Significance
Dendritic cells (DCs) ingest bacteria at sites of infection, signal the presence of invaders via phagosomal Toll-like receptors (TLRs), and present bacterial antigens to the adaptive immune system. We show that TLR signaling from maturing phagosomes in DCs stimulates the formation of membrane tubules that facilitate content transfer with other signaling phagosomes and thereby promote optimal presentation of phagocytosed antigens. The phagosomal tubules are thus functionally distinct from those of lysosomes and link innate immune signaling to enhanced adaptive immune responses.
Keywords: phagocytosis, Toll-like receptors, major histocompatibility complex, inflammation, microtubules
Abstract
Dendritic cells (DCs) phagocytose large particles like bacteria at sites of infection and progressively degrade them within maturing phagosomes. Phagosomes in DCs are also signaling platforms for pattern recognition receptors, such as Toll-like receptors (TLRs), and sites for assembly of cargo-derived peptides with major histocompatibility complex class II (MHC-II) molecules. Although TLR signaling from phagosomes stimulates presentation of phagocytosed antigens, the mechanisms underlying this enhancement and the cell surface delivery of MHC-II–peptide complexes from phagosomes are not known. We show that in DCs, maturing phagosomes extend numerous long tubules several hours after phagocytosis. Tubule formation requires an intact microtubule and actin cytoskeleton and MyD88-dependent phagosomal TLR signaling, but not phagolysosome formation or extensive proteolysis. In contrast to the tubules that emerge from endolysosomes after uptake of soluble ligands and TLR stimulation, the late-onset phagosomal tubules are not essential for delivery of phagosome-derived MHC-II–peptide complexes to the plasma membrane. Rather, tubulation promotes MHC-II presentation by enabling maximal cargo transfer among phagosomes that bear a TLR signature. Our data show that phagosomal tubules in DCs are functionally distinct from those that emerge from lysosomes and are unique adaptations of the phagocytic machinery that facilitate cargo exchange and antigen presentation among TLR-signaling phagosomes.
Professional phagocytes take up large particles, such as bacteria, by phagocytosis and submit them to an increasingly harsh environment during phagosome maturation (1). Phagocytes concomitantly alert the immune system that an invader is present via signaling programs initiated by pattern recognition receptors, such as Toll-like receptors (TLRs) (2). Conventional dendritic cells (DCs) also alter and optimize phagosome maturation and TLR-signaling programs to preserve bacterial antigens for loading onto MHC class I and class II (MHC-II) molecules and optimize cytokine secretion to stimulate and direct T-cell responses to the invading agent (3, 4). DC presentation of soluble antigen is facilitated by TLR-driven tubulation of lysosomes that harbor MHC-II–peptide complexes and by consequent fusion of tubulovesicular structures with the plasma membrane (5–7); however, little is known about the mechanism by which signaling pathways influence the formation or presentation of phagosome-derived MHC-II–peptide complexes, key processes in the adaptive immunity to bacterial pathogens.
TLRs respond to microbial ligands at the plasma membrane and in intracellular stores (8). TLR stimulation at the plasma membrane, endosomes, or phagosomes elicits distinct signaling pathways via two sets of adaptors, TIRAP (or MAL)-MyD88 and TRAM-TRIF (8, 9), which induce proinflammatory cytokine secretion and other downstream responses. TLRs such as TLR2 and TLR4 are recruited to macrophage and DC phagosomes at least partly from an intracellular pool (10–13), and signal autonomously from phagosomes independent of plasma membrane TLRs (11, 14, 15). Autonomous phagosomal signaling from TLRs or Fcγ receptors enhances the degradation of phagocytosed proteins and assembly of MHC-II with their derived peptides (14–16). Phagosomal TLR signaling has been proposed to also promote the reorganization of phagosome-derived MHC-II-enriched compartments (MIICs) to favor the delivery of MHC-II–peptide complexes to the plasma membrane (17), analogous to TLR-stimulated formation of tubules from MIICs/lysosomes (18–20) that fuse with the plasma membrane (7) and extend toward the immunologic synapse with T cells (5). Tubules emerge from phagosomes in macrophages shortly after phagocytosis and likely function in membrane recycling during early phagosome maturation stages (21–23), but tubules at later stages that might facilitate the presentation of phagosome-derived MHC-II–peptide complexes have not been reported previously. Moreover, a role for TLR signaling in formation of phagosome-derived tubules has not been established.
Herein we show that in DCs, maturing phagosomes undergo extensive tubulation up to several hours after phagocytosis, and that tubulation requires TLR and MyD88 signaling and an intact actin and microtubule cytoskeleton. Unlike lysosome tubulation, phagosome tubulation is not essential for MHC-II–peptide transport to the cell surface. Rather, it contributes to content exchange among phagosomes that carry a TLR signature, and thereby enhances presentation of phagocytosed antigens from potential pathogens.
Results
Tubules Emerge from Maturing Phagosomes in DCs.
