Abstract
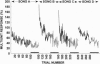
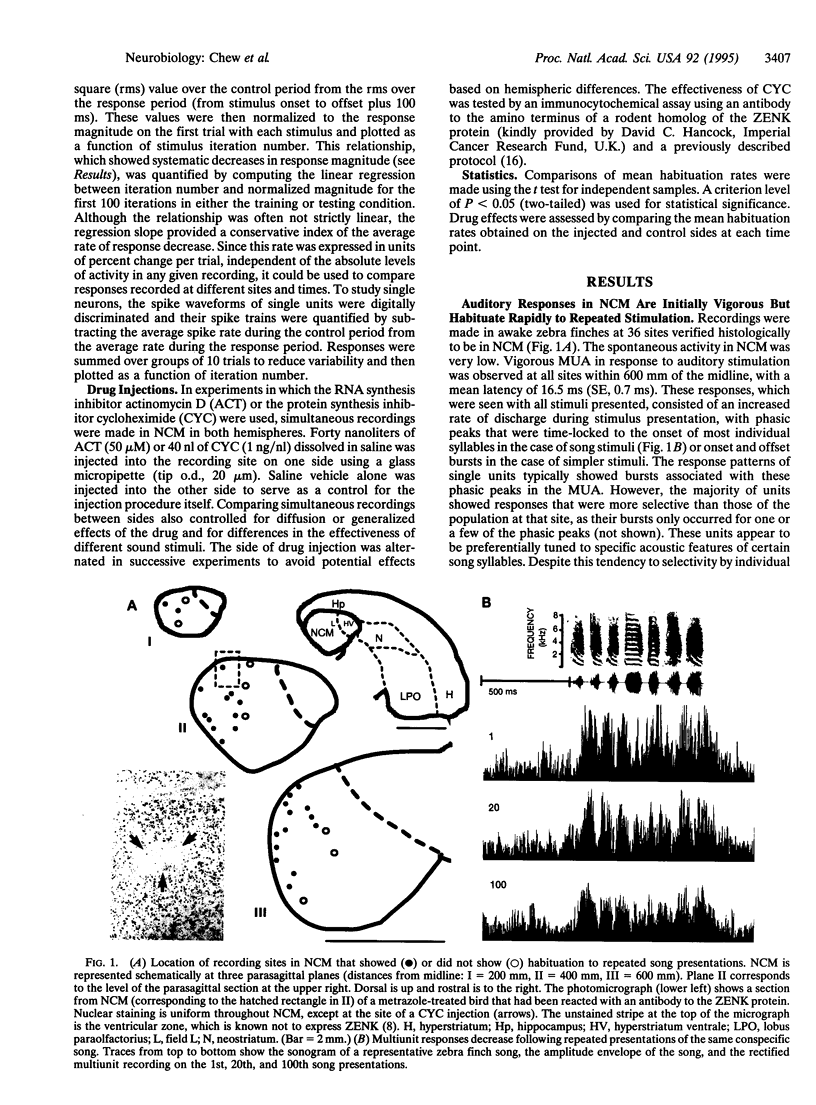
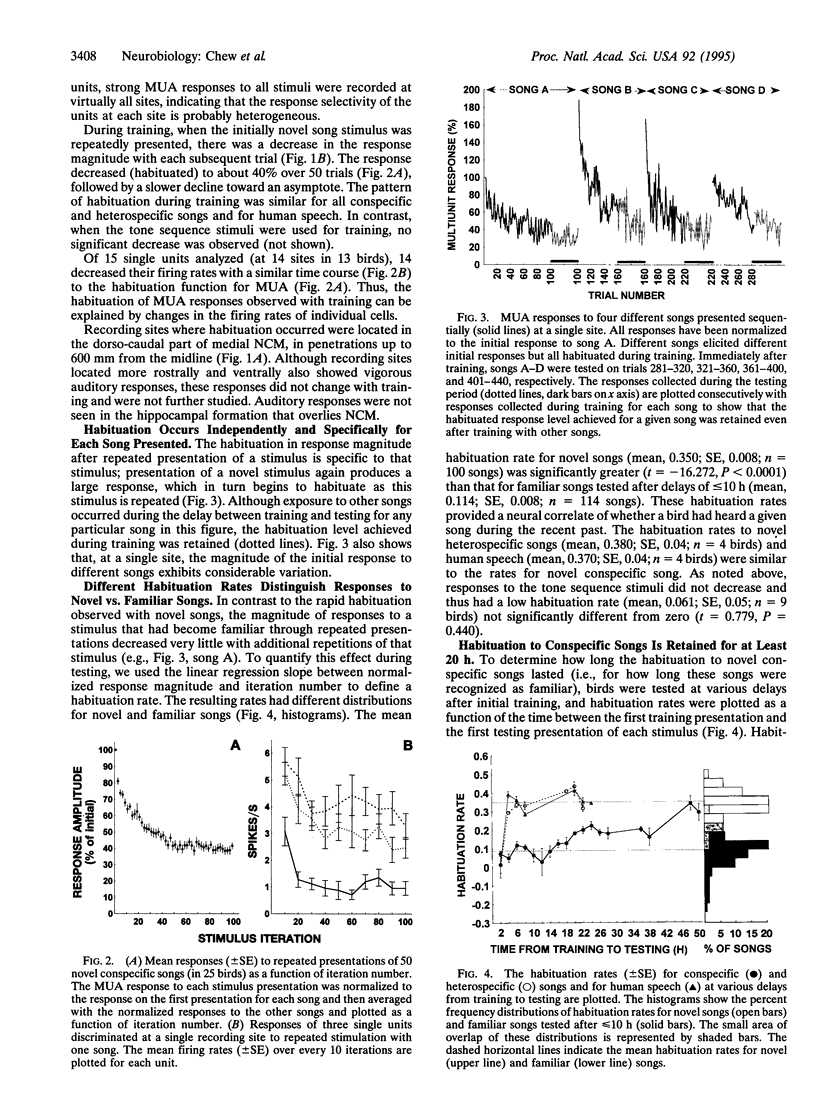
Earlier work showed that playbacks of conspecific song induce expression of the immediate early gene ZENK in the caudo-medial neostriatum (NCM) of awake male zebra finches and that this response disappears with repeated presentations of the same stimulus. In the present study, we investigated whether repetitions of a song stimulus also elicited a decrement in the electrophysiological responses in the NCM neurons of these birds. Multiunit auditory responses in NCM were initially vigorous, but their amplitude decreased (habituated) rapidly to repeated stimulation, declining to about 40% of the initial response during the first 50 iterations. A similar time course of change was seen at the single unit level. This habituation occurred specifically for each song presented but did not occur when pure tones were used as a stimulus. Habituation to conspecific, but not heterospecific, song was retained for 20 h or longer. Injections of inhibitors of protein or RNA synthesis at the recording site did not affect the initial habituation to a novel stimulus, but these drugs blocked the long-term habituation when injected at 0.5-3 h and at 5.5-7 h after the first exposure to the stimulus. Thus, at least two waves of gene induction appear to be necessary for long-lasting habituation to a particular song.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. C., Dragunow M., Tate W. P. The role of immediate early genes in the stabilization of long-term potentiation. Mol Neurobiol. 1991;5(2-4):297–314. doi: 10.1007/BF02935553. [DOI] [PubMed] [Google Scholar]
- Alberini C. M., Ghirardi M., Metz R., Kandel E. R. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994 Mar 25;76(6):1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Cole A. J., Saffen D. W., Baraban J. M., Worley P. F. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989 Aug 10;340(6233):474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Davis H. P., Squire L. R. Protein synthesis and memory: a review. Psychol Bull. 1984 Nov;96(3):518–559. [PubMed] [Google Scholar]
- Doupe A. J. A neural circuit specialized for vocal learning. Curr Opin Neurobiol. 1993 Feb;3(1):104–111. doi: 10.1016/0959-4388(93)90043-x. [DOI] [PubMed] [Google Scholar]
