How cells direct motion in response to environmental stimuli has long fascinated biologists. Chemotaxis, the migration guided by chemical gradients, is a fundamental property of many cells and plays important roles in physiology and pathological conditions. One of the best-studied models for chemotaxis is the social amoeba Dictyostelium discoideum. In nutrient-deprived environments, Dictyostelium cells initiate a developmental program that allows them to aggregate and form fruiting bodies. During this process, cells periodically secrete cAMP, which functions as a chemoattractant to guide their migration. In a field of cells, periodic waves of cAMP are initiated from an aggregation center every ∼6 min and sweep out in concentric circles or spirals. As the waves approach cells, they first experience a spatial gradient, with the high side facing the center. Thereafter, because the spatial profile of the waves is symmetric (1), as the peak of the wave passes cells are faced with an equivalent but oppositely directed gradient (Fig. 1). Despite this change of direction, the overall movement of cells is toward the center. How chemotactic cells are able to sense the approaching wave but appear to ignore it as it moves away is known as the “back of the wave” problem, and has perplexed the field for some time. Two possible explanations have been proposed. The first explanation relies on the fact that cells adapt—or cease to respond—to constant levels of stimuli (2). Therefore, cells are more sensitive during the rising phase of the wave, when the concentration of the chemoattractant is increasing over time, and lose sensitivity at the back of the wave when the concentration is declining. The second explanation notes that over time cells develop an intrinsic polarity with well-defined anterior and posterior regions, and this polarity allows cells to maintain their direction when the guidance cue fluctuates (3). The relative importance of each process in allowing cells to move unidirectionally in periodic waves has not been known, although both suggest that in addition to the spatial profile, cells make use of the temporal information of the concentration. In PNAS, Skoge et al. address this question through careful analysis of migration and the corresponding signaling activities of cells responding to spatiotemporal patterns of cAMP generated by a novel microfluidic device (4). The authors show that cells display a memory that persists beyond—but is modulated by—the adaptation process. This interplay between memory and adaptation allows cells to move against the gradient in the back of the wave.
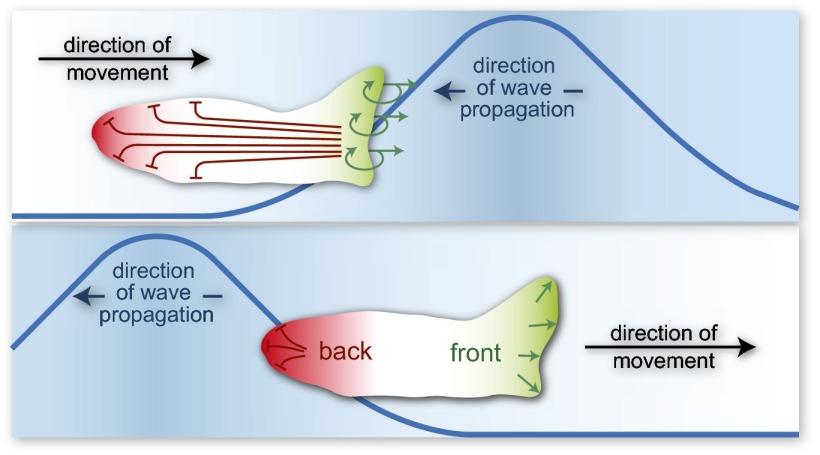
Fig. 1.
Development of polarity during wave propagation. This cartoon illustrates how polarity can develop during chemoattractant wave propagation, allowing the cell to maintain directional motility even as it moves away from the receding wave. (Top) The upper panel shows the cell as it moves in the direction of the approaching wave. Receptors at the front of the cell have higher occupancy, triggering complementary responses. A local positive feedback loop (green arrows) amplifies the signal at the front, and leads to cell movement in this direction. At the same time, long-range inhibition suppresses the back of the cell (red blunt lines). Over time, the cell develops well-defined “front” and “back” regions. (Bottom) The lower panel shows the cell state after the wave has passed. The higher receptor occupancy at the back is unable to overcome the lower sensitivity caused by polarity. The net effect is for movement to continue in a consistent direction.