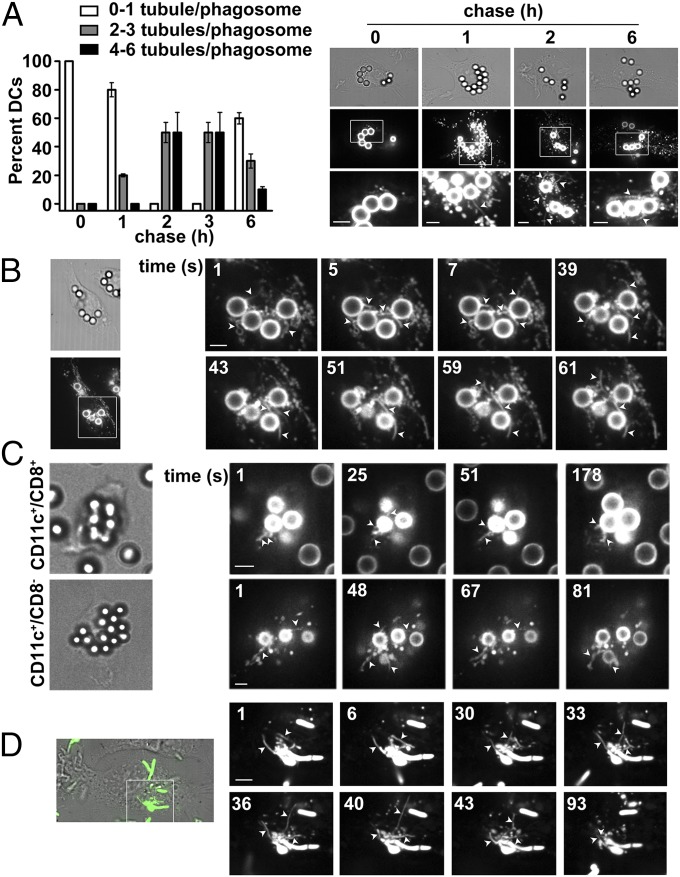
To investigate phagosomal tubulation in DCs, we exploited latex beads coated with Texas red-conjugated ovalbumin (OVA) and lipopolysaccharide (LPS; LPS/OVA-TxR) as phagocytic cargo (11, 24). Wild type (WT) C57BL/6 bone marrow-derived DCs (BMDCs) were pulsed with beads for 30 min, and the fate of phagocytosed OVA-TxR was monitored by live cell microscopy at different chase times. TxR-labeled tubules (referred to here as “phagotubules”) emanated from phagosomes starting at 1 h of chase and peaked in number and length by 2–3 h, when two to six phagotubules emanated from each phagosome within ∼90% of cells during 60 s of imaging (Fig. 1 A and B and Movies S1 and S2). Although the few tubules observed at 1 h were short (<2 μm), most of the tubules at 2–3 h were 6–8 μm long. Tubule formation from phagosomes decreased by 6 h of chase. Similar TxR-labeled tubules were observed in purified CD8+ and CD8− subsets from CD11c+ splenic DCs after phagocytosis of LPS/OVA-TxR beads (Fig. 1C and Fig. S1A). Thus, long phagotubules are prominent features of phagosomes at a late stage of maturation in DCs, and are distinguishable from the tubules that emanate from macrophage phagosomes early after phagocytosis (21, 22).
Fig. 1.
Tubules emerge from phagosomes in DCs. WT BMDCs (A, B, and D) or splenic CD8+ or CD8− DCs (C) were pulsed with LPS/OVA-TxR beads (A–C) or E. coli-EGFP (D) for 30 min, chased as indicated, and analyzed by live cell imaging. Arrowheads indicate phagotubules. (A) (Left) Tubules emerging from phagosomes over 2 min were quantified per phagosome at each chase time (10 cells per experiment, five independent experiments). The percentage of cells with the indicated average number of tubules/phagosome is shown. (Right) Representative frames from movies at indicated chase times. (Upper) Differential interference contrast (DIC) images emphasizing the beads. (Middle) TxR fluorescence. (Lower) Magnified insets from boxed region of the middle row. (B) Frame sequence of a representative movie after 2.5 h of chase. (C) Frames from representative movies showing DIC and TxR images after 2.5 h of chase. (D) Merged DIC and GFP fluorescence image from a single frame (Left) and frame sequence of representative movie of GFP fluorescence (Right) after a 2-h chase. (Scale bars: 2.6 μm.)
To test whether phagotubules form using a natural cargo, we analyzed BMDCs by live cell microscopy following a pulse with live Escherichia coli expressing EGFP (E. coli-EGFP). As with latex beads, phagotubules emanated from fluorescent bacteria after 2 h of chase (Fig. 1D and Movie S3). Thus, phagotubule formation is a general physiological feature of late phagosomes harboring LPS-coated particles in DCs. Subsequent experiments were performed with latex beads at 2.5 h of chase.
Phagosome Tubulation Requires TLR4-MyD88 Signaling.
