Abstract
Delivery of therapeutics into the brain is impeded by the presence of the blood-brain barrier (BBB) which restricts the passage of polar and high molecular weight compounds from the bloodstream and into brain tissue. Some direct delivery success in humans has been achieved via implantation of transcranial catheters; however this method is highly invasive and associated with numerous complications. A less invasive alternative would be to dose the brain through a surgically implanted, semipermeable membrane such as the nasal mucosa that is used to repair skull base defects following endoscopic transnasal tumor removal surgery in humans. Drug transfer though this membrane would effectively bypass the BBB and diffuse directly into the brain and cerebrospinal fluid. Inspired by this approach, a surgical approach in mice was developed that uses a donor septal mucosal membrane engrafted over an extracranial surgical BBB defect. This model has been shown to effectively allow the passage of high molecular weight compounds into the brain. Since numerous drug candidates are incapable of crossing the BBB, this model is valuable for performing preclinical testing of novel therapies for neurological and psychiatric diseases.
Keywords: Medicine, Issue 89, drug delivery, mucosa membrane, blood-brain barrier, neurosurgery, transnasal, mouse model
Introduction
The treatment of neurological and psychiatric disease is severely hindered by the presence of the blood-brain barrier (BBB) which prevents over 95% of all potential pharmaceutical agents from reaching the central nervous system1-3. For example, Glial Derived Neurotrophic Factor (GDNF) has been shown to be effective in treating Parkinson's Disease when injected directly into the brain, however is ineffective when delivered systemically because it cannot penetrate the BBB4-6.
Numerous approached have been developed to try to circumvent this problem. Improvement in systemic delivery of neurotheraputics has been demonstrated by using drug conjugates containing antibodies selective for transport proteins located on the brain capillary endothelium; however this method has not been shown to be applicable for a broad range of pharmaceuticals7,8. Additionally, osmotic opening of the BBB has been used clinically, however this method suffers from systemic drug dosing as opposed to a more direct delivery to the brain region of interest9. Substantial effort has been put into optimizing transnasal delivery in the hopes of directly targeting the brain10-12. Although some success has been achieved, conclusive results have only been obtained for drugs that possess endogenous receptors, such as insulin13,14. Furthermore the mechanism of transnasal delivery has been controversial with evidence suggesting indirect entry into the brain via olfactory neuron uptake or through the bloodstream11. Direct, transcranial delivery using implantable catheters has been achieved, however this procedure is highly invasive and associated with numerous complications15,16. To date, there is no general, minimally invasive method to deliver high molecular weight compounds into the brain.
Presented herein is a murine surgical procedure that creates a semipermeable interface with the brain. This is accomplished by engrafting a mucosal membrane explant17 over a surgical craniotomy defect in a mouse. Using this procedure it has been shown that soluble compounds up to 500 kDa can be delivered into the central nervous system (directly into brain parenchyma as well as into cerebrospinal fluid) in both a time and molecular weight dependent fashion18. This method of bypassing the BBB is a model for skull base defect repairs in humans which uses vascularized mucosal grafts to repair holes in the skull following transnasal endoscopic surgery19,20.
Protocol
Prior to surgery make sure all procedures to be done are approved by IACUC and any additional ethical or legal authorities and use humane animal treatment practices. This includes using sterile surgery conditions, anesthetizing the mouse using IACUC approved method, lubricating mice eyes with vet ointment during surgery, and providing postsurgical care. Do not proceed with surgery if there is any question whether aspects of the procedure are approved. All procedures performed herein were approved by the Boston University Institutional Animal Care and Use Committee.
1. Preparation of Animals and Surgical Supplies
Autoclave all surgical instrument that will be used during the surgery.
Make sure all techniques that are to be performed are approved by the animal regulatory agencies.
2. Harvesting of the Mucosal Graft
Chose a genetically identical mouse of similar age as the experimental mouse and euthanize it in an IACUC approved method (here: isoflurane asphyxiation followed by cervical dislocation).
Using surgical scissors, remove the skin around the nasal region of mouse head exposing the skull.
With a pneumatic drill, mark with three lines two of which laterally flank the nasal region and a third in line with the eyes which connects the two lines perpendicularly.
