Significance
Infectious proteins (prions) are capable of encoding genetic information by templating their conformation, just as DNA templates its sequence. The mechanism of this templating has not been clear. We provide definitive proof that the architecture of amyloid of the prion domain of yeast prion protein Sup35p is a folded in-register parallel β-sheet, and our data identify some of the sites of folds in the sheet. This architecture naturally suggests a templating mechanism based on favorable interactions among aligned side chains of identical amino acids. This is the only mechanism suggested to date for such a conformation templating.
Keywords: [PSI+] prion, solid-state NMR, dipolar recoupling, amyloid, Sup35
Abstract
The [PSI+] prion is a self-propagating amyloid of the translation termination factor, Sup35p, of Saccharomyces cerevisiae. The N-terminal 253 residues (NM) of this 685-residue protein normally function in regulating mRNA turnover but spontaneously form infectious amyloid in vitro. We converted the three Ile residues in Sup35NM to Leu and then replaced 16 single residues with Ile, one by one, and prepared Ile-1-13C amyloid of each mutant, seeding with amyloid formed by the reference sequence Sup35NM. Using solid-state NMR, we showed that 10 of the residues examined, including six between residues 30 and 90, showed the ∼0.5-nm distance between labels diagnostic of the in-register parallel amyloid architecture. The five scattered N domain residues with wider spacing may be in turns or loops; one is a control at the C terminus of M. All mutants, except Q56I, showed little or no [PSI+] transmission barrier from the reference sequence, suggesting that they could assume a similar amyloid architecture in vitro when seeded with filaments of reference sequence Sup35NM. Infection of yeast cells expressing the reference SUP35 gene sequence with amyloid of several mutants produced [PSI+] transfectants with similar efficiency as did reference sequence Sup35NM amyloid. Our work provides a stringent demonstration that the Sup35 prion domain has the folded in-register parallel β-sheet architecture and suggests common locations of the folds. This architecture naturally suggests a mechanism of inheritance of conformation, the central mystery of prions.
Prions are infectious proteins, mostly self-propagating amyloids. Amyloid is a filamentous polymer, rich in β-sheet structure, in which the β-strands run perpendicular to the long axis of the filament and the hydrogen bonds joining β-strands to make a sheet are along the long axis of the filament. In mammals, prions are uniformly lethal diseases, caused by amyloid formation of the PrP protein. In yeast and fungi, prions are not uniformly fatal and have widely varying effects (reviewed in ref. 1). Perhaps the most remarkable feature of prions is that they have strains or variants, distinct self-propagating forms of the same protein, analogous to alleles of a gene, each relatively stably propagated. The existence of different self-propagating prion variants implies an array of self-propagating structures, each based on the same protein sequence. Because each prion variant is self-propagating, and each variant represents a different amyloid structure/conformation, there must be some mechanism by which the protein can template its conformation. This mechanism must operate for each of the many conformations that are possible for a given prion.
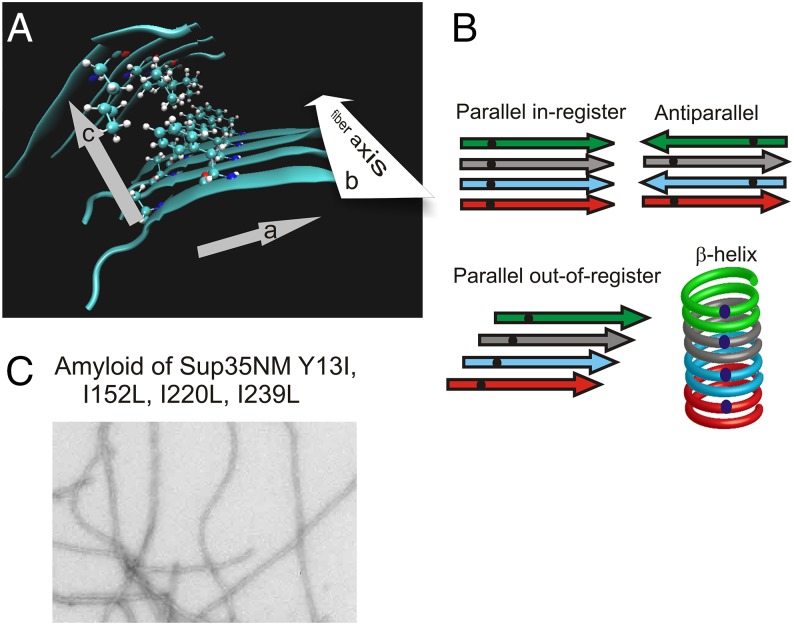
An amino acid residue in a β-sheet can have interactions in three dimensions (Fig. 1A): (a) along the peptide chain; (b) with the residues it faces within the β-sheet but perpendicular to the peptide chain, including the residues to which its main chain N–H and >C = O are hydrogen bonded; and (c) with residues located in the direction perpendicular to the β-sheet, possibly another β-sheet, interacting with the distal part of the side chain. The distance between residues having the relation (b), between adjacent β-strands in the β-sheet, is generally 0.47–0.48 nm. The distance between adjacent sheets, as in (c), is about 1.0 nm.
Fig. 1.
Amyloid structure. (A) An amino acid residue in amyloid has interactions in three directions: (a) along the peptide chain, (b) between aligned residues in the β-sheet along the fibril axis but perpendicular to the peptide chain (∼0.48 nm distant), and (c) with residues in an adjacent β-sheet (∼1.0 nm distant). Based on a model of Aβ amyloid (4). (B) Types of β-sheet. The dots represent a single 13C-labeled atom in each protein molecule. The expected separation is ∼0.48 nm for in-register parallel, ∼0.94 nm for antiparallel, and >0.9 nm for β-helix. (C) Amyloid fibers of Ile-1-13C–labeled Sup35NM with mutations Y13I, I152L, I220L, and I239L.
Given that the filament is a regular/repeating structure, the molecules in a filament can have any of several architectures (Fig. 1B). Most β-sheets in soluble enzymes are antiparallel, with adjacent β-strands running in opposite directions (Fig. 1B). Labeling a single atom in each molecule and measuring the distance between nearest neighbors will usually produce a distance of >0.9 nm (the distance between strands in a β-sheet is ∼0.47 nm) for an antiparallel β-sheet. A parallel β-sheet can be in-register or out-of-register. In-register means that each residue in one molecule is aligned with the same residue in the neighboring molecule, producing lines of identical residues along the long axis of the filament. Labeling a single atom in each molecule will give a distance between labels of ∼0.48 nm if the filament has the parallel in-register β-sheet architecture (Fig. 1B). For an out-of-register β-sheet, the distance will be greater, depending on the register shift (Fig. 1B). In a β-helix, the distance between single labels will be >0.9 nm if each molecule constitutes two turns of the helix, and even greater if each molecule comprises more than two turns (Fig. 1B).
The first demonstration of an in-register parallel amyloid structure was by Benzinger et al. studying the 10–35 fragment of the Aβ peptide, and established the approach for proving this architecture (2). The peptide was synthesized with a single 13C atom as the carbonyl carbon of one residue. Amyloid was assembled from each such synthetic peptide, and the distance between labels (necessarily in different molecules because there was only one label per molecule) was measured by solid-state NMR using dipolar recoupling. The distance between labeled carbonyl carbons was ∼0.5 nm regardless of which residue was labeled, establishing the in-register parallel architecture for this peptide.