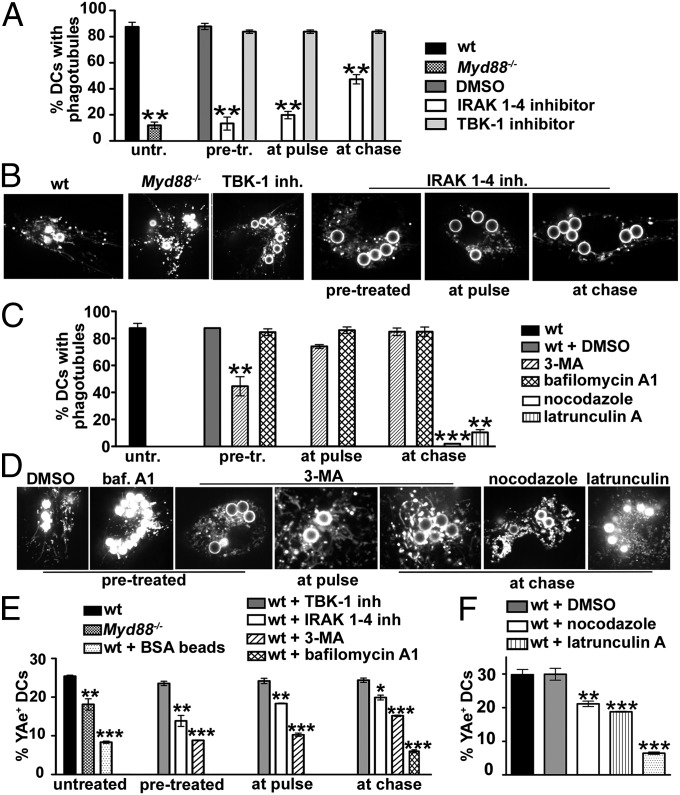
Because TLR signaling induces tubulation from endolysosomes in DCs (6), we probed its role in phagotubule formation. As reported previously (25, 26), pretreatment of WT BMDCs with IRAK-1/4 inhibitor (9) specifically inhibited MyD88-dependent signaling (Fig. S1 B and C), whereas the TBK-1 inhibitor BX795, but not IRAK-1/4 inhibitor, effectively impaired IRF3 phosphorylation downstream of TRAM-TRIF (Fig. S1D). Inhibitors were then added to WT BMDCs either 2 h before a 30-min pulse with LPS/OVA-TxR beads (“pretreatment”) or at the time of the pulse (“at pulse”) to block signaling from both the plasma membrane and phagosomes, or at the start of a 2.5-h chase following the pulse (“at chase”) to impede only phagosomal signaling. IRAK-1/4 inhibitor impaired phagotubule formation by 84% for pretreatment, by 76% at pulse, and by 46% at chase (Fig. 2 A and B and Movie S4), suggesting that optimal tubulation requires both surface and phagosomal MyD88-dependent LPS signaling. Consistently, phagotubules were detected in only 12 ± 3% of Myd88−/− BMDCs (compared with 87.5 ± 4% of WT) that were stimulated with LPS/OVA-TxR beads (Fig. 2 A and B and Movie S4). In contrast, the addition of BX795 to WT DCs at any time did not affect phagosome tubulation. Consistently, the addition of poly(I:C) (which stimulates only the TRAM-TRIF pathway via TLR3) to the LPS/OVA-TxR beads in IRAK-1/4 inhibitor-treated WT cells did not rescue phagosome tubulation (Fig. S2A and Movie S5), although it did restore IL-6 secretion (Fig. S2B). These data indicate that tubule formation from phagosomes in response to LPS in DCs requires signaling through the TLR4-MyD88 axis but not through the TRAM-TRIF pathway, and can be uncoupled from proinflammatory cytokine secretion.
Fig. 2.
Phagosome tubulation requires TLR4-MyD88 signaling and an intact cytoskeleton and has a moderate impact on MHC-II-antigen presentation. BMDCs from WT or Myd88−/− mice were untreated or pretreated, treated at pulse or at chase with vehicle (DMSO) or inhibitors as indicated, and pulsed with LPS/OVA-TxR beads, Eα beads, or LPS/BSA beads. (A–D) BMDCs pulsed with LPS/OVA-TxR beads and treated as indicated were analyzed by live cell imaging after a 2.5-h chase. (A and C) The percentage of DCs that showed phagotubules during 2 min of analysis was calculated for 10 cells per experiment, five independent experiments. (B and D) Frames from representative movies of cells treated as indicated. (E and F) Cells treated as indicated, pulsed with Eα beads (or LPS/BSA beads as a control), and chased for 4 h were labeled for surface MHC-II:Eα52–68 by YAe antibody and analyzed by flow cytometry. Shown are the percentages of positive cells. Error bars represent mean ± SD.
Phagosome Tubulation Requires Actin and Microtubule Integrity, but Not Complete Phagolysosome Maturation.
Because phagotubules were detected by TxR released from LPS/OVA-TxR beads, we tested whether their formation required phagosomal proteolysis, a consequence of phagosome maturation (11, 16). DCs were treated with 3-methyladenine (3-MA), an inhibitor of class III phosphatidylinositol 3-kinases that disrupts early stages of phagosome maturation and acquisition of proteolytic activity (27–30). DCs pretreated with 3-MA generated 50 ± 7% fewer phagotubules than vehicle-treated cells, but treatment at the pulse or during the chase had only a modest to insignificant effect (Fig. 2 C and D and Movie S6). Thus, phagotubule formation was impaired much less by 3-MA than by IRAK-1/4 inhibitor or by loss of MyD88 expression, and likely in part reflects inefficient detection owing to reduced TxR release from the beads. Pretreatment with bafilomycin A1, an inhibitor of the proton vacuolar ATPase, also had no effect on phagotubule formation (Fig. 2 C and D and Movie S6). Nevertheless, 3-MA or bafilomycin A1 treatment substantially impaired phagosomal proteolytic activity, measured as degradation of bead-associated OVA (Fig. S3 A and B). In comparison, the impairment of OVA proteolysis in IRAK-1/4 inhibitor-treated or Myd88−/− or Tlr4−/− DCs was less pronounced (Fig. S3 A and B), despite the dramatic effect on phagotubule formation (Fig. 2 A and B). These observations suggest that tubulation is independent of classical measures of phagosome maturation.