Drill down ventrally in order to separate the nasal septum from the surrounding tissue. A wider path will prevent damage to the mucosal membrane however it will also make it more difficult to isolate the membrane. A narrow cut closer to midline is recommended.
Use scissors to cut the septum free from any tissue adhered to it and store it in a sterile saline solution. At this time the graft can be cleaned up to remove any connected tissue. The ideal situation is to have undamaged mucosal membranes exposed on both sides of the cartilage septum. One graft can supply membrane for two mice provided the surface area of the membrane is sufficient to cover the craniotomy sites. It is recommended that the graft is used as quickly as possible and the researcher proceeds to step 3 as soon as the graft is isolated.
3. Surgical Implantation of Mucosal Graft
Using standard, aseptic murine surgical procedures, anesthetize and mount a mouse in the stereological frame. Use approximately 2% isoflurane in pure oxygen using a rodent anesthesia machine.
Immobilize the mouse in a stereotaxic apparatus with ear bars and a nose holder. Apply ophthalmic ointment to the eyes and scrub the scalp with betadine and 75% ethanol for three rounds. Using either scissors or a hair trimmer, remove the fur on the head. Expose the skull with a razor blade and level the head. Perform a craniotomy above the location of the brain that will be dosed. For example, when targeting the striatum cut a 1.25 mm diameter circular hole in the skull (centered at AP: 1.00 mm; ML: 0.88 mm) using a pneumatic drill. Wet the drilled area with sterile saline and use a razor blade to remove the skull.
Carefully remove the dura using the tip of a needle. Additionally, this can be accomplished by applying a minimal amount of tissue adhesive to the moist dural surface. Once this layer has hardened, lateral motion with a razor blade tip can be used to remove the membrane.
Place the mucosal membrane above the brain surface taking extreme care to keep the epithelial side facing away from the wound. This is best done by transferring the entire septum on to the surface of the skull adjacent to the craniotomy site with tweezers. Using the tip of a pair of surgical scissors, pull the membrane off of the cartilage and onto the skull and brain surface. Do not let the membrane dry out or touch it with any absorbent material. The graft should generously overlap all bony edges of the craniotomy site.
Cover the graft with a sterile piece of nitrile. This acts to prevent adhesion of the skin to the graft during healing. The nitrile needs to be large enough to cover the entire mucosal membrane. Trim excessive membrane if necessary. Avoid any motion of the nitrile once it has come into contact with the graft.
Close the skin with a running 5-0 sterile suture and let the mouse recover for 3-7 days before proceeding to the next step. Take care not to perturb the nitrile barrier or the mucosal graft during skin closure.
4. Administration of Dosing Solution
After securing the anesthetized mouse in the stereotaxic frame, cut the suture with scissors and remove excess skin around the skull.
Remove the nitrile barrier and clean the surface of the skull. Use sterile saline and cotton swabs to clean the area until the graft is visible. It may be necessary to cut the graft with a razor if has grown larger than the desired surface area.
If the experiment will be longer than a few days, it is wise to implant at least two skull screws to reinforce the head implant.
Place the well above the graft so that the edges are in contact with the skull. Apply cyanoacrylate adhesive at the junction between the well and the skull. Fill the well with sterile saline and check to make sure there are no leaks. Wells are made from cut syringe needles.
Apply bone cement on the skull to secure the well in place.
Remove the saline from the well with a pipette. Wash the well several times to verify that adhesive has not leaked in. Add the desired solution; in this case 50 μl of fluorescent dextran is used. It is expected that water soluble compounds of a similar polarity will behave the same as dextran. Delivery of hydrophobic compounds or suspensions has not been explored with this method.
Cap the top of the well by using a circular piece of nitrile secured to the top using cyanoacrylate adhesive. Make sure the adhesive does not come in contact with the well contents.
5. Analysis of Transmucosal Delivery
After the desired amount of time has passed, anesthetize the mouse and exchange the well contents with a solution of Evan's blue dye. This dye is used to verify that the graft was intact.
After 30 min, anesthetize the mouse heavily, remove the dye solution, and euthanize via decapitation.
