Significance
Caenorhabditis elegans studies have contributed greatly to our understanding of the temporal regulation of development. These studies led to the discovery of microRNAs (miRNAs) and revealed that a succession of stage-specifically expressed miRNAs act as switches to guide life-stage transitions in cell fates. These miRNAs are conserved to humans and cause disease states when misexpressed, highlighting the importance of their temporal regulation. Much is known about the biogenesis and function of miRNAs, but how these processes are temporally sculpted is not well understood. This study reports that LIN-42, the C. elegans homolog of the circadian rhythm protein Period, regulates timing of miRNA expression by modulating transcription. These findings further our understanding of the functions of developmental timekeepers and regulation of miRNAs.
Keywords: C. elegans, lin-42, miRNA, let-7, heterochronic
Abstract
MicroRNAs (miRNAs) are small RNAs that regulate gene expression posttranscriptionally via the 3′ UTR of target mRNAs and were first identified in the Caenorhabditis elegans heterochronic pathway. miRNAs have since been found in many organisms and have broad functions, including control of differentiation and pluripotency in humans. lin-4 and let-7–family miRNAs regulate developmental timing in C. elegans, and their proper temporal expression ensures cell lineage patterns are correctly timed and sequentially executed. Although much is known about miRNA biogenesis, less is understood about how miRNA expression is timed and regulated. lin-42, the worm homolog of the circadian rhythm gene period of flies and mammals, is another core component of the heterochronic gene pathway. lin-42 mutants have a precocious phenotype, in which later-stage programs are executed too early, but the placement of lin-42 in the timing pathway is unclear. Here, we demonstrate that lin-42 negatively regulates heterochronic miRNA transcription. let-7 and the related miRNA miR-48 accumulate precociously in lin-42 mutants. This defect reflects transcriptional misregulation because enhanced expression of both primary miRNA transcripts (pri-miRNAs) and a let-7 promoter::gfp fusion are observed. The pri-miRNA levels oscillate during larval development, in a pattern reminiscent of lin-42 expression. Importantly, we show that lin-42 is not required for this cycling; instead, peak amplitude is increased. Genetic analyses further confirm that lin-42 acts through let-7 family miRNAs. Taken together, these data show that a key function of lin-42 in developmental timing is to dampen pri-miRNAs levels, preventing their premature expression as mature miRNAs.
Metazoan development uses both positional and temporal information to pattern life stages from the single-cell embryo to the reproductive adult. In Caenorhabditis elegans, the temporal component of postembryonic patterning is conveyed through the heterochronic gene regulatory circuit (see ref. 1 for review). A core theme of the heterochronic circuit is that a series of microRNA (miRNA) switches promote transitions from one stage to the next by negatively regulating key factors that direct stage-specific programs, thereby allowing successive temporal fates to be executed.
Five conserved miRNAs, lin-4 and the let-7 family members, let-7, miR-48, miR-84, and miR-241, play prominent roles in the circuit (2–6). lin-4 appears first, midway through the L1 larval stage and guides the transition from the L1 to L2 stage (7). miR-48, miR-84, and miR-241 amass in the L2 and control the transition to the L3 stage, when let-7 levels rise dramatically and govern the switch to the L4. Mutations in these miRNAs cause the relevant transition to fail to advance. This process results in, for example, repetition of the L1-stage pattern in lin-4 mutants or L2-stage patterns in mir-48/84/241 mutants, consistent with the sequential expression of these miRNAs. Deciphering this temporal control mechanism then simplifies, to a large extent, to understanding the timing of miRNA accumulation.
miRNA biogenesis occurs via a multistep process that provides many opportunities for controlling miRNA abundance (see ref. 8 for review). Briefly, most miRNA genes are transcribed as long primary transcripts (pri-miRNA) that are cleaved in the nucleus by the Microprocessor complex. This step generates an ∼70-nt precursor (pre-miRNA) with a hairpin structure that is exported from the nucleus and processed by the Dicer endonuclease into an ∼22-nt heteroduplex. The mature miRNA strand from the duplex is retained in a miRNA-induced silencing complex and guides it to mRNA targets for silencing. Although much is known about miRNA biogenesis, how this process is temporally sculpted to control development is less well understood. One gene with a prominent role in timing miRNA expression is lin-28. The RNA-binding protein LIN-28 negatively regulates let-7 by blocking processing, a function that is conserved in mammals (9–13). In addition, transcription factors encoded by the heterochronic genes daf-12 and hbl-1 have been implicated in modulating miRNA transcription (14–16). However, given the complex temporal expression patterns of heterochronic miRNA genes, there are likely to be other factors involved in this process.
LIN-42, the C. elegans homolog of the fly and mammalian circadian clock protein Period (17), is a candidate regulator of heterochronic miRNAs. Loss of lin-42 function causes a precocious heterochronic phenotype in which the adult hypodermal program occurs one stage too early, as opposed to the reiteration of larval programs observed in miRNA mutants. In addition, lin-42 acts antagonistically to daf-12 to time cell fate decisions, and one role of daf-12 is to augment expression of let-7 family miRNAs (14, 15), suggesting that lin-42 might oppose miRNA function.
Here, we demonstrate that lin-42 is indeed a negative regulator of heterochronic miRNA expression. let-7 and miR-48 accumulate precociously in lin-42(loss-of-function) [lin-42(lf)] animals, and genetic analysis places these miRNAs downstream of lin-42. The early appearance of these miRNAs likely reflects transcriptional misregulation, because aberrant expression of both pri-miRNAs and a let-7 promoter::gfp fusion are observed in lin-42 mutants. Interestingly, the levels of all heterochronic pri-miRNAs oscillate during larval development, in a pattern reminiscent of lin-42 expression (17). Importantly, we show that lin-42 is not required for pri-miRNA cycling. Instead, peak amplitude is increased in the absence of lin-42. Taken together, these results indicate that a key role for lin-42 in developmental timing is to dampen miRNA transcript oscillations, preventing premature accumulation of mature miRNAs.
Results
let-7 miRNA Accumulates Precociously in lin-42(ve11) Mutants.