The full-size Aβ is a peptide of 40–42 residues. Tycko’s group used a similar approach to show that amyloid of Aβ40 has an in-register parallel β-sheet architecture, and long range intramolecular cross-peaks showed that the sheet was folded along the long axis of the filaments (refs. 3 and 4, reviewed in ref. 5). Moreover, filament mass per length data implied that these folded sheets were associated to form filaments with either two or three molecules per layer (6, 7). Different filament formation conditions can also produce minor differences in the conformation of the monomer (8–10), but seeding can fix the amyloid form (6). Notably, seeding recombinant Aβ peptide with brain material of different Alzheimer’s disease patients produced different amyloid forms, with the same form produced by seeds from different parts of the brain of a single patient (11). This suggests that there may be different forms of Alzheimer’s disease based on different self-propagating amyloid variants, a notion now supported by animal studies (12).
Most other pathologic human amyloids have been found to have the in-register parallel β-sheet architecture. Amylin/IAPP (type 2 diabetes), alpha-synuclein (Parkinson’s disease), and β2-microglobulin (dialysis-induced amyloidosis) all follow this pattern (13, 14). An 11-residue peptide from transthyretin also forms an in-register parallel β-sheet structure (15), but full-length transthyretin (senile systemic amyloidosis) breaks the rule in that amyloid formed in vitro does not have this architecture (16, 17).
The [Het-s] prion of Podospora anserina is responsible for a heterokaryon incompatibility reaction, a phenomenon widespread in filamentous fungi that limits cell–cell fusion to the closest relatives to block the spread of fungal viruses (18, 19). Infectious amyloid formed by the prion domain of the HET-s prion protein has a β-helix structure, with two helical turns per molecule and three β-sheets forming the sides of the coil (20, 21). There is only a single prion variant of [Het-s] and the amyloid forms an unique structure in vitro with very narrow peaks in 2D solid-state NMR experiments, enabling complete assignments and structural determination (20, 21).
The yeast prions [URE3], [PSI+], and [PIN+] (22, 23) are amyloids of Ure2p, Sup35p, and Rnq1p, respectively, with a restricted region (the prion domain) of each responsible for both the prion properties and the amyloid structure (24–33). Amyloid of the Ure2p prion domain (residues 1–89) was shown by solid-state NMR to be in-register parallel using preparations with [13C]carbonyl labeling of either the two Leu or the four Val residues or [13C]methyl labeling of the single Ala residue (34). These residues are scattered through the prion domain, and the result with the single Ala residue implies that the nearest neighbor distance measured was an intermolecular distance. This result was confirmed by solid-state NMR studies of full-length Ure2p labeled with Ile (three residues in the prion domain) (35) and by electron spin resonance (36).
Sup35p consists of a Q/N-rich N-terminal domain (N, residues 1–123), a highly charged middle domain (M, residues 124–253), and the C-terminal domain essential for translation termination (C, residues 254–685). The Sup35p prion domain includes the N domain (residues 1–123) and an unknown part of the M domain, depending on how “prion domain” is defined. Residues 1–61 fused to GFP are sufficient, in amyloid form, to transmit several different prion variants by protein transformation (31), but some variants require up to residue 137 (37) and residues in the M domain are part of the barrier to [PSI+] prion transmission between wild strains of Saccharomyces cerevisiae (38). H–D exchange experiments indicate stable structures in the M domain in the amyloid of some [PSI+] variants (39). Of the eight Leu residues (seven of which are in M), solution NMR suggests that only four are fully unstructured (39). In agreement, solid-state NMR experiments suggested that four of the eight Leu residues are in parallel in-register structures (40).
We previously showed that amyloid of Sup35NM having all Tyr carbonyl carbons labeled with 13C showed signal decay rates in dipolar recoupling experiments indicative of nearest neighbor 13C at a distance of ∼0.5 nm (40, 41). Dilution of this Tyr-labeled sample with unlabeled Sup35NM and then formation of amyloid resulted in a dramatic increase in the nearest neighbor distance, exactly as predicted if the nearest neighbor were on another molecule. We inferred that the Sup35NM amyloid has an in-register parallel architecture, and data with Sup35NM amyloid labeled with Phe-1-13C, Leu-1-13C or Ala-3-13C were consistent with this conclusion. However, one could imagine that although the nearest neighbor of a given Tyr was on another molecule, it was not the same Tyr. There are 20 Tyr residues in Sup35N (and none in Sup35M), making this a real possibility. We therefore sought a definitive proof of the in-register parallel architecture of Sup35NM. In addition, there continues to be a view that Sup35NM amyloid has a β-helix architecture (42–45), and a more rigorous test was therefore important to resolve this central issue.
Results
To test the in-register parallel model for amyloid of Sup35NM, it would be ideal to have singly 13C-labeled molecules so that nearest neighbor distances would necessarily be intermolecular. At 253 residues, Sup35NM is too long to synthesize. Instead, we eliminated the only three Ile residues, all in the M domain (residues 152, 220, and 239) to do Ile scanning mutagenesis. Ile is a suitable choice for this role because adding labeled Ile to the culture completely suppresses endogenous synthesis, there is no incorporation into other amino acids, there are only three Ile residues in Sup35NM to change to the nearly identical Leu, and Ile is an amino acid compatible with prion formation (46).
It would be ideal to have amyloid preparations with uniform conformation for structural studies. Although efforts have been made using specific Sup35NM filament formation conditions (30) or seeding using Sup35p or Ure2p prion amyloid isolated from cells (31, 35, 47), the relatively broad peaks found in 2D solid-state NMR experiments (35, 47) and nonexponential H–D exchange curves (39) indicate considerable heterogeneity of filaments made in vitro. Even single [PSI+] clones show segregation of prion variants and frequent mutation, indicating the existence of a cloud of prion variants in vivo (48). Furthermore, single amino acid changes can, in many cases, block [PSI+] prion propagation (49, 50). Thus, we can only hope to approach this ideal.
Single isoleucine residues were introduced throughout the N domain, and one control at residue 239, presumed to be outside the amyloid-forming region. For each mutant, Ile-1-13C–labeled protein was produced in Escherichia coli for NMR studies. Electron micrographs of amyloid preparations showed filaments similar to those previously described for Sup35NM (28) (Fig. 1C). To ensure that the amyloid whose structure we are studying is representative of prion amyloid in vivo, we took several steps. First, the mutant SUP35NM sequences were fused with the normal SUP35C and used to replace the normal protein in vivo. Using cytoduction (cytoplasmic mixing), [PSI+] was transferred into these cells from a strain with the wild-type Sup35p, and the transmission efficiency was measured (Table 1). We found a high transmission frequency for most of the mutants, with the cytoductants showing intensity of phenotype and stability of propagation similar to that seen with the wild-type recipient. Q56I, which resulted in a barrier to transmission, was presumed to block formation of the structure and was not studied further. As described previously, Sup35 natural variants Δ19 and E9 largely blocked transmission of [PSI+] (38). The second approach to ensuring normal prion amyloid structures was to seed filament formation by mutant protein with 1% of sonicated amyloid filaments composed of the wild-type Sup35NM sequence. The third method was to transfect filaments formed from wild-type and mutant Sup35NM (seeded with wild-type Sup35NM filaments) into yeast expressing the wild-type Sup35p and assess the efficiency of transfection and the array of prion variants formed (Table 2). Filaments of wild-type Sup35NM produced mostly strong, stable [PSI+] transfectants curable by growth on guanidine. Filaments of mutant proteins produced [PSI+] transfectants at efficiencies within twofold that of the wild-type sequence, and most were strong variants, but many were unstable (Table 2). Although all mutants could form [PSI+] prions in vivo, the results suggest that the mutations often selected certain variants or even altered the amyloid conformation, as could be expected from previous results (49–51).