Manually remove the implant and remove the brain using surgical scissors. Be careful to keep the graft in place.
Once removed, flash-freeze the brain in a solution of isobutane cooled in a dry ice bath.
Embed the brain in Optimal Cutting Temperature (OCT) solution and slice at 50 μm.
Place the desired slices directly on a microscope slide.
Image the slice using a florescence microscope as soon as the OCT solution has dried.
Representative Results
Obtaining a large enough nasal septum explant is crucial for the subsequent steps. This can be accomplished by drilling at the location on the donor mouse's skull shown in Figure 1a. Cutting along this path will produce an explant of sufficient the size as shown in Figure 1b. If the drilling depth is not deep enough, the graft will be truncated and it will be hard to obtain a large enough membrane to cover the brain surface. Drilling more laterally than the suggested path is not advised because more tissue will remain on the nasal septum, and the next step will be more difficult. Prior to the transfer of the mucosal membrane, the surface of the septum needs to be cleaned of all excess connective tissue. Once this is done, the mucosal membrane should be visible on the surface of the cartilage. Figure 1c shows what the tissue looks like after cleaning.
Prior to transferring the membrane, a craniotomy and durotomy need to be performed to expose the brain surface. Only after the post-durotomy bleeding has stopped can the membrane be transferred. Figure 2a shows what the surgery site should look like before continuing to the next step. Transferring the mucosal membrane from the cartilage onto the brain surface is the most technically challenging step of the entire surgical procedure and should be done carefully. If the harvested septum is large enough, the surface area of the mucosal graft should be large enough to cover the brain surface. Once transferred, the surgical area should looks similar to that in Figure 2b. It is important to make sure that the side of the membrane that is in contact with the cartilage is the same one that is in contact with the brain surface. If the membrane gets flipped over or folded over itself, it should be discarded and another one should be used. Once this step is complete, the scalp can be sutured so that the mouse can recover and the graft can heal. In order to prevent any adverse reactions from the graft coming in contact with the inner surface of the scalp, a piece of protective nitrile should be placed above to the surgical site. As shown in Figure 2c, the inserted piece should be large enough to cover the membrane but not large enough to impede suturing the skin closed.
During the recovery period, substantial tissue in-growth occurs of the surrounding periosteum which has to be removed prior to dosing the membrane. After reopening the scalp, sterile cotton swabs and saline solution will need to be used to clean the site until it looks similar to that in Figure 3a. We have not observed any substantial immune response; however this has not been confirmed histologically. The graft may need to be cut with a razor if it has grown across the surface of the skull. At this stage the well that will contain the dosing solution should be implanted above the graft. Several skull screws can be put in at this step if long term mechanical stability of the implant is a concern. Make sure the well is positioned so that it does not touch the graft. Once in place, cyanoacrylate adhesive can be applied to glue it to the skull. To prevent the adhesive from coming in contact with the graft it should be applied to the surface of the well and pushed downward to seal the gap. Once the well is secure, it should be filled temporarily with saline to both hydrate the graft and check for leaks. The adhered well should look like that in Figure 3b and thesolutionshould be clear indicating that it does not contain any adhesive. Any leaks will be obvious because the fluid level in the well will drop. If leaks are found they need to be fixed with more adhesive. The well is then reinforced with bone cement. At this point the saline in the well can be removed with a pipette and replaced with the desired dosing solution. When covering the well with a piece of nitrile care should be taken to make sure the adhesive does not contact the solution in the well. At the end of the entire procedure, the mouse head should look like that in Figure 3c. The bone cement is slightly translucent therefore if the contents of the well are light sensitive, dye or ink can be added to the surface of the dried cement or to the cement mixture to prevent photodegradation of the well contents.