Consistent with the requirement for microtubules in forming and stabilizing lysosomal tubules in DCs (31, 32) and for actin dynamics in MHC-II transport to the plasma membrane (20), phagotubules were nearly ablated when cells were treated at chase with either nocodazole to depolymerize microtubules or latrunculin B to destabilize actin (Fig. 2 C and D and Movie S6). Latrunculin treatment also led to the accumulation of TxR-labeled vesicles). Nocodazole treatment also partially impaired OVA degradation on OVA-TxR beads, as expected (33, 34), whereas latrunculin B treatment did not (Fig. S3C). Thus, both actin and microtubule dynamics are needed for phagotubule formation/stabilization, but not substantially for phagolysosome maturation.
Phagosomal Tubulation Is Not Essential for Surface Delivery of MHC-II–Peptide Complexes.
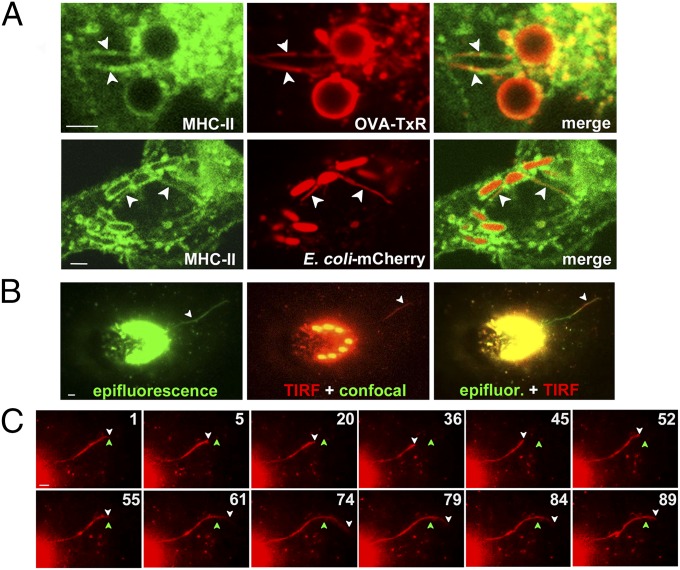
To test whether phagotubules, like endolysosome-derived tubules in DCs (5–7), are conduits for MHC-II trafficking, we exposed MHC-II–EGFP–expressing BMDCs (5) to LPS/OVA-TxR beads. TxR-labeled tubules overlapped with tubules harboring MHC-II–EGFP by live cell microscopy (Fig. 3A and Movie S7), indicating that MHC-II derived from phagosomes is present on phagotubules. To test whether phagotubule formation correlated with MHC-II–peptide surface expression, we pulsed cells with latex beads coated with recombinant Eα protein and then quantified cell surface MHC-II/Eα peptide complexes 4 h later using flow cytometry with the YAe antibody (11, 35). Typically, YAe labeled 25–30% of WT DCs after exposure to Eα beads, but not to beads coated with BSA (Fig. 2 E and F). As expected from their effects on proteolysis, both 3-MA and bafilomycin A1 inhibited Eα presentation to background levels (Fig. 2E); the mean fluorescence intensity of positive cells was constant, as shown previously (11, 30). In contrast, despite its profound effects on phagotubule formation, IRAK-1/4 inhibitor added at pulse or at chase reduced Eα presentation by only 25–30%, similar to its effect on OVA degradation (Fig. S3 A and B). A comparable moderate reduction was observed in Myd88−/− DCs (Fig. 2E) despite an 88 ± 3% reduction in phagotubule formation (Fig. 2 A and B). Furthermore, latrunculin and nocodazole treatments, both of which nearly completely blocked phagotubule formation, also had mild effects on Eα presentation (Fig. 2F), possibly attributable to impaired proteolysis (Fig. S3C). These data show that MHC-II presentation of phagosome-derived antigens can proceed to a great extent in the absence of phagotubules.
Fig. 3.
Phagotubules contain MHC-II molecules, but do not fuse with the plasma membrane. (A) WT MHC-II–GFP–expressing BMDCs were pulsed with LPS/OVA-TxR beads (Upper) or E. coli-expressing mCherry (Lower), chased for 2.5 h, and analyzed by live cell imaging. Shown is a magnified region from a frame of a representative movie (Upper) and a still image (Lower). Arrowheads indicate OVA-TxR or mCherry (red) on MHC-II–GFP–positive phagotubules (green). (B and C) WT BMDCs were pulsed with LPS/OVA-TxR beads, chased for 2.5 h, and analyzed by live cell imaging using epifluorescence and confocal and TIRF microscopy. (B) Shown are epifluorescence alone (green, Left), TIRF plane (red, Center) and confocal (green, Center; note the beads), and merged epifluorescence (green) and TIRF (red) images (Right) from a representative movie. (C) Frame sequence of the same movie in the TIRF plane, cropped to highlight the prominent phagotubule. The white arrowhead indicates the distal end of the extending phagotubule; the green arrowhead, a reference point for tubule extension and retraction over time. (Scale bars: 2.6 μm.)
To test whether phagotubules fuse with the plasma membrane, we exploited total internal reflection fluorescence (TIRF) microscopy (36). DCs pulsed with LPS/OVA-TxR beads were imaged 2.5 h later by alternating TIRF (to visualize phagotubules within 200 nm of the plasma membrane) with epifluorescence microscopy (to detect all tubules) and confocal microscopy (to visualize phagosomes). Whereas numerous tubules were detected by epifluorescence (green in Fig. 3 B and C and Movie S8), only rare long tubules (>8 μm long) were observed near the plasma membrane (red in Fig. 3 B and C and Movie S8). These tubules often retracted without fusing with the plasma membrane or losing their contents (Fig. 3C and Movie S8). In more than 15 movies in which phagotubules were detected by TIRF, we observed no obvious fusion events (indicated by flashes of fluorescence followed by signal dissipation). In contrast, we readily detected plasma membrane fusion of TxRed-labeled tubulovesicular structures derived from endolysosomes at 2 h after uptake of soluble LPS/OVA-TxR (Fig. S4), as described previously (7). Thus, phagotubules are functionally distinct from the tubules that emerge from endolysosomes, do not contact the cell surface in DCs, and are not likely conduits for the cell surface delivery of phagosome-derived MHC-II–peptide complexes.
