Significance
Our study shows that pyruvate kinase M2 (PKM2), an alternatively spliced variant of the pyruvate kinase gene, mediates epithelial–mesenchymal transition (EMT), which is critical for cancer cells to acquire invasive potential. Our study demonstrates that EMT stimulates nuclear translocation of PKM2 and decreases epithelial cadherin transcription (a requirement for EMT induction). Our results also demonstrate that PKM2 interacts with the transcriptional factor TGF-β–induced factor homeobox 2, which induces the deacetylation of histone H3, resulting in repressed E-cadherin expression. The precise understanding of nuclear PKM2 function suggests the potential for a model preventing cancer metastasis.
Keywords: pyruvate kinase M2, epithelial–mesenchymal transition, colorectal cancer, invasion, transforming growth factor-β–induced factor homeobox 2
Abstract
Pyruvate kinase M2 (PKM2) is an alternatively spliced variant of the pyruvate kinase gene that is preferentially expressed during embryonic development and in cancer cells. PKM2 alters the final rate-limiting step of glycolysis, resulting in the cancer-specific Warburg effect (also referred to as aerobic glycolysis). Although previous reports suggest that PKM2 functions in nonmetabolic transcriptional regulation, its significance in cancer biology remains elusive. Here we report that stimulation of epithelial–mesenchymal transition (EMT) results in the nuclear translocation of PKM2 in colon cancer cells, which is pivotal in promoting EMT. Immunoprecipitation and LC-electrospray ionized TOF MS analyses revealed that EMT stimulation causes direct interaction of PKM2 in the nucleus with TGF-β–induced factor homeobox 2 (TGIF2), a transcriptional cofactor repressor of TGF-β signaling. The binding of PKM2 with TGIF2 recruits histone deacetylase 3 to the E-cadherin promoter sequence, with subsequent deacetylation of histone H3 and suppression of E-cadherin transcription. This previously unidentified finding of the molecular interaction of PKM2 in the nucleus sheds light on the significance of PKM2 expression in cancer cells.
Colorectal cancer (CRC) is the second most common cancer in the world, with more than 1.2 million new cases and about 600,000 deaths annually (1). Cancerous cells exploit a cancer-specific glycolytic system known as the Warburg effect (also referred to as aerobic glycolysis), which involves rapid glucose uptake and preferential conversion to lactate, despite an abundance of oxygen (2, 3). The precise mechanism underpinning aerobic glycolysis was unclear for a long time. However, in 2008, pyruvate kinase M2 (PKM2) gained attention when its expression was shown to be required for the maintenance of aerobic glycolysis (4). PKM2 is an alternatively spliced variant of the PKM gene that regulates the final rate-limiting step of glycolysis. PKM2 is expressed during embryonic development, but it is generally not expressed in most adult tissues. However, its counterpart, PKM1, is exclusively expressed in adult tissues. PKM2 has been shown to be reactivated in tumor development (5, 6). In cancer cells, PKM2 expression allows the diversion of glycolytic flux into the pentose phosphate pathway associated with attenuated pyruvate kinase activity, thereby meeting the biosynthetic demands for rapid proliferation (3).
Investigations about the nuclear function of PKM2 arose after elucidation of the PKM2 metabolic function. It was identified that in cancer cells, PKM2 can translocate into the nucleus and function as a transcriptional cofactor in response to several extracellular signals, including EGF and hypoxia, subsequently activating CYCLIN D1, C-MYC, or hypoxia-inducible factor 1α (HIF-1α) (7, 8). Particularly in the hypoxic condition, PKM2 interacts with HIF-1α and participates in a positive feedback loop, thereby enhancing HIF-1α transactivation and reprogramming glucose metabolism by regulating the expression of glycolysis-associated enzymes (8). This finding suggested that the PKM2 nuclear function may operate upstream of metabolic regulation and that the resultant metabolic reprogramming and oncogene activation by PKM2 work cooperatively to promote cancer cell proliferation and tumor growth.
In addition to proliferation maintenance and growth suppression prevention, invasion and metastasis have also been targeted as hallmarks of cancer (9). In the invasion process, cancer cells acquire the ability to dissociate from the bulk of the tumor and to migrate into the surrounding stroma, which is regulated by epithelial–mesenchymal transition (EMT) (9, 10). During EMT, cancer cells lose their cell-to-cell contacts by inhibiting epithelial cadherin (E-cadherin; encoded by CDH1) expression and acquiring mesenchymal markers. This process is physiologically important during embryogenesis and is required for in utero development. Given that PKM2 expression and EMT are common to both tumorigenesis and development, PKM2 may affect EMT within cancer cells. However, the significance of PKM2 during EMT or invasion is yet to be investigated.
