Abstract
Background
Connexin43 (Cx43) is an integral membrane protein that forms intercellular channels called gap junctions. Intercellular communication in the eye lens relies on an extensive network of gap junctions essential for the maintenance of lens transparency. The association of Cx43 with cholesterol enriched lipid raft domains was recently demonstrated. The objective of this study is to assess if products of cholesterol oxidation (oxysterols) affect gap junction intercellular communication (GJIC).
Results
Primary cultures of lens epithelial cells (LEC) were incubated with 7-ketocholesterol (7-Keto), 25-hydroxycholesterol (25-OH) or cholesterol and the subcellular distribution of Cx43 was evaluated by immunofluorescence confocal microscopy. The levels of Cx43 present in gap junction plaques were assessed by its insolubility in Triton X-100 and quantified by western blotting. The stability of Cx43 at the plasma membrane following incubation with oxysterols was evaluated by biotinylation of cell surface proteins. Gap junction intercellular communication was evaluated by transfer of the dye Lucifer yellow. The results obtained showed that 7-keto induces an accumulation of Cx43 at the plasma membrane and an increase in intercellular communication through gap junction. However, incubation with cholesterol or 25-OH did not lead to significant alterations on subcellular distribution of Cx43 nor in intercellular communication. Data further suggests that increased intercellular communication results from increased stability of Cx43 at the plasma membrane, presumably forming functional gap-junctions, as suggested by decreased solubility of Cx43 in 1% Triton X-100. The increased stability of Cx43 at the plasma membrane seems to be specific and not related to disruption of endocytic pathway, as demonstrated by dextran uptake.
Conclusions
Results demonstrate, for the first time, that 7-keto induces an increase in gap junction intercellular communication, that is most likely due to an increased stability of protein at the plasma membrane and to increased abundance of Cx43 assembled in gap junction plaques.
Background
Gap junction channels (GJ) consist of two connexons that are located at the plasma membrane of two adjacent cells. Each connexon is composed of six subunits, the connexins. These channels allow passage of small molecules, with a molecular mass below 1 kDa, such as small metabolites, ions, and second messengers [1]. The physiological importance of intercellular communication through gap junctions is well illustrated in the eye lens where inner fiber cells fully depend on a complex network of gap junctions for nutrition and signalling [2,3]. The lens is an avascular organ containing a central mass of fiber cells covered by an anterior monolayer of epithelial cells. In the lens, the gap junctions allow the passage of small molecules between metabolically active epithelial cells, which produces most of the ATP used by the lens, and the fully differentiated fiber cells that present low metabolic activity. At the equatorial region of the lens, epithelial cells exit cell cycle and undergo significant morphological and biochemical changes that result in the formation of fully differentiated fiber cells, where virtually all organelles, including the nuclei, are absent [4]. Three connexin genes are expressed in the vertebrate lens; α1 (Cx43) connexin that is expressed mostly in epithelial cells [5]; α3 (Cx46) and α8 (Cx50) connexins which are expressed in fiber cells [6,7].
Lens plasma membrane is unique among eukaryotic cell membranes due to its extremely high content of cholesterol and deficit of polyunsaturated fatty acids. In fact, lens membranes contain the highest cholesterol content of any known biological membrane [8,9]. As the main unsaturated lipid present in lens membranes, cholesterol is prone to oxidation yielding a variety of oxidation products. Some of these cholesterol oxides (or oxysterols) were shown to be increased in human cataractous lenses [10], 7-ketocholesterol being the predominant oxysterol present in human cataracts.
Accumulation of oxysterols on plasma membrane may alter intercellular communication by a variety of mechanisms most of which are unclear. For example, oxysterols may alter lipid bilayer order and, therefore, affect intercellular communication [11]. Recently, it was shown that Cx43 at the plasma membrane is localised in specialised domains called cavoelae [12]. The presence of products of cholesterol oxidation on caveolae disturbs the function of such domains [13-15]. Moreover, Cx43 trafficking, assembly, and turnover are regulated by multiple mechanisms, including those mediated by the cytoskeleton [16-18]. Cholesterol oxides were shown to disrupt cytoskeleton network organisation, through a mechanism that involves activation of Rho GTPases [19].
The objective of this study is to evaluate if oxysterols alter intercellular communication through Cx43 gap-junctions in lens epithelial cells.
Results
7-keto stabilises Cx43 at the plasma membrane
Cholesterol has been shown to increase gap junction assembly and cell-cell communication. In this study we evaluated the effect of the products of cholesterol oxidation on subcellular distribution of Cx43 and GJIC in LEC.
