Significance
Phosphorus is an important nutrient for living organisms. Phosphorus is generally considered to bear a 5+ oxidation state, but several lower redox states have been reported, including the toxic gas phosphine. We show here that the lower redox states of phosphorus are common in Florida water samples, and that based on the global concentration of phosphine, we might expect to see 5−15% of all dissolved phosphorus in a lower redox state.
Keywords: phosphorus, redox chemistry, phosphonates, element cycling, biogeochemistry
Abstract
The element phosphorus (P) controls growth in many ecosystems as the limiting nutrient, where it is broadly considered to reside as pentavalent P in phosphate minerals and organic esters. Exceptions to pentavalent P include phosphine—PH3—a trace atmospheric gas, and phosphite and hypophosphite, P anions that have been detected recently in lightning strikes, eutrophic lakes, geothermal springs, and termite hindguts. Reduced oxidation state P compounds include the phosphonates, characterized by C−P bonds, which bear up to 25% of total organic dissolved phosphorus. Reduced P compounds have been considered to be rare; however, the microbial ability to use reduced P compounds as sole P sources is ubiquitous. Here we show that between 10% and 20% of dissolved P bears a redox state of less than +5 in water samples from central Florida, on average, with some samples bearing almost as much reduced P as phosphate. If the quantity of reduced P observed in the water samples from Florida studied here is broadly characteristic of similar environments on the global scale, it accounts well for the concentration of atmospheric phosphine and provides a rationale for the ubiquity of phosphite utilization genes in nature. Phosphine is generated at a quantity consistent with thermodynamic equilibrium established by the disproportionation reaction of reduced P species. Comprising 10–20% of the total dissolved P inventory in Florida environments, reduced P compounds could hence be a critical part of the phosphorus biogeochemical cycle, and in turn may impact global carbon cycling and methanogenesis.
Life as we know it is dependent on phosphate esters, which act in metabolism as energy-storing polyphosphates and cofactors, in replication and transcription as the backbone of RNA and DNA, and in cell structure as phospholipids. Phosphate minerals are the ultimate source of phosphate in the biosphere. However, most phosphate minerals are poorly soluble and slow to dissolve at neutral pH and at room temperature; hence phosphorus (P) is the limiting nutrient in many ecosystems. Phosphorus cycling is especially slow compared with carbon and nitrogen cycling (1).
Although inorganic phosphate and phosphate esters (P5+) are viewed as the prevalent compounds in nature, phosphonates, with C−P bonds, are ubiquitous, comprising up to 25% of the dissolved organic P in some natural samples (2). The P in phosphonates has a stronger potential for electron sharing than the P in phosphates, based on the electronegativity difference between C and P (2.5–2.2) compared with O (3.5) and P in phosphates. With a greater potential for electron sharing, the formal oxidation state of P in phosphonates is thus less than +5; hence phosphonates represent a reduced oxidation state P (hereafter, reduced P) speciation in the environment. Phosphonates appear to be critical to some biogeochemical pathways, including a role for methylphosphonate in aerobic methanogenesis in marine environments (3, 4). Ties between biogeochemical cycles, such as between P in phosphonates and C as methane, demonstrate that a thorough understanding of P geochemistry is necessary for understanding the biogeochemical cycling of other elements, such as carbon and nitrogen (5).
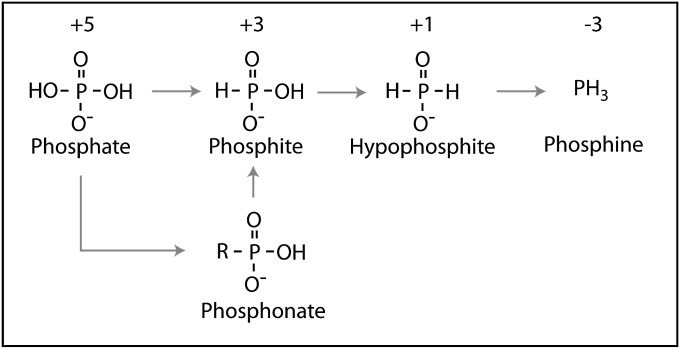
With the exception of the phosphonates, P is generally considered to be a redox-insensitive element as the reduction reaction is highly endergonic, and requires more free electrons than normally exist in the environment (6, 7). However, the reduced P compounds phosphite, hypophosphite, and phosphine are known to occur in nature (Fig. 1), and have origins that range from nonbiological (8, 9) to biological (10, 11). Phosphite and hypophosphite can be used by many microorganisms as sole P sources, suggesting there must be an environmental source of these compounds (12).
Fig. 1.
Phosphorus compounds with proposed transformations denoted by arrows. Oxidation state is shown above.
In contrast to phosphite and hypophosphite, which are accessible as nutrients, phosphine is toxic to many organisms, although it is also a ubiquitous trace atmospheric gas that occurs at concentrations of about 1 ng/m3 on average (13). Variations in phosphine concentration are significant: the concentration of PH3 in low-PH3 environments is a factor of more than 10,000 less than those in high-PH3 natural environments. The origin of phosphine may be linked to metal corrosion (14) in some environments, but in others, it is linked to microbial activity (15, 16). The link between phosphine and the other reduced P compounds, and to the organic phosphonates, is unclear, and is explored using thermodynamic models in Modeling of Relationship Between Phosphine, Phosphite, and Hypophosphite.
Between 10% and 67% of culturable bacteria are capable of using phosphite as a sole P source (12), including the critical primary producer Prochlorococcus (17). The ability of microbes to use reduced P as a sole P source suggests that a fraction of soluble P in the environment may be in a reduced oxidation state (18), possibly greater than 10% given the rate of occurrence of phosphite-using genes (12). To this end, we analyzed 32 Florida freshwater samples from six locations and at a variety of depths in the water column (Table 1; see Methods) for P speciation using high-performance liquid chromatography to separate P species coupled to detection with an inductively coupled plasma mass spectrometer (HPLC-ICP-MS). This method has been used previously to identify low-redox state P in Archean rocks, and the current work optimized the conditions for P speciation analysis in freshwater (19) (see Methods).
