated in a rotary evaporator and quantitatively combined before final evaporation to dryness and dissolution in a measured volume of deionized H2O for analysis of GB and DMG by the TLC method described above. The total amount spotted on the plates was varied as necessary for the unknown to be within the linear range of the GB and DMG standard curves.
COG5598 Phylogenetic Tree and Sequence Acquisition.
The DSY3156 sequenced was used as a query in a BLASTp search of the Microbial Genome database maintained at the National Center for Biotechnology Information (75). Full-length pyrrolysine-containing proteins were identified using the same database but with a tBLASTn using Methanosarcina barkeri MttB as a query. These entire ORFs were translated to include the pyrrolysine residues and then added to the database to give 900 sequences. The larger dataset was reduced with the ElimDupes program (hcv.lanl.gov/content/sequence/ELIMDUPES/elimdupes.html), yielding 515 sequences that were at a maximum 90% similar to each other, except all copies of MttB homologs from D. hafniense Y51 were retained. The truncated dataset was aligned with MUSCLE (79) using default setting in MEGA6 (80). This dataset was tested for the highest Bayesian information criterion (BIC), corrected Akaike information criterion (AICc), and maximum likelihood values (InL) in MEGA6. Molecular phylogeny was inferred using the maximum likelihood method based on the LG model (81) with a discrete gamma distribution used to estimate rates among sites [five categories (+G = 3.4371)]. Gaps and missing data were given a 95% cutoff and then partially deleted from the analysis of the 515 sequences. There were a total of 439 residue positions present in the final analysis. The tree is presented with a log likelihood value of −249096.7688 and was initialized using the neighbor-joining method to a pairwise distance matrix calculated from the JTT model (82) and was computed for 1,000 bootstrap repetitions. The tree was drawn to scale with the scale bar representing 0.5 substitutions per site. A radiation tree is presented with larger groups being collapsed, with bootstrap percentages being located at nodes where applicable; those values less than 50% are hidden. Groups of interest were colored green and red, and large assignments of phylum or orders that make up groups were clustered together.
Assay of MethylCbl:THF Methylation by DSY3157.
Spectral determination of the rate of THF methylation by methylCbl was performed essentially as described by Ragsdale and coworkers (83) using the photodiode array spectrophotometer described above. Briefly, spectra were collected every minute after initiation of the reaction, and the THF-dependent demethylation of methylCbl was monitored at 525 nm using Δε = 8.6 mM−1⋅cm−1). Assays were conducted in a 0.2-cm path length cuvette containing 0.58 mL of assay volume at 37 °C with a nitrogen gas phase. The complete assay contained 0.42 mM methylCbl, 0.87 mM THF, 6.7 mM DTT, and 22.4 μg of MtgA (Fig. S3B) in 20 mM potassium phosphate buffer, pH 7.0. The production of methyl-THF was confirmed using HPLC. Aliquots of the spectral assays (20 μL) were injected onto a 250 × 4.6-mm Varian Microsorb MV-100 C18 column on a Dionex UltiMate 3000 HPLC system. The column was eluted isocratically with 7% (vol/vol) acetonitrile in 30 mM potassium phosphate buffer, pH 3.0, at 1 mL/min, and monitored at 272 nm. The standards included THF, which eluted at ∼9.5 min and methyl-THF (Santa Cruz Biotechnology), which eluted at ∼12.5 min in this system.
Supplementary Material
Acknowledgments
We thank Dr. Andor Kiss and the Center for Bioinformatics and Functional Genomics at Miami University for the use of the ÄKTA chromatography system, Nanodrop spectrophotometers, Agilent BioAnalyzer, and the Bio-Rad CFX Connect thermocycler. We thank Dr. Mei-Chin Lai and her laboratory for very helpful discussions and protocols used for thin-layer chromatography of GB and its products. We thank Dr. Kari Green and Dr. Liwen Zhang in the Mass Spectrometry and Proteomics Facility at The Ohio State University for performing preliminary proteomic analysis of cell-free extracts. This work was supported by funding from the US Department of Energy, Office of Science, Office of Basic Energy Sciences through Grant DE-FG0202-91ER200042 (to J.A.K.) and Computational Bridges to Experiments (COMBREX) Subaward 4500000088 (to D.J.F.), as well as internal funding from Miami University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409642111/-/DCSupplemental.