Table 1.
Cytoduction test of mutant protein compatibility
| Cytoductants | ||||
| Donor | Recipient | Sup35 allele | [PSI+], % | Total |
| 779-6A [PSI+] ρ+ SUP35reference | 4830 ρo | Reference | 95 | 57 |
| Δ19 | 1 | 114 | ||
| E9 | 5 | 80 | ||
| 3I→3L, Y13I | 80 | 15 | ||
| 3I→3L, Q22I | 100 | 73 | ||
| 3I→3L, Y29I | 74 | 50 | ||
| 3I→3L, Y35I | 84 | 135 | ||
| 3I→3L, Y46I | 91 | 105 | ||
| 3I→3L, N48I | 99 | 197 | ||
| 3I→3L, G51I | 80 | 60 | ||
| 3I→3L, Y55I | 84 | 68 | ||
| 3I→3L, Q56I | 15 | 39 | ||
| 3I→3L, G58I | 92 | 26 | ||
| 3I→3L, Q62I | 98 | 62 | ||
| 3I→3L, Q71I | 83 | 123 | ||
| 3I→3L, Q73I | 100 | 35 | ||
| 3I→3L, Y82I | 95 | 87 | ||
| 3I→3L, Q90I | 82 | 152 | ||
| 3I→3L,Y101I | 89 | 18 | ||
| 3I→3L, N109I | 96 | 96 | ||
| 3I→3L, L126I | 93 | 42 | ||
| 3I→3L, H225I | 94 | 33 | ||
| 3I→3L, 239I | 97 | 88 | ||
Cytoplasmic mixing (cytoduction) was used to introduce [PSI+] from the donor strain (779-6A) expressing the reference Sup35p into the recipient strain 4830 carrying Sup35p with the indicated sequence. Cytoductants were scored for transmission of the [PSI+] prion.
Table 2.
Transfection of yeast with mutant Sup35NM amyloid filaments
| Amyloid | Weak stable [PSI+] | Strong unstable [PSI+] | Strong stable [PSI+] | [PSI+]/μg amyloid |
| Wild type | 0 | 2 | 89 | 14 |
| H2O | 0 | 0 | 0 | 0 |
| Y35I | 0 | 1 | 93 | 34 |
| N48I | 0 | 69 | 20 | 12 |
| Q71I | 0 | 23 | 60 | 36 |
| Wild type | 0 | 0 | 50 | 13 |
| Y46I | 8 | 44 | 27 | 26 |
| Wild type | 4 | |||
| Q62I | 0 | 59 | 31 | 2 |
| Y101I | 0 | 39 | 54 | 4 |
| Wild type | 5 | |||
| Y13I | 0 | 74 | 20 | 5 |
| N48I | 2 | 68 | 23 | 6 |
| Q90I | 0 | 84 | 6 | 5 |
| N109I | 2 | 34 | 58 | 17 |
| H2O | 0 | 0 | 0 | 0 |
Ade+ clones were checked for guanidine-curability, for strength of the prion (Ade+) phenotype on 1/2 YPD and for prion stability in subclones.
One-Dimensional Solid-State NMR.
Detecting the single 13C label per molecule (Ile-1-13C) in a 253-residue protein is challenging; with 356 other carbonyl groups, 1.1% of which have the natural abundance 13C, there is a total of 3.92 natural abundance 13C residues. In principle, the specific label may constitute only ∼20% of the total carbonyl-13C. We used the aliphatic 13C signal as a measure of natural abundance in each amyloid sample, and with the ratio of aliphatic to carbonyl 13C signal from an unlabeled amyloid sample determined the amount of natural abundance carbonyl 13C to subtract to give the signal derived from the Ile-1-13C label. This correction was applied to adjust the dipolar recoupling experiments for natural abundance signal (Methods). This method was also applied directly to 1D spectra, subtracting the Fourier-transformed spectrum of unlabeled Sup35NM fibrils from spectra of labeled fibrils to cancel the aliphatic region, with the remaining carbonyl peak(s) attributable to the Ile-1-13C label (Fig. 2). In all samples the carbonyl peak was narrowed after subtracting the natural abundance signal and showed a chemical shift indicative of β-sheet structure (Table 3). Rehydration of several samples showed significant narrowing of the carbonyl peaks and again chemical shifts typical of β-sheet structure (Table 3 and Fig. S1).
Fig. 2.
Solid-state NMR spectra of Sup35NM amyloid samples. Spectra of Sup35NM labeled with Ile-1-13C at single sites (or unlabeled to measure the natural abundance signal) were recorded at 9.39 T at 9 kHz MAS. Each protein has the I152L, I220L, and I239L mutations (except the I239 sample, which has only the first two changes) as well as a single other residue changed to Ile. All (except the “unlabeled” sample, which is Sup35NM Y29I) were fully labeled at the indicated single site with Ile-1-13C. The Fourier-transformed signal from the unlabeled samples was subtracted from the indicated labeled sample signals to eliminate the aliphatic signal and, therefore, the natural abundance part of the carbonyl signal. The carbonyl chemical shift determined from these corrected spectra was consistently within 0.2 ppm of that determined from the raw spectra. The peak between 100 and 150 ppm is background signal from plastic in the probe.
Table 3.
Analysis of 13C solid-state NMR spectra
| Chemical shift, ppm | FWHM, ppm | ||
| Sup35NM sample | Raw | Net | Net |
| Y13I | 172.99 | 172.83 | 3.4 |
| Q22I | 172.96 | 172.98 | 2.2 |
| Y29I | 172.28 | 172.19 | 3.8 |
| Y35I | 172.52 | 172.16 | 3.7 |
| Y46I | 173.12 | 173.19 | 3.5 |
| Y46I wet | 172.7 | 2.2 | |
| N48I | 172.60 | 172.25 | 4.1 |
| N48I wet | 172.2 | 2.6 | |
| G51I | 172.97 | 172.98 | 4.6 |
| G51I wet | 173.0 | 3.4 | |
| G58I | 172.75 | 172.93 | 4.3 |
| Q62I | 172.35 | 172.10 | 4.6 |
| Q71I | 172.96 | 172.94 | 4.0 |
| Y73I | 173.38 | ||
| Y82I | 172.75 | 172.75 | 3.7 |
| Y82I wet | 173.0 | 2.8 | |
| Q90I | 173.28 | 173.45 | 3.0 |
| Y101I | 173.15 | 173.19 | 3.5 |
| N109I | 172.92 | 172.95 | 5.8 |
| I239 | 172.16 | 172.46 | 4.1 |
| Unlabeled | 172.98 | 6.7 | |
Each sample was labeled with Ile-1-13C (except for the ‟unlabeled” sample) and a 1D solid-state NMR spectrum was obtained on the dry sample and, in several cases, after rehydration (wet). Chemical shifts determined from the uncorrected 1D spectra are in the ‟raw” column, whereas those after subtraction of the natural abundance signal are shown as ‟net.” Only net peak widths are shown, except for the unlabeled sample. The Ile carbonyl 13C chemical shift for random coil conformation is 174.7, and a significantly lower carbonyl chemical shift is characteristic of β-sheet structure (64).