Since its introduction, microfluidics has been seen as a natural tool for studying cell migration (5). The ability to apply precisely controlled, temporally and spatially varying stimuli, while simultaneously tracking cell dynamics, allows biologists to probe cellular responses to chemoattractants with precision previously unavailable. The device used in Skoge et al.’s (4) study creates a spatial bell-shaped profile of cAMP that sweeps across the length of the chamber, thus being able to mimic the cAMP wave seen by cells, but also having the ability to alter the period between the wave peaks. This device also allows quick reversal or removal of the gradient. Using this device, Dictyostelium cells were exposed to cAMP waves with a period similar to those produced by aggregating cells under starvation condition. As expected, the overall direction of migration was opposite to that of wave propagation. Remarkably, during the down phase of waves when the gradient was reversed, cells continued to move with the same bearing until the wave completely passed by (Fig. 1). As the interval between the wave peaks was increased, with a concomitant decrease in the speed of cAMP waves, the cells eventually responded to the reverse gradient at the back of the wave by changing the direction of movement. However, the net displacement path remained unchanged because of the more efficient movement in the forward direction. Next, Skoge et al. (4) took a closer look at the effect of gradient removal and reversal. Intriguingly, when the mean cAMP concentration was kept the same, cells maintained their direction of motility and signaling activities long after the gradient was eliminated. In fact, cells could even overcome a shallow gradient in the reverse direction. However, reduction in the mean cAMP concentration led to suppression of directional memory. These observations are consistent with a model in which the directional memory is modulated by an adaptive mechanism.
How can a cell develop a memory? A number of cell-signaling systems that display memory rely on a hysteretic, bistable circuit (6), and Skoge et al. (4) suggest that such a circuit can recreate their experimental observations. For a system to be bistable, two things are required: a positive feedback loop, which can take the form of a double-negative feedback loop, and a sigmoidal or ultrasensitive component. Frequently, for polarity, a global negative feedback is also present. In this scheme, as the wave front approaches the cell, positive feedback reinforces the sensed signal at the site of higher receptor occupancy (Fig. 1). At the same time, an inhibitory signal from the front desensitizes the rear of the cell. These complementary signals establish well-defined front and back regions in the polarized cell, representing the two states of a bistable system. As the wave passes, these states persist so that the new chemoattractant gradient is not sufficiently strong to alter the direction of the cell’s intrinsic polarity.
There are several putative positive feedback loops in the chemotactic signaling network in Dictyostelium. The first loop involves the phosphoinositide metabolism that is downstream of Ras: Ras activates PI3K, which phosphorylates PI(4,5)P2 to create PI(3,4,5)P3. The phosphatase responsible for the reverse reaction, PTEN, has a PI(4,5)P2 binding motif (7). Thus, activation of PI3K accelerates PI(3,4,5)P3 formation, reducing its substrate PI(4,5)P2, and hence the binding sites for PTEN, leading to further increases in PI(3,4,5)P3. This loop has formed the basis of models describing bistable polarity in Dictyostelium (8). Another proposed feedback loop involving Ras and PI3K relies on actin-dependent binding of PI3K to the membrane (9). Both of these models place PI3K as an important element of the positive feedback loop. Moreover, there is evidence that the response of PI3K to the chemoattractant cAMP gradient is sigmoidal (10). Interestingly, cells lacking PI3K display chemotaxis, but at much lower efficiency than wild-type cells (11), are consistent with models of cells that are able to sense gradients but cannot develop intrinsic polarity (12). These results suggest that PI3K has a role in developing polarity. In neutrophils, chemoattractant-mediated sensing triggers two mutually antagonistic “frontness” and “backness” pathways involving Rho GTPases (13), and models describing these interactions display polarization (14). To limit the effects of positive feedback, negative feedback is also required. The origin of this inhibition is unknown, but tension has been suggested as a likely source during polarity (15).
The results presented suggest that the cell is able to integrate extrinsic (chemoattractants) and intrinsic (memory/polarity) information (16). How this is accomplished is unclear, but one possibility is directly through the G protein-coupled receptor used to bind cAMP. Having asymmetrically distributed receptor components allows the polarity to bias the directional signal; this is the basis for polarity in the pheromone response of Saccharomyces cerevisiae (17). Although
Skoge et al. report persistent memory of Ras activation in cells treated with latrunculin, a commonly used inhibitor for actin polymerization.
Dictyostelium’s G protein-coupled receptor components are uniformly distributed around the cell surface, Gβ is overrepresented at the front of highly polarized cells (18). Although the observed asymmetry is small, chemotactic cells display great amplification of the stimulus, so that a small (∼5%) difference in G-protein subunits could have a large effect on the localization of downstream molecules.