Phagosomal Tubules Favor Content Transfer Between Phagosomes.
Phagotubules often contact other phagosomes, as seen by live cell imaging after phagocytosis of mixtures of LPS/OVA beads conjugated to either TxR or Alexa Fluor 488 (AF488; Fig. 4A and Movie S9). Thus, we considered whether the tubules potentiate “cargo exchange” among phagosomes. One phagosome can bear only one latex bead (16); thus, if content exchange occurs after phagocytosis of multiple LPS-coated beads with different labels, then phagosomes with one label will gradually acquire the other label. A reduction in double-labeled phagosomes from cells treated with agents that interfere with tubulation would support a requirement for tubules in content exchange.
Fig. 4.
Phagotubules facilitate content transfer between LPS-containing phagosomes. WT BMDCs pulsed with a 1:1 mixture of LPS/OVA-TxR or LPS/OVA-647 beads and LPS/OVA-AF488 beads were chased as indicated. (A) DCs chased for 2.5 h were analyzed by live cell imaging. (Left) DIC (Upper) and corresponding immunofluorescence image (Lower) from a frame of a representative movie. (Right) Magnification of the boxed region from a frame sequence of the same movie. Arrowheads indicate green phagotubules that contact red phagosomes (arrows). (B) DCs were chased as indicated, after which intact phagosomes were isolated and analyzed by flow cytometry. (Left) Forward scatter (FSC) and side scatter (SSC) plots showing a gated region (box) representing single phagosomes. (Center) Dot plots of a gated region from each chase time point in a representative of three experiments. The percentage of bicolored phagosomes (upper right quadrant) is indicated in red. (Right) Mixing control. Phagosomes were isolated from DCs pulsed with either LPS/OVA-AF488 beads or LPS/OVA-AF647 beads alone, chased for 4 h, and then mixed before homogenization. (C and D) Untreated Myd88−/−, Tlr4−/−, or WT DCs or WT DCs treated with vehicle or inhibitors as indicated were chased for 4 h, and isolated phagosomes were analyzed by flow cytometry as in B. (C) The percentage of phagosomes bearing both AF488 and AF647 labels was quantified (mean ± SD) in five independent experiments. (D) Representative experiment.
Accordingly, BMDCs were pulsed with beads coated with LPS/OVA-AF647 (“red beads”), LPS/OVA-AF488 (“green beads”), or both (Fig. S5A), such that ∼80% of cells phagocytosed both red and green beads (Fig. S5B). Cells were then chased for different times, and phagosomes were isolated and analyzed by flow cytometry (11, 16). Consistent with content exchange at late stages of phagosome maturation, bicolored single phagosomes accumulated during the chase, peaking at 23 ± 1% of phagosomes by 4 h (Fig. 5B, upper right quadrants on flow cytometry plots), substantially later than phagosome fusion induced by overexpression of constitutively active RAB5 (37), and correlating with the kinetics of phagotubule formation. Bicolored phagosomes reflected intracellular exchange before fractionation, because only 4 ± 1% of phagosomes were bicolored when isolated from BMDCs that had separately phagocytosed either red or green beads, chased for 4 h, and mixed before fractionation (Fig. 4B, mixing control). Bead degradation independent of phagocytosis, evaluated by incubating beads in cell culture media at 37 °C in parallel to the chase, was insignificant (1%; Fig. S5A). Even more robust phagosome content exchange was observed using freshly isolated splenic DCs (Fig. S5 E and F). These data indicate that quantifiable content exchange occurs among LPS-containing phagosomes within individual DCs.
Fig. 5.
Content transfer among phagosomes enhances antigen presentation. (A–C) WT BMDCs treated as indicated were pulsed with a 1:1 mixture of LPS/Eα/OVA beads (Eα:OVA ratio on beads, 1:1) and either uncoated beads or LPS/OVA beads. (A) DCs were chased for 4 h, labeled with YAe antibody, and analyzed by flow cytometry. The percentage of YAe+ DCs is shown (mean ± SD; four independent experiments). Values for populations receiving different cohorts of beads and treated with IRAK-1/4 inhibitor or nocodazole were not statistically different. (B) Cells were analyzed after the pulse by flow cytometry for FSC and SSC relative to nonpulsed cells. The percentage of cells that phagocytosed beads is indicated. (C) Representative flow cytometry histograms of DCs surface-labeled for MHC-II and CD40 after a pulse and a 4-h chase. (D–F) DCs pulsed with a 1:1 ratio of LPS/OVA/BSA beads (OVA:BSA at the indicated ratios) and either uncoated or LPS/BSA-coated beads were washed and cocultured with unlabeled (D and E) or CFSE-labeled (F) OT-II T cells. D. OT-II T-cell activation after 16 h was measured by CD69 expression (Left) or IL-2 secretion (Right; arbitrary units). Values are mean ± SD; n = 3. (E) Representative flow cytometry plots showing CD69 expression (y-axis; shown are percentages of CD69+ cells) on vβ5+ (x-axis) gated OT-II cells. (F) After 3 d of coculture, T-cell proliferation was assessed by CFSE dilution on the gated vβ5+ population. (Left) Division index; mean ± SD, n = 3. (Right) Representative flow cytometry histograms, with the percentages of vβ5+ cells within each CFSE dilution population indicated.