Table 1.
Sample locations, characteristics, and reduced P contents given as percentages of TDP
| Sample | Depth, cm | ORP, mV | pH | TDP, mg/L | H2PO2− | HPO32− | PO43- |
| River Front Park, 28° 4' 12.25” N, 82° 22' 39.41” W | |||||||
| A-1 | 0 | 213 | 8.1 | 0.3 | 2 (±1) | 3 (±0.5) | 95 (±1.5) |
| A-2 | 15 | 213 | 8.1 | 0.3 | 2 (±1) | 1 (±0.5) | 97 (±1.5) |
| A-3-g | 35 | n.d. | n.d. | 0.2 | 2 (±1) | 0 (±0.3) | 98 (±1) |
| B-1 | 0 | 169 | 8.46 | 0.08 | 8 (±4) | 3 (±2) | 89 (±6) |
| B-2 | 20 | 168 | 8.46 | 0.3 | 2 (±1) | 1 (±1) | 97 (±2) |
| B-3-g | 41 | n.d. | n.d. | 0.2 | 21 (±1) | 5 (±0.5) | 74 (±0.6) |
| Pithalachascotee River, 28° 14' 26.15” N, 82° 40' 29.52” W | |||||||
| A-1 | 0 | 8 | 6.49 | 0.2 | 7 (±1) | 11 (±3) | 82 (±8) |
| A-2-g | 24 | 2 | 6.44 | 0.2 | 1 (±2) | 10 (±3) | 89 (±8) |
| B-1 | 0 | 6 | 6.49 | 0.2 | 6 (±1) | 14 (±2) | 80 (±8) |
| B-2 | 22 | 11 | 6.5 | 0.2 | 18 (±1) | 7 (±2) | 75 (±7) |
| B-3-g | 44 | 13 | 6.51 | 0.2 | 3 (±2) | 9 (±3) | 88 (±8) |
| Hillsborough River, 28° 1' 14.14” N, 82° 27' 13.94” W | |||||||
| A-1 | 0 | 64 | 8 | 0.09 | 3 (±2) | 10 (±6) | 87 (±17) |
| A-2 | 20 | 62 | 7.88 | 1.5 | BDL | BDL | 100 (±1) |
| A-3-g | 39 | 62 | 7.84 | 0.04 | 6 (±5) | 20 (±12) | 74 (±20) |
| B-1 | 0 | 92 | 7.93 | 0.06 | 4 (±2) | 15 (±9) | 81 (±20) |
| B-2 | 14 | 90 | 7.85 | 0.04 | BDL | 24 (±14) | 76 (±18) |
| B-3-g | 28 | 88 | 7.51 | 0.01 | BDL | BDL | 100 (±20) |
| Pemberton Creek Retention Pond, 28° 2' 4.74” N, 82° 15' 30.71” W | |||||||
| A-1 | 0 | 167 | 6.5 | 0.15 | 18 (±2) | 33 (±3) | 49 (±9) |
| A-2-g | 15 | −31 | 6.4 | 0.18 | 8 (±1) | 33 (±3) | 59 (±8) |
| B-1 | 0 | 195 | 6.5 | 0.16 | 10 (±2) | 33 (±3) | 57 (±8) |
| B-2-g | 17 | −12 | 6.1 | 0.22 | 23 (±1) | 25 (±2) | 52 (±6) |
| Muck Pond, 28° 13' 44.27” N, 82° 42' 83” W | |||||||
| A-1 | 0 | 22 | 6.51 | 0.05 | 5 (±3) | 11 (±6) | 84 (±17) |
| A-2 | 18 | 41 | 6.49 | 0.06 | 4 (±3) | 11 (±6) | 85 (±17) |
| A-3-g | 36 | 36 | 6.44 | 0.05 | 5 (±3) | 9 (±6) | 86 (±17) |
| B-1 | 0 | 11 | 6.76 | 0.05 | 5 (±3) | 11 (±6) | 84 (±17) |
| B-2 | 20 | 7 | 6.69 | 0.06 | 19 (±3) | 9 (±5) | 72 (±16) |
| B-3-g | 41 | 5 | 6.65 | 0.05 | 12 (±3) | 9 (±5) | 79 (±17) |
| River Front Swamp, 28° 4' 12.81” N, 82° 22' 51.73” W | |||||||
| A-1 | 0 | −138 | 5.73 | 0.3 | 3 (±1) | 30 (±2) | 67 (±5) |
| A-2-g | 10 | −162 | 5.74 | 0.3 | 8 (±1) | 16 (±2) | 76 (±5) |
| B-1 | 0 | 56 | 5.73 | 0.3 | BDL | BDL | 100 (±5) |
| B-2 | 9 | 52 | 5.73 | 0.3 | 11 (±1) | 10 (±2) | 79 (±5) |
| B-3-g | 19 | −31 | 5.7 | 0.3 | 21 (±1) | 17 (±1) | 62 (±4) |
Samples noted with “g” are from associated groundwater. Each sample was run in duplicate when possible, and errors (in parentheses) are reported as relative percent differences between runs, multiplied by the average percentage. BDL, below detection limit; n.d., not determined; TDP, total dissolved phosphorus.
Florida Water Analysis
Phosphite and hypophosphite were detected in many Florida water samples (Table 1). In several samples, the phosphite and hypophosphite account for more than 25% of the total dissolved P (Fig. 2). The samples with lower oxidation-reduction potential, lower pH, and standing or stagnant water tended to bear the most reduced P, although this is not universally true (11). The oxidation-reduction potential (Eh) and the percentage of reduced P are negatively correlated (R = 0.24 for hypophosphite and 0.41 for phosphite), as are pH and reduced P (R = 0.51 for hypophosphite and 0.57 for phosphite). These correlations are consistent with studies of phosphite in Lake Taihu (11). These results suggest that reduced P compounds are common to many environments in the hydrosphere, although given the limited geographical scope of the present study, extending these results to estimate the global quantity of reduced P is unreasonable. To this end, we use phosphine gas as a proxy for the global reduced P production.