The study leaves several important questions unanswered. If memory is the result of a bistable circuit, why do cells forget the direction of the gradient when the waves are sufficiently spaced apart? Additionally, an intriguing finding is that although polarity is usually thought to be dependent on cytoskeleton, Skoge et al. (4) report persistent memory of Ras activation in cells treated with latrunculin, a commonly used inhibitor for actin polymerization. Is the observed memory a process distinct from polarity? Finally, Skoge et al. argue for a modular network, whereby a local-excitation, global-inhibition mechanism (19) is coupled to a memory module. How does this network integrate with other known constituents, including excitable signaling and cytoskeletal oscillatory components (20)? There is no doubt, however, that the continued combination of novel experimental techniques with mathematical modeling, as demonstrated by Skoge et al. (4), will be crucial in answering these questions.
Footnotes
The authors declare no conflict of interest.
See companion article on page 14448 in issue 40 of volume 111.
References
- 1.Tomchik KJ, Devreotes PN. Adenosine 3′,5′-monophosphate waves in Dictyostelium discoideum: A demonstration by isotope dilution—Fluorography. Science. 1981;212(4493):443–446. doi: 10.1126/science.6259734. [DOI] [PubMed] [Google Scholar]
- 2.Devreotes PN, Steck TL. Cyclic 3′,5′ AMP relay in Dictyostelium discoideum. II. Requirements for the initiation and termination of the response. J Cell Biol. 1979;80(2):300–309. doi: 10.1083/jcb.80.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: Distinctions between directional sensing and polarization. J Biol Chem. 2003;278(23):20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- 4.Skoge M, et al. Cellular memory in eukaryotic chemotaxis. Proc Natl Acad Sci USA. 2014;111(40):14448–14453. doi: 10.1073/pnas.1412197111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Jeon N, et al. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat Biotechnol. 2002;20(8):826–830. doi: 10.1038/nbt712. [DOI] [PubMed] [Google Scholar]
- 6.Ferrell JE., Jr Self-perpetuating states in signal transduction: Positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14(2):140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 7.Iijima M, Huang YE, Devreotes P. Temporal and spatial regulation of chemotaxis. Dev Cell. 2002;3(4):469–478. doi: 10.1016/s1534-5807(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 8.Gamba A, et al. Diffusion-limited phase separation in eukaryotic chemotaxis. Proc Natl Acad Sci USA. 2005;102(47):16927–16932. doi: 10.1073/pnas.0503974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol. 2004;167(3):505–518. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janetopoulos C, Ma L, Devreotes PN, Iglesias PA. Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc Natl Acad Sci USA. 2004;101(24):8951–8956. doi: 10.1073/pnas.0402152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosgraaf L, Keizer-Gunnink I, Van Haastert PJ. PI3-kinase signaling contributes to orientation in shallow gradients and enhances speed in steep chemoattractant gradients. J Cell Sci. 2008;121(Pt 21):3589–3597. doi: 10.1242/jcs.031781. [DOI] [PubMed] [Google Scholar]
- 12.Shi C, Huang CH, Devreotes PN, Iglesias PA. Interaction of motility, directional sensing, and polarity modules recreates the behaviors of chemotaxing cells. PLOS Comput Biol. 2013;9(7):e1003122. doi: 10.1371/journal.pcbi.1003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114(2):201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 14.Onsum MD, Rao CV. Calling heads from tails: The role of mathematical modeling in understanding cell polarization. Curr Opin Cell Biol. 2009;21(1):74–81. doi: 10.1016/j.ceb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houk AR, et al. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148(1-2):175–188. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samadani A, Mettetal J, van Oudenaarden A. Cellular asymmetry and individuality in directional sensing. Proc Natl Acad Sci USA. 2006;103(31):11549–11554. doi: 10.1073/pnas.0601909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson CL, Konopka JB, Hartwell LH. S. cerevisiae α pheromone receptors activate a novel signal transduction pathway for mating partner discrimination. Cell. 1991;67(2):389–402. doi: 10.1016/0092-8674(91)90190-a. [DOI] [PubMed] [Google Scholar]
- 18.Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 2000;287(5455):1034–1036. doi: 10.1126/science.287.5455.1034. [DOI] [PubMed] [Google Scholar]
- 19.Levchenko A, Iglesias PA. Models of eukaryotic gradient sensing: Application to chemotaxis of amoebae and neutrophils. Biophys J. 2002;82(1 Pt 1):50–63. doi: 10.1016/S0006-3495(02)75373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CH, Tang M, Shi C, Iglesias PA, Devreotes PN. An excitable signal integrator couples to an idling cytoskeletal oscillator to drive cell migration. Nat Cell Biol. 2013;15(11):1307–1316. doi: 10.1038/ncb2859. [DOI] [PMC free article] [PubMed] [Google Scholar]