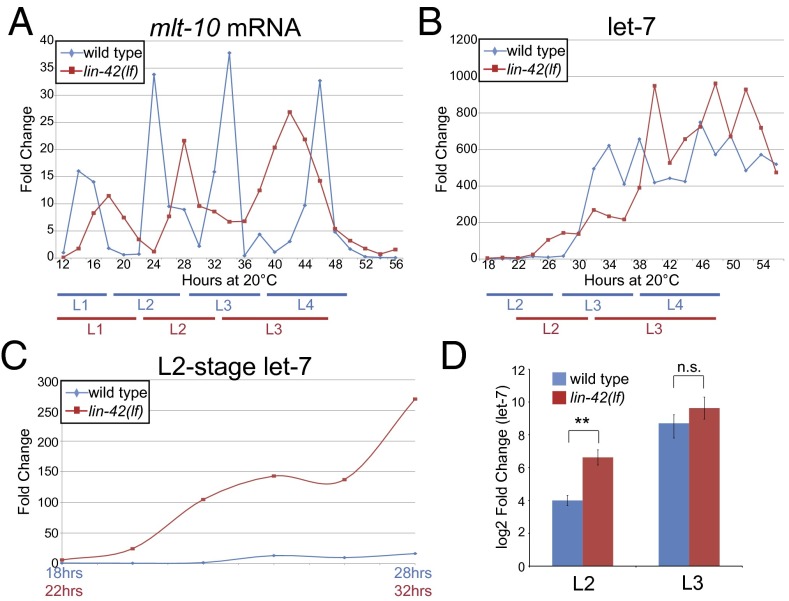
miRNAs play prominent roles in the heterochronic gene pathway, directing stage-to-stage transitions in temporal cell fates. Genetic data discussed above suggest that lin-42 could function to modulate accumulation of miRNAs that act in the timing pathway. To test this idea, let-7 levels were compared between synchronized populations of wild-type and lin-42(ve11) animals using Taqman miRNA quantitative RT-PCR (qRT-PCR) (Fig. 1 and Figs. S1 and S2). Because these populations are not directly comparable based on absolute time from hatching, as a result of lengthening of larval stages in lin-42 mutants (18), we used the cyclical expression pattern of mlt-10 as an internal control to align stages. Molting defective-10, or mlt-10, encodes a nematode-specific protein that is required for molting (19), and its message level peaks once per larval stage in wild-type animals, ∼4 h before the onset of lethargus at 20 °C (Fig. 1A). This temporal profile is similar to that reported for a Pmlt-10::gfppest reporter (20) used to monitor molting in individual lin-42 mutant animals (18).
Fig. 1.
let-7 accumulates precociously in lin-42 mutants. (A) A representative biological replicate of mlt-10 mRNA levels in wild type and lin-42(lf) mutants from 12 to 56 h after starved L1s were placed on food. All time points were normalized to wild type at 12 h. Here and in subsequent figures, approximate stages based on mlt-10 expression are indicated with blue bars for wild-type and red bars for lin-42(lf). (B) let-7 levels in wild type and lin-42(lf) mutants in the 18- to 56-h samples analyzed in A, normalizing to wild type at 18 h. The let-7 assay was specific and the data are representative of three biological replicates (Figs. S1 and S2). (C) The L2-stage data from B graphed using the mlt-10 profiles to align developmentally similar time points. (D) Graph of let-7 levels at the time of mlt-10 peaks during the L2 and L3 stages averaged over three biological replicates. For example, the let-7 levels at 24 h in wild type and 28 h in lin-42(lf) were used from the biological replicate shown in A and B. For statistical purposes, log2(fold-change) values are shown. **P = 0.002, n.s., not significant, Welch’s t test. Error bars represent SD.
mlt-10 message levels also oscillate in lin-42(ve11) mutants (Fig. 1A), but the period length increases, consistent with the observed developmental delay. Strikingly, lin-42(ve11) mutants only progress through three larval stages (with three corresponding mlt-10 peaks) in the time that wild-type animals complete four and become adults. The mlt-10 peak time point in a stage is considered to be developmentally similar between strains and is used as an alignment guidepost.
lin-42(ve11) mutants were used for most of our studies because they are well characterized, have a strong heterochronic defect, and the mlt-10 profiles (Fig. 1A) indicate we can achieve a reasonably high level of developmental synchrony, at least through the early larval stages. lin-42(ve11), hereafter referred to as lin-42(lf), contains a premature stop codon but is not a null allele (Fig. S3A) (21).
In wild-type animals, let-7 was not detectable until the animals approached the L1 molt, and remained low through the L2. During the L3 stage, let-7 levels rapidly increased (Fig. 1B) and remained high throughout subsequent development, in agreement with previous northern analyses (2, 6, 13, 22). In contrast, in lin-42(lf) mutants, let-7 levels increased dramatically during the early L2 and continued to rise until the early to mid-L3, when they became more equivalent to wild-type levels and were somewhat variable (Fig. 1B). To more directly compare the let-7 expression pattern between strains, the data were aligned based on mlt-10 expression and plotted to focus on the dynamics of the L2 stage (Fig. 1 A and C). let-7 levels rose nearly 150-fold by the mid-L2 in lin-42(lf) mutants, whereas they remained just above background in wild-type animals. This result is reproducible and statistically significant across multiple biological replicates (Fig. 1D and Fig. S3B). Therefore, one function of lin-42 is to temporally restrict let-7 levels during early larval development.
miR-48 Levels Are Increased in L1-Stage lin-42(lf) Mutants.