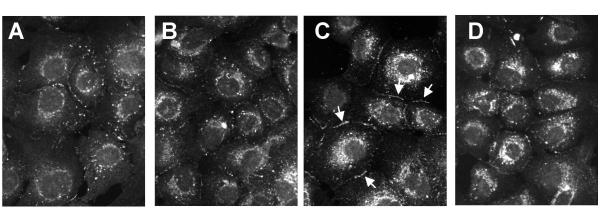
To investigate the effects of cholesterol oxides on subcellular distribution of Cx43, primary cultures of lens epithelial cells were incubated with 20 μg/ml cholesterol, 7-keto or 25-OH, for 3 hours. Cells incubated with 0.2% ethanol were used as controls. The cells were fixed and stained with antibodies directed against Cx43 and imaged by immunofluorescence confocal microscopy. In control cells Cx43 staining appeared as small punctate spots at the plasma membrane and cell-cell interfaces (Figure 1A). After 3 hours of incubation with 7-keto the abundance of Cx43 at the plasma membrane (as evaluated by the number of punctate staining) was higher (Figure 1C) while incubation with cholesterol (Figure 1B) or 25-OH (Figure 1D) did not result in major alterations on Cx43 staining at plasma membrane. A perinuclear staining was also observed following incubation with both 7-keto or 25-OH.
Figure 1.
Effect of oxysterols on subcellular distribution of Cx43. LEC were treated with 20 μg/ml cholesterol (B), 7-keto (C) or 25-OH (D) for 3 hours. Cells incubated in 0.2% ethanol were used as controls (A). The cells were fixed and stained with antibodies directed against Cx43 and imaged by confocal microscopy.
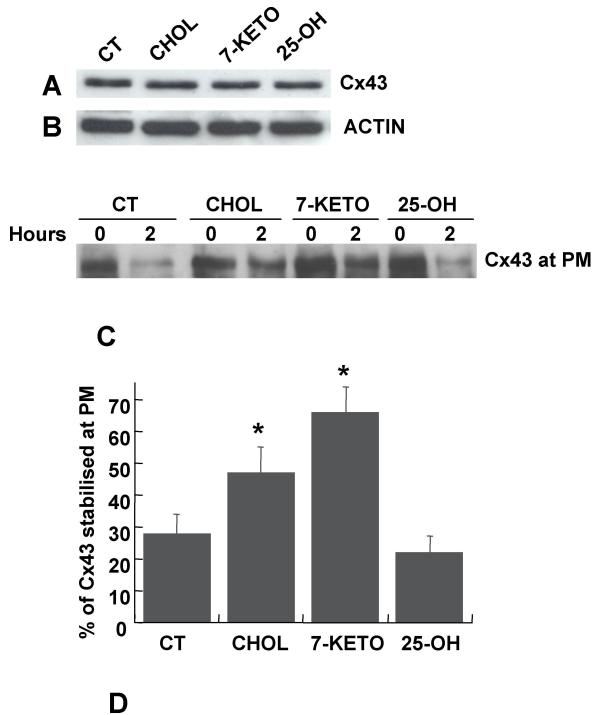
There is a variety of factors reported to affect the amount of Cx43 at the plasma membrane. These are often cell specific and lead to either recruitment of Cx43 to the plasma membrane or to stabilisation of the protein at the membrane. The observation that the total amount of Cx43 does not change following treatment with 7-keto (Figure 2A) strongly suggests that Cx43 is not up regulated in response to cholesterol oxides.
Figure 2.
Effect of oxysterols on Cx43 stability at the plasma membrane. The total amount of Cx43 following treatment with 20 μg/ml cholesterol, 7-keto or 25-OH, for 3 hours, was determined by western blot using antibodies directed against Cx43 (A). Actin on the same samples is included to demonstrate comparable loading of the lanes (B). LEC were treated with 20 μg/ml cholesterol (lane 3), 7-keto (lane 5) or 25-OH (lane 7) for 30 min prior biotinylation. Cells incubated in 0.2% ethanol were used as controls (lane 1). To determine the stability of Cx43 at the plasma membrane, the biotinylated cells were returned to the incubator for an additional 2 hours in DMEM, in the absence (lane 2) or presence of cholesterol (lane 4), 7-keto (lane 6) or 25-OH (lane 8). After extraction in situ by incubation in DPBS containing 0.5% Triton X-100, the biotinylated surface Cx43 was detected with antibodies directed against Cx43 following isolation with Neutravidin beads (C). The results obtained are depicted in a graph and correspond to the Cx43 remaining at the plasma membrane 2 hours after biotinylation (D). Each bar represents means ± SD of three independent experiments. * – indicates statistically significant differences from controls (p < 0.01).
In order to determine if accumulation of Cx43 at the plasma membrane following incubation with 7-keto was associated with an increased stabilisation of the protein at the membrane, cell-surface proteins were labelled with biotin. The cells were incubated in the absence or presence of 20 μg/ml cholesterol, 7-keto or 25-OH, for 30 minutes, prior to biotinylation. The stabilisation of Cx43 at the plasma membrane was evaluated as the amount of biotinylated Cx43 remaining at the cell surface 2 hours after biotinylation (Figure 2C). The internalised Cx43 was washed out from the cells following permeabilisation with 0.5% Triton X-100 [20]. In controls, the levels of Cx43 at the plasma membrane decreased about 70%, while in cells incubated with 7-keto the decrease was only of about 37% (Figure 2D). Cells incubated with cholesterol or 25-OH showed a decrease on the amount of Cx43 at the plasma membrane of about 53% and 80%, respectively (Figure 2D).