References
- 1.Heijthuijsen JHFG, Hansen TA. Betaine fermentation and oxidation by marine Desulfuromonas strains. Appl Environ Microbiol. 1989;55(4):965–969. doi: 10.1128/aem.55.4.965-969.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler C, Bremer E, Krämer R. The BCCT family of carriers: From physiology to crystal structure. Mol Microbiol. 2010;78(1):13–34. doi: 10.1111/j.1365-2958.2010.07332.x. [DOI] [PubMed] [Google Scholar]
- 3.Oren A. Formation and breakdown of glycine betaine and trimethylamine in hypersaline environments. Antonie van Leeuwenhoek. 1990;58(4):291–298. doi: 10.1007/BF00399342. [DOI] [PubMed] [Google Scholar]
- 4.Peluso G, et al. Carnitine: An osmolyte that plays a metabolic role. J Cell Biochem. 2000;80(1):1–10. doi: 10.1002/1097-4644(20010101)80:1<1::aid-jcb10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Bremer J. Carnitine: Metabolism and functions. Physiol Rev. 1983;63(4):1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 6.Prell J, Poole P. Metabolic changes of rhizobia in legume nodules. Trends Microbiol. 2006;14(4):161–168. doi: 10.1016/j.tim.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 7.King GM. Metabolism of trimethylamine, choline, and glycine betaine by sulfate-reducing and methanogenic bacteria in marine sediments. Appl Environ Microbiol. 1984;48(4):719–725. doi: 10.1128/aem.48.4.719-725.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muñoz-Clares RA, Díaz-Sánchez AG, González-Segura L, Montiel C. Kinetic and structural features of betaine aldehyde dehydrogenases: Mechanistic and regulatory implications. Arch Biochem Biophys. 2010;493(1):71–81. doi: 10.1016/j.abb.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Wargo MJ. Homeostasis and catabolism of choline and glycine betaine: Lessons from Pseudomonas aeruginosa. Appl Environ Microbiol. 2013;79(7):2112–2120. doi: 10.1128/AEM.03565-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wargo MJ, Ho TC, Gross MJ, Whittaker LA, Hogan DA. GbdR regulates Pseudomonas aeruginosa plcH and pchP transcription in response to choline catabolites. Infect Immun. 2009;77(3):1103–1111. doi: 10.1128/IAI.01008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wargo MJ, Szwergold BS, Hogan DA. Identification of two gene clusters and a transcriptional regulator required for Pseudomonas aeruginosa glycine betaine catabolism. J Bacteriol. 2008;190(8):2690–2699. doi: 10.1128/JB.01393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meskys R, Harris RJ, Casaite V, Basran J, Scrutton NS. Organization of the genes involved in dimethylglycine and sarcosine degradation in Arthrobacter spp.: Implications for glycine betaine catabolism. Eur J Biochem. 2001;268(12):3390–3398. doi: 10.1046/j.1432-1327.2001.02239.x. [DOI] [PubMed] [Google Scholar]
- 14.Casaitė V, Povilonienė S, Meškienė R, Rutkienė R, Meškys R. Studies of dimethylglycine oxidase isoenzymes in Arthrobacter globiformis cells. Curr Microbiol. 2011;62(4):1267–1273. doi: 10.1007/s00284-010-9852-6. [DOI] [PubMed] [Google Scholar]
- 15.Smith LT, Pocard JA, Bernard T, Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988;170(7):3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen NC, et al. Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J. 2010;24(8):2804–2817. doi: 10.1096/fj.09-143651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naumann E, Hippe H, Gottschalk G. Betaine: New oxidant in the Stickland reaction and methanogenesis from betaine and L-alanine by a Clostridium sporogenes- Methanosarcina barkeri coculture. Appl Environ Microbiol. 1983;45(2):474–483. doi: 10.1128/aem.45.2.474-483.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreesen JR. Glycine metabolism in anaerobes. Antonie van Leeuwenhoek. 1994;66(1-3):223–237. doi: 10.1007/BF00871641. [DOI] [PubMed] [Google Scholar]