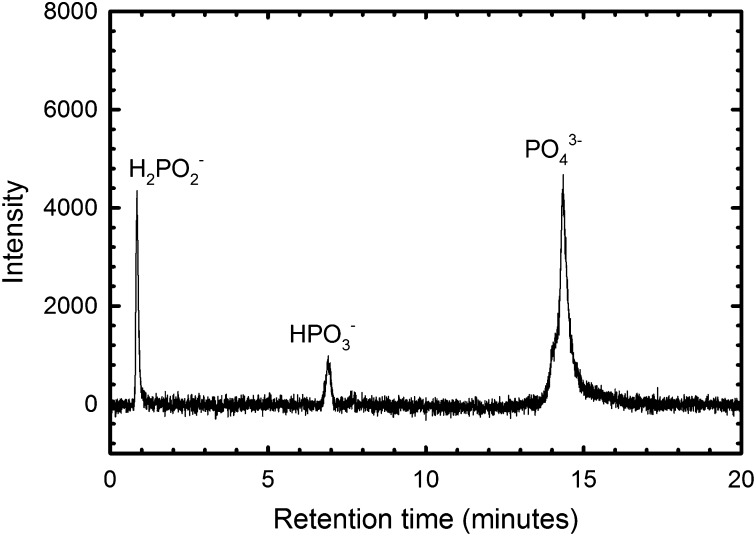
Fig. 2.
HPLC-ICP-MS spectrum of water from central Florida (River Front Park B-3-g, Tampa) showing hypophosphite (∼0.8 min), phosphite (∼7 min), and phosphate (∼14 min) peaks. The concentration of each P species was estimated from the peak height using standards with concentrations of 10−7 M, 5 × 10−7 M, 10−6 M, 5 × 10−6 M, and 10−5 M of each P species. Peak heights varied linearly with concentration (R2 = 0.999, 0.9996, and 0.998 for hypophosphite, phosphite, and phosphate, respectively), and the linear relationship between concentration and peak height was used to calculate individual P species concentration. Both reduced P compounds combined comprise about 26% of the total P (0.2 mg/L) in this sample.
Modeling of Relationship Between Phosphine, Phosphite, and Hypophosphite
Phosphite and hypophosphite may be globally relevant to the P biogeochemical cycle. Proof of their relevance would require a global water-sampling campaign with P speciation analysis. However, the gas phosphine has been measured across the globe and is known as a trace atmospheric constituent. We use this gas as a proxy to estimate the global redox chemistry of P. We propose that phosphite and hypophosphite generated by microbial activity are the ultimate source of phosphine in the atmosphere. To demonstrate the efficacy of reduced P compounds in the production of phosphine, the speciation of P was determined using thermodynamic models with varying pH, total P content, temperature, and starting concentrations of different P oxyanions (see Methods). These models at present consider only dissolved anions and ignore formation of most aqueous complexes with cations, and are hence most applicable to freshwater systems.
No other common reducing agent can produce phosphine at the concentration at which it is observed in nature, as reduction from phosphate is energetically unfeasible unless phosphite or hypophosphite are present in the environment. Phosphite and hypophosphite generate phosphine and phosphate from disproportionation reactions:
| [1] |
| [2] |
Phosphine is the major volatile product of these reactions. Phosphine is oxidized by O2 and UVB radiation (20); hence its nighttime concentration is closest to equilibrium, which is reached quickly (13, 21, 22). At equilibrium, the phosphine yield predicted by disproportionation (Figs. 3 and 4) matches those of the gaseous phosphine analyses in natural samples (21, 22), if more than 5% of the dissolved P bears a lower redox state. A disproportionation reaction may be justified as hypophosphite disproportionates to phosphine on heating (14), and also when participating in metal redox reactions (23).
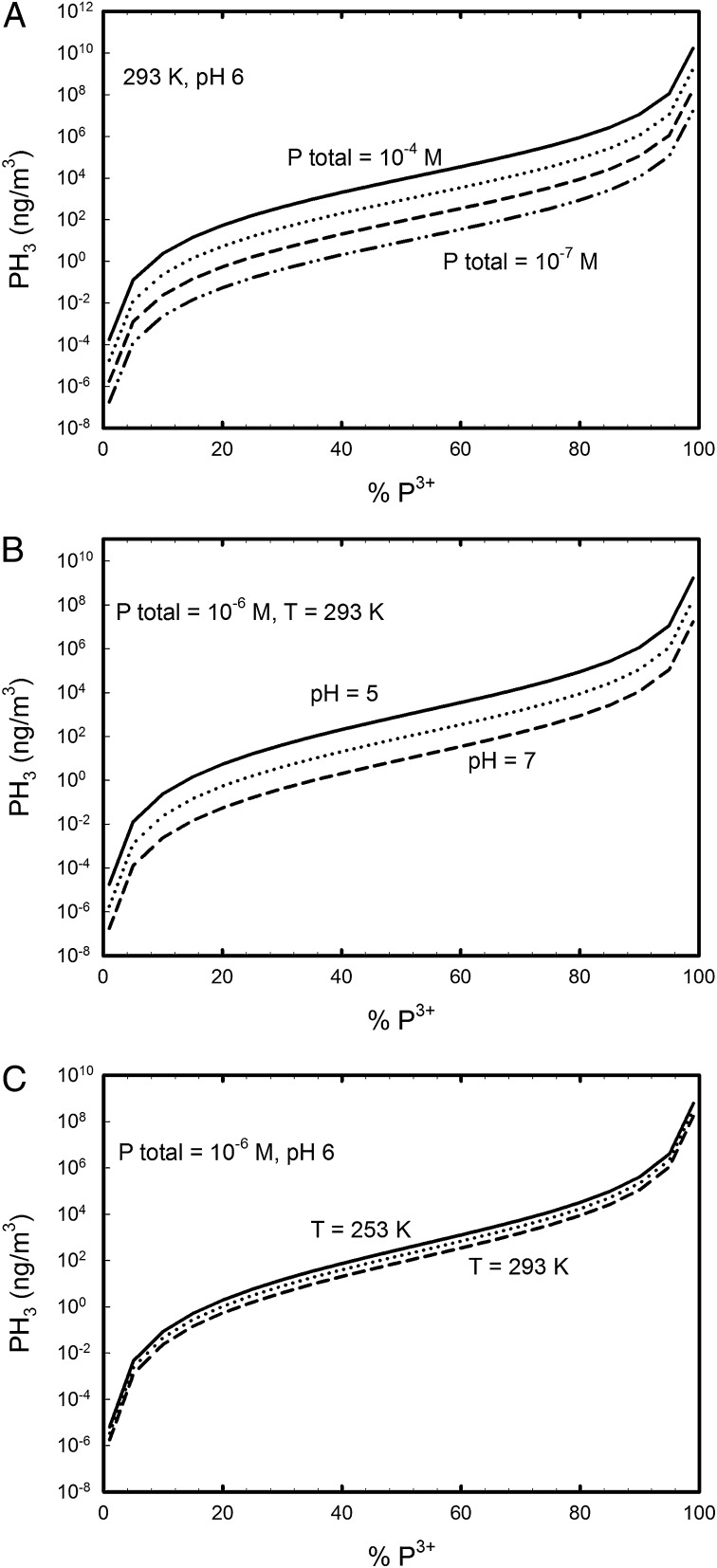
Fig. 3.