Stabilisation of Cx43 at plasma membrane is not related to a decrease in endocytosis
Endocytosis of dextran is often used as a functional test for the endocytic pathway [20-22]. To demonstrate that stabilisation of Cx43 induced by 7-keto results from a specific effect on the protein, rather than a general effect on endocytic pathway, the uptake of RITC-dextran was evaluated in LEC in culture (Figure 3). The internalised fluorescently labelled dextran was assessed by confocal microscopy. For negative controls, cells were incubated at 4°C to inhibit endocytosis (Figure 3E). Results show that endocytosis is slightly enhanced in cells treated with 7-keto (Figure 3C) or cholesterol (Figure 3B), as revealed by the increased fluorescence inside the cells. Conversely, endocytosis was not affected following treatment with 25-OH (Figure 3D). Despite the increase in endocytosis following treatment with 7-keto, Cx43 accumulates at the plasma membrane, indicating that the effect of 7-keto is most likely associated with stabilisation of Cx43 at the plasma membrane.
Figure 3.
Effect of oxysterols on the endocytic pathway. Lens epithelial cells were incubated with RITC-dextran for 30 min, at 37°C, following incubation with 20 μg/ml cholesterol (B), 7-keto (C) or 25-OH (D) for 2 hours. Cells incubated with 0.2% ethanol were used as controls (A). The cells were incubated for an additional 30 min in the absence of the dye, and subsequently fixed with 4% PFA and imaged by fluorescence confocal microscopy. For negative controls, cells were incubated at 4°C to inhibit endocytosis (E).
7-keto increases intercellular communication through gap junctions
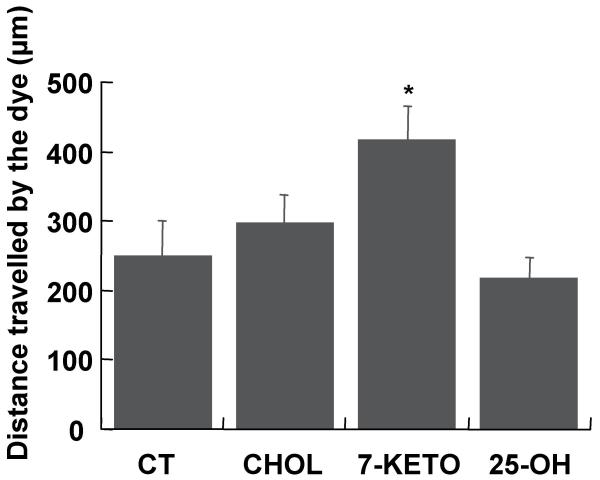
The functional implications of Cx43 redistribution following incubation with 7-keto was investigated in primary cultures of lens epithelial cells by scrape loading/dye transfer assays. The intercellular communication was quantified as the distance travelled by the dye Lucifer yellow 8 min after loading of the dye. The results obtained are presented in an histogram (Figure 4). Incubation of LEC with 7-keto resulted in a 57% increase in GJIC, as compared to controls, while incubation with cholesterol did not significantly affect intercellular communication. On the other hand, when cells were incubated with 25-OH there was a slight decrease in intercellular communication through gap junction.
Figure 4.
Effect of oxysterols in intercellular communication through gap-junctions. LEC were treated with 20 μg/ml cholesterol (B), 7-keto (C) or 25-OH (D) for 3 hours. The cells were assayed for intercellular communication by Lucifer yellow dye transfer, following scrape loading. The intercellular communication was evaluated as the average distance travelled by the dye Lucifer yellow along the monolayer and is represented as an histogram. The values are the average of three individual experiments ± SD. * – indicates statistically significant differences from controls (p < 0.05).
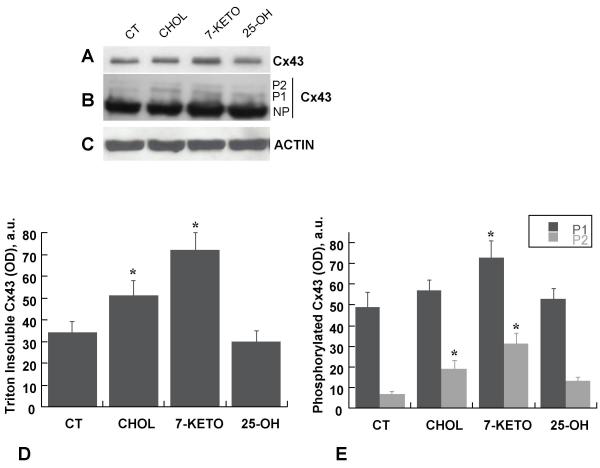
7-keto induces partition of Cx43 into Triton X-100 insoluble fraction and accumulation of phosphorylated form of Cx43
Formation of functional gap junctions requires incorporation of Cx43 into gap junction plaques. Thus, by increasing intercellular communication 7-keto should consistently stabilise Cx43 in GJ. To confirm this hypothesis primary cultures of lens epithelial cells were incubated with 20 μg/ml cholesterol, 25-OH or 7-keto, for 3 hours. Cells incubated with ethanol 0.2% were used as control. Phosphorylation of Cx43 and its assembly into GJ plaques results in an increased insolubility in 1% Triton X-100 [23]. The results presented in Figure 5A and 5D showed that incubation with 7-keto led to a 2-fold increase in the amount of Triton X-100 insoluble Cx43 (Figure 5A, lane 3, and 5D). Incubation of cells with cholesterol resulted in a 53% increase on the amount of Cx43 partitioned into the Triton insoluble fraction (Figure 5A, lane 2, and 5D) while cells incubated with 25-OH presented a decrease of about 20% (Figure 5A, lane 4, and 5D).