- 19.Meyer M, Granderath K, Andreesen JR. Purification and characterization of protein PB of betaine reductase and its relationship to the corresponding proteins glycine reductase and sarcosine reductase from Eubacterium acidaminophilum. Eur J Biochem. 1995;234(1):184–191. doi: 10.1111/j.1432-1033.1995.184_c.x. [DOI] [PubMed] [Google Scholar]
- 20.Möller B, Ossmer R, Howard BH, Gottschalk G, Hippe H. Sporomusa, a new genus of Gram-negative anaerobic bacteria including Sporomusa sphaeroides spec. nov. and Sporomusa ovata spec. nov. Arch Microbiol. 1984;139:388–396. [Google Scholar]
- 21.Müller E, Fahlbusch K, Walther R, Gottschalk G. Formation of N,N-dimethylglycine, acetic acid, and butyric acid from betaine by Eubacterium limosum. Appl Environ Microbiol. 1981;42(3):439–445. doi: 10.1128/aem.42.3.439-445.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang WH, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35(14):904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Pas BA, et al. A Desulfitobacterium strain isolated from human feces that does not dechlorinate chloroethenes or chlorophenols. Arch Microbiol. 2001;175(6):389–394. doi: 10.1007/s002030100276. [DOI] [PubMed] [Google Scholar]
- 25.Villemur R, Lanthier M, Beaudet R, Lépine F. The Desulfitobacterium genus. FEMS Microbiol Rev. 2006;30(5):706–733. doi: 10.1111/j.1574-6976.2006.00029.x. [DOI] [PubMed] [Google Scholar]
- 26.Hao B, et al. A new UAG-encoded residue in the structure of a methanogen methyltransferase. Science. 2002;296(5572):1462–1466. doi: 10.1126/science.1069556. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan G, James CM, Krzycki JA. Pyrrolysine encoded by UAG in Archaea: Charging of a UAG-decoding specialized tRNA. Science. 2002;296(5572):1459–1462. doi: 10.1126/science.1069588. [DOI] [PubMed] [Google Scholar]
- 28.Gaston MA, Jiang R, Krzycki JA. Functional context, biosynthesis, and genetic encoding of pyrrolysine. Curr Opin Microbiol. 2011;14(3):342–349. doi: 10.1016/j.mib.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nonaka H, et al. Complete genome sequence of the dehalorespiring bacterium Desulfitobacterium hafniense Y51 and comparison with Dehalococcoides ethenogenes 195. J Bacteriol. 2006;188(6):2262–2274. doi: 10.1128/JB.188.6.2262-2274.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krzycki JA. Function of genetically encoded pyrrolysine in corrinoid-dependent methylamine methyltransferases. Curr Opin Chem Biol. 2004;8(5):484–491. doi: 10.1016/j.cbpa.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Naidu D, Ragsdale SW. Characterization of a three-component vanillate O-demethylase from Moorella thermoacetica. J Bacteriol. 2001;183(11):3276–3281. doi: 10.1128/JB.183.11.3276-3281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Studenik S, Vogel M, Diekert G. Characterization of an O-demethylase of Desulfitobacterium hafniense DCB-2. J Bacteriol. 2012;194(13):3317–3326. doi: 10.1128/JB.00146-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson DJ, Jr, Gorlatova N, Grahame DA, Krzycki JA. Reconstitution of dimethylamine:coenzyme M methyl transfer with a discrete corrinoid protein and two methyltransferases purified from Methanosarcina barkeri. J Biol Chem. 2000;275(37):29053–29060. doi: 10.1074/jbc.M910218199. [DOI] [PubMed] [Google Scholar]