Modeled phosphine atmospheric concentration calculated with respect to percent of total dissolved inorganic P as phosphite in freshwater, with the remainder phosphate. These calculations calculate the quantity of phosphine in air (ng/m3) resulting from disproportionation of phosphite (reaction 1), which is dependent on pH, temperature, amount of P as phosphite, and total P. (A) Dependence on PH3 concentration on total phosphorus in solution, with constant temperature and pH. (B) Dependence on pH. (C) Dependence on temperature.
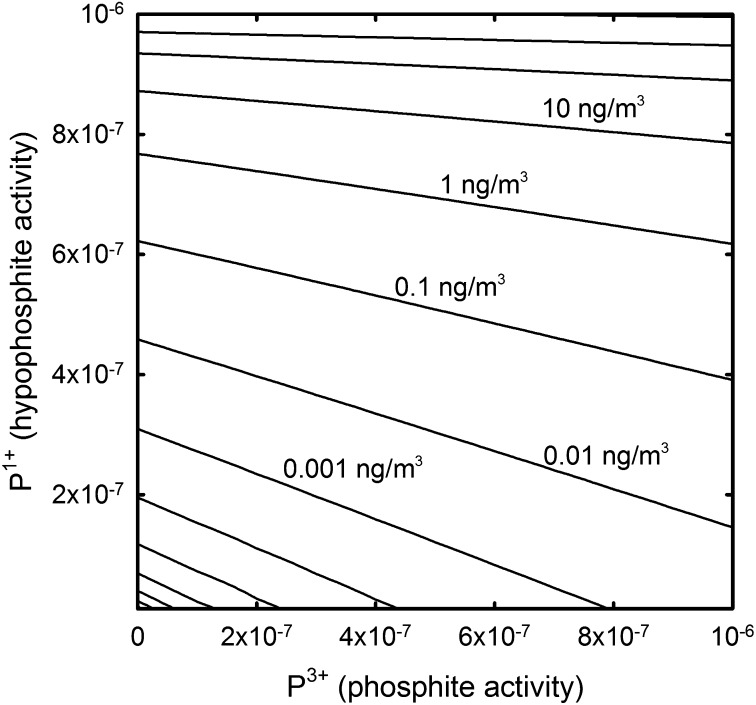
Fig. 4.
Contour plot illustrating PH3 atmospheric concentration (ng/m3) dependence on phosphite and hypophosphite activity in water, assuming a pH of 7.2, temperature of 298 K, and total free dissolved inorganic phosphate activity of 10−6. PH3 abundance is solved for using the HSC chemistry program (see Methods) and follows predictions from reactions 1 and 2. The phosphite and hypophosphite concentrations from Fig. 2 would be in equilibrium with between 1 and 10 ng/m3 of phosphine, consistent with the measured PH3 concentrations from the Florida Everglades (52).
Phosphine serves as a tracer of the total quantity of reduced P compounds in the environment. Since the majority of the earth is covered by oceans, the phosphine content of oceanic air can be used as a proxy for the total dissolved P bearing a reduced oxidation state. The model results discussed above, although not necessarily considering all P species possible within ocean water, can be considered a first step toward estimating the total amount of reduced P in the oceans. The phosphine content of oceanic air ranges from less than 0.01 ng/m3 to more than 100 ng/m3 (21, 24). Even larger PH3 concentrations have been reported in nonoceanic environments, but some of these may be due to phosphide corrosion or have another anthropogenic source (25). In biogases, PH3 may become extremely abundant, up to fractions of a gram per cubic meter (25). Such large variations in PH3 pressure are most consistent with slight variations in reduced P content of water (Fig. 4). A doubling of hypophosphite concentration can result in a 100-fold increase of phosphine content in the associated air column. Phosphine production is primarily dependent on the concentration of phosphite and hypophosphite, but is also dependent on pH, temperature, and total dissolved P concentration. If the majority of the reduced P occurs as hypophosphite, then more PH3 should be expected than if the reduced P occurs as phosphite.
If 1 ng/m3 represents the average global atmospheric PH3 concentration, and if the total dissolved inorganic P concentration is set to cellular levels (10−4 M), then using the predicted PH3 production shown in Figs. 3 and 4, we propose between 5% and 15% of reactive dissolved P could be in reduced form in aqueous environments. This steady-state concentration of reduced P indicates active maintenance of reduced P in the environment, as reduced P can be oxidized to phosphate by microbes under relatively short time scales (26, 27). Phosphine production is generally known to be highest in high P environments (28), under lower pH conditions (29), and can be high in arctic regions (30), consistent with the thermodynamics of the disproportionation reaction.