Figure 5.
Effect of oxysterols on Cx43 phosphorylation and solubility in Triton X-100. LEC were incubated with 20 μg/ml cholesterol (lane 2), 7-keto (lane 3) or 25-OH (lane 4) for 3 hours. The Triton X-100 insoluble fractions of cell lysates were separated by SDS-PAGE, transferred to PVDF membranes and probed with antibodies directed against Cx43 (A). To allow quantification of phosphorylated forms of Cx43 the film of was overexposed (B). Actin on the same samples is included to demonstrate comparable loading of the lanes (C). The intensity of the bands was determined by laser scanning of the films followed by quantitative densitometric analysis and the results obtained are depicted in a graph (D and E). The values are the average of three individual experiments ± SD. * – indicates statistically significant differences from controls (p < 0.05).
Incorporation of Cx43 into GJ is associated with phosphorylation of the protein [23-25]. The presence of phosphorylated Cx43 in the Triton insoluble fraction was determined by immunoblotting using polyclonal antibodies that recognise the phosphorylated forms of Cx43 (Figure 5B). Three major forms of Cx43 are often recognised by immunoblotting: the non-phosphorylated (NP) form and two slower migrating bands corresponding to the phosphorylated species (P1 and P2). However, in Figure 5B, due to overexposure of the film, the non-phosphorylated form could not be quantified. Thus, only the phosphorylated forms P1 and P2 were considered and quantified (Figure 5B and 5E). The results serve our purpose by showing that the amount of phosphorylated Cx43 increased in cells incubated with 7-keto (Figure 5B, lane 3) suggesting its assembly into gap junction plaques.
Discussion
In the present study we demonstrate, for the first time, that 7-keto can modulate gap junction intercellular communication, probably through a mechanism that involves stabilisation of Cx43 at the plasma membrane. Data presented in this study shows that incubation of LEC with 7-keto leads to an accumulation of the protein at the plasma membrane and to an increase of cell-cell communication. Additionally, an increased partition of Cx43 into Triton X-100 insoluble fraction after treatment with 7-keto suggests that Cx43 is incorporated into gap junctions. This hypothesis is further supported by the observation that the amount of total Cx43 is not altered following incubation with 7-keto. Such changes in subcellular distribution may thus account for the increased intercellular communication. On the other hand, the biotinylation experiments showed that the rate of endocytosis of Cx43 is significantly reduced following incubation with 7-keto. Biotin labels mainly non-junctional Cx43, therefore, taken together the experiments of biotinylation and solubility in Triton X-100 suggest a role for 7-keto in stabilisation of Cx43 at the plasma membrane. Moreover, at least part of Cx43 stabilised at the plasma membrane is likely to be incorporated into functional gap junctions, as revealed by the increased intercellular communication. Thus, it is conceivable that the accumulation of Cx43 into gap junctions induced by 7-keto is a consequence of an increase in Cx43 at the plasma membrane available to form gap junction plaques. It is not likely that perinuclear accumulation of Cx43 following treatment with 7-keto or 25-OH can account for the accumulation of Cx43 at the plasma membrane. Indeed, although both 7-keto and 25-OH lead to an accumulation of Cx43 in perinuclear region it is only 7-keto that stabilises Cx43 at the plasma membrane.
The findings in this report further suggest that 7-keto effects on Cx43 endocytosis are rather specific and most likely involve activation of specific signalling pathways. This hypothesis is supported by two main observations: (1) the effects of 7-keto cannot be mimicked by other cholesterol oxides such as 25-OH and (2) the effects of 7-keto are exerted specifically on Cx43 as the endocytic pathway is not inhibited (internalisation of dextran is not diminished). Taken together these observations suggest that the effects of 7-keto reported in this paper are not the result of unspecific cholesterol oxidation at the plasma membrane but rather reflect a physiological role for 7-keto in LEC intercellular communication. Presumably, a similar effect is also of significance in other cells and tissues, where changes in intercellular communication are likely to compromise cell or tissue function.
The effects of cholesterol oxidation on endocytosis are controversial. In fact, Chow et al. have shown that OxLDL (rich in 7-keto) promote endocytosis in endothelial cells [26], while Borsum showed a decrease of endocytic pathway induced by OxLDL [27]. The results presented in this report indicate that 7-keto slightly enhances endocytosis in LEC. This emphasises that Cx43 stabilisation is a specific phenomena unrelated to endocytic pathway.