Dipolar Recoupling Experiments.
The distance of a single labeled atom, the carbonyl 13C of the lone isoleucine in each molecule, to the nearest neighbor 13C is measured using the PITHIRDS-CT method of dipolar recoupling (52). The rate of signal decay in this experiment is proportional to 1/r3, where r is the distance being measured. Because of this relation, the measurement is only useful within the range of 0.2–0.8 nm, but this is exactly the range to test for the expected ∼0.5-nm spacing between identical atoms in different molecules of an in-register parallel β-sheet.
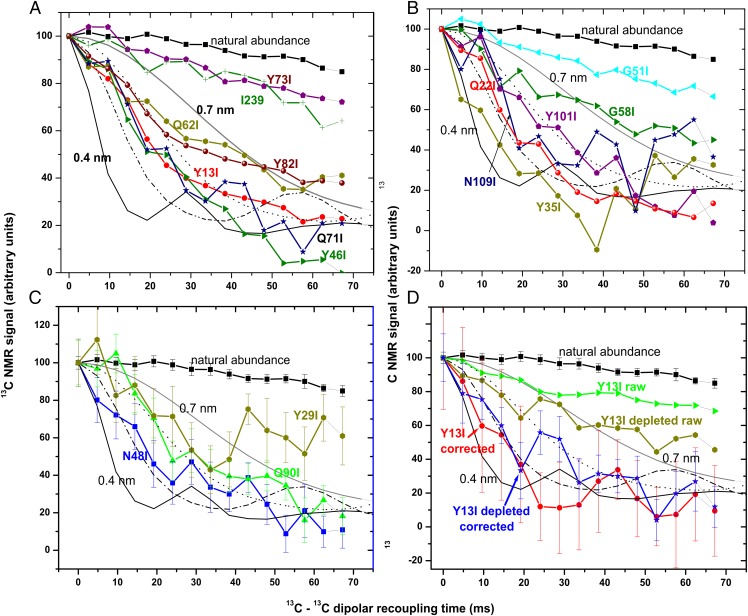
One control was the natural abundance 13C in amyloid of unlabeled Sup35NM. Because only 1.1% of 13C atoms are labeled, the nearest neighbor distance should be >>1.0 nm. Indeed, there is only a very gradual decay of the signal in the PITHIRDS-CT experiment for this sample. As another control, labeling residue I239, which is naturally isoleucine, and is beyond the prion domain by all measures, shows a decay indicative of >0.8 nm. We carried out PITHIRDS-CT experiments with 15 other samples, each with a single site labeled with Ile-1-13C (Fig. 3 A–C). Many residues showed the rapid decay indicative of in-register parallel β-sheet structure. Y13I, Q22I, Y35I, Y46I, N48I, Q71I, and N109I are clearly in this group. Q62I, Y82I, Y101I, and Q90I show slightly slower signal decay, suggestive of in-register parallel structure and far too rapid for a β-helix. The slightly slower decay for these four samples may be due to errors in the large correction for natural abundance 13C, the presence of some protein not in amyloid form, or a mixture of prion variants. The Y29I sample shows rapid initial decay followed by a high plateau, consistent with this residue being parallel in-register in some fibrils, but not others. I239, G51I, G58I, and Y73I are clearly not parallel in-register. The error bars in these experiments are necessarily quite wide because, as described above and in Methods, one is subtracting a large natural abundance signal and setting the difference to 100 at the 0 recoupling time.
Fig. 3.
Measurements of 13C-13C dipole–dipole couplings of Sup35NM samples singly labeled with Ile-1-13C. The PITHIRDS-CT method (52) was used with 20-kHz MAS spinning in a 9.4 T magnet and sample cooling to maintain room temperature. Simulated curves for PITHIRDS-CT results with linear chains of 13C atoms separated by 0.4 (—), 0.5 (– –⋅), 0.6 (⋅⋅⋅), or 0.7 (gray—) nm are shown. (A) Amyloid filaments of Sup35NM Y13I, Y46I, Q62I, Q71I, Y73I, Y82I, and I239 (each having the I152L, I220L, and I239L, except for the I239 sample) singly labeled with Ile-1-13C are compared with unlabeled filaments. Error bars are not shown in A or B for clarity but are generally 5–12%. (B) Amyloid filaments of Sup35NM Y35I, Q22I, N109I, Y101I, G58I, and G51I were analyzed. (C) Results for N48I, Q90I, and Y29I are shown. (D) Sup35NM Y13I was singly labeled with Ile-1-13C either with glucose containing the 1.1% natural abundance 13C (Y13I raw and Y13I corrected) or with ∼99.93% 13C-depleted glucose (Y13Id raw and Y13I depleted corrected). Curves labeled “raw” are not corrected for natural abundance 13C, whereas the natural abundance 13C signal has been subtracted from those labeled “corrected.”
To confirm the validity of our correction for natural abundance 13C, we prepared one mutant, Y13I, with Ile-1-13C, using either the usual dextrose containing 1.1% natural abundance isotope, or 13C-depleted dextrose, so that the natural abundance 13C would be a smaller proportion of the total. The 1D spectrum shows the expected lower proportion of natural abundance 13C in the aliphatic region (Fig. 2). From these data, we calculate that the fibers made with 13C-depleted dextrose had only 0.24% 13C, or ∼0.85 residues per Sup35NM molecule. The corresponding raw PITHIRDS-CT data show a more robust decay, and when corrected for this natural abundance 13C, a result similar to that of the Y13I sample made with the usual dextrose, but with a smaller variance (Fig. 3D).
Discussion
Our previous claim that Sup35NM amyloid has the in-register parallel β-sheet architecture relied on our demonstration that the distance from a 13C carbonyl label on a Tyr residue to another 13C carbonyl Tyr in a different molecule was about 0.5 nm. However, because each molecule had 20 labeled Tyrs, that data did not prove it was the same Tyr in the neighboring molecule that was nearby. Y35 in one molecule might have been near Y13 in another molecule. Here, using singly labeled molecules, we show that this cannot be the case at least for five Tyr residues and five other residues scattered through the N domain. These data prove the in-register parallel model for the N domain.
If the N domain, which is largely in β-sheet form, were a single flat sheet, it would appear as fibers more than 0.35 nm/residue × ∼100 residues = 35 nm wide, but the diameter of filaments of Sup35NM amyloid is only 11.5 ± 1.5 nm (28) or 9 ± 1 nm (41) wide. We infer that the β-sheet must be multiply folded. We propose that residues not showing the ∼0.5-nm spacing are in the turns or loops present at each fold (Fig. 4), because residues in turns or loops are not as tightly constrained by main chain H-bonds to the neighboring molecules to be close to the corresponding residues. Transfection experiments (Table 2) and solid-state NMR lineshapes (Fig. S1) indicate that our mutant Sup35NM fiber samples are not structurally homogeneous, so we cannot determine a unique secondary structure for Sup35NM in these fibers. It is possible that residues showing decay rates slightly above the 0.5-nm level are parallel in-register in some fibers, but in a turn or loop in others. Thus, our data confirm the presence of turns of the β-strands (i.e., folds in the β-sheet).