In the present study, we demonstrate that PKM2 translocates into the nucleus during EMT and acts as a transcription cofactor that inhibits CDH1 expression. PKM2 interacts with TGF-β–induced factor homeobox 2 (TGIF2), which recruits histone deacetylase 3 (HDAC3) to the promoter sequence of E-cadherin, thereby promoting histone H3 lysine 9 (H3K9) deacetylation and CDH1 expression down-regulation.
Results
EMT Induction Elicits Nuclear Translocation of PKM2.
For the induction of EMT, we cultured colon cancer cells in a medium with TGF-β1 and EGF, as described previously (Fig. 1A) (11–14). The SW480 cells changed morphology from epithelial to fibroblastic-like and spindle-shaped in a time-dependent manner (Fig. 1B). Consistent with this observation, CDH1 transcript expression was suppressed, whereas the expression levels of the vimentin (VIM), zinc finger e-box binding homeobox 1 (ZEB1), and snail family zinc finger 2 (SNAI2) genes were increased (Fig. 1C). PK gene expression was induced in the EMT condition, with preferential expression of PKM2 compared with PKM1 (Fig. 1D). Western blot analysis indicated that the induction of EMT resulted in decreased CDH1 expression, increased VIM expression, and up-regulated PKM2 (Fig. 1E). We confirmed that the expression and secretion of endogenous TGF-β1 was minimal in SW480 (Fig. S1 A and B).
Fig. 1.
PKM2 translocates into the nucleus during EMT. (A) Schematic representation of the procedure for EMT induction. The cells incubated for 48 h after seeding are defined as pre-EMT, and the cells cultured with 2.5 ng/mL TGF-β1 and 10 ng/mL EGF are defined as post-EMT. (B) Photomicrographs of the morphological change in SW480 cells. The cells were stained using the Diff-Quik Kit (Sysmex Corp.). The number of hours indicates the period since EMT induction was initiated. (Scale bar, 100 μm.) (C) Relative transcript (mRNA) levels of CDH1, VIM, ZEB1, and SNAI2 after induction of EMT for 0, 48, and 96 h. The values at 0 h (pre-EMT) have been normalized to 1, and the data are expressed as fold. (D) Relative mRNA levels of PKM1, PKM2, and pyruvate kinase (total PK) after induction of EMT for 0, 48, and 96 h. (E) Western blot assays of E-cadherin, vimentin, and PKM2 expression in pre-EMT and post-EMT cells. Post-EMT cells were harvested at 72 h. (F) Western blot assays of PKM1, PKM2, α-tubulin, and histone H3 in nuclear and cytoplasmic lysates prepared from SW480 cells. With normalization to cytoplasmic tubulin or nuclear histone H3 blots, the relative intensities of PKM2 blots are shown in comparison with those in the pre-EMT condition. (G) SW480 cells were treated with dimethyl suberimidate for 30–60 min, immediately followed by whole cell lysis. The monomer and dimer states of PKM2 were analyzed by Western blot assay. Columns represent the average of at least three independent experiments; error bars represent the SD of the mean from triplicate results. *P < 0.05.
To determine the intracellular localization of proteins, cytoplasmic and nuclear fractions were separated from the EMT-induced cells and Western blot analysis was performed. The data indicated that, although the EMT condition stimulated an increase in cytoplasmic PKM2, nuclear PKM2 was augmented compared with levels in the pre-EMT state (Fig. 1F). Immunocytochemistry and immunofluorescence intensity quantification confirmed the increase in nuclear PKM2 (Fig. S2 A–D). In addition, we confirmed that nuclear PKM2 was also increased in HCT116 cells under the same EMT condition (Fig. S2E) and that the expression of EMT markers was increased in murine Pkm2 knock-in, compared with Pkm1 knock-in, mesencymal cells, as well as other human cancer cells (Fig. S1 C and D).
Previous studies showed that EGF stimulation increased nuclear PKM2 (7) and indicated that cytoplasmic PKM2 functions with tetramer formation, whereas nuclear PKM2 functions with dimer formation. Given that the large hydrophobic hole at the nucleotide binding site is buried in tetrameric PKM2 structure, which becomes accessible in dimer form (15), the dimer formation may provide a protein binding ability. We studied the status of PKM2 during EMT and found that simultaneous stimulation by TGF-β1 and EGF, in comparison with either alone, resulted in increased expression of an ∼120-kDa complex, corresponding to dimeric PKM2 (Fig. 1G and Fig. S3). The present study demonstrated that PKM2 nuclear translocation was stimulated in the EMT condition, suggesting a unique function of PKM2 in the nucleus.
PKM2 Expression Is Required to Induce EMT.