Two main pathways for Cx43 internalisation have been suggested: via clathrin-coated pits [28,29] and via caveolae [12]. Caveolae are membrane lipid rafts rich in cholesterol and sphingolipids involved in signal transduction [30-32], endocytosis [33] and cholesterol transport [34,35]. Indeed, connexins, including Cx43, were shown to specifically target to lipid raft/caveolae and directly interact with caveolin-1, a membrane protein that acts as a scaffolding protein to cluster lipids and signalling molecules within caveolae [12]. More recently, Lin et al. showed that phosphorylation of Cx43 regulates the distribution of the protein within caveolae in lens epithelial cells [36]. Disruption of caveolae following incubation with cholesterol oxidase was reported to affect the activity of various membrane receptors sequestrated in these membrane domains, including the endothelin receptor type A [37], GLUT4 [38] and the platelet-derived growth factor receptor (PDGFR) [15]. The presence of oxysterols, namely 7-keto, on caveolae has been shown to interfere with enzymes that concentrate in such membrane domains [14,39]. Additionally, in vitro studies demonstrated that 7-keto interacts with caveolin [40]. Therefore, disturbing caveolae structure and/or function, 7-keto may affect proteins involved in regulating the stability of Cx43 at the plasma membrane. The identity of such proteins is largely unknown as are the mechanisms whereby Cx43 is extracted from the plasma membrane, endocytosed and eventually degraded. Protein kinases are likely candidates to assist in regulation of Cx43 stability and endocytosis. Indeed, C-terminal domain of Cx43 was shown to be phosphorylated by a variety of kinases including protein kinase C (PKC) [41], v-Src [42], mitogen-activated protein kinase (MAPK) [43,44] or casein kinase I [25]. We and others have shown that hyperphosphorylation of Cx43 is associated with decreased GJIC and with increased protein degradation [24,45,46]. However, recent studies reported an increased GJIC following phosphorylation of Cx43. For example, it was shown that casein kinase 1 directly phosphorylated Cx43, promoting gap junction assembly [25] while FGF upregulated intercellular communication between lens epithelial cells through activation of extracellular signal-regulated kinase (ERK) [46]. Initial phosphorylation of Cx43 is normally involved in assembly of the protein into gap junction plaques [23-25]. Significantly 7-keto was shown to activate a variety of protein kinases [14,48]. It is thus conceivable that the presence of 7-keto at the plasma membrane may enhance Cx43 assembly and formation of gap junction plaques by a mechanism that probably involves phosphorylation of Cx43. The importance of cholesterol oxidation in the lens is not without significance. Indeed, cholesterol is the most abundant lipid in lens membranes and we have shown before that cholesterol oxides are present at detectable levels in the lens and more so in human cataracts [10]. Although the ratio of cholesterol/phospholipid is higher in the inner regions of the lens [9], where the oxysterols are most likely accumulate, this does not exclude that cholesterol oxides in fiber cells may still affect function of lens epithelial cells nor that cholesterol oxides may interfere with intercellular communication through gap junctions formed by Cx46/Cx50. in inner regions of the lens. We have previously shown that cholesterol oxides affect the organisation of cytoskeletal network in LEC, through a mechanism mediated by Rho GTPases [19]. Since cytoskeleton plays a major role in the trafficking and recruitment of connexins into gap junction [16-18] it is conceivable that the effect of 7-keto on GJIC may be caused by a mechanism involving cytoskeleton disruption. Although data presented in this report does not allow to demonstrate a mechanism whereby 7-keto increases Cx43 stability at the plasma membrane, it certainly provides a new insight into the effects of cholesterol oxides in LEC and into its physiological implications in intercellular communication. The inner layers of the lens fully depend on a complex network of gap junctions through which nutrients and signal molecules produced in the outer layers reach the inner fibers. Through a model similar to that described for Cx43 in lens epithelial cells, upregulation of intercellular communication through gap junctions formed by Cx46/Cx50 in deeper cortical regions of the lens may compromises lens transparency leading to formation of cataract. Additionally, the increase of intercellular communication induced by 7-keto may also disrupt a variety of highly regulated events, including differentiation of LEC into fibers. Several studies have shown that connexins play an important role on lens differentiation and development [46,50-52]. For example, recent data obtained in our laboratory showed that 7-keto induces differentiation of LEC, by an unknown mechanism [53]. Thus, by interfering with GJIC, cholesterol oxides may affect highly regulated, differentiation program and compromise normal lens growth and transparency, contributing to the cataratogenic process.
Conclusions
The results obtained in this study show for the first time that 7-keto induces an increase in GJIC, that is most likely due to an increased stability of the protein at the plasma membrane and to an increased abundance of Cx43 assembled into gap junction plaques. The upregulation of GJIC in the lens may disrupt a variety of highly regulated events, including differentiation of LEC into fibers, that ultimately compromises lens transparency leading to formation of cataract.
Methods
Cell culture
For primary cultures of LEC eyeballs were removed from adult bovines and the anterior capsule of the lens, with the attached epithelium, was cut along the equator and cultured in a 24 well-plate containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Gibco BRL Life Technologies, Inchinnan, UK), 100 i.u./ml penicillin, 100 μg/ml streptomycin, at 37°C with 5% CO2. The epithelial cells were then allowed to spread out from the capsule into the plate. The cells were kept in culture for 48 hours before treatments. At this stage they reached 90% confluence. 1 × 106 cells were plated onto 60 mm Petri dishes.
Antibodies and reagents
Both rabbit polyclonal and mouse monoclonal anti-Cx43 antibodies were obtained from Zymed (San Francisco, CA, USA). Unless otherwise noted, all other reagents were from Sigma. Oxysterols were dissolved in ethanol.