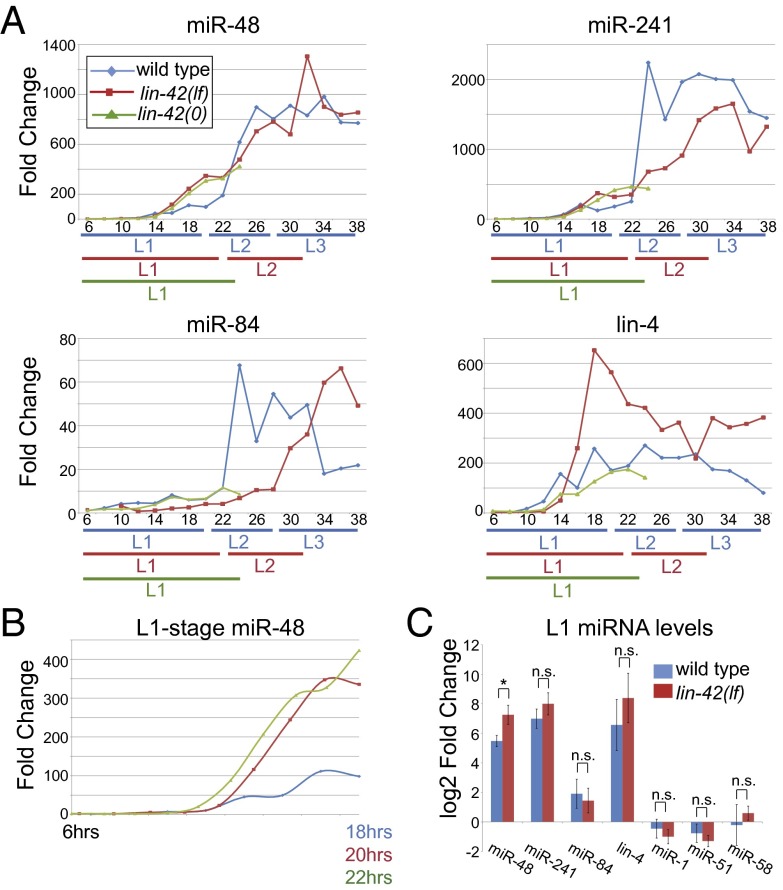
Four additional miRNAs act earlier than let-7 in the timing pathway: lin-4 and the let-7 miRNA family members miR-48, miR-241, and miR-84 (2, 4, 5). To test whether any of these miRNAs are aberrantly expressed in lin-42 mutants, we focused on their dynamics in early larval stages when they are up-regulated in wild-type animals, again using mlt-10 expression to assess synchrony and stage lengths. The wild-type expression patterns of the early-acting miRNAs closely match their profiles established by prior Northern blot analyses (2, 5, 7, 22). lin-4 levels rise in the L1 stage, whereas miR-48, miR-84, and miR-241 levels increase later, rising dramatically in the L2 (Fig. 2A).
Fig. 2.
miR-48 levels are elevated in lin-42 mutants. (A) Fold-change in levels of miR-48, miR-241, miR-84, and lin-4 in wild type, lin-42(lf), and lin-42(0) animals normalized to wild type at 6 h. The qRT-PCR assays were specific, and the miRNA accumulation patterns were similar between biological replicates (Figs. S1 and S2). Representative of ≥3 biological replicates, except for lin-42(0), where n = 2. (B) Graph of miR-48 data for the L1 stage from A aligned for stage length. (C) Average miRNA levels during the L1 stage at the time point of the mlt-10 expression peak (Fig. 1D and Fig. S5A). n = 3 biological replicates. *P < 0.05, n.s., not significant P > 0.05, Welch’s t test.
Among the early-acting let-7-family miRNAs, only miR-48 levels showed a reproducible and statistically significant precocious increase in lin-42(lf) mutants (Fig. 2 and Fig. S3B), and this difference was most pronounced in the late L1 (Fig. 2B). We also examined animals homozygous for a lin-42 null allele (lin-42(0) (Materials and Methods) and obtained similar results (Fig. 2 A and B). lin-42(0) mutants have a strong molting defect, which results in L2 arrest of most animals, limiting the analysis to the L1 stage.
miR-241 and miR-84 profiles were similar between lin-42 mutants and wild type, aside from the developmental delay (Fig. 2 A and C). lin-4 miRNA levels increased dramatically during the L1 stage in lin-42 mutants, as in wild-type animals (Fig. 2A), but were more variable than all other miRNAs examined. When averaged over multiple biological replicates, lin-4 levels were slightly higher in lin-42(lf) mutants versus wild type from the L1 to the L2, but these differences were not statistically significant (P > 0.05, two-way ANOVA) (Fig. S3B). Levels of three miRNAs not in the heterochronic gene pathway did not change significantly in L1-stage lin-42(lf) mutants (Fig. 2C).
pri-miRNA Transcript Levels Oscillate Through Larval Development.
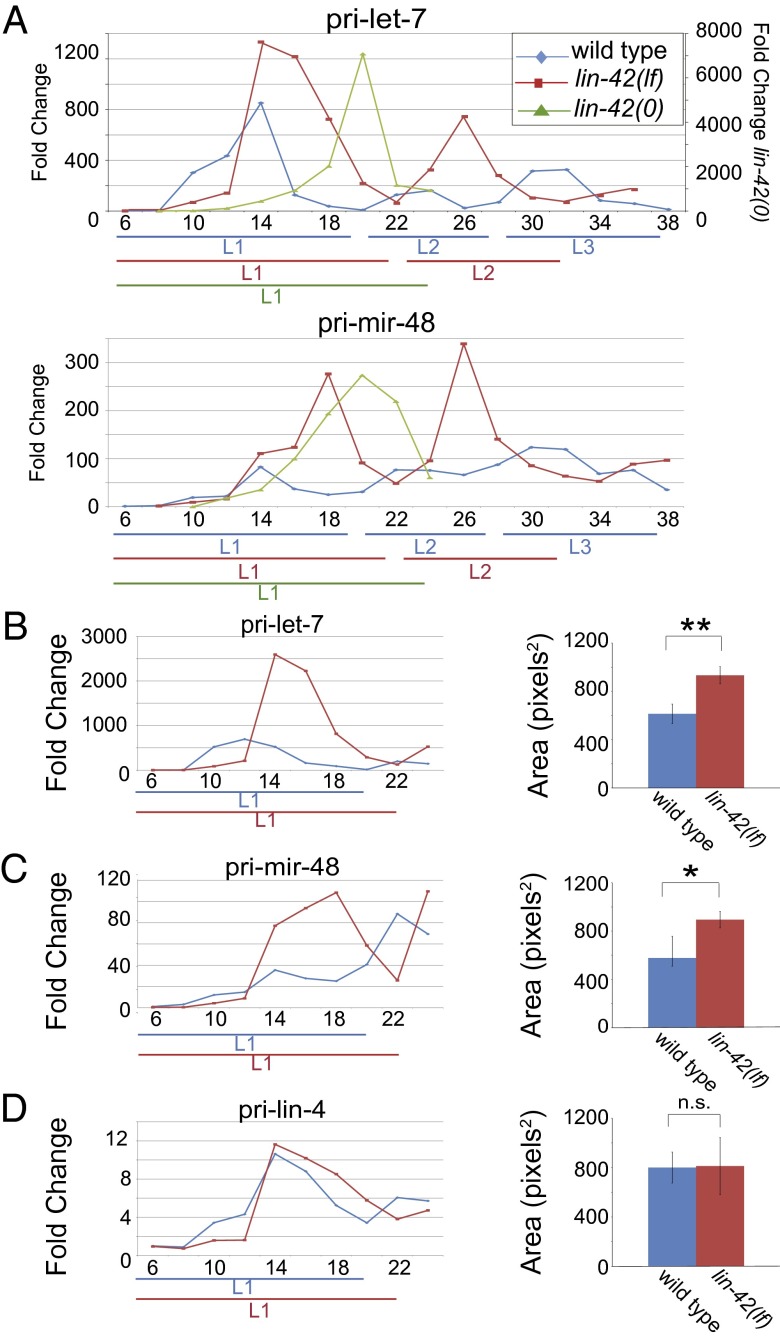
The miRNA biogenesis process provides multiple opportunities for regulation. Because LIN-42 is nuclear (21) and related to period proteins of flies and mammals, which control gene expression by inhibiting transcriptional activators (23), one possibility is that the misregulation of let-7 and miR-48 observed in lin-42 mutants reflects transcriptional derepression. To test this idea, qRT-PCR assays were developed to track the primary transcript levels of miRNA genes in the timing pathway (Fig. S1). The expression level of each pri-miRNA cycled during wild-type larval development (Fig. 3A and Fig. S4), generally peaking once per stage. This finding is consistent with a previous study that revealed oscillation of pri–let-7 levels (13).