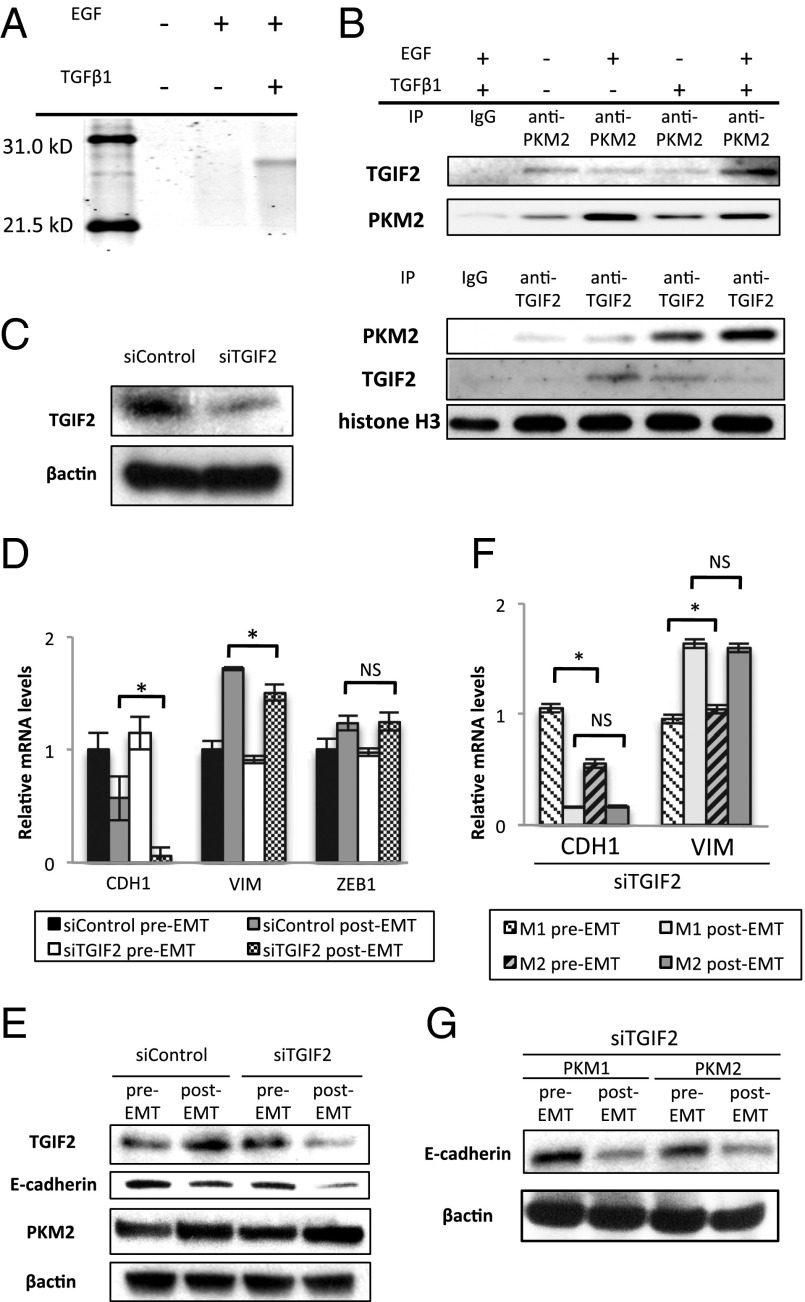
To investigate the causative role of PKM2 in EMT induction, we cultured cells with endogenous PKM2 inhibition by small interfering RNA (siRNA) knockdown (KD) under EMT conditions. We used the siRNA targeting system, which reportedly inhibits PKM2 without any off-target effects on other genes (16), and the results indicate that the most effective siRNA sequence could inhibit transcriptional and translational levels of PKM2, whereas those of PKM1 were increased (Fig. S4 A and B). PKM2 KD failed to induce spindle-shaped morphological changes under EMT conditions (Fig. 2A). Expression analysis indicated that PKM2 KD prevented CDH1 down-regulation, although VIM expression persisted (Fig. 2B), suggesting a role for PKM2 in CDH1 transcription. Fifty percent reductions in glucose or glutamine in the medium did not have significant effects on EMT marker expression (Fig. S5A), suggesting distinct effects on EMT and metabolism.
Fig. 2.