Discussion
The ubiquity of microbial phosphite utilization provides some confirmation of our analyses and thermodynamic models. The genes for reduced P utilization are prominent in microbial organisms, and indicate a global redox cycle for P with production of phosphite at a level such that there is evolutionary pressure to be able to use these reduced forms of P (18). Hypophosphite and phosphite both participate in biochemical pathways (31, 32), including metabolism (33). The production of phosphine is also likely biological: Phosphine is produced by microbial activity (16), and when an organic phosphonate is a substrate, phosphine production is increased (28), along with increased methane production. The effect of phosphite and hypophosphite on phosphine production is unknown.
Phosphite and hypophosphite could be produced as byproducts of microbial phosphonate biosynthesis, as the reduction of phosphate from phosphoenolpyruvate by H2 to give pyruvate and phosphite is exergonic (ATP can also generate reduced P; see Methods for both calculations). Phosphoenolpyruvate is the key precursor to phosphonates in C−P biosynthesis, and we hypothesize that phosphite may be generated by rearrangement and reduction of this compound (34), or from degradation of other phosphonates.
Microbial phosphine generation is coupled to methanogenesis (16, 28). Aerobic methanogenesis begins with methylphosphonate as the methane precursor (35–37). This process likely proceeds by glycyl radical intermediate (38), with the radical transferred to the phosphate. Under slightly oxidizing to mildly reducing conditions (< +0.1 V at pH 7), a phosphate radical can rearrange to a phosphite radical (7); hence aerobic methanogenesis can result in phosphite production, requiring enzymes for phosphite utilization in the oligotrophic sea, which are present in Prochlorococcus (17). These reduced P compounds exist in equilibrium with PH3 gas, which may be generated in the intracellular medium. Phosphine is soluble in both hydrocarbons and water, and hence it will diffuse out of cells, passing through the cell membrane. The thermodynamic results also suggest that before the oxidation of the earth’s phosphorus (19), PH3 may have been an important constituent of the early earth’s atmosphere, with a partial pressure of up to a few millibars. The concentration of phosphine in the early Archean atmosphere would have depended on oceanic pH, and our estimate is based upon a concentration of phosphite in the ocean of 10−4 M (99% reduced), which although high, is consistent with analytical results of extracts from Archean carbonates (19).
We acknowledge that much is still unknown about the links between phosphine, phosphite, hypophosphite, and organic phosphonates. The generation of phosphite from phosphonates or phosphoenolpyruvate has not been demonstrated. It is also unknown whether the sequential reduction of phosphite to hypophosphite and ultimately to phosphine is microbially mediated. However, our results demonstrate that the concentrations of phosphite and hypophosphite present in the Florida environment are sufficient to supply phosphine at a quantity consistent with both thermodynamic predictions (see Modeling of Relationship Between Phosphine, Phosphite, and Hypophosphite) and air analyses from prior work (13, 20–22, 24, 25, 28–30). Phosphine has been known for some time to be a trace gas with possibly some relevance to global biogeochemical P cycling. However, its origin has been unclear. We propose here the most likely source of phosphine is the disproportionation of reduced phosphorus oxyacids, which have P−H bonds and significant reducing potential, and are found in water samples from at least seven locations (ref. 11; Table 1). The microbial ability to use these reduced P compounds is widespread, implying a significant presence of phosphite and hypophosphite in the environment.
Methods
The Center for Geochemical Analysis at the University of South Florida houses a PerkinElmer S200 High Performance Liquid Chromatograph capable of coupling to a PerkinElmer Elan DRC II inductively coupled plasma mass spectrometer (HPLC-ICP-MS). General methods were modified from those used for P speciation with ion chromatography (IC) and IC-electrospray mass spectrometry (39) and optimized for HPLC-ICP-MS. This work used a Dionex IonPac AS17C chromatographic column with an AG17 Guard Column fitted on the HPLC. Experiment runs eluted 50 μL of sample with a mobile phase consisting of a linear gradient using a starting concentration of KOH of 3.5 mM for the first 2 min and then ramping up to 35 mM over the course of 10 min and then remaining at 35 mM for the last 10 min at a 1.0 mL/min flow rate. The ICP-MS was run at 1300 W radio frequency power to effectively ionize P, which has a high first ionization potential. Nebulizer flow and lens voltage was adjusted to optimize for maximal signal intensity on P. All standard samples and blanks were mixed or prepared using 18 MΩ water or doubly distilled 18 MΩ water, distilled acids, and American Chemical Society grade or better solid reagents. Calibration and single species standards were mixed just before analysis.
P was analyzed as PO at mass 47 made by a reaction cell in line to the detector. This cell is generally used to eliminate interferences with the aid of a reaction gas to either combine the species of interest into a molecular species at a different mass or by reaction with the interfering species. This was done to reduce interference from 15N16O. While the total abundance of 15N is low, the atmospheric interface on the ICP nearly assures that a substantial signal from 15N16O will always be present, and must be mitigated.
Standards were created from commercially available hypophosphorous acid, phosphoric acid, and phosphorous acid (Fisher Scientific). These standards were diluted in analytical grade 18MΩ DI-H2O. Standards were stored in brown glass or opaque high density polyethylene (HDPE) containers for no more than 4 d at 4 °C and then discarded. Hypophosphite and phosphite were observed as narrow peaks that elute at ∼1.0 min and 7.1 min, respectively, and phosphate tended to be a broader peak that was retained in the chromatographic column until 14.3 min with a tail that extended to ∼15–16 min (Fig. 2). No hypophosphite and phosphite peaks are observed for the blank. Peak height varied linearly with concentration, and these standards were used to calibrate peak height to calculate the concentration of P compounds in samples collected from the sites listed in Locations. Each sample was run in duplicate when possible, and errors are reported as relative percent differences between runs, multiplied by the average percentage (error is shown between parentheses in Table 1).
Locations.