Fig. 3.
pri–let-7 and pri–mir-48 levels cycle and are increased in lin-42(lf) mutants. All samples are normalized to wild type at 6 h. (A) pri–let-7 (Top) and pri–mir-48 (Bottom) levels in wild type, lin-42(lf), and lin-42(0) populations. (B–D) pri-miRNA levels in L1-stage wild type and lin-42(lf) mutants (Left). pri-miRNA expression during the L1 stage from ≥3 biological replicates was calculated as the area under each curve in pixels-squared using ImageJ software (Right) (40). (B) pri–let-7. (C) pri–mir-48. (D) pri-lin-4. Data graphed in B and C are technical replicates of that shown in A, except that 2× total RNA was used in the assay. **P < 0.01, *P < 0.05, Welch’s t test.
pri–let-7 and pri–lin-4 temporal profiles were similar to each other (Fig. S4 A and B), with coordinate peak amplitudes at—or just before—each mlt-10 expression peak. The similar temporal patterns of lin-4 and let-7 transcription is surprising, given the marked dissimilarity in the temporal accumulation of their miRNA products (6, 7). mir-48 and mir-241 also exhibited comparable cyclical pri-miRNA patterns (Fig. S4 C and D). Given their relatively close proximity in the genome (∼1.7 kb apart), coexpression of these two miRNAs in a single primary transcript could be partly or wholly responsible for the resemblance of their accumulation patterns. However, there is some evidence for transcriptional contributions from independent start sites (24). Finally, the expression pattern of pri–mir-84 differed from that of pri–mir-48/241 (Fig. S4E), even though there is temporal coordination in their mature miRNA accumulation profiles (Fig. 2A). Although pri–mir-48/241 levels peak during midlarval stages, pri–mir-84 levels are highest near the molt and peak out of phase with the other pri-miRNAs analyzed, suggesting that mir-84 is controlled by a different regulatory regime than that of its paralogs.
Primary Transcript Levels Cycle in the Absence of lin-42.
The observed oscillations of heterochronic pri-miRNA levels are intriguing in light of the well-established cyclical lin-42 expression pattern (17, 18, 21) and the role of period proteins in maintaining the rhythmic expression of circadian-regulated genes in other organisms (25, 26). These relationships prompted us to ask whether lin-42 function is required for pri-miRNA cycling. Unexpectedly, pri-miRNA transcript levels oscillate in lin-42(lf) mutants, peaking once per larval stage, with a temporal profile similar to that observed in wild type (Fig. 3A and Fig. S5). Because hypomorphic lin-42 alleles leave one of three transcription units intact (Fig. S3A) (18, 21), it is essential to examine pri-miRNA levels in a null mutant background to rule out a role for LIN-42 function. Even in lin-42(0) mutants, heterochronic pri-miRNAs levels cycled once during the L1 stage (Fig. 3A and Fig. S5). Although we cannot exclude the possibility that the observed profile was affected by the health of the strain, the majority of these animals progress through the L1 and arrest development in the late L2 stage. Thus, we conclude lin-42 activity is not required to generate a cycle of pri–let-7 or pri–mir-48 expression.
pri–let-7 and pri–mir-48 Levels Are Increased in lin-42 Mutants.
Although lin-42 function is dispensable for generation of oscillatory pri-miRNA expression patterns, peak amplitude was increased for some pri-miRNAs in the mutants (Fig. 3 and Fig. S5). To estimate the relative expression of each gene in wild-type versus mutant animals, the areas under the L1-stage curves were compared. The L1 stage was chosen because during this stage, lin-42(lf) populations are most synchronous with wild type, and misexpression of miRNAs is first apparent. A significant increase in the area under the pri–let-7 and pri–mir-48 curves was observed in lin-42(lf) compared with wild-type animals (Fig. 3 B and C), supporting a role for lin-42 in dampening let-7 and mir-48 transcription. In contrast, the area under the pri–lin-4 curve was not significantly greater in lin-42(lf) than in wild type, similar to mature lin-4 miRNA levels (Figs. 2C and 3D). pri–mir-241 expression was elevated in the L1 stage of some experiments, but this effect was not statistically significant when averaged over several biological replicates (Fig. S5), similar to miR-241 levels.
Plet-7::gfppest Expression Is Elevated and Precocious in the Seam of lin-42(lf) Mutants.