PKM2 is required for EMT induction. (A) Phase-contrast photomicrographs of SW480 cells transfected with siControl or siPKM2 after EMT induction for 48 h. (B) Relative transcript (mRNA) levels of CDH1 and VIM after EMT induction in cells transfected with siControl or siPKM2 for 48 h. (C) Western blot assays of E-cadherin, vimentin, PKM2, and β-actin expression in pre-EMT and post-EMT cells. Post-EMT cell samples were harvested at 72 h. With normalization to β-actin as a control, the relative intensities of E-cadherin and vimentin are shown in comparison with those in the control pre-EMT condition. Note that siPKM2 knockdown works efficiently in post-EMT cells. (D) Invasive behavior of SW480 cells treated with siControl or siPKM2. (E) Schematic procedure for establishing PKM1 OE or PKM2 OE SW480 cells. (F) Western blot assays of PKM1, PKM2, and β-actin expression in WT SW480 cells, cells stably expressing shRNA constructs targeting pyruvate kinase (shPK), and shPK cells overexpressing either PKM1 or PKM2 constructs. (G) Relative mRNA levels of CDH1, VIM, and ZEB1 after EMT induction in PKM1 OE or PKM2 OE SW480 cells for 72 h. (H) Western blot assays of E-cadherin, vimentin, and β-actin expression in PKM1 OE and PKM2 OE cells. Post-EMT cell samples were harvested at 72 h. Column values = average of at least three independent experiments; error bars represent SD from the mean of triplicate experiments. *P < 0.05.
Western blot analysis indicated that PKM2 KD hindered CDH1 loss and VIM gain compared with the control (Fig. 2C). Inhibition of EMT by PKM2 KD resulted in a significant reduction in in vitro cellular invasiveness (Fig. 2D). The assessment of mothers against decapentaplegic homolog 2 (SMAD2) and ERK, which are downstream effectors of TGF-β1 and EGF signaling, indicated that PKM2 KD disturbed the phosphorylation process (Fig. S5B).
To minimize the effect of an alternative exon and to focus on the function of PKM2 in the nucleus, we established PKM1- and PKM2-overexpressing (OE) cell lines (PKM1 and PKM2 OE in Fig. 2E). In brief, we transfected the cells with a small hairpin RNA (shRNA) vector targeting the common region in PK and then introduced an overexpression vector of PKM1 or PKM2 cDNA without a complementary sequence to the shRNA (Fig. 2F). We cultured the established cells in EMT-inducing conditions. The results demonstrated a greater decrease in CDH1 expression and greater increase in VIM and ZEB1 expression in PKM2 OE cells compared with that in PKM1 OE cells (Fig. 2G and Fig. S4C). Consistent results were obtained by Western blot analysis (Fig. 2H). These results indicate that PKM2 expression is necessary for EMT induction.
Nuclear PKM2 Binds to TGIF2 and Represses CHD1 Expression.
Nuclear PKM2 reportedly binds to and phosphorylates STAT3 through its function as a protein kinase (15). The observation that nuclear PKM2 increased during EMT led us to consider the possibility that PKM2 may interact with other transcription factors. To validate this hypothesis, fractions pulled-down with the PKM2 antibody were subjected to LC-electrospray ionized TOF MS analyses. The result showed that nuclear PKM2 was coimmunoprecipitated with TGIF2 and that this binding was detectable when both EGF and TGFβ1 were added to the culture (Fig. 3A). These findings were confirmed by immunoprecipitation, followed by Western blot analysis (Fig. 3B). The EMT stimulation resulted in the significant increase of TGIF2 expression (Fig. S6A). TGIF2 KD did not show significant alterations of PKM2 expression regardless of EMT induction (Fig. 3E and Fig. S6B). We could not detect an association of PKM1 with TGIF2 in the nucleus (Fig. S7A), which further supports the cytoplasmic localization of PKM1 (Fig. 1F).
Fig. 3.
Interaction between nuclear PKM2 and TGIF2 mediates EMT induction. (A) Polyacrylamide gel electrophoresis of proteins immunoprecipitated with anti-PKM2 antibody in the nucleic lysate of cells cultured under normal conditions, with EGF alone, or with TGF-β1 and EGF. The band detected in samples of cells stimulated with TGF-β1 and EGF was excised and analyzed by MS. (B) Western blot assays of immunoprecipitated samples of nucleic lysates with anti-PKM2 or anti-TGIF2 antibody. Samples were harvested after the cells were treated as indicated for 72 h. (C) Western blot assays of TGIF2 and β-actin expression in cells transfected with siControl or siTGIF2. (D) Relative transcript (mRNA) levels of CDH1, VIM, and ZEB1 after induction of EMT in cells transfected with siControl or siTGIF2 for 72 h. (E) Western blot analysis of TGIF2, E-cadherin, PKM2, and β-actin expression in pre-EMT and post-EMT cells transfected with siControl or siTGIF2. Post-EMT samples were harvested at 72 h, when siRNA inhibition was profound. (F) Relative mRNA levels of CDH1 and VIM after EMT induction in PKM1 OE and PKM2 OE cells. Post-EMT samples were harvested at 72 h. (G) Western blot analysis of E-cadherin and β-actin after EMT induction in PKM1 OE and PKM2 OE cells transfected with siTGIF2. Post-EMT samples were harvested at 72 h. Column values = average of at least three independent experiments; error bars represent SD from the mean of triplicate experiments. *P < 0.05.