Thirty-two individual water samples were collected from six different freshwater locations in the Tampa Bay area between the months of November 2012 and March 2013 (Table 1). Samples were collected from two sites at least 5 m apart at each location (A or B). At each site, water was collected from the surface and beneath the surface as groundwater. Groundwater samples were collected by hammering a PVC tube with a metal rod insert into the base of the water body, and the rod was removed. Water was filtered through a 0.45-μm in-line filter. If depth was sufficient, a midpoint sample was also collected (denoted as depth in centimeters on Table 1), with temperature, pH, and redox potential (ORP) also determined, where available. These locations were chosen to be representative of freshwater from rivers and ponds/swamps in the Tampa Bay area, FL based on their land use and land cover. The average water temperature of these sample locales was 20.0 ± 2.5 °C.
Samples were collected from two rivers in three locations. Two of the river locations are found on the Hillsborough River, Hillsborough County, FL. The first, River Front Park, is situated within a recreational park lying on the northern edge of Tampa, FL, part of the Tampa Bay watershed. The second location on the Hillsborough River is east of the point where Sulfur Springs merges with the river. This site is located within Tampa, FL, and samples the Floridan Aquifer (pH ∼8), which has been influenced by urbanization. The third river sampled was the Pithalachascotee River in Pasco County, FL. The chosen collection points from this river lie southwest of pastures and northeast of a nursery and horse stable.
The pond/swamp locations consist of a retention pond, a man-made pond, and a swamp. The retention pond located in Seffner, FL is a shallow, stagnant pond resulting from surface water runoff that has been allowed to flow unobstructed from the surrounding residential properties. In Pasco County, FL, the man-made pond sampled was created as a result of providing fill dirt needed for the foundation of the residence on the property. Swamp water samples were collected from the River Front Park, Hillsborough County, FL, due west of the sampled river location. This location is representative of a natural, reduced environment that has been less influenced by anthropogenic activity than the other two sites.
Samples with low water velocity (ponds/swamps) showed the highest quantity of reduced P (up to 50%, average of 27%). Samples collected from groundwater (noted with a “g” on Table 1) had a higher reduced P content (22%) than surface water (18%). River samples tended to have less reduced P (up to 26%, average of 13%). As this is an initial study with limited geographical scope, an extension to other environments around the globe should be seen as preliminary, although phosphine concentrations suggest that the reduction seen in west central Florida is not exceptional.
Thermodynamic Calculations.
Data for organophosphates are from Alberty (40–42). We evaluate the direction of reactions using both equilibrium and nonequilibrium chemistry coupled to the law of mass balance. Equilibrium computations have been carried out using the program HSC (version 7.1, Outokompu Research Oy). This code uses the Gibbs energy solver (43) to determine equilibrium concentrations, and has been used previously to constrain sulfur chemistry in the solar system (44, 45). The behavior of aqueous species in water is approximated using the Davies model (extended Debye−Hückel), the semiempirical Pitzer model (with binary interactions only), and Harvie’s modification of the Pitzer model (binary and ternary parameters), using the HSC Chem Aqua module. The code allows for the injection or removal of species, computing the resulting changes in solution chemistry with respect to time. Reaction kinetics are ignored in this study, as we assume the reactions of note are rapid.
Reactions investigated include (i) the disproportionation reactions described earlier (reactions 1 and 2) and (ii) the formation of phosphite, hypophosphite, and phosphine from phosphate, adenosine triphosphate, and phosphoenol pyruvate, and sought to determine the direction of phosphorus redox reactions in biochemical systems. The disproportionation reactions were determined by theoretical equilibrium chemistry.
The mass balance of a bulk assembly consisting of the gases N2 (1 mol initially) was solved for the abundance of PH3 using components of an aqueous solution consisting of 1 L of H2O, in addition to H+, H3PO2, H2PO2−, H3PO3, H2PO3−, HPO32−, H3PO4, H2PO4−, HPO42−, PO43−, and OH−. The solution was “buffered” by addition of an excess of Na2S (0.18 moles) and H2S (aq, at 0.41 mol), which established an equilibrium between H2S and HS− (aq) with a pH of 7.2, since sulfur was not allowed to oxidize (46). The total ionic strength of this solution was hence 0.6, similar to ocean water. The concentration of phosphate (initially as H3PO4) was set to 10−6 M, close to an average oceanic concentration (47), and the initial concentrations of H3PO3 and H3PO2 were varied from 10−8 M to 10−6 M, and the equilibrium mole fraction of PH3 was determined. The initial temperature was kept at 25 °C with a pressure of 1 atm. The addition of magnesium (0.05 M as MgCl2) to form the Mg−phosphate aqueous complexes Mg3(PO4)2 (aq) and MgHPO4(aq) was also investigated, with no production of these species noted or influencing PH3 concentration.
Nonequilibrium chemistry energy and mass balances are calculated using
where ΔG0 is the Gibbs Free Energy at equilibrium and Q is the reaction quotient (products over reactants). A ΔG less than zero implies a spontaneous reaction, and suggests that a biochemical reaction may proceed toward products when reactants are in excess. For these reactions, we determined the Gibbs free energy using intracellular concentrations (48–51) of adenosine, adenosine monophosphate (AMP, and its protonated forms), adenosine diphosphate (ADP and its protonated forms), adenosine triphosphate (ATP and its protonated forms), and gaseous PH3, as well as phosphate (as H2PO42−, HPO42−). Reduced P as phosphite (H3PO3, H2PO3−, HPO32−) and hypophosphite (H2PO2−) were assumed to be initially present at about 10−8 M, and pH and H2 were variables. The temperature and pressure of these reactions were kept at 298 K and 1 atm.
Reactions studied include the reduction of phosphate by the hydration of phosphate and polyphosphate bonds of ATP to ADP, AMP, and adenosine, and of phosphoenolpyruvate to pyruvate. These were compared with potential redox potentials of phosphate leading to phosphite, hypophosphite, and phosphine at intracellular conditions. Three reactions are spontaneous (ΔG < −50 kJ/mol) under cellular conditions: reduction of phosphate to phosphite by (i) the hydration of phosphoenolpyruvate, and the transformation of ATP to (ii) AMP or (iii) adenosine.