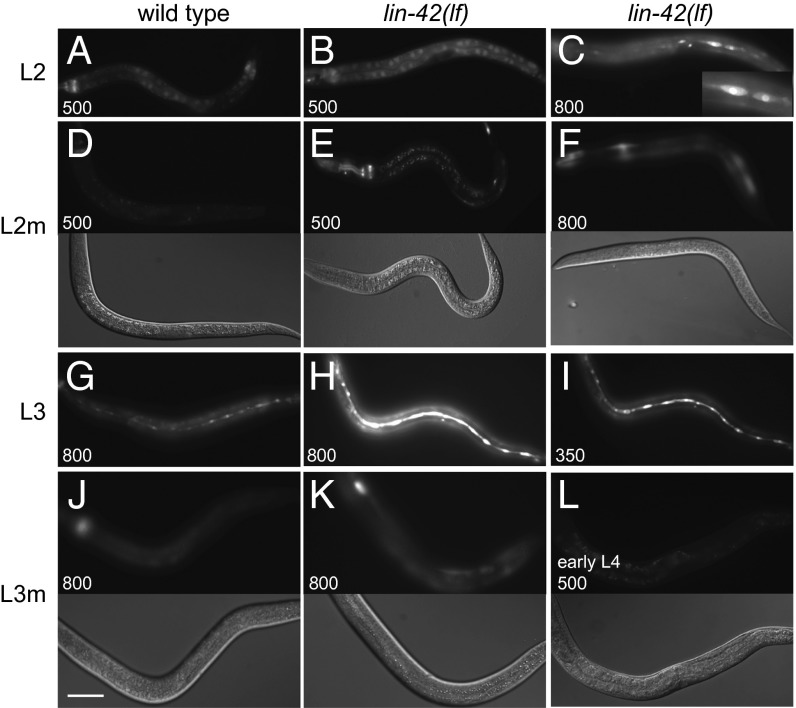
Although total pri–let-7 and pri–mir-48 levels are increased in lin-42(lf) mutants relative to wild type when synchronized populations are examined by qRT-PCR, a limitation of this approach is that transcript levels are averaged throughout the animal. To simultaneously visualize the spatial and temporal dynamics of let-7 expression associated with loss of lin-42 function, we generated animals transgenic for an integrated Plet-7::gfp reporter encoding a rapidly-degraded GFP variant (GFPPEST) (27) (SI Materials and Methods). The spatial pattern and onset of Plet-7::gfppest expression in wild type was similar to previous reports using standard gfp fusions (22, 28, 29), with expression observed in a variety of tissues (hypodermis, intestine, body wall muscle, pharynx, vulva, and nervous system) beginning in different larval stages. For example, gut and pharyngeal expression began in the L1, whereas hypodermal seam cells, a site of relevance for the lin-42 heterochronic phenotype, were not observed until the L3 stage (Fig. 4 and Fig. S6). Notably, use of gfppest revealed cyclical expression in all tissues examined. GFP was generally detected in multiple tissues late in each larval stage, but was weak or absent from molting animals (Fig. 4 D and J).
Fig. 4.
Plet-7::gfppest expression is elevated and detected early in lin-42 mutants. Plet-7::gfppest expression during midlarval development in wild-type and lin-42(lf) animals with exposure times indicated (ms). (A and B) Expression in the gut and pharynx of wild-type and lin-42(lf) animals, respectively. (C) GFP is precociously expressed in the seam of L2-stage lin-42(lf) mutants. (Inset) Magnification of two seam cells. GFP is not detected in the seam of wild type at this stage. (D–F) Fluorescence (Top) and DIC (Bottom) images of L2-molt stage animals. Plet-7::gfppest expression decreases during the L2 molt of wild type and lin-42(lf). Some GFP remains detectable in the pharynx and body wall muscle (BWM), particularly in the mutant. (G–I) Expression is detected in the seam of L3 stage wild-type animals and with greater intensity in lin-42(lf) mutants. (J and K) Fluorescence (Top) and DIC (Bottom) images of L3-molt stage animals. Plet-7::gfppest expression is not detected in the seam of L3m-stage animals, although BWM and pharynx expression remains visible in the mutant. (L) An early-L4 stage lin-42(lf) mutant lacks detectable GFP. (A–L) Scale bar, 50 μm.
Plet-7::gfppest expression cycled in lin-42(lf) mutants, with decreased intensity in molting animals becoming undetectable by the beginning of the next stage, similar to what was observed in wild-type animals (Fig. 4 E, F, K, and L). However, two differences were apparent in lin-42 mutants. First, the intensity of GFP fluorescence was elevated in lin-42(lf) mutants relative to wild type in all tissues and at all stages where it was detected (Fig. 4 A, B, G, and H), consistent with the increased levels of pri–let-7 revealed by qRT-PCR. Second, Plet-7::gfppest expression was detected precociously in hypodermal seam cells of all lin-42(lf) mutants analyzed (n = 22), appearing during the L2-stage, a time when pharyngeal and intestinal Plet-7::gfppest expression was readily evident in both mutant and wild-type animals (Fig. 4 B and C). On average, GFP was detected in 11.2 ± 3.4 seam cells per lateral side of late L2-stage lin-42(lf) animals (n = 8) but was not detected at this stage in wild type (n = 15). Therefore, not only does lin-42 play a global role in dampening let-7 transcription, but it also functions specifically in the L2 seam to prevent let-7 expression. The early seam expression likely contributes to the lin-42 precocious phenotype and helps explain the multiple genetic interactions between lin-42 and heterochronic genes that function in the L2 hypodermis, such as daf-12.
let-7 Family Genes Act Downstream of lin-42 in the Timing Pathway.
The increased levels of let-7 and miR-48 in lin-42 mutants suggest that these miRNAs are downstream of lin-42 in the heterochronic gene pathway, a hypothesis we tested with genetic epistasis. Mutations in heterochronic genes alter the timing of hypodermal seam cell terminal differentiation, which occurs during the final (L4) larval molt in wild type and results in generation of characteristic adult cuticle containing ridges (“alae”). lin-42 mutants have a precocious phenotype in which adult cuticle is synthesized one stage early, during the L3 molt (17). In contrast, let-7 mutants and mir-48 mir-241 double-mutants have retarded phenotypes, inappropriately synthesizing larval cuticle during the final molt (2, 6). We analyzed lin-42(0); let-7(0) double-mutants and observed mutual suppression (Table 1), similar to experiments carried out with lin-42 hypomorphic alleles or lin-42(RNAi) rather than a null (6, 21). At the L3 molt, the precocious alae phenotype of lin-42(0) single-mutants was largely suppressed by loss of let-7, and furthermore, when alae were detected in the double-mutant, they were weak and indistinct compared with lin-42(0). At the L4 molt, the let-7(0) retarded phenotype was strongly suppressed by the loss of lin-42; most animals had full-length alae similar to wild-type animals. One interpretation of this genetic result, supported by our molecular analysis, is that let-7 acts downstream but is not the only output of lin-42 activity.