Melhuish et al. (17) revealed that TGIF2 is a transcriptional repressor that suppresses TGF-β–responsive gene expression by binding to TGF-β–activated SMADs. First, we performed TGIF2 KD, followed by EMT induction (Fig. 3C and Fig. S6B). TGIF2 KD enhanced the decrease in both the transcriptional and the translational levels of CDH1 expression (Fig. 3 D and E). To analyze the difference in the effect of TGIF2 KD in cells expressing either PKM1 or PKM2, we performed TGIF2 KD on PKM1 OE and PKM2 OE cells, followed by EMT induction. Interestingly, the decrease in CDH1 expression and increase in VIM expression were similar at the transcriptional and translational levels after EMT induction in both cell lines (Fig. 3 F and G). These results indicate that the augmented sensitivity to EMT induction in PKM2 OE cells is abrogated under TGIF2 suppression. These data further suggest that nuclear PKM2 responds to EMT stimulation and interacts with TGIF2 to mediate EMT induction downstream of PKM2.
PKM2 and TGIF2 Recruit HDAC3 to the CDH1 Promoter to Repress Transcription.
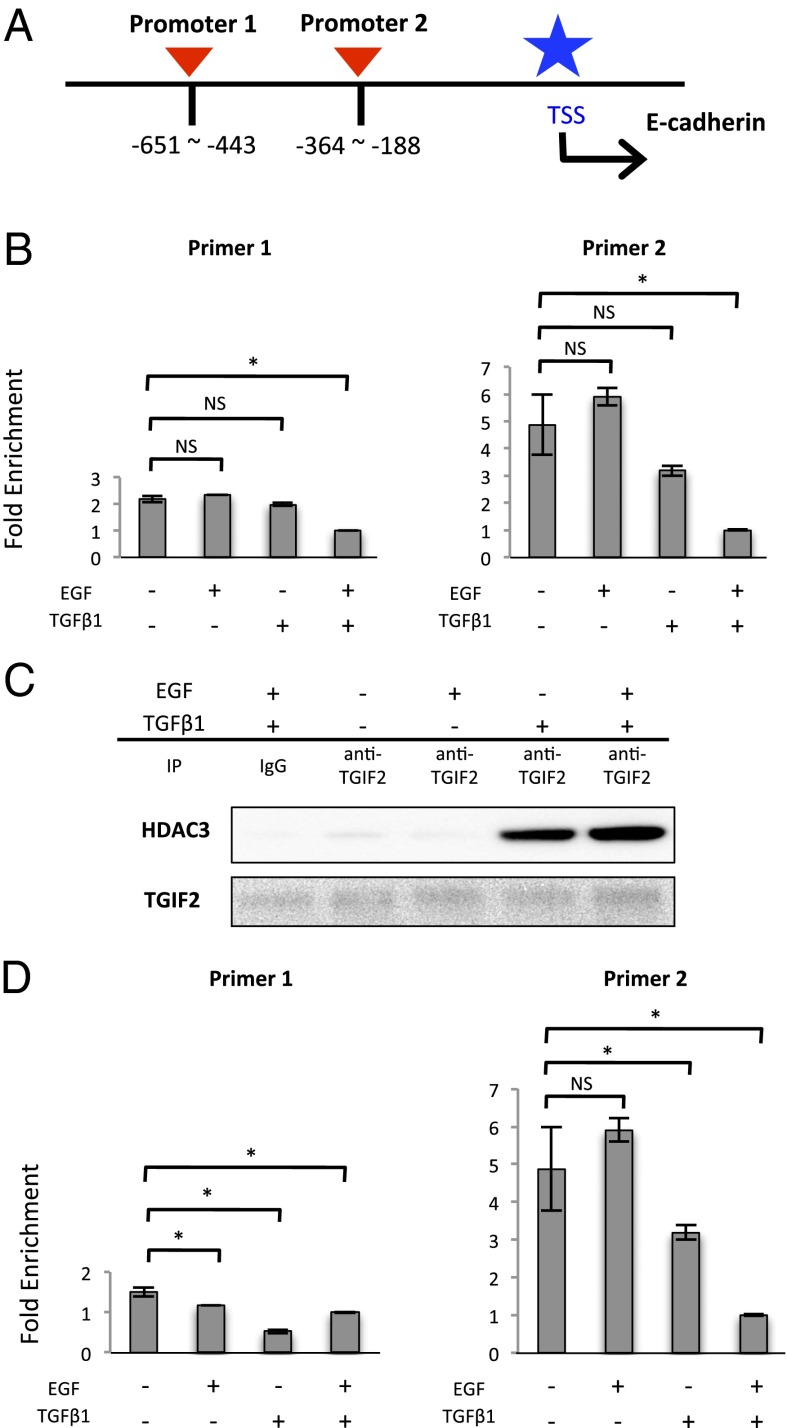
TGIF2 is a transcriptional factor that regulates TGF-β signal transduction (17). Based on the above findings, we hypothesized that TGIF2 could bind to the CDH1 promoter and activate CDH1 expression in the epithelial state. To examine this hypothesis, we performed a ChIP quantitative PCR (qPCR) assay using two sets of primers located in the CDH1 promoter sequence region (Fig. 4A). We found depressed binding of TGIF2 to the CDH1 promoter region during EMT (Fig. 4B).