- 35.Sauer K, Thauer RK. Methanol:coenzyme M methyltransferase from Methanosarcina barkeri: Substitution of the corrinoid harbouring subunit MtaC by free cob(I)alamin. Eur J Biochem. 1999;261(3):674–681. doi: 10.1046/j.1432-1327.1999.00355.x. [DOI] [PubMed] [Google Scholar]
- 36.Goulding CW, Postigo D, Matthews RG. Cobalamin-dependent methionine synthase is a modular protein with distinct regions for binding homocysteine, methyltetrahydrofolate, cobalamin, and adenosylmethionine. Biochemistry. 1997;36(26):8082–8091. doi: 10.1021/bi9705164. [DOI] [PubMed] [Google Scholar]
- 37.Hippler B, Thauer RK. The energy conserving methyltetrahydromethanopterin:coenzyme M methyltransferase complex from methanogenic archaea: Function of the subunit MtrH. FEBS Lett. 1999;449(2-3):165–168. doi: 10.1016/s0014-5793(99)00429-9. [DOI] [PubMed] [Google Scholar]
- 38.Klock HE, Lesley SA. The Polymerase Incomplete Primer Extension (PIPE) method applied to high-throughput cloning and site-directed mutagenesis. Methods Mol Biol. 2009;498:91–103. doi: 10.1007/978-1-59745-196-3_6. [DOI] [PubMed] [Google Scholar]
- 39.Soares JA, et al. The residue mass of L-pyrrolysine in three distinct methylamine methyltransferases. J Biol Chem. 2005;280(44):36962–36969. doi: 10.1074/jbc.M506402200. [DOI] [PubMed] [Google Scholar]
- 40.Chistoserdova L, Kalyuzhnaya MG, Lidstrom ME. The expanding world of methylotrophic metabolism. Annu Rev Microbiol. 2009;63:477–499. doi: 10.1146/annurev.micro.091208.073600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auclair J, Lépine F, Parent S, Villemur R. Dissimilatory reduction of nitrate in seawater by a Methylophaga strain containing two highly divergent narG sequences. ISME J. 2010;4(10):1302–1313. doi: 10.1038/ismej.2010.47. [DOI] [PubMed] [Google Scholar]
- 42.Hao B, et al. Reactivity and chemical synthesis of L-pyrrolysine- the 22(nd) genetically encoded amino acid. Chem Biol. 2004;11(9):1317–1324. doi: 10.1016/j.chembiol.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Fougère F, Le Rudulier D. Uptake of glycine betaine and its analogues by bacteroids of Rhizobium meliloti. J Gen Microbiol. 1990;136(1):157–163. doi: 10.1099/00221287-136-1-157. [DOI] [PubMed] [Google Scholar]
- 44.Boncompagni E, Osteras M, Poggi MC, le Rudulier D. Occurrence of choline and glycine betaine uptake and metabolism in the family Rhizobiaceae and their roles in osmoprotection. Appl Environ Microbiol. 1999;65(5):2072–2077. doi: 10.1128/aem.65.5.2072-2077.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandon K, et al. The Sinorhizobium meliloti glycine betaine biosynthetic genes (betlCBA) are induced by choline and highly expressed in bacteroids. Mol Plant Microbe Interact. 2003;16(8):709–719. doi: 10.1094/MPMI.2003.16.8.709. [DOI] [PubMed] [Google Scholar]
- 46.Goldmann A, et al. Betaine use by rhizosphere bacteria: Genes essential for trigonelline, stachydrine, and carnitine catabolism in Rhizobium meliloti are located on pSym in the symbiotic region. Mol Plant Microbe Interact. 1991;4(6):571–578. doi: 10.1094/mpmi-4-571. [DOI] [PubMed] [Google Scholar]