Table 1.
Genetic interaction of lin-42(0) and let-7-family genes
| Genotype* | Animals with L3-molt alae (%) | Animals with L4-molt alae (%) | ||||||||
| Full | Partial† | None | Quality‡ | n | Full | Partial† | None | Quality‡ | n | |
| Wild-type (N2) | 0 | 0 | 100 | +++ | 10 | 100 | 0 | 0 | +++ | 10 |
| lin-42 | 66 | 32 | 2 | +++ | 87 | ND | ND | ND | ND | |
| let-7 unc-3 | ND | ND | ND | ND | 11 | 26 | 63 | + | 38 | |
| lin-42; let-7 unc-3 | 0 | 15 | 85 | ++/+ | 62 | 83 | 14 | 3 | +++ | 59 |
| lin-42; unc-3 | 74 | 24 | 3 | +++ | 34 | ND | ND | ND | ND | |
| lin-42; mir-48 | 0 | 35 | 65 | +++/+ | 43 | ND | ND | ND | ND | |
| lin-42; mir-241 | 85 | 15 | 0 | +++ | 26 | ND | ND | ND | ND | |
| lin-42; mir-84 | 73 | 27 | 0 | +++/++ | 22 | ND | ND | ND | ND | |
| mir-48 mir-241 | ND | ND | ND | ND | 2 | 88 | 19 | ++ | 42 | |
| lin-42; mir-48 mir-241 | 0 | 0 | 100 | — | 25 | 97 | 3 | 0 | +++ | 29 |
| mir-48 mir-241; mir-84 | ND | ND | ND | ND | 0 | 57 | 43 | ++/+ | 35 | |
| lin-42; mir-48 mir-241; mir-84 | 0 | 2 | 98 | + | 41 | 83 | 17 | 0 | +++ | 35 |
| lin-42; mir-48 mir-241; let-7 unc-3 | 0 | 13 | 87 | + | 30 | 19 | 22 | 59 | + | 37 |
ND, not determined.
The following null alleles were used: lin-42(ox461), let-7(mn112), mir-48 mir-241(nDf51), mir-48(n4097), mir-84(n4037), mir-241(n4316).
Partial indicates that ≥1 of 16 seam cells scored per animal lacked alae in the overlying cuticle, resulting in gaps.
Alae varied in quality between strains but was of consistent quality within strains, from +++ (wild-type quality) to + (poor, indistinct alae quality).
The three early-acting let-7 family members, mir-48, mir-241, and mir-84, also promote temporal transitions in seam cells and have identical “seed” sequences to let-7, suggesting they may share targets (2, 5) and could be additional outputs of lin-42 activity. Although individual deletions of these miRNAs result in little or no heterochronic defect (2), we tested whether they could suppress the lin-42(0) phenotype. lin-42(0); mir-241(0) and lin-42(0); mir-84(0) double-mutants are phenotypically similar to lin-42(0) mutants, indicating that misexpression of these miRNAs makes little contribution to the lin-42 mutant phenotype (Table 1). In contrast, deletion of mir-48 strongly suppressed the precocious phenotype of lin-42(0) mutants. These results support our molecular studies that revealed significant misregulation of mir-48 and let-7, but not mir-241 or mir-84, in lin-42 mutants.
The suppression of let-7(0) by lin-42(0) may reflect increased mir-48 expression compensating for the absence of let-7. Indeed, overexpression of mir-48 from multicopy arrays can suppress let-7(0) (5). To test whether let-7 paralogs are required for suppression of let-7(0) by lin-42(0), we analyzed lin-42(0); nDf51; let-7(0) mutants. nDf51 is a small deficiency that removes both mir-48 and mir-241 (2). At the L4 molt, the quadruple-mutant exhibited a strong retarded phenotype similar to let-7(0) single-mutants (Table 1). Importantly, this genetic result demonstrates that lin-42 functions through these miRNAs to regulate developmental timing; the absence of lin-42 does not alter seam development when let-7 and mir-48 are also missing. Thus, genetic analyses place let-7-family miRNAs, particularly let-7 and mir-48, downstream of lin-42 as key mediators of seam cell temporal fates.
Discussion
Five miRNAs play critical roles in regulating developmental timing in C. elegans by accumulating sequentially and repressing earlier-stage gene-expression patterns to promote a succession of temporal cell fates. We demonstrate that lin-42, the C. elegans homolog of the circadian rhythm gene period, negatively regulates miRNA biogenesis, ensuring the appropriate expression of these temporal regulators. In lin-42 mutants, the primary transcripts of let-7 and mir-48 are elevated, leading to precocious and enhanced accumulation of their miRNA products. The intensity of a Plet-7::gfppest reporter is increased in lin-42 mutants during larval stages, further supporting a model wherein LIN-42, similar to period proteins, acts as a transcriptional repressor.
Our work complements and extends two recent reports (30, 31) that also identify lin-42 as a negative regulator of miRNA transcription. The three studies reach this conclusion using diverse approaches, analyzing a variety of mutant alleles and focusing on different larval stages. Our studies examine early stages (L1–L3), an interval when lin-42 function in the seam is supported by genetic interactions (21), whereas the other studies largely centered on later stages, spanning the emergence of the hypodermal phenotype at the L3 molt in lin-42 mutants. Despite these differences, each study concludes that dampening let-7 transcription is a key output of LIN-42 function, indicating that this is not a stage-restricted function, and the observed defects are not allele specific.
Detailed analysis of early-stage dynamics uncovered precocious accumulation of let-7 and miR-48 during the L1 and L2 stages. Temporal misregulation was also observed with a Plet-7::gfppest reporter in a tissue of relevance to the lin-42 heterochronic phenotype; hypodermal seam cells expressed the reporter early, beginning in the L2 rather than the L3 as in wild type. We did not detect significant increases in expression of the let-7-family members mir-84 and mir-241, or lin-4, although others found these to be elevated in lin-42 mutants in late larval stages and adults (30, 31). Importantly, our epistasis analysis using null alleles revealed that let-7 family miRNAs act downstream of lin-42, and that misregulation of let-7 and mir-48 is the principle factor responsible for the precocious hypodermal phenotype of lin-42 mutants. In the case of lin-4, we observed a trend for increased accumulation in lin-42 mutants. However, lin-4 levels were variable relative to let-7 family miRNAs assayed in the same samples, and the differences were not statistically significant. pri–lin-4 levels were also similar between the strains. It is possible that lin-4 levels are less sensitive to lin-42 mutation during early larval development because a Plin-4::gfppest reporter analysis showed the greatest difference at the late L3–L3m stage (30).