Fig. 4.
A possible model of Sup35NM amyloid. Residues in blue are found to have in-register parallel structure and those in red are not. The Ile residues in green were changed to Leu in all mutants as was Ile239, except when labeling Ile239 itself. The data suggest that each molecule in the in-register β-sheet contributes several β-strand segments and several turns or loops. The underlined regions may be β-strands and possible loop/turn regions are shown.
Paradoxically, alteration of Q56, which seems to be in a loop or turn based on the results with Q51 and Q58, shows a barrier to transmission of [PSI+], but similar alteration of other residues located in in-register regions do not produce such a barrier. Resolution of this paradox will require more detailed examination of the structure in this region, and it remains possible that this residue is not actually in a loop.
Krishnan and Lindquist proposed that Sup35NM amyloid was a β-helix including residues 30–90, based on chemical modification of cysteine-substituted mutants with pyrene maleimide, a large fluorescent probe (43), and single fiber stretching experiments (44). Our data are incompatible with the claim that residues 30–90 are in a β-helix form. Six of the 10 residues examined in this region show the ∼0.5-nm spacing incompatible with this architecture. We suggest that the other four are in loops or turns. In addition, the mass per length of a β-helical fiber is less than 0.5 molecules per 0.47 nm, but that of amyloid fibers of Sup35NM or full-length Sup35p is consistently ∼1.0 (7, 53), ruling out the β-helix model.
Hydrogen–deuterium exchange experiments showed protection from exchange varying with amyloid formation conditions and with location in the sequence, with most N residues examined more protected than most M residues (39). Luckgei et al. and Schutz et al. have been able to make NMR assignments for residues 4–27 of amyloid of Sup35NM (54) and residues 2–30 of full-length Sup35p (55) and find that most residues in this domain are in β-sheet. Another study examined the chemical shifts and dynamic features of different amino acid types, finding that the N domain is more rigid than the M domain (47). None of these studies deals with the issue of which type of β-sheet forms the amyloid core of Sup35NM.
The folded in-register parallel β-sheet architecture naturally suggests an explanation for the heritability of protein conformation and the existence of multiple heritable prion variants (1, 56). The favorable interactions between identical side chains—hydrogen bonds between Q, N, S, or T side chains or hydrophobic interactions between F, Y, W, I, L, or V side chains—can occur only if the peptides are in-register. Therefore, these interactions keep the structure in-register. Charge repulsion between identical K, R, E, or D residues would impair this structure, but such residues are few in the Ure2p, Sup35p, and Rnq1p prion domains shown to have this architecture. The same interactions force the prion domain of a molecule newly joining the end of the filament to have the same conformation as the last molecule on the end of the filament, and thus have its folds/loops in the same locations as the other molecules in that filament. We suggest that different prion variants have folds in different locations, but once formed the conformation is rather faithfully propagated by this mechanism (1, 56).
The yeast prions [URE3], [PSI+], and [PIN+] each have multiple prion variants in vivo and form heterogeneous/polymorphic amyloid in vitro. Seeding with filaments isolated from cells can reduce the variability of filaments to some extent (30–32, 47), and specific filament formation conditions may favor one group of variants over another (39). However, this approach may be limited by the known existence of a “cloud” of prion variants in a single strain (48). Further progress in obtaining more detailed structural information on yeast prion amyloid filaments will depend on development of new methods to obtain homogeneous preparations.
Methods
Strains and Media.
Media are as described (57). Strain 779–6A [MATα kar1-1 ade2-1 SUQ5 his3 leu2 trp1 ura3 [PSI+] (58)] was from Dan Masison, National Institutes of Health, and strain 4830 [MATa kar1-1 ade2-1 SUQ5 leu2 trp1 ura3 lys2 sup35::kanMX p1215 (CEN URA3 PSUP35SUP35C)] has been described (38).
Plasmid Constructs.
Plasmid p1399 is pET21a(+) (EMD Millipore) into which SUP35NM with a His6 tag was inserted with codons optimized for E. coli (Gene Art, Life Technologies). Using the QuikChange Lightning Multi-Site-Directed Mutagenesis Kit (210515; Agilent Technologies), we simultaneously made I152L, I220L, and I239L, converting all of the Ile residues in Sup35NM to Leu making p1426. Single residues were then changed to Ile in individual plasmid constructs using the QuikChange Lightning Site-Directed Mutagenesis Kit (210519; Agilent Technologies). These plasmids were used for protein production in E. coli. For testing the biological properties of mutant proteins in yeast, we used the InFusion kit (639649; Clontech) to transfer the SUP35NM mutants, without the His6 tag, into p1422 (LEU2 CEN PSUP35 BamHI NdeI AUG SUP35C), producing an in-frame fusion with SUP35C under control of the SUP35 promoter on a single-copy plasmid.
Cytoduction.
Transfer of cytoplasm from cell to cell without altering nuclei or plasmids uses the kar1-1 mutation, which interferes with nuclear fusion (59). The [PSI+] ρ+ donor strain 779–6A (carrying a strong stable [PSI+]) was mixed in twofold excess over the [psi-] ρο strain 4830 with a derivative of p1422 as the only source of Sup35p. After 7 h of mating on a rich plate, mating mixtures were streaked for single colonies on plates selecting against the donor strain. Colonies that were respiration-competent and were not diploids were tested for growth on –Ade plates to determine if [PSI+] had been successfully transmitted. Recipients carrying either the reference (S288C) Sup35, the deletion of residues 59–77 in the oligopeptide repeat region of Sup35N (Δ19), or the “E9” allele (N109S with several M domain differences) (38) were used as positive and negative controls.
Expression, Labeling, and Purification of Sup35NM.
Proteins were expressed in E. coli strain BL21-CodonPlus (DE3) RIPL (Agilent Technologies) growing in synthetic complete medium containing 100 μg/mL of ampicillin (41). At OD550 at 0.5, cells were collected and resuspended in the same medium but with isoleucine replaced with 100 mg/L isoleucine-1-13C (Cambridge Isotope Laboratories), incubated with shaking at 37 °C for 15 min, then made 1 mM in isopropyl β-d-1-thiogalactopyranoside. After 4–6 h further growth, cells were harvested, lysed by addition of 8 M guanidine containing 100 mM Tris⋅Cl (pH 8.0) and 150 mM NaCl with one “complete, EDTA-free” protease inhibitor tablet (Roche). After overnight incubation at 4 °C, the extract was spun at 30,000 rpm in a 45Ti Beckman rotor for 1 h. The supernatant was applied to a 4-mL (packed volume) NiNTA column (Qiagen), washed with 200 mL of 8 M urea 0.1 M Tris⋅Cl (pH 8.0) and 150 mM NaCl, then with 10 mL of the same buffer with 10 mM imidazole and eluted with the same buffer containing 200 mM imidazole. Protein-containing fractions were immediately applied to a PD-10 desalting column equilibrated with 5 mM K PO4 (pH 7.2) and 150 mM NaCl. The eluate was immediately seeded with 1% wt/wt of wild-type Sup35NM filaments and incubated at room temperature without agitation to allow filament formation. Filaments were washed twice with water, dried by lyophilization, and packed in 3.2-mm-thick-walled zirconium oxide rotors for solid-state NMR. Filaments stained with uranyl acetate were examined using an FEI Morgagni transmission electron microscope operating at 80 kV.