Fig. 4.
TGIF2 binds to the CDH promoter and recruits HDAC3 during EMT. (A) Schematic diagram showing the positions of two sets of primers designed to cover the promoter region of the CDH1 gene. (B) ChIP assays were performed with IgG and anti-TGIF2 antibody, followed by qPCR (mean ± SD, n = 3). ChIP samples were harvested from the nucleic lysate of SW480 cells treated as indicated for 72 h. (C) Western blot assays of immunoprecipitated samples of nucleic lysate with anti-TGIF2 antibody. Each sample was harvested after the cells were treated as indicated for 72 h. (D) ChIP assays were performed with IgG and anti-acetylated H3K9 antibody, followed by qPCR (mean ± SD, n = 3). ChIP samples were harvested from the nucleic lysate of SW480 cells treated as indicated for 72 h. Column values = average of at least three independent experiments; error bars represent SD from the mean of triplicate experiments. *P < 0.05.
TGIF2 can control transcription by recruiting HDAC in response to TGF-β signaling (17) and PKM2 can associate with HDAC3 in the nucleus (7). To investigate whether TGIF2 can bind to HDAC3 during EMT, we performed immunoprecipitation followed by Western blot analysis and found an association between TGIF2 and HDAC3 under EMT induction (Fig. 4C and Figs. S7B and S8). To examine the acetylation status of histone H3 in the CDH1 promoter region, we performed ChIP qPCR and found that binding of acetylated H3K9 to the CDH1 promoter was decreased under EMT conditions (Fig. 4D). Furthermore, to understand how the PKM2–TGIF2–HDAC3 complex can bind to the CDH1 promoter, additional ChIP qPCR analysis was performed. The data indicated that similar to the binding of TGIF2, the binding of PKM2 and HDAC3 to the CDH1 promoter was reduced during EMT (Fig. S9 A and B). Given that the TGIF2 protein bound to PKM2 and HDAC3 during EMT (Figs. 3B and 4C and Fig. S7B), the present study demonstrates that nuclear PKM2 plays a role in the TGIF2-dependent control of CHD1 expression and that EGF induces formation of the PKM2–TGIF2–HDAC3 complex, followed by histone deacetylation, thus resulting in suppressed CDH1 expression. TGF-β1 may modulate the association of this complex, although H3K9 was deacetylated (Fig. 5D).
Fig. 5.
The immunohistochemistry. (A) Staining at the invasive front, showing an inverse correlation between PKM2, E-cadherin, and TGIF2 expression. (Scale bar, 100 μm.) (B) The representative cases are shown for staining for PKM2, TGIF2, and E-cadherin. Invasive fronts of tumors were stained by anti-PKM2, anti-E-cadherin, and anti-TGIF2 antibodies, and the intensities were assigned to positive and negative groups. With regard to TGIF2 staining, under the microscopic observation, cases with more than 50% of cells stained in nucleus were designated as positive, whereas the others were negative. (C) The 10 positive and 10 negative cases for cellular PKM2 were examined for nuclear TGIF2 and membranous E-cadherin. (D) Theoretical model illustrating the functional roles of PKM2 and TGIF2 in regulating CDH1 transcription during EMT.
PKM2 Expression in the Deepest Tumor Regions Correlated with CRC Metastasis.
To investigate the clinical significance of PKM2 expression in cancer metastasis, we immunohistochemically analyzed clinical CRC samples. Staining was assessed in the deepest tumor regions where the CRC invasion begins (18, 19). The PKM2 staining intensities were assigned to positive and negative groups (Fig. 5 A–C). The correlations between PKM2 expression and clinicopathological factors are summarized in Table S1. PKM2-positive staining was significantly correlated with metastasis to lymph nodes and distant organs. To further understand the clinical significance of PKM2 in CRC, we analyzed the GSE17536 database of the gene expression array and patient prognosis. To study the specific effect of PKM2 in the array database, we analyzed expression of both PK and its splicing factor hnRNPA2, because hnRNPA2 stimulates the splicing to PKM2 (20, 21). As expected, cases with high PK and high hnRNPA2 expression showed a poorer prognosis than other groups; the difference in prognosis was apparent in stages III and IV with metastasis (Fig. S10 A and B). The data confirmed that PKM2 can enhance the ability of cancer cells to metastasize in primary cancer tissues.