After the desired period of waiting time, the brain should be analyzed to discover the effects of the dosing. The brain should be treated differently depending on what is being examined. If the purpose of the procedure is to analyze the presence of any chemical added to the well, (such as described in the protocol section above) then it is recommended to do a flash freezing of the brain to preserve the administered compounds. Figure 4 shows a representative outcome of using this approach. Dosing with a 40 kDa fluorescent polymer (tetramethyrhodamine conjugated dextran) for 24 hours shows noticeable diffusion into the brain tissue. Our previous results with dextran show diffusion into the brain tissue is both time and molecular weight dependent18. Smaller molecules diffuse into the brain parenchyma to a greater extent than larger ones, and a larger dosing time results in a larger amount of diffusion for all molecular weights that we tested. If flash freezing is done it is important to mount the slides after sectioning and not expose them to any solution. Any liquid will solubilize the administered compound and diffuse it across the surface area of the brain slice. If immunohistochemistry is to be performed on the brain then it is recommended to perfuse the mouse to fix the brain tissue.
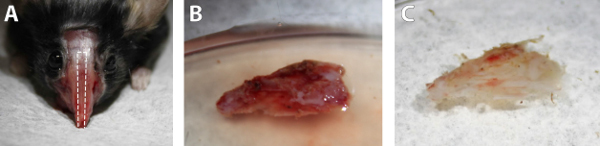 Figure 1. Images of the nasal septum harvesting procedure. A) The location of the drilling site on the skull of a euthanized mouse. The dotted lines indicate the perimeter to drill. B) The nasal septum directly after surgical removal. C) The nasal septum after removing excess tissue. Click here to view larger image.
Figure 1. Images of the nasal septum harvesting procedure. A) The location of the drilling site on the skull of a euthanized mouse. The dotted lines indicate the perimeter to drill. B) The nasal septum directly after surgical removal. C) The nasal septum after removing excess tissue. Click here to view larger image.
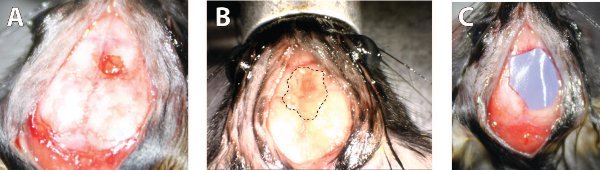 Figure 2. Surgical images of the engrafting process. A) The surgical site following craniotomy and durotomy. The image is representative of what the skull should look like prior to placing the graft upon it. B) Placement of the mucosa graft on to the craniotomy site. The dotted line indicates the perimeter of the graft. The image shows the desired surface area of the graft. C) Implantation of the protective nitrile barrier. The size of the material as shown is large enough to cover the graft but not large enough to interfere with suturing. Click here to view larger image.
Figure 2. Surgical images of the engrafting process. A) The surgical site following craniotomy and durotomy. The image is representative of what the skull should look like prior to placing the graft upon it. B) Placement of the mucosa graft on to the craniotomy site. The dotted line indicates the perimeter of the graft. The image shows the desired surface area of the graft. C) Implantation of the protective nitrile barrier. The size of the material as shown is large enough to cover the graft but not large enough to interfere with suturing. Click here to view larger image.
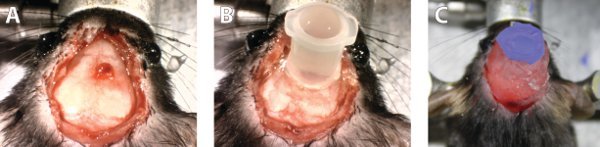 Figure 3. Surgical images of the drug dosing process. A) An example of what the healed mucosal graft looks like following rigorous cleaning of the surgical site. B) Image of well attached to the skull. The interface of the skull and well is secured with cyanoacrylate adhesive and the well is filled with sterile saline. The stabile solution level indicates no leaks from the well and the clarity of the solution indicates no contamination of the solution with adhesive. C) The image of the completed surgery. The well contains the dosing solution and is securely capped with a nitrile barrier. Bone cement is in place to rigidify the well implant. Click here to view larger image.
Figure 3. Surgical images of the drug dosing process. A) An example of what the healed mucosal graft looks like following rigorous cleaning of the surgical site. B) Image of well attached to the skull. The interface of the skull and well is secured with cyanoacrylate adhesive and the well is filled with sterile saline. The stabile solution level indicates no leaks from the well and the clarity of the solution indicates no contamination of the solution with adhesive. C) The image of the completed surgery. The well contains the dosing solution and is securely capped with a nitrile barrier. Bone cement is in place to rigidify the well implant. Click here to view larger image.