Our work also adds to the increasing wealth of data showing that the cyclical pri-miRNA expression profiles are often uncoupled from their mature miRNA patterns. This finding is particularly true of let-7: its primary transcript is strongly expressed during the mid-L1 stage, two stages before the accumulation of its miRNA. This uncoupling is in part because of action of the LIN-28 RNA-binding protein blocking processing (12, 13, 32). Therefore, lin-42 and lin-28 work in concert to regulate let-7, acting at the transcription and processing steps, respectively, and increased pri–let-7 levels in lin-42 mutants could overwhelm the ability of LIN-28 to inhibit processing. The L2 is likely to be particularly sensitive to the amount of pri–let-7 present because LIN-28 levels dramatically decline during this stage (33). Freed from inhibition, the excess pri-miRNA could result in precocious accumulation of let-7.
lin-42 also has a highly dynamic expression pattern with mRNA and protein levels cycling once per larval stage and in a broad variety of tissues (17, 18, 21). Given that LIN-42 is nuclear, and that period proteins act as transcriptional repressors that program oscillatory expression patterns, LIN-42 was a prime candidate to establish the pri-miRNA profiles. Testing whether lin-42 is required for these cyclical patterns has been confounded by the fact that all described alleles leave one of three lin-42 transcription units intact. We performed a definitive experiment by examining pri-miRNA expression using a lin-42–null allele that deletes all transcription units. Surprisingly, the levels of the two pri-miRNAs examined, pri–let-7 and pri–mir-48, still cycled but their peak amplitudes were increased in the absence of lin-42. Thus, lin-42 dampens, rather than programs, miRNA oscillations, acting as a buffer to ensure that miRNAs are expressed at the appropriate levels and in the correct temporal window.
The broad somatic expression lin-42, together with described nonheterochronic functions, including control of molting and inhibition of dauer formation (18, 34), suggest its reach extends beyond transcriptional regulation of heterochronic miRNAs. Indeed, genome-wide studies implicate lin-42 in modulation of both nonheterochronic miRNA genes as well as protein coding genes (30, 31), and it will be interesting to partition these targets among lin-42 functions. Cyclical expression patterns linked to the molting cycle have emerged as a common theme in C. elegans larval development and include a large proportion of the transcriptome (35, 36), including the heterochronic miRNAs. An important goal for the future is to decipher how these cyclical patterns are generated and aligned with the molting cycles. Given the roles for lin-42 in both molting and developmental timing, it may act to integrate these two developmental pathways.
Materials and Methods
Nematode Maintenance and Strains.
C. elegans strains were cultured at 20 °C using standard methods (37). lin-42(ox461) is a null allele, generated by mosDEL technology (38), in which the lin-42 coding region has been replaced with unc-119(+). lin-42(ox461); nDf51; let-7(mn112) unc-3(e151) animals were maintained under conditions of “hbl-1(low RNAi)” (12), which weakly suppresses the lethality caused by let-7(0). To score, eggs were isolated from adults grown on low hbl-1(RNAi) plates and grown on nematode growth medium (NGM) plates seeded with Escherichia coli OP50. let-7 suppression was not observed in this generation. Strain names and details on genetics can be found in SI Materials and Methods and Table S1.
Transgenic Strains and Fluorescence Microscopy.
The Plet-7::gfppest reporter contains 3.2 kb of DNA extending 5′ of the pre-let-7 hairpin. Additional details about generation and analysis of transgenic animals can be found in SI Materials and Methods.
qRT-PCR.
Starvation-synchronized L1s were grown on 10-cm OP50 seeded NGM plates at a density of ∼5,000 to ∼10,000 animals per plate. Biological replicates were performed with animals from independent hypochlorite treatments and grown on different days. Quantitative Taqman miRNA and gene-expression assays were performed as directed (Life Technologies) using Trizol to extract total RNA (Table S2). All quantitative PCR reactions were assayed in triplicate on an Eppendorf Realplex Thermocycler and Realplex 2.0 software. The sample used for normalization was run on every plate. U18 RNA was used as an internal control for miRNA experiments. ama-1 was used for normalization of pri-miRNAs and mlt-10 expression. Data were analyzed using the ΔΔCt method (39) and statistical analyses were performed using R. Additional details can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Tamar Resnick for thoughtful comments on the manuscript. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by Grant R01GM50227 from the NIH (to A.E.R.); a grant from the Minnesota Medical Foundation (to A.E.R.); and NIH Predoctoral Training Grant 5T32HD007480 (to K.A.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414856111/-/DCSupplemental.