Discussion
In the present study, we demonstrated that nuclear PKM2 interacts with TGIF2 during EMT, which is pivotal in promoting the transition into the mesenchymal cancer cell phenotype. Consequently, we propose a model for the nuclear PKM2 function in response to EMT stimulation (Fig. 5D). Under epithelial conditions, histone H3 is acetylated on the CDH1 promoter region and CDH1 is transcribed where TGIF2 should serve as an active transcription factor. Once the EMT signal stimulates transformation of the cancer cell, a PKM2 fraction enters the nucleus and associates with TGIF2. We assume that this association will alter the conformation of TGIF2 or its associated complexes, effectively loosening the binding between TGIF2 and the CDH1 promoter sequence to allow the recruitment of HDAC3 and subsequent histone H3 deacetylation. CDH1 expression is suppressed as a consequence of the down-regulated promoter activity. In this context, nuclear PKM2 serves as a transcriptional cofactor regulating TGIF2 behavior.
Few reports have investigated the significance of TGIF2 in cancer. In ovarian cancer, TGIF2 is reportedly amplified and overexpressed (22), whereas a comparison between colorectal adenoma and colorectal carcinoma revealed that TGIF2 expression is increased only in the latter (23). Further, TGIF2 has been shown to interact with TGF-β–activated SMADs and be able to repress the activation of TGF-β–responsive transcription (17). The present study demonstrated that TGIF2 affects CDH1 expression through the regulation of promoter activity in which TGIF2 is supposed to function as an activating transcription factor.
TGF-β1 is a multifunctional cytokine that has dual and opposing roles in controlling cell fate. In the early stages of cancer, TGF-β1 induces growth arrest and apoptosis, exerting tumor-suppressive effects, whereas in later stages, TGF-β1 enhances tumor progression by provoking a variety of malignancy-related responses, including EMT (24–26). This paradox remains unsolved despite numerous studies addressing the issue. However, based on the results in the present study, we propose that the interaction between PKM2 and TGIF2 may offer a plausible explanation. In normal cells, PK expression is exclusively shifted to PKM1, but on TGF-β signaling, TGIF2 can suppress transcription downstream of the SMAD signal. Conversely, in cancer cells abundantly expressing PKM2, PKM2 translocates and is bound to TGIF2 in the nucleus, thereby reversing TGF-β signal transduction. Further investigation is necessary to determine the significance of TGIF2 expression and the precise mechanism underlying this interaction.
Nuclear PKM2 forms a dimer and functions as a protein kinase, whereas cytoplasmic PKM2 forms a tetramer and functions as a pyruvate kinase (15). In the present study, the dimeric form of PKM2 was increased, suggesting that the protein kinase activity of PKM2 is enhanced during EMT. PKM2 translocates into the nucleus in response to variable signals, of which, the EGF–ERK pathway is the most investigated (7, 27). Interestingly, TGIF2 is phosphorylated in response to EGF signaling (17). Given that EGF induces nuclear translocation of PKM2, PKM2 may function as a dimeric protein kinase in the nucleus, phosphorylating TGIF2. However, the phosphorylation status of TGIF2 was not addressed in our study. Gao et al. (15) demonstrated that PKM2 interacts with STAT3 to control downstream gene expression in SW480 cells. Thus, it is conceivable that the molecular interaction of PKM2 is highly context dependent, with cell fate determined by how nuclear PKM2 regulates gene expression.
PKM2 has both metabolic and nonmetabolic functions, which are essential in the cytoplasm and nucleus, respectively. Increasing evidence has suggested that nuclear PKM2 binds to numerous transcriptional factors, thereby conferring cells with advanced malignant potential. The present study determined that PKM2 significantly influences EMT induction by modulating CDH1 expression, thus providing a molecular basis for EMT acquisition. Future cancer treatments may be able to target the inhibition of nuclear PKM2.
Methods
Cell Lines and Culture.
The human colorectal cancer cell lines, SW480 and HCT116, were obtained from ATCC, and CaR-1 was obtained from JCRB. These cell lines were grown in DMEM (Sigma-Aldrich) supplemented with 10% (vol/vol) FBS (Thermo Fisher Scientific), 100 U/mL penicillin, and 100 U/mL streptomycin (Life Technologies) and grown at 37 °C in a humidified incubator with 5% CO2.
EMT Induction.
Cells were seeded at a concentration of 5.0 × 104 cells/mL and incubated in a humidified atmosphere (37 °C and 5% CO2) in standard medium for 48 h, after which they were treated with TGF-β1 (2.5 ng/mL; Sigma-Aldrich). Next, they were incubated with MEM supplemented with 10 ng/mL FBS-free EGF (Sigma-Aldrich), 100× insulin-transferring selenium (ITS; Life Technologies), and 50 nmol/L hydrocortisone (Tokyo Kasei) for 48–96 h.