Acknowledgments
The manuscript benefited from helpful comments from two anonymous reviewers, and from discussion with Mark Rains. This work was jointly supported by National Science Foundation (NSF) and the NASA Astrobiology Program, under the NSF Center for Chemical Evolution Grant CHE-1004570 (to J.M.S. and M.A.P.), and by the NASA Exobiology and Evolutionary Biology Program Grant NNX14AN96G (to M.A.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Lajtha K, Schlesinger WH. The biogeochemistry of phosphorus cycling and phosphorus availability along a desert soil chronosequence. Ecology. 1988;69(1):24–39. [Google Scholar]
- 2.Hilderbrand RL. Role of phosphonates in living systems. CRC Press; Boca Raton, FL: 1983. [Google Scholar]
- 3.Beversdorf LJ, White AE, Björkman KM, Letelier RM, Karl DM. Phosphonate metabolism of Trichodesmium IMS101 and the production of greenhouse gases. Limnol Oceanogr. 2010;55(4):1768–1778. [Google Scholar]
- 4.Metcalf WW, et al. Synthesis of methylphosphonic acid by marine microbes: A source for methane in the aerobic ocean. Science. 2012;337(6098):1104–1107. doi: 10.1126/science.1219875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redfield AC. The biological control of chemical factors in the environment. Am Sci. 1958;46(3):205–221. [PubMed] [Google Scholar]
- 6.Roels J, Verstraete W. Biological formation of volatile phosphorus compounds. Bioresour Technol. 2001;79(3):243–250. doi: 10.1016/s0960-8524(01)00032-3. [DOI] [PubMed] [Google Scholar]
- 7.Pasek MA. Rethinking early Earth phosphorus geochemistry. Proc Natl Acad Sci USA. 2008;105(3):853–858. doi: 10.1073/pnas.0708205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasek M, Block K. Lightning-induced reduction of phosphorus oxidation state. Nat Geosci. 2009;2(8):553–556. [Google Scholar]
- 9.Pech H, et al. Detection of geothermal phosphite using high-performance liquid chromatography. Environ Sci Technol. 2009;43(20):7671–7675. doi: 10.1021/es901469t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pech H, et al. Elucidating the redox cycle of environmental phosphorus using ion chromatography. J Chromatogr Sci. 2011;49(8):573–581. doi: 10.1093/chrsci/49.8.573. [DOI] [PubMed] [Google Scholar]
- 11.Han C, et al. Phosphite in sedimentary interstitial water of Lake Taihu, a large eutrophic shallow lake in China. Environ Sci Technol. 2013;47(11):5679–5685. doi: 10.1021/es305297y. [DOI] [PubMed] [Google Scholar]
- 12.Stone BL, White AK. Most probable number quantification of hypophosphite and phosphite oxidizing bacteria in natural aquatic and terrestrial environments. Arch Microbiol. 2012;194(3):223–228. doi: 10.1007/s00203-011-0775-9. [DOI] [PubMed] [Google Scholar]
- 13.Glindemann D, Edwards M, Liu JA, Kuschk P. Phosphine in soils, sludges, biogases and atmospheric implications—A review. Ecol Eng. 2005;24(5):457–463. [Google Scholar]
- 14.Roels J, Verstraete W. Occurrence and origin of phosphine in landfill gas. Sci Total Environ. 2004;327(1-3):185–196. doi: 10.1016/j.scitotenv.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Rutishauser BV, Bachofen R. Phosphine formation from sewage sludge cultures. Anaerobe. 1999;5(5):525–531. [Google Scholar]
- 16.Jenkins RO, Morris TA, Craig PJ, Ritchie AW, Ostah N. Phosphine generation by mixed- and monoseptic-cultures of anaerobic bacteria. Sci Total Environ. 2000;250(1-3):73–81. doi: 10.1016/s0048-9697(00)00368-5. [DOI] [PubMed] [Google Scholar]
- 17.Martínez A, Osburne MS, Sharma AK, DeLong EF, Chisholm SW. Phosphite utilization by the marine picocyanobacterium Prochlorococcus MIT9301. Environ Microbiol. 2012;14(6):1363–1377. doi: 10.1111/j.1462-2920.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- 18.Karl DM. Microbially mediated transformations of phosphorus in the sea: New views of an old cycle. Annu Rev Mar Sci. 2014;6:279–337. doi: 10.1146/annurev-marine-010213-135046. [DOI] [PubMed] [Google Scholar]
- 19.Pasek MA, Harnmeijer JP, Buick R, Gull M, Atlas Z. Evidence for reactive reduced phosphorus species in the early Archean ocean. Proc Natl Acad Sci USA. 2013;110(25):10089–10094. doi: 10.1073/pnas.1303904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu R, et al. Tropospheric phosphine and its sources in coastal Antarctica. Environ Sci Technol. 2006;40(24):7656–7661. doi: 10.1021/es061601e. [DOI] [PubMed] [Google Scholar]
- 21.Glindemann D, Bergmann A, Stottmeister U, Gassmann G. Phosphine in the lower terrestrial troposphere. Naturwiss. 1996;83(3):131–133. [Google Scholar]
- 22.Gassmann G, Van Beusekom JEE, Glindemann D. Offshore atmospheric phosphine. Naturwiss. 1996;83(3):129–131. [Google Scholar]
- 23.Prins R, Bussell ME. Metal phosphides: Preparation, characterization and catalytic reactivity. Catal Lett. 2012;142(12):1413–1436. [Google Scholar]
- 24.Zhu R, et al. Phosphine in the marine atmosphere along a hemispheric course from China to Antarctica. Atmos Environ. 2007;41(7):1567–1573. [Google Scholar]
- 25.Dévai I, Felföldy L, Wittner I, Plósz S. Detection of phosphine: New aspects of the phosphorus cycle in the hydrosphere. Nature. 1988;333(6171):343–345. [Google Scholar]