- 47.Gao M, et al. sinI- and expR-dependent quorum sensing in Sinorhizobium meliloti. J Bacteriol. 2005;187(23):7931–7944. doi: 10.1128/JB.187.23.7931-7944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barra L, et al. Interrelations between glycine betaine catabolism and methionine biosynthesis in Sinorhizobium meliloti strain 102F34. J Bacteriol. 2006;188(20):7195–7204. doi: 10.1128/JB.00208-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welsh DT. Ecological significance of compatible solute accumulation by micro-organisms: From single cells to global climate. FEMS Microbiol Rev. 2000;24(3):263–290. doi: 10.1111/j.1574-6976.2000.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 50.Keller MD, Kiene RP, Matrai PA, Bellows WK. Production of glycine betaine and dimethylsulfoniopropionate in marine phytoplankton. I. Batch cultures. Mar Biol. 1999;135:237–248. [Google Scholar]
- 51.Hanson BT, Hewson I, Madsen EL. Metaproteomic survey of six aquatic habitats: Discovering the identities of microbial populations active in biogeochemical cycling. Microb Ecol. 2014;67(3):520–539. doi: 10.1007/s00248-013-0346-5. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y. Comparative genomics of methylated amine utilization by marine Roseobacter clade bacteria and development of functional gene markers (tmm, gmaS) Environ Microbiol. 2012;14(9):2308–2322. doi: 10.1111/j.1462-2920.2012.02765.x. [DOI] [PubMed] [Google Scholar]
- 53.Newton RJ, et al. Genome characteristics of a generalist marine bacterial lineage. ISME J. 2010;4(6):784–798. doi: 10.1038/ismej.2009.150. [DOI] [PubMed] [Google Scholar]
- 54.Reisch CR, et al. Novel pathway for assimilation of dimethylsulphoniopropionate widespread in marine bacteria. Nature. 2011;473(7346):208–211. doi: 10.1038/nature10078. [DOI] [PubMed] [Google Scholar]
- 55.Lidbury I, Murrell JC, Chen Y. Trimethylamine N-oxide metabolism by abundant marine heterotrophic bacteria. Proc Natl Acad Sci USA. 2014;111(7):2710–2715. doi: 10.1073/pnas.1317834111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geng H, Belas R. Molecular mechanisms underlying roseobacter-phytoplankton symbioses. Curr Opin Biotechnol. 2010;21(3):332–338. doi: 10.1016/j.copbio.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 57.Moran MA, et al. Ecological genomics of marine Roseobacters. Appl Environ Microbiol. 2007;73(14):4559–4569. doi: 10.1128/AEM.02580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mou X, Hodson RE, Moran MA. Bacterioplankton assemblages transforming dissolved organic compounds in coastal seawater. Environ Microbiol. 2007;9(8):2025–2037. doi: 10.1111/j.1462-2920.2007.01318.x. [DOI] [PubMed] [Google Scholar]
- 59.Sun J, et al. One carbon metabolism in SAR11 pelagic marine bacteria. PLoS ONE. 2011;6(8):e23973. doi: 10.1371/journal.pone.0023973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilbert JA, Mühling M, Joint I. A rare SAR11 fosmid clone confirming genetic variability in the ‘Candidatus Pelagibacter ubique’ genome. ISME J. 2008;2(7):790–793. doi: 10.1038/ismej.2008.49. [DOI] [PubMed] [Google Scholar]
- 61.Swan BK, et al. Potential for chemolithoautotrophy among ubiquitous bacteria lineages in the dark ocean. Science. 2011;333(6047):1296–1300. doi: 10.1126/science.1203690. [DOI] [PubMed] [Google Scholar]
- 62.Watkins AJ, Roussel EG, Parkes RJ, Sass H. Glycine betaine as a direct substrate for methanogens (Methanococcoides spp.) Appl Environ Microbiol. 2014;80(1):289–293. doi: 10.1128/AEM.03076-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.L'Haridon S, Chalopin M, Colombo D, Toffin L. Methanococcoides vulcani sp. nov., a marine methylotrophic methanogen that uses betaine, choline and N,N-dimethylethanolamine for methanogenesis, isolated from a mud volcano, and emended description of the genus Methanococcoides. Int J Syst Evol Microbiol. 2014;64(Pt 6):1978–1983. doi: 10.1099/ijs.0.058289-0. [DOI] [PubMed] [Google Scholar]