ImmunofIuorescence
Cells grown on glass coverslips were fixed with 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS), containing Ca2+ and Mg2+. The samples were then washed with PBS, permeabilised with 1% v/v Triton X-100 in PBS, and blocked with goat serum (1:10) for 20 min. Incubation with primary antibodies proceeded for 1 hour at room temperature. The samples were then washed three times with PBS before incubation with Texas Red isothiocyanate (TRITC) conjugated secondary antibodies for 1 hour at room temperature. The specimens were rinsed in PBS and mounted with Glycergel (Dako, Glostrup, Denmark). All solutions were made up in 0.2% w/v BSA (Sigma) containing 0.02% sodium azide (Sigma, St Louis, MO, USA) in PBS. For controls, primary antibodies were omitted.
Western blotting
To isolate the Triton X-100 soluble fraction, cells were rinsed with PBS at 4°C, scraped and ressuspended in lysis buffer (190 mM NaCI, 50 mM Tris-HCI, 6 mM EDTA, 1% Triton X-100, Roche protease inhibitor cocktail Complete Mini (Roche, Mannheim, Germany), 2 mM PMSF, 10 mM iodacetamide, 50 mM NaF, 500 μM NaVO4), pH 8.3. Following incubation on ice for 30 min, samples were ultra-centrifuged for 50 min at 100 000 g at 4°C and the supernatant collected (Triton X-100 soluble fraction), while the pellet was ressuspended in lysis buffer containing 1% SDS and solubilised by sonication (Triton X-100 insoluble fraction). After denaturation with Laemmli buffer for 30 min, at 37°C the proteins were separated by SDS-PAGE, transferred to PVDF membranes and probed with antibodies to Cx43.
Dextran uptake
Lens epithelial cells grown on glass coverslips were incubated with 10 mg/ml rhodamine isothiocyanate RITC-dextran (Mr 10 200 Da) in DMEM for 30 min, at 37°C. Cells were then rinsed with PBS and incubated with fresh DMEM without dextran and returned to the incubator for an additional 30 min. The cells were subsequently rinsed with PBS and fixed with 4% PFA and imaged by fluorescence confocal microscopy.
Dye transfer assay for gap junctional intercellular communication
Lens epithelial cells grown on glass coverslips were assayed for gap junction-mediated intercellular coupling as described by Le and Musil [49]. Briefly, the culture medium from a confluent monolayer of LEC was removed and saved. The cells were rinsed three times with Hank's balanced salt solution containing 1% bovine serum albumin (HBC), after which a 27-gauge needle was used to create multiple scrapes through the cell monolayer in the presence of Dulbecco's phosphate buffered saline containing 0.5% rhodamine-dextran and 0.5% lucifer yellow. After exactly 1 minute, the culture was rinsed three times with HBC and then incubated for an additional 8 min in the saved culture medium to allow the loaded dye to transfer to adjoining cells. The cells were then rinsed, fixed with 4% paraformaldehyde and imaged on a fluorescence microscope under UV light.
Biotinylation of cell surface proteins
LEC grown on 60 mm culture dishes were rinsed twice with 5 ml of ice-cold PBS containing 0.5 mM MgCl2 and 1 mM CaCl2, followed by the addition of 3 ml of the same ice-cold solution containing 1 mg/ml of freshly added SULFO-NHS-SS-biotin (Pierce, Rockford, IL, USA). After 30 min at 4°C, to stop subcellular trafficking, the medium was discarded and the plates were washed 3 times with PBS containing 0.5 mM MgCl2, 1 mM CaCl2 and 100 mM glycine. The cells were scraped in RIPA buffer (50 mM Tris-HCI, 150 mM NaCI, 5 mM EGTA, containing 1% Triton, 0.5% DOC, 0.1% SDS and supplemented with protease inhibitor cocktail, 2 mM PMSF, 10 mM iodoacetamide, 10 mM NaF and 500 μM Na3VO4; pH 7.5). After 15 min on ice the cells were sonicated and the homogenates were centrifuged at 14000 rpm, for 10 minutes. To determine the stability of Cx43 at the plasma membrane, the biotinylated cells were incubated for an additional 2 hours in DMEM, at 37°C, in the absence or presence of cholesterol or oxysterols. The culture medium was then rejected and the cells extracted in situ by incubation in DPBS containing 0.5% Triton X-100, for 10 minutes, at room temperature. The samples were then processed as described above. The protein content of the supernatants was determined and the same quantity of protein was transferred to 1.5 ml Eppendorf microfuge tubes containing 200 μl of Neutravidin beads (Pierce, Rockford, IL, USA). After 2 h of incubation at 4°C under agitation, the beads were washed four times with RIPA buffer. The final pellets were resuspended in 150 μl 2x Laemmli buffer and incubated 1 h at 37°C. The beads were pelleted and the solubilised proteins were separated by SDS-PAGE, transferred to PVDF membranes and probed with antibodies directed against Cx43.
Statistics
All results are representative of at least three experiments. Data are expressed as a sample mean ± standard deviation (SD). Different samples were compared by using the Student's t test and two-tailed probability (P).