- 26.White AK, Metcalf WW. Microbial metabolism of reduced phosphorus compounds. Annu Rev Microbiol. 2007;61:379–400. doi: 10.1146/annurev.micro.61.080706.093357. [DOI] [PubMed] [Google Scholar]
- 27.Schink B, Friedrich M. Phosphite oxidation by sulphate reduction. Nature. 2000;406(6791):37. doi: 10.1038/35017644. [DOI] [PubMed] [Google Scholar]
- 28.Han SH, Zhuang YH, Zhang HX, Wang ZJ, Yang JZ. Phosphine and methane generation by the addition of organic compounds containing carbon-phosphorus bonds into incubated soil. Chemosphere. 2002;49(6):651–657. doi: 10.1016/s0045-6535(02)00401-0. [DOI] [PubMed] [Google Scholar]
- 29.Ding L, et al. Effect of pH on phosphine production and the fate of phosphorus during anaerobic process with granular sludge. Chemosphere. 2005;59(1):49–54. doi: 10.1016/j.chemosphere.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Wu M, Wang Q, Geng J, Yang X. The determination of atmospheric phosphine in Ny-Ålesund. Chin Sci Bull. 2010;55(16):1662–1666. [Google Scholar]
- 31.Yang K, Metcalf WW. A new activity for an old enzyme: Escherichia coli bacterial alkaline phosphatase is a phosphite-dependent hydrogenase. Proc Natl Acad Sci USA. 2004;101(21):7919–7924. doi: 10.1073/pnas.0400664101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feingersch R, et al. Potential for phosphite and phosphonate utilization by Prochlorococcus. ISME J. 2012;6(4):827–834. doi: 10.1038/ismej.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schink B, Thiemann V, Laue H, Friedrich MW. Desulfotignum phosphitoxidans sp. nov., a new marine sulfate reducer that oxidizes phosphite to phosphate. Arch Microbiol. 2002;177(5):381–391. doi: 10.1007/s00203-002-0402-x. [DOI] [PubMed] [Google Scholar]
- 34.Freeman S, Irwin WJ, Schwalbe CH. Synthesis and hydrolysis studies of phosphonopyruvate. J Chem Soc Perkin Trans. 1991;2(2):263–267. [Google Scholar]
- 35.Karl DM, et al. Aerobic production of methane in the sea. Nat Geosci. 2008;1(7):473–478. [Google Scholar]
- 36.Dyhrman ST, et al. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature. 2006;439(7072):68–71. doi: 10.1038/nature04203. [DOI] [PubMed] [Google Scholar]
- 37.Kamat SS, Williams HJ, Dangott LJ, Chakrabarti M, Raushel FM. The catalytic mechanism for aerobic formation of methane by bacteria. Nature. 2013;497(7447):132–136. doi: 10.1038/nature12061. [DOI] [PubMed] [Google Scholar]
- 38.Buckel W. Bacterial methanogenesis proceeds by a radical mechanism. Angew Chem Int Ed Engl. 2013;52(33):8507–8509. doi: 10.1002/anie.201304593. [DOI] [PubMed] [Google Scholar]
- 39.Ivey MM, Foster KL. Detection of phosphorus oxyanions in synthetic geothermal water using ion chromatography-mass spectrometry techniques. J Chromatogr A. 2005;1098(1-2):95–103. doi: 10.1016/j.chroma.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 40.Alberty RA, et al. Recommendations for nomenclature and tables in biochemical thermodynamics. Pure Appl Chem. 1994;66(8):1641–1666. [Google Scholar]
- 41.Alberty RA. Thermodynamics of the hydrolysis of adenosine triphosphate as a function of temperature, pH, pMg, and ionic strength. J Phys Chem B. 2003;107(44):12324–12330. [Google Scholar]
- 42.Alberty RA, Goldberg RN. Standard thermodynamic formation properties for the adenosine 5′-triphosphate series. Biochemistry. 1992;31(43):10610–10615. doi: 10.1021/bi00158a025. [DOI] [PubMed] [Google Scholar]
- 43.White WB, Johnson SM, Dantzig GB. 1957. Chemical equilibrium in complex mixtures (RAND Corporation, Santa Monica, CA), P-1059; reprinted (1958) J Chem Phys 28:751–755.
- 44.Pasek MA, et al. Sulfur chemistry with time-varying oxygen abundance during Solar System formation. Icarus. 2005;175(1):1–14. [Google Scholar]
- 45.Pasek MA, Greenberg R. Acidification of Europa’s subsurface ocean as a consequence of oxidant delivery. Astrobiology. 2012;12(2):151–159. doi: 10.1089/ast.2011.0666. [DOI] [PubMed] [Google Scholar]
- 46.Gull M, Pasek MA. Is struvite a prebiotic mineral? Life. 2013;3(2):321–330. doi: 10.3390/life3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benitez-Nelson CR. The biogeochemical cycling of phosphorus in marine systems. Earth Sci Rev. 2000;51(1-4):109–135. [Google Scholar]
- 48.Alberty RA. Thermodynamics of Biochemical Reactions. Wiley; New York: 2005. [Google Scholar]
- 49.Dobson GP, Hitchins S, Teague WE., Jr Thermodynamics of the pyruvate kinase reaction and the reversal of glycolysis in heart and skeletal muscle. J Biol Chem. 2002;277(30):27176–27182. doi: 10.1074/jbc.M111422200. [DOI] [PubMed] [Google Scholar]
- 50.Nelson DL, Cox MM. Lehninger Principles of Biochemistry. WH Freeman; New York: 2005. [Google Scholar]
- 51.Bowman E, McQueney M, Barry RJ, Dunaway-Mariano D. Catalysis and thermodynamics of the phosphoenolpyruvate/phosphonopyruvate rearrangement. Entry into the phosphonate class of naturally occurring organophosphorus compounds. J Am Chem Soc. 1988;110(16):5575–5576. [Google Scholar]
- 52.Devai I, Delaune RD. Evidence for phosphine production and emission from Louisiana and Florida marsh soils. Org Geochem. 1995;23(3):L277–L279. [Google Scholar]