Supplementary Material
Acknowledgments
We thank the members of our laboratories for helpful discussions; Idea Consultants, Inc. (Osaka, Japan) and Olympus Co. (Tokyo, Japan) for technical assistance; Lewis C. Cantley for providing the lentiviral shRNA and retroviral expression vector; and H. Miyoshi for providing the packaging plasmids.
Footnotes
Conflict of interest statement: This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology; a Grant-in-Aid from the Third Comprehensive 10-year Strategy for Cancer Control, Ministry of Health, Labor, and Welfare; a grant from the Kobayashi Cancer Research Foundation; a grant from the Princess Takamatsu Cancer Research Fund, Japan; a grant from the National Institute of Biomedical Innovation; and a grant from the Osaka University Drug Discovery Funds. A.H. is a research fellow of the Japan Society for the Promotion of Science. Partial support was received from Taiho Pharmaceutical Co., Ltd. (to J.K., M.M., and H.I.), Chugai Co., Ltd., Yakult Honsha Co., Ltd., Merck Co., Ltd., Takeda Science Foundation, and Takeda Medical Research Foundation (to M.K., N.N., M.M., and H.I.) through institutional endowments.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407717111/-/DCSupplemental.
References
- 1.Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 5.Hacker HJ, Steinberg P, Bannasch P. Pyruvate kinase isoenzyme shift from L-type to M2-type is a late event in hepatocarcinogenesis induced in rats by a choline-deficient/DL-ethionine-supplemented diet. Carcinogenesis. 1998;19(1):99–107. doi: 10.1093/carcin/19.1.99. [DOI] [PubMed] [Google Scholar]
- 6.Elbers JR, et al. Pyruvate kinase activity and isozyme composition in normal fibrous tissue and fibroblastic proliferations. Cancer. 1991;67(10):2552–2559. doi: 10.1002/1097-0142(19910515)67:10<2552::aid-cncr2820671027>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Yang W, et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480(7375):118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo W, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145(5):732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29(6):1092–1095. doi: 10.1093/carcin/bgn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rees JR, Onwuegbusi BA, Save VE, Alderson D, Fitzgerald RC. In vivo and in vitro evidence for transforming growth factor-beta1-mediated epithelial to mesenchymal transition in esophageal adenocarcinoma. Cancer Res. 2006;66(19):9583–9590. doi: 10.1158/0008-5472.CAN-06-1842. [DOI] [PubMed] [Google Scholar]
- 12.Yokobori T, et al. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res. 2013;73(7):2059–2069. doi: 10.1158/0008-5472.CAN-12-0326. [DOI] [PubMed] [Google Scholar]
- 13.Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273(4 Pt 2):F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- 14.Strutz F, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61(5):1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45(5):598–609. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg MS, Sharp PA. Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J Exp Med. 2012;209(2):217–224. doi: 10.1084/jem.20111487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melhuish TA, Gallo CM, Wotton D. TGIF2 interacts with histone deacetylase 1 and represses transcription. J Biol Chem. 2001;276(34):32109–32114. doi: 10.1074/jbc.M103377200. [DOI] [PubMed] [Google Scholar]
- 18.Lugli A, Karamitopoulou E, Zlobec I. Tumour budding: A promising parameter in colorectal cancer. Br J Cancer. 2012;106(11):1713–1717. doi: 10.1038/bjc.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueno H, et al. Novel risk factors for lymph node metastasis in early invasive colorectal cancer: A multi-institution pathology review. J Gastroenterol. 2014;49(9):1314–1323. doi: 10.1007/s00535-013-0881-3. [DOI] [PubMed] [Google Scholar]
- 20.Clower CV, et al. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci USA. 2010;107(5):1894–1899. doi: 10.1073/pnas.0914845107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463(7279):364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imoto I, et al. Amplification and overexpression of TGIF2, a novel homeobox gene of the TALE superclass, in ovarian cancer cell lines. Biochem Biophys Res Commun. 2000;276(1):264–270. doi: 10.1006/bbrc.2000.3449. [DOI] [PubMed] [Google Scholar]
- 23.Lips EH, et al. Integrating chromosomal aberrations and gene expression profiles to dissect rectal tumorigenesis. BMC Cancer. 2008;8:314. doi: 10.1186/1471-2407-8-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci USA. 2003;100(15):8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian M, Schiemann WP. The TGF-beta paradox in human cancer: An update. Future Oncol. 2009;5(2):259–271. doi: 10.2217/14796694.5.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahimi RA, Leof EB. TGF-beta signaling: A tale of two responses. J Cell Biochem. 2007;102(3):593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- 27.Yang W, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. 2012;14(12):1295–1304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.