Solid-State NMR.
Solid-state NMR experiments were carried out at 100.4 MHz 13C frequency (9.4 T) on a Varian InfinityPlus spectrometer and 100.8 MHz 13C frequency on a Bruker Avance spectrometer with lyophilized amyloid samples packed in thick-walled 3.2-mm zirconium oxide rotors using a magic angle spinning (MAS) NMR probe (Varian). Sample temperatures were maintained at ∼24 °C during PITHIRDS-CT measurements by cooling with cold nitrogen gas. One-dimensional 13C NMR spectra used 9-kHz MAS with 1.5-ms 1H-13C cross-polarization (60) and 100-MHz two-pulse phase-modulated 1H decoupling (61). Dipolar recoupling experiments were carried out at an MAS frequency of 20 kHz using the PITHIRDS-CT method (52) and spin-lock detection (62). T2 values were measured for each sample by varying the dipolar recoupling period but with no recoupling, and in all cases >20% of the initial spin-locked 13C NMR signal remained after 76.8 ms, implying T2 relaxation times greater than 48 ms for carbonyl sites under PITHIRDS-CT conditions.
One-dimensional NMR spectra were recorded of amyloid of each mutant Sup35NM labeled with Ile-1-13C and one that had only natural abundance 13C. The unlabeled sample and several labeled samples were rehydrated by addition of 4 μL of water, and 1D spectra were again obtained. Signal in the aliphatic region could come only from natural abundance 13C, whereas that in the carbonyl region was a combination of natural abundance and the single residue of fully labeled Ile-1-13C. The ratio of aliphatic to carbonyl signal in the unlabeled sample compared with the same ratio for the Ile-1-13C labeled samples allowed us to measure the fraction of the carbonyl signal due to the Ile-1-13C label. The slow rate of decay of magnetization of natural abundance carbonyl 13C in the PITHIRDS-CT experiment was measured using amyloid of Sup35NM Y29I lacking any added label. This was used to correct the PITHIRDS-CT results for the labeled samples as follows.
P3lab(t) = PITHIRDS-CT signal from a labeled sample.
P3NA(t) = PITHIRDS-CT signal from natural abundance sample (unlabeled).
Starting with integrals of the carbonyl (CO, 182–167 ppm) and aliphatic (AL, 73–4 ppm) regions of the 1D spectra of labeled (lab) and unlabeled (NA for natural abundance) samples, potentially from different numbers of scans and slightly different sample sizes, we first rescale the labeled sample values to the NA scale by assuming the aliphatic signal in both cases arises solely from NA 13C.
Let 1DCONA = raw integral of carbonyl region of 1D spectrum of the unlabeled sample.
1DALNA = raw integral of aliphatic region of 1D spectrum of the unlabeled sample.
1DCOlab = raw integral of carbonyl region of 1D spectrum of the labeled sample.
1DALlab = raw integral of aliphatic region of 1D spectrum of the labeled sample.
Multiply both 1DCOlab and 1DALlab by [1DALNA/1DALlab] to rescale.
From the rescaled CO signal of the labeled sample, subtract 1DCONA to get the part that is due to the label = [1DALNA/1DALlab]*1DCOlab – 1DCONA = 1DCOlab2.
The fraction of 1DCOlab that is from the label is flabCO = 1DCOlab2/1DCOlab.
The fraction of 1DCOlab that is from NA 13C is 1− flabCO = flabNA.
The part of the PITHIRDS-CT signal of the labeled amyloid that was due to the label (and not the natural abundance), scaled to 100 at t = 0 is
The noise estimates for carbonyl peak integrals were made by integrating similar frequency intervals above and below the carbonyl frequency and calculating the SD (σ). The SD of differences between labeled (lab) and unlabeled (NA) samples was calculated by , where flabCO is the fraction of the raw carbonyl signal due to the label (see above).
Transfection of Amyloid.
Recipient cells (779-6A [psi-]) were grown overnight at 30 °C in yeast extract-peptone-dextrose-adenine medium (YPAD). One milliliter of culture was used to inoculate 50 mL YPAD and cells were grown for two to three doublings (5 h) at 30 °C. Cells were washed once with 20 mL water, twice with 25 mL ST buffer [1 M sorbitol and 10 mM Tris⋅HCl (pH 7.5)] and resuspended in 5 mL ST buffer. Cells were protoplasted for 40 min at 30 °C by addition of 20 µL lyticase (5 U/µL Sigma L5263 in 50% glycerol). Protoplasts were collected by centrifugation at 216 × g (1,000 rpm in a Sorvall Legend T centrifuge equipped with a Sorvall Heraeus 75006434 rotor) for 3 min, washed two times with 10 mL STC buffer [1 M sorbitol, 10 mM Tris⋅HCl (pH 7.5), and 10 mM CaCL2)] and resuspended in 1 mL STC buffer. To reduce shearing protoplasts were resuspended by gentle rocking of the tube (50-mL polypropylene tubes; Corning).
Amyloid fibril suspensions were sonicated on ice three times for 45 s (duty cycle 40%, output 4) using a Branson 250 sonifier equipped with a microtip. When previously lyophilized amyloid fibrils were used, 45-s sonications were done up to 10 times and the duty cycle was raised to 90%. Between sonications samples were kept on ice. Amyloid filaments were used at a concentration of ∼6 µg/µL. To 100 µL of protoplasts was added 1 µL of single-strand DNA (calf sperm DNA, 10 µg/µL), 4 µL of pRS316 plasmid (URA3 CEN, 0.9 µg/µL) (63),and 7 µL of amyloid filament suspension. The protoplast/DNA/protein mixture was incubated for 10 min at room temperature. Next, 900 µL PTC buffer was added and the mixture was incubated for 20 min at room temperature. Protoplasts were then collected by centrifugation for 3 min in a microcentrifuge at 250 × g (1,600 rpm, Eppendorf 5415R with F-45-24-11 rotor). To the pellet was added 200 µL SOS buffer and the protoplasts were left to recover for 30 min at 30 °C. Recovered protoplasts were pipetted into 10 mL of CS+A.1-U or CS+A5-U medium kept at 50 °C. To reduce shearing the ends of pipette tips were cut off. Fourteen-milliliter round-bottom polypropylene tubes (BD Falcon) were used. The solution was mixed by inverting the tubes and poured directly into Petri dishes containing 20 mL of the same solidified medium. Plates were incubated for 6 d at 30 °C.