- Fahy F. L., Riches I. P., Brown M. W. Neuronal activity related to visual recognition memory: long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp Brain Res. 1993;96(3):457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Li L., Miller E. K., Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophysiol. 1993 Jun;69(6):1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- Margoliash D., Fortune E. S. Temporal and harmonic combination-sensitive neurons in the zebra finch's HVc. J Neurosci. 1992 Nov;12(11):4309–4326. doi: 10.1523/JNEUROSCI.12-11-04309.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies H. In search of cellular mechanisms of memory. Prog Neurobiol. 1989;32(4):277–349. doi: 10.1016/0301-0082(89)90024-5. [DOI] [PubMed] [Google Scholar]
- Mello C. V., Clayton D. F. Differential induction of the ZENK gene in the avian forebrain and song control circuit after metrazole-induced depolarization. J Neurobiol. 1995 Jan;26(1):145–161. doi: 10.1002/neu.480260112. [DOI] [PubMed] [Google Scholar]
- Mello C. V., Clayton D. F. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994 Nov;14(11 Pt 1):6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. V., Vicario D. S., Clayton D. F. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. K., Li L., Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991 Nov 29;254(5036):1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- Müller C. M., Leppelsack H. J. Feature extraction and tonotopic organization in the avian auditory forebrain. Exp Brain Res. 1985;59(3):587–599. doi: 10.1007/BF00261351. [DOI] [PubMed] [Google Scholar]
- Nguyen P. V., Abel T., Kandel E. R. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994 Aug 19;265(5175):1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Stokes T. M., Leonard C. M. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976 Feb 15;165(4):457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Saini K. D., Leppelsack H. J. Cell types of the auditory caudomedial neostriatum of the starling (Sturnus vulgaris). J Comp Neurol. 1981 May 10;198(2):209–229. doi: 10.1002/cne.901980203. [DOI] [PubMed] [Google Scholar]
- Simpson H. B., Vicario D. S. Early estrogen treatment of female zebra finches masculinizes the brain pathway for learned vocalizations. J Neurobiol. 1991 Oct;22(7):777–793. doi: 10.1002/neu.480220711. [DOI] [PubMed] [Google Scholar]
- Sobotka S., Ringo J. L. Stimulus specific adaptation in excited but not in inhibited cells in inferotemporal cortex of macaque. Brain Res. 1994 May 16;646(1):95–99. doi: 10.1016/0006-8993(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Vicario D. S. Motor mechanisms relevant to auditory-vocal interactions in songbirds. Brain Behav Evol. 1994;44(4-5):265–278. doi: 10.1159/000113581. [DOI] [PubMed] [Google Scholar]
- Vicario D. S., Yohay K. H. Song-selective auditory input to a forebrain vocal control nucleus in the zebra finch. J Neurobiol. 1993 Apr;24(4):488–505. doi: 10.1002/neu.480240407. [DOI] [PubMed] [Google Scholar]