- 64.Goodchild A, et al. A proteomic determination of cold adaptation in the Antarctic archaeon, Methanococcoides burtonii. Mol Microbiol. 2004;53(1):309–321. doi: 10.1111/j.1365-2958.2004.04130.x. [DOI] [PubMed] [Google Scholar]
- 65.Andreesen JR. Glycine reductase mechanism. Curr Opin Chem Biol. 2004;8(5):454–461. doi: 10.1016/j.cbpa.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci USA. 2012;109(52):21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heinzmann SS, et al. Stability and robustness of human metabolic phenotypes in response to sequential food challenges. J Proteome Res. 2012;11(2):643–655. doi: 10.1021/pr2005764. [DOI] [PubMed] [Google Scholar]
- 68.Kirsch SH, Herrmann W, Rabagny Y, Obeid R. Quantification of acetylcholine, choline, betaine, and dimethylglycine in human plasma and urine using stable-isotope dilution ultra performance liquid chromatography-tandem mass spectrometry. J Chrom B. 2010;878(32):3338–3344. doi: 10.1016/j.jchromb.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 69.Borrel G, et al. Phylogenomic data support a seventh order of methylotrophic methanogens and provide insights into the evolution of methanogenesis. Genome Biol Evol. 2013;5(10):1769–1780. doi: 10.1093/gbe/evt128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brugère JF, et al. Archaebiotics: Proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes. 2014;5(1):5–10. doi: 10.4161/gmic.26749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burke SA, Krzycki JA. Involvement of the “A” isozyme of methyltransferase II and the 29-kilodalton corrinoid protein in methanogenesis from monomethylamine. J Bacteriol. 1995;177(15):4410–4416. doi: 10.1128/jb.177.15.4410-4416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kremer JD, Cao X, Krzycki J. Isolation of two novel corrinoid proteins from acetate-grown Methanosarcina barkeri. J Bacteriol. 1993;175(15):4824–4833. doi: 10.1128/jb.175.15.4824-4833.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lai MC, Yang DR, Chuang MJ. Regulatory factors associated with synthesis of the osmolyte glycine betaine in the halophilic methanoarchaeon Methanohalophilus portucalensis. Appl Environ Microbiol. 1999;65(2):828–833. doi: 10.1128/aem.65.2.828-833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 75.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 76.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 77.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 78.Kreft JU, Schink B. O-demethylation by the homoacetogenic anaerobe Holophaga foetida studied by a new photometric methylation assay using electrochemically produced cob(I)alamin. Eur J Biochem. 1994;226(3):945–951. doi: 10.1111/j.1432-1033.1994.00945.x. [DOI] [PubMed] [Google Scholar]
- 79.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le SQ, Gascuel O. An improved general amino acid replacement matrix. Mol Biol Evol. 2008;25(7):1307–1320. doi: 10.1093/molbev/msn067. [DOI] [PubMed] [Google Scholar]
- 82.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8(3):275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 83.Roberts DL, Zhao S, Doukov T, Ragsdale SW. The reductive acetyl coenzyme A pathway: Sequence and heterologous expression of active methyltetrahydrofolate:corrinoid/iron-sulfur protein methyltransferase from Clostridium thermoaceticum. J Bacteriol. 1994;176(19):6127–6130. doi: 10.1128/jb.176.19.6127-6130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.