We then assessed whether inhibitors of phagotubule formation also block phagosome content exchange. None of the inhibitors altered DC phagocytic activity (Fig. S5 B and C). Treatment with protease inhibitors, 3-MA, or bafilomycin strongly inhibited content transfer (Fig. 4 C and D), likely reflecting inhibition of OVA degradation (Fig. S3 A and B) and consequent failure to release bound fluorophores. IRAK-1/4 inhibitor, which moderately affected OVA degradation but strongly inhibited tubule formation (Fig. 2 and Fig. S3), reduced phagosome content exchange by >50%, even when added during the chase after phagocytosis (Fig. 4 C and D). Consistently, phagosome content exchange in Myd88−/− and Tlr4−/− DCs, in which phagotubule formation was dramatically impaired, was reduced by 50 ± 1% and 40 ± 1%, respectively, compared with WT DCs (Fig. 4 C and D). Moreover, both actin and microtubule integrity were required for optimal content transfer between phagosomes (Fig. 4 C and D), as they were for tubulation (Fig. 2 C and D). The requirements for actin dynamics, microtubule integrity, and MyD88-dependent signaling in tubule formation were more absolute than in content exchange, indicating that some degree of transfer occurs even in the absence of tubules. Nevertheless, these data suggest that phagotubules facilitate MyD88-dependent content transfer between phagosomes.
TLR-Dependent Phagosomal Cross-Talk Enhances MHC-II Presentation.
We next tested whether phagotubule-dependent phagosomal cross-talk would enhance MHC-II presentation, perhaps by exposing MHC-II molecules from multiple TLR signaling phagosomes to potential peptide sources. BMDCs were pulsed simultaneously with two cohorts of beads, one cohort coated with LPS, Eα, and OVA (Eα/ΟVA beads) and the other either uncoated (not expected to activate TLRs) or coated with LPS and OVA but not Eα (OVA beads) to further stimulate interphagosomal exchange. DCs were left untreated or treated at pulse with IRAK-1/4 inhibitor or at chase with nocodazole, both of which prevent phagotubule formation (Fig. 2), and MHC-II:Eα surface expression was detected by flow cytometry using YAe. In DCs exposed to Eα/ΟVA beads and OVA beads, Eα presentation was significantly enhanced relative to DCs exposed to Eα/OVA beads and uncoated beads (Fig. 5A). This enhancement was not related to increased expression of MHC-II molecules or the costimulatory molecule CD40 during the chase or to increased phagocytic uptake (Fig. 5 B and C), and did not reflect altered OVA proteolysis (16). Moreover, whereas Eα presentation was only mildly impaired by both inhibitors in cells exposed to Eα/ΟVA beads and uncoated beads—consistent with results shown in Fig. 2E—the inhibitors ablated the increased presentation observed in cells exposed to additional OVA (LPS-containing) beads (Fig. 5A).
To determine whether the enhanced MHC-II presentation by additional LPS-containing beads led to an enhanced T-cell response, we exploited OVA323–339-specific/I-Ab–restricted OT-II T cells and OVA as an antigen. DCs were pulsed with beads coated with LPS, OVA, and BSA (OVA/BSA beads) together with either BSA beads (containing LPS but lacking OVA) or uncoated beads, and then cocultured with OT-II cells. T-cell activation, measured by CD69 expression and IL-2 secretion (Fig. 5 D and E), and T-cell proliferation, measured by carboxyfluorescein succinimidyl ester (CFSE) dilution (Fig. 5F), were significantly enhanced in cocultures with DCs pulsed with both types of LPS-coated beads compared with DCs exposed to OVA/BSA beads and uncoated beads. These data indicate that cross-talk among TLR-signaling phagosomes that carry different cargo enhances MHC-II presentation and T-cell responses.
Discussion
We have shown that in DCs, maturing phagosomes emit long tubules that harbor luminal phagosome contents several hours after phagocytosis. These late-onset tubules are distinct from tubules previously shown to emanate from early phagosomes and that function in recycling phagosomal membrane contents (22, 23). Tubule formation requires intact microtubules and actin dynamics and MyD88-dependent TLR signaling, but not phagolysosome formation or extensive proteolysis. The late-onset tubules are not as long as those that emerge from lysosomes after TLR stimulation (5, 7) and, unlike the lysosomal tubules, they are not essential for the delivery of phagosome-derived MHC-II–peptide complexes to the plasma membrane. Rather, our data suggest that they promote MHC-II presentation by facilitating exchange of contents among distinct TLR-signaling phagosomes. Our data suggest that phagotubules in DCs are functionally distinct from those that emerge from lysosomes and are unique adaptations of the DC phagocytic machinery.