PTC buffer: 20% (wt/vol) PEG8000, 10 mM Tris⋅HCl (pH 7.5), and 10 mM CaCL2
SOS buffer: 1 M sorbitol, 7 mM CaCL2, 1/3 YPD (per 1 L: 3.3 g yeast extract, 6.7 g peptone, and 6.7 g glucose)
CS+A.1-U: 1 M sorbitol, 0.67% yeast nitrogen base, 2% glucose, 1× complete amino acid mix, 0.1 mg/L adenine sulfate, and 20 g/L agar
CS+A5-U: 1 M sorbitol, 0.67% yeast nitrogen base, 2% glucose, 1× complete amino acid mix, 5 mg/L adenine sulfate, and 20 g/L agar
10× complete amino acid mix (per 1 L): 200 mg Met, 500 mg Tyr, 500 mg Ile, 500 mg Leu, 500 mg Phe, 1,000 mg Glu acid, 2,000 mg Thr, 1,000 mg Asp, 1,500 mg Val, 4,000 mg Ser, 200 mg Arg, 200 mg His, 300 mg Lys, and 300 mg Trp
The 10× complete amino acid mix was filter-sterilized before adding to autoclaved CS media containing the other components.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417974111/-/DCSupplemental.
References
- 1.Wickner RB, et al. Amyloids and yeast prion biology. Biochemistry. 2013;52(9):1514–1527. doi: 10.1021/bi301686a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benzinger TL, et al. Propagating structure of Alzheimer’s beta-amyloid(10-35) is parallel beta-sheet with residues in exact register. Proc Natl Acad Sci USA. 1998;95(23):13407–13412. doi: 10.1073/pnas.95.23.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paravastu AK, Leapman RD, Yau WM, Tycko R. Molecular structural basis for polymorphism in Alzheimer’s β-amyloid fibrils. Proc Natl Acad Sci USA. 2008;105(47):18349–18354. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petkova AT, Yau WM, Tycko R. Experimental constraints on quaternary structure in Alzheimer’s beta-amyloid fibrils. Biochemistry. 2006;45(2):498–512. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tycko R. Solid-state NMR studies of amyloid fibril structure. Annu Rev Phys Chem. 2011;62:279–299. doi: 10.1146/annurev-physchem-032210-103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petkova AT, et al. Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science. 2005;307(5707):262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, Thurber KR, Shewmaker F, Wickner RB, Tycko R. Measurement of amyloid fibril mass-per-length by tilted-beam transmission electron microscopy. Proc Natl Acad Sci USA. 2009;106(34):14339–14344. doi: 10.1073/pnas.0907821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meinhardt J, Sachse C, Hortschansky P, Grigorieff N, Fändrich M. Abeta(1-40) fibril polymorphism implies diverse interaction patterns in amyloid fibrils. J Mol Biol. 2009;386(3):869–877. doi: 10.1016/j.jmb.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertini I, Gonnelli L, Luchinat C, Mao J, Nesi A. A new structural model of Aβ40 fibrils. J Am Chem Soc. 2011;133(40):16013–16022. doi: 10.1021/ja2035859. [DOI] [PubMed] [Google Scholar]
- 10.Lopez del Amo JM, et al. An asymmetric dimer as the basic subunit in Alzheimer’s disease amyloid β fibrils. Angew Chem Int Ed Engl. 2012;51(25):6136–6139. doi: 10.1002/anie.201200965. [DOI] [PubMed] [Google Scholar]
- 11.Lu JX, et al. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell. 2013;154(6):1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watts JC, et al. Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2014;111(28):10323–10328. doi: 10.1073/pnas.1408900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luca S, Yau W-M, Leapman R, Tycko R. Peptide conformation and supramolecular organization in amylin fibrils: Constraints from solid-state NMR. Biochemistry. 2007;46(47):13505–13522. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margittai M, Langen R. Fibrils with parallel in-register structure constitute a major class of amyloid fibrils: Molecular insights from electron paramagnetic resonance spectroscopy. Q Rev Biophys. 2008;41(3-4):265–297. doi: 10.1017/S0033583508004733. [DOI] [PubMed] [Google Scholar]
- 15.Debelouchina GT, et al. Higher order amyloid fibril structure by MAS NMR and DNP spectroscopy. J Am Chem Soc. 2013;135(51):19237–19247. doi: 10.1021/ja409050a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serag AA, Altenbach C, Gingery M, Hubbell WL, Yeates TO. Arrangement of subunits and ordering of beta-strands in an amyloid sheet. Nat Struct Biol. 2002;9(10):734–739. doi: 10.1038/nsb838. [DOI] [PubMed] [Google Scholar]
- 17.Bateman DA, Tycko R, Wickner RB. Experimentally derived structural constraints for amyloid fibrils of wild-type transthyretin. Biophys J. 2011;101(10):2485–2492. doi: 10.1016/j.bpj.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coustou V, Deleu C, Saupe S, Begueret J. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc Natl Acad Sci USA. 1997;94(18):9773–9778. doi: 10.1073/pnas.94.18.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saupe SJ. The [Het-s] prion of Podospora anserina and its role in heterokaryon incompatibility. Semin Cell Dev Biol. 2011;22(5):460–468. doi: 10.1016/j.semcdb.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Ritter C, et al. Correlation of structural elements and infectivity of the HET-s prion. Nature. 2005;435(7043):844–848. doi: 10.1038/nature03793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasmer C, et al. Amyloid fibrils of the HET-s(218-289) prion form a beta solenoid with a triangular hydrophobic core. Science. 2008;319(5869):1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 22.Wickner RB. [URE3] as an altered URE2 protein: Evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264(5158):566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 23.Derkatch IL, Bradley ME, Hong JY, Liebman SW. Prions affect the appearance of other prions: The story of [PIN(+)] Cell. 2001;106(2):171–182. doi: 10.1016/s0092-8674(01)00427-5. [DOI] [PubMed] [Google Scholar]
- 24.Ter-Avanesyan MD, Dagkesamanskaya AR, Kushnirov VV, Smirnov VN. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [psi+] in the yeast Saccharomyces cerevisiae. Genetics. 1994;137(3):671–676. doi: 10.1093/genetics/137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masison DC, Wickner RB. Prion-inducing domain of yeast Ure2p and protease resistance of Ure2p in prion-containing cells. Science. 1995;270(5233):93–95. doi: 10.1126/science.270.5233.93. [DOI] [PubMed] [Google Scholar]
- 26.Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD. In vitro propagation of the prion-like state of yeast Sup35 protein. Science. 1997;277(5324):381–383. doi: 10.1126/science.277.5324.381. [DOI] [PubMed] [Google Scholar]
- 27.King C-Y, et al. Prion-inducing domain 2-114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proc Natl Acad Sci USA. 1997;94(13):6618–6622. doi: 10.1073/pnas.94.13.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glover JR, et al. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell. 1997;89(5):811–819. doi: 10.1016/s0092-8674(00)80264-0. [DOI] [PubMed] [Google Scholar]