List of abbreviations
25-OH, 25-hydroxycholesterol; 7-keto, 7-ketocholesterol; Cx, connexin; eNOS, endothelial nitric-oxide synthase; ERK, extracellular signal-regulated kinase; FGF, fibroblast growth factor; GJ, Gap junctions; GJIC, gap junction intercellular communication; LEC, lens epithelial cells; MAPK, mitogen-activated protein kinase; NP, non-phosphorylated; OxLDL; oxidised low density lipoproteins; PDGFR, platelet-derived growth factor receptor; PKC, protein kinase C
Competing interests
None declared.
Authors' contributions
HG designed and conceived most of the experiments and drafted the manuscript; SC performed biotinylation experiments and other experimental procedures; PP participated in the experimental design and coordination of the research.
Acknowledgments
Acknowledgement
This work was supported by grants from Portuguese Foundation for Science and Technology – FCT (Programme POCTI and FEDER).
Contributor Information
Henrique Girão, Email: hgirao@ibili.uc.pt.
Steve Catarino, Email: scatarino@ibili.uc.pt.
Paulo Pereira, Email: ppereira@ibili.uc.pt.
References
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/S0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Rae JL, Bartling C, Rae J, Mathias RT. Dye transfer between cells of the lens. J Membr Biol. 1996;150:89–103. doi: 10.1007/s002329900033. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;77:21–49. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- Menko AS. Lens epithelial cell differentiation. Exp Eye Res. 2002;75:485–490. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- Beyer E, Kistler J, Paul D, Goodenough D. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DL, Ebihara L, Takemoto LJ, Swenson Kl, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage- gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell. 1992;3:711–720. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenka PS. Lens lipids. Curr Eye Res. 1984;3:1337–1359. doi: 10.3109/02713688409007421. [DOI] [PubMed] [Google Scholar]
- Li LK, So L, Spector A. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J Lipid Res. 1985;26:600–609. [PubMed] [Google Scholar]
- Girao H, Mota MC, Ramalho J, Pereira P. Cholesterol oxides accumulate in human cataracts. Exp Eye Res. 1998;66:645–652. doi: 10.1006/exer.1998.0465. [DOI] [PubMed] [Google Scholar]
- Verhagen JC, ter Braake P, Teunissen J, van Ginkel G, Sevanian A. Physical effects of biologically formed cholesterol oxidation products on lipid membranes investigated with fluorescence depolarization spectroscopy and electron spin resonance. J Lipid Res. 1996;37:1488–1502. [PubMed] [Google Scholar]
- Schubert AL, Schubert W, Spray DC, Lisanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- Pike LJ, Miller JM. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem. 1998;273:22298–22304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- Myers SJ, Stanley KK. Src family kinase activation in glycosphingolipid-rich membrane domains of endothelial cells treated with oxidised low density lipoprotein. Atherosclerosis. 1999;143:389–397. doi: 10.1016/S0021-9150(98)00331-1. [DOI] [PubMed] [Google Scholar]
- Liu P, Wang P, Michaely P, Zhu M, Anderson RG. Presence of oxidized cholesterol in caveolae uncouples active platelet-derived growth factor receptors from tyrosine kinase substrates. J Biol Chem. 2000;275:31648–31654. doi: 10.1074/jbc.M004599200. [DOI] [PubMed] [Google Scholar]
- Thomas T, Jordan K, Laird DW. Role of cytoskeletal elements in the recruitment of Cx43-GFP and Cx26-YFP into gap junctions. Cell Commun Adhes. 2001;8:231–236. doi: 10.3109/15419060109080729. [DOI] [PubMed] [Google Scholar]
- Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci USA. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss C, Meller K. Microinjected anti-actin antibodies decrease gap junctional intercellular commmunication in cultured astrocytes. Exp Cell Res. 2002;281:197–204. doi: 10.1006/excr.2002.5652. [DOI] [PubMed] [Google Scholar]
- Girão H, Pereira P, Ramalho J, Quinlan R, Prescott A. Cholesterol oxides mediated changes in cytoskeletal organisation involves Rho GTPases. Exp Cell Res. 2003;291:502–513. doi: 10.1016/j.yexcr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Govindarajan R, Zhao S, Song XH, Guo RJ, Wheelock M, Johnson KR, Mehta PP. Impaired trafficking of connexins in androgen-independent human prostate cancer cell lines and its mitigation by alpha-catenin. J Biol Chem. 2002;277:50087–50097. doi: 10.1074/jbc.M202652200. [DOI] [PubMed] [Google Scholar]
- Song JC, Rangachari PK, Matthews JB. Opposing effects of PKCalpha and PKCepsilon on basolateral membrane dynamics in intestinal epithelia. Am J Physiol Cell Physiol. 2002;283:C1548–1556. doi: 10.1152/ajpcell.00105.2002. [DOI] [PubMed] [Google Scholar]
- Kauppi M, Simonsen A, Bremnes B, Vieira A, Callaghan J, Stenmark H, Olkkonen VM. The small GTPase Rab22 interacts with EEA1 and controls endosomal membrane trafficking. J Cell Sci. 2002;115:899–911. doi: 10.1242/jcs.115.5.899. [DOI] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe P, Lau A. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- Chow SE, Lee RS, Shih SH, Chen JK. Oxidized LDL promotes vascular endothelial cell pinocytosis via a prooxidation mechanism. FASEB J. 1998;12:823–830. doi: 10.1096/fasebj.12.10.823. [DOI] [PubMed] [Google Scholar]