Our data confirm earlier observations that phagosomal TLR signaling stimulates proinflammatory cytokine secretion (10, 11) and proteolytic activity (14), antigen processing (15), and MHC-II presentation from phagosomes (11). However, whereas global TLR signaling in DCs induces lysosomal tubulation that traffics endolysosome-derived MHC-II–peptide complexes to the cell surface (6, 7), we show that phagosomal TLR4 signaling stimulates the formation of tubules that are not required for cell surface access of phagosome-derived MHC-II–peptide complexes. Phagosomal tubule formation and antigen presentation were uncoupled using pharmacologic agents or gene targeting to block TLR signaling (in which phagotubules did not form but antigen presentation was largely intact) or phagolysosomal fusion (in which phagotubules were intact but antigen presentation was blocked). Moreover, phagotubules were not observed to fuse with the plasma membrane under conditions in which fusion of endolysosome-derived tubules was readily apparent. These observations suggest that phagosome-derived MHC-II–peptide complexes are delivered to the plasma membrane by other means via vesicles or content transfer to lysosomes, as also has been observed in the endolysosomal system (20, 38).
Phagosomal tubules formed with kinetics similar to that of phagosomal TLR4 and MyD88 recruitment following phagocytosis (10, 11), required MyD88 signaling through IRAK-1/4, and did not require TRAM-TRIF signaling through TBK-1 and IRF3. Moreover, TLR4 signaling events that stimulated phagotubule formation were independent of those that led to proinflammatory cytokine secretion, and were not substituted by signals downstream of MyD88-independent TLR3. These findings indicate that tubule formation from phagosomes is not the result of generalized TLR signaling and occurs on phagosomes carrying specific microbial patterns, such as LPS, supporting the concept that phagosomal contents elicit specific responses (39).
Relative to previously described endolysosomal tubules, phagosomal tubules were shorter in length and delayed in appearance relative to cargo uptake. Moreover, whereas lysosomal tubules fuse with the plasma membrane and extend toward the immunologic synapse (5, 7), phagosomal tubules were rarely detected near the plasma membrane. Rather, the tubules often contacted other phagosomes, correlating with content exchange among multiple phagosomes within a DC. A mechanism of tubule-based content exchange supports earlier observations that phagosome-derived tubules in RAW cells contact lysosomes (21), and that lysosomal tubules facilitate phagosome–lysosome fusion (40, 41). Although fusion between Leishmania-containing phagosomes has been previously observed in macrophages expressing a constitutively active form of RAB5, and RAB5 regulates kiss-and-run fusion between phagosomes and early endosomes (37), the events described here occur much later during phagosome maturation, are regulated by TLR signaling, and uniquely allow for exchange of contents that require intraphagosomal hydrolysis for their release. Efficient content exchange between phagosomes had the same requirements as phagotubule formation, including MyD88- and IRAK-1/4–dependent TLR signaling and intact microtubule and actin networks. Thus, although it is possible that tubulation and content exchange are distinct coregulated events, these data support the notion that the tubules are in part responsible for content exchange.
What are the functional consequences of phagosome content exchange? We found that MHC-II presentation of a fixed quantity of phagocytosed antigen by DCs was enhanced by the presence of additional phagosomes carrying a different antigen and a TLR stimulus. The enhanced presentation in turn enhanced the activation and proliferation of naïve antigen-specific T cells. The additional signaling phagosomes did not alter antigen proteolysis or generally increase cellular activation status in DCs, and although we cannot exclude the possibility of other potential TLR-dependent mechanisms, our data indicate that phagosomal cross-talk correlates with, and is likely responsible for, the enhanced MHC-II presentation. We speculate that content transfer between phagosomes engaged in TLR signaling favors the access of cargo-derived peptides to a larger pool of MHC-II molecules. This cross-talk may be of special importance during an infection, and would prevent pathogen immune escape by maximizing the presentation of MHC-II–peptide complexes from phagosomes containing different arrays of pathogen-derived peptides and MHC-II molecules.
Our results also suggest refinements to the concept of autonomous phagosome signaling (14, 16). The exchange of contents only among phagosomes that harbor a TLR ligand suggests that although phagosomes that lack signaling ligands remain isolated, those that bear signaling ligands might form an interacting network that optimizes pathogen sensing and presentation to the adaptive immune system.
Experimental Procedures
SI Experimental Procedures provides details on antibodies, pharmacologic treatments, other reagents, mouse strains, cell culture, and procedures.
Mice and Cell Culture.
Sex- and age-matched mice at 6–12 wk of age were used in all experiments. The mice were housed and bred under pathogen-free conditions in accordance with University of Pennsylvania Institutional Animal Care and Use Committee-approved protocols.
Statistical Analyses.
Statistical significance for experimental samples relative to untreated WT control (unless stated otherwise) was determined using the unpaired Student t test and ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary Material
Acknowledgments
We thank S. Grinstein for helpful discussions; M. Pepper Pew, M. Jenkins, A. Rudensky, J. Liboon, S. Ross, S. Akira, A. Ávalos, H. Ploegh, E. Lien, K. Moody, E. Behrens, C. Hunter, M. Chou, J. Burkhardt, and C. López for generous gifts of reagents; A. Stout for assistance with spinning disk microscopy; and R. Botelho for critical reading of the manuscript. This work was supported by National Institutes of Health Grants R01 EY015625 (to M.S.M.) and R21 AI092398 (to A.R.M. and M.S.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412998111/-/DCSupplemental.