References
- 1.Rougvie AE, Moss EG. Developmental transitions in C. elegans larval stages. Curr Top Dev Biol. 2013;105:153–180. doi: 10.1016/B978-0-12-396968-2.00006-3. [DOI] [PubMed] [Google Scholar]
- 2.Abbott AL, et al. The let-7 MicroRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Dev Cell. 2005;9(3):403–414. doi: 10.1016/j.devcel.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambros V. A hierarchy of regulatory genes controls a larva-to-adult developmental switch in C. elegans. Cell. 1989;57(1):49–57. doi: 10.1016/0092-8674(89)90171-2. [DOI] [PubMed] [Google Scholar]
- 4.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Jones-Rhoades MW, Lau NC, Bartel DP, Rougvie AE. Regulatory mutations of mir-48, a C. elegans let-7 family MicroRNA, cause developmental timing defects. Dev Cell. 2005;9(3):415–422. doi: 10.1016/j.devcel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Reinhart BJ, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 7.Feinbaum R, Ambros V. The timing of lin-4 RNA accumulation controls the timing of postembryonic developmental events in Caenorhabditis elegans. Dev Biol. 1999;210(1):87–95. doi: 10.1006/dbio.1999.9272. [DOI] [PubMed] [Google Scholar]
- 8.Finnegan EF, Pasquinelli AE. MicroRNA biogenesis: Regulating the regulators. Crit Rev Biochem Mol Biol. 2013;48(1):51–68. doi: 10.3109/10409238.2012.738643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagan JP, Piskounova E, Gregory RI. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol. 2009;16(10):1021–1025. doi: 10.1038/nsmb.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heo I, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138(4):696–708. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Piskounova E, et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147(5):1066–1079. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vadla B, Kemper K, Alaimo J, Heine C, Moss EG. lin-28 controls the succession of cell fate choices via two distinct activities. PLoS Genet. 2012;8(3):e1002588. doi: 10.1371/journal.pgen.1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Wynsberghe PM, et al. LIN-28 co-transcriptionally binds primary let-7 to regulate miRNA maturation in Caenorhabditis elegans. Nat Struct Mol Biol. 2011;18(3):302–308. doi: 10.1038/nsmb.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bethke A, Fielenbach N, Wang Z, Mangelsdorf DJ, Antebi A. Nuclear hormone receptor regulation of microRNAs controls developmental progression. Science. 2009;324(5923):95–98. doi: 10.1126/science.1164899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammell CM, Karp X, Ambros V. A feedback circuit involving let-7-family miRNAs and DAF-12 integrates environmental signals and developmental timing in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009;106(44):18668–18673. doi: 10.1073/pnas.0908131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roush SF, Slack FJ. Transcription of the C. elegans let-7 microRNA is temporally regulated by one of its targets, hbl-1. Dev Biol. 2009;334(2):523–534. doi: 10.1016/j.ydbio.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon M, Gardner HF, Miller EA, Deshler J, Rougvie AE. Similarity of the C. elegans developmental timing protein LIN-42 to circadian rhythm proteins. Science. 1999;286(5442):1141–1146. doi: 10.1126/science.286.5442.1141. [DOI] [PubMed] [Google Scholar]
- 18.Monsalve GC, Van Buskirk C, Frand AR. LIN-42/PERIOD controls cyclical and developmental progression of C. elegans molts. Curr Biol. 2011;21(24):2033–2045. doi: 10.1016/j.cub.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 19.Meli VS, Osuna B, Ruvkun G, Frand AR. MLT-10 defines a family of DUF644 and proline-rich repeat proteins involved in the molting cycle of Caenorhabditis elegans. Mol Biol Cell. 2010;21(10):1648–1661. doi: 10.1091/mbc.E08-07-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frand AR, Russel S, Ruvkun G. Functional genomic analysis of C. elegans molting. PLoS Biol. 2005;3(10):e312. doi: 10.1371/journal.pbio.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tennessen JM, Gardner HF, Volk ML, Rougvie AE. Novel heterochronic functions of the Caenorhabditis elegans period-related protein LIN-42. Dev Biol. 2006;289(1):30–43. doi: 10.1016/j.ydbio.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Esquela-Kerscher A, et al. Post-embryonic expression of C. elegans microRNAs belonging to the lin-4 and let-7 families in the hypodermis and the reproductive system. Dev Dyn. 2005;234(4):868–877. doi: 10.1002/dvdy.20572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C, Bae K, Edery I. PER and TIM inhibit the DNA binding activity of a Drosophila CLOCK-CYC/dBMAL1 heterodimer without disrupting formation of the heterodimer: A basis for circadian transcription. Mol Cell Biol. 1999;19(8):5316–5325. doi: 10.1128/mcb.19.8.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruesi WS, Core LJ, Waters CT, Lis JT, Meyer BJ. Condensin controls recruitment of RNA polymerase II to achieve nematode X-chromosome dosage compensation. eLife. 2013;2:e00808. doi: 10.7554/eLife.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardin PE, Hall JC, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990;343(6258):536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- 26.Hardin PE, Hall JC, Rosbash M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci USA. 1992;89(24):11711–11715. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, et al. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem. 1998;273(52):34970–34975. doi: 10.1074/jbc.273.52.34970. [DOI] [PubMed] [Google Scholar]
- 28.Johnson SM, Lin SY, Slack FJ. The time of appearance of the C. elegans let-7 microRNA is transcriptionally controlled utilizing a temporal regulatory element in its promoter. Dev Biol. 2003;259(2):364–379. doi: 10.1016/s0012-1606(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 29.Kai ZS, Finnegan EF, Huang S, Pasquinelli AE. Multiple cis-elements and trans-acting factors regulate dynamic spatio-temporal transcription of let-7 in Caenorhabditis elegans. Dev Biol. 2013;374(1):223–233. doi: 10.1016/j.ydbio.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perales R, King DM, Aguirre-Chen C, Hammell CM. LIN-42, the Caenorhabditis elegans PERIOD homolog, negatively regulates microRNA transcription. PLoS Genet. 2014;10(7):e1004486. doi: 10.1371/journal.pgen.1004486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Wynsberghe PM, et al. The Period protein homolog LIN-42 negatively regulates microRNA biogenesis in C. elegans. Dev Biol. 2014;390(2):126–135. doi: 10.1016/j.ydbio.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehrbach NJ, et al. LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nat Struct Mol Biol. 2009;16(10):1016–1020. doi: 10.1038/nsmb.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seggerson K, Tang L, Moss EG. Two genetic circuits repress the Caenorhabditis elegans heterochronic gene lin-28 after translation initiation. Dev Biol. 2002;243(2):215–225. doi: 10.1006/dbio.2001.0563. [DOI] [PubMed] [Google Scholar]
- 34.Tennessen JM, Opperman KJ, Rougvie AE. The C. elegans developmental timing protein LIN-42 regulates diapause in response to environmental cues. Development. 2010;137(20):3501–3511. doi: 10.1242/dev.048850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendriks GJ, Gaidatzis D, Aeschimann F, Großhans H. Extensive oscillatory gene expression during C. elegans larval development. Mol Cell. 2014;53(3):380–392. doi: 10.1016/j.molcel.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Kim Dh, Grün D, van Oudenaarden A. Dampening of expression oscillations by synchronous regulation of a microRNA and its target. Nat Genet. 2013;45(11):1337–1344. doi: 10.1038/ng.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frøkjaer-Jensen C, et al. Targeted gene deletions in C. elegans using transposon excision. Nat Methods. 2010;7(6):451–453. doi: 10.1038/nmeth.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.