- Borsum T, Henriksen T, Reisvaag A. Oxidized low density lipoprotein can reduce the pinocytic activity in cultured human endothelial cells as measured by cellular uptake of [14C]sucrose. Atherosclerosis. 1985;58:81–96. doi: 10.1016/0021-9150(85)90057-7. [DOI] [PubMed] [Google Scholar]
- Larsen WJ, Tung HN, Murray SA, Swenson CA. Evidence for the participation of actin microfilaments and bristle coats in the internalization of gap junction membrane. J Cell Biol. 1979;83:576–578. doi: 10.1083/jcb.83.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naus CC, Hearn S, Zhu D, Nicholson BJ, Shivers RR. Ultrastructural analysis of gap junctions in C6 glioma cells transfected with connexin43 cDNA. Exp Cell Res. 1993;206:72–84. doi: 10.1006/excr.1993.1122. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am J Physiol. 1998;275:L843–851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- Razani B, Schlegel A, Lisanti MP. Caveolin proteins in signaling, oncogenic transformation and muscular dystrophy. J Cell Sci. 2000;113:2103–2109. doi: 10.1242/jcs.113.12.2103. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/S0092-8674(01)00472-X. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Mclntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science. 1996;274:239–242. doi: 10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- Fielding PE, Fielding CJ. Plasma membrane caveolae mediate the efflux of cellular free cholesterol. Biochemistry. 1995;34:14288–14292. doi: 10.1021/bi00044a004. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Ying Y, Donzell WC, Anderson RG. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- Lin D, Zhou J, Zelenka PS, Takemoto DJ. Protein kinase Cgamma regulation of gap junction activity through caveolin-1-containing lipid rafts. Invest Ophthalmol Vis Sci. 2003;44:5259–5268. doi: 10.1167/iovs.03-0296. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Ninomiya H, Miwa S, Masaki T. Cholesterol oxidation switches the internalization pathway of endothelin receptor type A from caveolae to clathrin-coated pits in Chinese hamster ovary cells. J Biol Chem. 2000;275:6439–6446. doi: 10.1074/jbc.275.9.6439. [DOI] [PubMed] [Google Scholar]
- Shigematsu S, Watson RT, Khan AH, Pessin JE. The adipocyte plasma membrane caveolin functional/structural organization is necessary for the efficient endocytosis of GLUT4. J Biol Chem. 2003;278:10683–10690. doi: 10.1074/jbc.M208563200. [DOI] [PubMed] [Google Scholar]
- Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- Sleer LS, Brown AJ, Stanley KK. Interaction of caveolin with 7-ketocholesterol. Atherosclerosis. 2001;159:49–55. doi: 10.1016/S0021-9150(01)00486-5. [DOI] [PubMed] [Google Scholar]
- Solan JL, Fry MD, TenBroek EM, Lampe PD. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci. 2003;116:2203–2211. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]
- Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol. 2001;154:815–827. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GT, Lin R, Warn-Cramer BJ, Lau AF, Burt JM. Mechanism of v-Src- and mitogen-activated protein kinase-induced reduction of gap junction communication. Am J Physiol Cell Physiol. 2003;284:C511–520. doi: 10.1152/ajpcell.00214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem. 1998;273:9188–9196. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]
- Girao H, Pereira P. Phosphorylation of connexin 43 acts as a stimuli for proteasome-dependent degradation of the protein in lens epithelial cells. Mol Vis. 2003;9:24–30. [PubMed] [Google Scholar]
- Qin H, Shao Q, Igdoura SA, Alaoui-Jamali MA, Laird DW. Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J Biol Chem. 2003;278:30005–30014. doi: 10.1074/jbc.M300614200. [DOI] [PubMed] [Google Scholar]
- Le AC, Musil LS. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J Cell Biol. 2001;154:197–216. doi: 10.1083/jcb.200101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert V, Duverneuil L, Poupon S, Monier S, Le Guern N, Lizard G, Masson D, Lagrost L. The impairment of endothelium-dependent arterial relaxation by 7-ketocholesterol is associated with an early activation of protein kinase C. Br J Pharmacol. 2002;137:655–662. doi: 10.1038/sj.bjp.0704920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AC, Musil LS. Normal differentiation of cultured lens cells after inhibition of gap junction-mediated intercellular communication. Dev Biol. 1998;204:80–96. doi: 10.1006/dbio.1998.9030. [DOI] [PubMed] [Google Scholar]
- Gu S, Yu XS, Yin X, Jiang JX. Stimulation of lens cell differentiation by gap junction protein connexin 45.6. Invest Ophthalmol Vis Sci. 2003;44:2103–2111. doi: 10.1167/iovs.02-1045. [DOI] [PubMed] [Google Scholar]
- Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–320. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- Girao H, Shang F, Pereira P. 7-ketocholesterol stimulates differentiation of lens epithelial cells. Mol Vis. 2003;9:497–501. [PubMed] [Google Scholar]