References
- 1.Fairn GD, Grinstein S. How nascent phagosomes mature to become phagolysosomes. Trends Immunol. 2012;33(8):397–405. doi: 10.1016/j.it.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Kagan JC, Iwasaki A. Phagosome as the organelle linking innate and adaptive immunity. Traffic. 2012;13(8):1053–1061. doi: 10.1111/j.1600-0854.2012.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantegazza AR, Magalhaes JG, Amigorena S, Marks MS. Presentation of phagocytosed antigens by MHC class I and II. Traffic. 2013;14(2):135–152. doi: 10.1111/tra.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts C, West MA, Zaru R. TLR signalling regulated antigen presentation in dendritic cells. Curr Opin Immunol. 2010;22(1):124–130. doi: 10.1016/j.coi.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Boes M, et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418(6901):983–988. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- 6.Boes M, et al. T cells induce extended class II MHC compartments in dendritic cells in a Toll-like receptor-dependent manner. J Immunol. 2003;171(8):4081–4088. doi: 10.4049/jimmunol.171.8.4081. [DOI] [PubMed] [Google Scholar]
- 7.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418(6901):988–994. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 8.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: Regulation through compartmentalization. Nat Rev Immunol. 2009;9(8):535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Husebye H, et al. The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity. 2010;33(4):583–596. doi: 10.1016/j.immuni.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantegazza AR, et al. Adaptor protein-3 in dendritic cells facilitates phagosomal Toll-like receptor signaling and antigen presentation to CD4(+) T cells. Immunity. 2012;36(5):782–794. doi: 10.1016/j.immuni.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underhill DM, et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401(6755):811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 13.Wolf AJ, et al. Phagosomal degradation increases TLR access to bacterial ligands and enhances macrophage sensitivity to bacteria. J Immunol. 2011;187(11):6002–6010. doi: 10.4049/jimmunol.1100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from Toll-like receptors. Science. 2004;304(5673):1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 15.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440(7085):808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann E, et al. Autonomous phagosomal degradation and antigen presentation in dendritic cells. Proc Natl Acad Sci USA. 2012;109(36):14556–14561. doi: 10.1073/pnas.1203912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blander JM. Coupling Toll-like receptor signaling with phagocytosis: Potentiation of antigen presentation. Trends Immunol. 2007;28(1):19–25. doi: 10.1016/j.it.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Kleijmeer M, et al. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol. 2001;155(1):53–63. doi: 10.1083/jcb.200103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierre P, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388(6644):787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 20.Turley SJ, et al. Transport of peptide–MHC class II complexes in developing dendritic cells. Science. 2000;288(5465):522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 21.Harrison RE, Bucci C, Vieira OV, Schroer TA, Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: Role of Rab7 and RILP. Mol Cell Biol. 2003;23(18):6494–6506. doi: 10.1128/MCB.23.18.6494-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D, et al. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science. 2010;327(5970):1261–1264. doi: 10.1126/science.1184840. [DOI] [PubMed] [Google Scholar]
- 23.Damiani MT, Colombo MI. Microfilaments and microtubules regulate recycling from phagosomes. Exp Cell Res. 2003;289(1):152–161. doi: 10.1016/s0014-4827(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 24.Jancic C, et al. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9(4):367–378. doi: 10.1038/ncb1552. [DOI] [PubMed] [Google Scholar]
- 25.Dunne A, et al. IRAK1 and IRAK4 promote phosphorylation, ubiquitination, and degradation of MyD88 adaptor-like (Mal) J Biol Chem. 2010;285(24):18276–18282. doi: 10.1074/jbc.M109.098137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark K, Plater L, Peggie M, Cohen P. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: A distinct upstream kinase mediates Ser-172 phosphorylation and activation. J Biol Chem. 2009;284(21):14136–14146. doi: 10.1074/jbc.M109.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Florey O, Overholtzer M. Autophagy proteins in macroendocytic engulfment. Trends Cell Biol. 2012;22(7):374–380. doi: 10.1016/j.tcb.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanjuan MA, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450(7173):1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 29.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HK, et al. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32(2):227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollenbeck PJ, Swanson JA. Radial extension of macrophage tubular lysosomes supported by kinesin. Nature. 1990;346(6287):864–866. doi: 10.1038/346864a0. [DOI] [PubMed] [Google Scholar]
- 32.Mrakovic A, Kay JG, Furuya W, Brumell JH, Botelho RJ. Rab7 and Arl8 GTPases are necessary for lysosome tubulation in macrophages. Traffic. 2012;13(12):1667–1679. doi: 10.1111/tra.12003. [DOI] [PubMed] [Google Scholar]
- 33.Blocker A, Griffiths G, Olivo JC, Hyman AA, Severin FF. A role for microtubule dynamics in phagosome movement. J Cell Sci. 1998;111(Pt 3):303–312. doi: 10.1242/jcs.111.3.303. [DOI] [PubMed] [Google Scholar]
- 34.Desjardins M, Huber LA, Parton RG, Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124(5):677–688. doi: 10.1083/jcb.124.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudensky AYu, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr On the complexity of self. Nature. 1991;353(6345):660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 36.Axelrod D. Cell–substrate contacts illuminated by total internal reflection fluorescence. J Cell Biol. 1981;89(1):141–145. doi: 10.1083/jcb.89.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duclos S, Corsini R, Desjardins M. Remodeling of endosomes during lysosome biogenesis involves “kiss and run” fusion events regulated by rab5. J Cell Sci. 2003;116(Pt 5):907–918. doi: 10.1242/jcs.00259. [DOI] [PubMed] [Google Scholar]
- 38.Wubbolts R, et al. Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J Cell Biol. 1996;135(3):611–622. doi: 10.1083/jcb.135.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012;12(7):492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harding CV, Geuze HJ. Class II MHC molecules are present in macrophage lysosomes and phagolysosomes that function in the phagocytic processing of Listeria monocytogenes for presentation to T cells. J Cell Biol. 1992;119(3):531–542. doi: 10.1083/jcb.119.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121(5):1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.