- 29.Edskes HK, Gray VT, Wickner RB. The [URE3] prion is an aggregated form of Ure2p that can be cured by overexpression of Ure2p fragments. Proc Natl Acad Sci USA. 1999;96(4):1498–1503. doi: 10.1073/pnas.96.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428(6980):323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- 31.King CY, Diaz-Avalos R. Protein-only transmission of three yeast prion strains. Nature. 2004;428(6980):319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- 32.Brachmann A, Baxa U, Wickner RB. Prion generation in vitro: Amyloid of Ure2p is infectious. EMBO J. 2005;24(17):3082–3092. doi: 10.1038/sj.emboj.7600772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel BK, Liebman SW. “Prion-proof” for [PIN+]: Infection with in vitro-made amyloid aggregates of Rnq1p-(132-405) induces [PIN+] J Mol Biol. 2007;365(3):773–782. doi: 10.1016/j.jmb.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baxa U, et al. Characterization of β-sheet structure in Ure2p1-89 yeast prion fibrils by solid-state nuclear magnetic resonance. Biochemistry. 2007;46(45):13149–13162. doi: 10.1021/bi700826b. [DOI] [PubMed] [Google Scholar]
- 35.Kryndushkin DS, Wickner RB, Tycko R. The core of Ure2p prion fibrils is formed by the N-terminal segment in a parallel cross-β structure: Evidence from solid-state NMR. J Mol Biol. 2011;409(2):263–277. doi: 10.1016/j.jmb.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngo S, Gu L, Guo Z. Hierarchical organization in the amyloid core of yeast prion protein Ure2. J Biol Chem. 2011;286(34):29691–29699. doi: 10.1074/jbc.M111.269092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradley ME, Liebman SW. The Sup35 domains required for maintenance of weak, strong or undifferentiated yeast [PSI+] prions. Mol Microbiol. 2004;51(6):1649–1659. doi: 10.1111/j.1365-2958.2003.03955.x. [DOI] [PubMed] [Google Scholar]
- 38.Bateman DA, Wickner RB. [PSI+] Prion transmission barriers protect Saccharomyces cerevisiae from infection: Intraspecies ‘species barriers’. Genetics. 2012;190(2):569–579. doi: 10.1534/genetics.111.136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyama BH, Kelly MJ, Gross JD, Weissman JS. The structural basis of yeast prion strain variants. Nature. 2007;449(7159):233–237. doi: 10.1038/nature06108. [DOI] [PubMed] [Google Scholar]
- 40.Shewmaker F, Kryndushkin D, Chen B, Tycko R, Wickner RB. Two prion variants of Sup35p have in-register parallel β-sheet structures, independent of hydration. Biochemistry. 2009;48(23):5074–5082. doi: 10.1021/bi900345q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shewmaker F, Wickner RB, Tycko R. Amyloid of the prion domain of Sup35p has an in-register parallel β-sheet structure. Proc Natl Acad Sci USA. 2006;103(52):19754–19759. doi: 10.1073/pnas.0609638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kishimoto A, et al. beta-Helix is a likely core structure of yeast prion Sup35 amyloid fibers. Biochem Biophys Res Commun. 2004;315(3):739–745. doi: 10.1016/j.bbrc.2004.01.117. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan R, Lindquist SL. Structural insights into a yeast prion illuminate nucleation and strain diversity. Nature. 2005;435(7043):765–772. doi: 10.1038/nature03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong J, Castro CE, Boyce MC, Lang MJ, Lindquist S. Optical trapping with high forces reveals unexpected behaviors of prion fibrils. Nat Struct Mol Biol. 2010;17(12):1422–1430. doi: 10.1038/nsmb.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeSantis ME, Shorter J. Hsp104 drives “protein-only” positive selection of Sup35 prion strains encoding strong [PSI(+)] Chem Biol. 2012;19(11):1400–1410. doi: 10.1016/j.chembiol.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toombs JA, McCarty BR, Ross ED. Compositional determinants of prion formation in yeast. Mol Cell Biol. 2010;30(1):319–332. doi: 10.1128/MCB.01140-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frederick KK, et al. Distinct prion strains are defined by amyloid core structure and chaperone binding site dynamics. Chem Biol. 2014;21(2):295–305. doi: 10.1016/j.chembiol.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bateman D, Wickner RB. The [PSI+] prion exists as a dynamic cloud of variants. PLoS Genet. 2013;9(1):e1003257. doi: 10.1371/journal.pgen.1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King CY. Supporting the structural basis of prion strains: Induction and identification of [PSI] variants. J Mol Biol. 2001;307(5):1247–1260. doi: 10.1006/jmbi.2001.4542. [DOI] [PubMed] [Google Scholar]
- 50.Chang H-Y, Lin J-Y, Lee H-C, Wang H-L, King C-Y. Strain-specific sequences required for yeast [PSI+] prion propagation. Proc Natl Acad Sci USA. 2008;105(36):13345–13350. doi: 10.1073/pnas.0802215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DePace AH, Santoso A, Hillner P, Weissman JS. A critical role for amino-terminal glutamine/asparagine repeats in the formation and propagation of a yeast prion. Cell. 1998;93(7):1241–1252. doi: 10.1016/s0092-8674(00)81467-1. [DOI] [PubMed] [Google Scholar]
- 52.Tycko R. Symmetry-based constant-time homonuclear dipolar recoupling in solid state NMR. J Chem Phys. 2007;126(6):064506. doi: 10.1063/1.2437194. [DOI] [PubMed] [Google Scholar]
- 53.Diaz-Avalos R, King CY, Wall J, Simon M, Caspar DLD. Strain-specific morphologies of yeast prion amyloid fibrils. Proc Natl Acad Sci USA. 2005;102(29):10165–10170. doi: 10.1073/pnas.0504599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luckgei N, et al. The conformation of the prion domain of Sup35p in isolation and in the full-length protein. Angew Chem Int Ed Engl. 2013;52(48):12741–12744. doi: 10.1002/anie.201304699. [DOI] [PubMed] [Google Scholar]
- 55.Schutz AK, et al. Solid-state NMR sequential assignments of the amyloid core of full-length Sup35p. Biomol NMR Assign. 2014;8(2):349–356. doi: 10.1007/s12104-013-9515-1. [DOI] [PubMed] [Google Scholar]
- 56.Wickner RB, Edskes HK, Shewmaker F, Nakayashiki T. Prions of fungi: Inherited structures and biological roles. Nat Rev Microbiol. 2007;5(8):611–618. doi: 10.1038/nrmicro1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherman F. Getting started with yeast. In: Guthrie C, Fink GR, editors. Guide to Yeast Genetics and Molecular Biology. Academic; San Diego: 1991. pp. 3–21. [Google Scholar]
- 58.Jung G, Masison DC. Guanidine hydrochloride inhibits Hsp104 activity in vivo: A possible explanation for its effect in curing yeast prions. Curr Microbiol. 2001;43(1):7–10. doi: 10.1007/s002840010251. [DOI] [PubMed] [Google Scholar]
- 59.Conde J, Fink GR. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci USA. 1976;73(10):3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pines A, Gibby MG, Waugh JS. Proton-enhanced Nmr of dilute spins in solids. J Chem Phys. 1973;59(2):569–590. [Google Scholar]
- 61.Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. Heteronuclear decoupling in rotating solids. J Chem Phys. 1995;103:6951–6958. [Google Scholar]
- 62.Petkova AT, Tycko R. Sensitivity enhancement in structural measurements by solid state NMR through pulsed spin locking. J Magn Reson. 2002;155(2):293–299. doi: 10.1006/jmre.2002.2519. [DOI] [PubMed] [Google Scholar]
- 63.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wishart DS, Sykes BD, Richards FM. Relationship between nuclear magnetic resonance chemical shift and protein secondary structure. J Mol Biol. 1991;222(2):311–333. doi: 10.1016/0022-2836(91)90214-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.