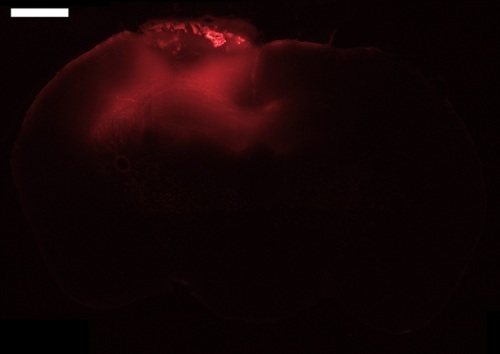 Figure 4. Fluorescent microscope image of a mouse brain slice following transmucosal dosing with 40 kDa tetramethylrhodamine conjugated dextran for 24 hr. The slice was taken at -1.06 mm bregma with a thickness of 50 µm. The mucosal graft is visible on the surface of the brain. Bar = 1 mm. Click here to view larger image.
Figure 4. Fluorescent microscope image of a mouse brain slice following transmucosal dosing with 40 kDa tetramethylrhodamine conjugated dextran for 24 hr. The slice was taken at -1.06 mm bregma with a thickness of 50 µm. The mucosal graft is visible on the surface of the brain. Bar = 1 mm. Click here to view larger image.
Discussion
The most difficult step of the procedure described herein is the successful transfer of an adequately sized mucosal membrane onto the brain surface. This step is made significantly easier if the harvested nasal septum is large enough and cleaned well. If the ventral portion of the septum is truncated, a new graft should be obtained. The drilling angle should be perpendicular to the mouse head to ensure that the mucosal membrane is not damaged by the drill. If a wider than recommended drilling path is taken it will be much harder to remove the extra tissue that is connected to the septum. Any large pieces of connective tissue should be removed with sharp scissor cuts rather than attempting to pull them loose. Smaller pieces of tissue can be removed by scraping with the tips of the scissors provided the tissue is not connected to the mucosal membrane. At the end of the cleaning process, the cartilage surface with the membrane still attached should be exposed. Occasionally the septal cartilage may become detached from the bone that it is connected to on the dorsal edge. If this happens it is still possible to use it in the next step however it is more challenging for two reasons. The bone is a convenient handle to hold the explant when cleaning and transferring it. Once removed, it is harder to maneuver the more delicate cartilage sheet. Secondly, the cartilage-bone junction is where the mucosal membrane is thickest and easiest to obtain when removing it from the cartilage. If the bone is severed from the cartilage this portion of membrane is lost.
Once the septal graft has been cleaned and the craniotomy and durotomy have been performed, the mucosal membrane can be placed over the exposed brain surface. This is best accomplished by positioning the explant adjacent to the craniotomy site. The membrane can then be pulled from the cartilage, onto the skull, and over the brain. Many things can go wrong during this process. The membrane can dry out, get torn, fold on top of itself, or bunch up. Also the membrane covering the other side of the septum can get damaged from rubbing against the skull. It is also possible that in attempting to remove the upward facing mucosal membrane, one removes the membranes from both sides at the same time. In order to prevent all of these problems it is crucial to move the membrane slowly and always observe both sides of the septum. The best strategy is to use the tips of the scissors to dislodge the membrane from the cartilage sheet before trying to slide it off. If any portion of the membrane remains attached, it is possible that the elasticity of the tissue will retract after pulling on it. By using small, tugging motions the membrane can be detached from the septum. Only then will it readily slide off. The membrane is too thin to be able to be handled with tweezers; it can only be pulled from one surface to another. If it gets flipped over or folded over itself, it should be discarded and another one should be obtained.
If the graft covers the entire surface area of the exposed brain, the process was successful and the scalp can be sutured so that the surgery site can heal. A nitrile barrier must be put in place so that the skin does not come in contact with the membrane. Care must be taken so that pressure is not exerted on the nitrile when suturing otherwise the graft position may move. Also take care not to suture into the nitrile. The best way to make sure the material obeys is to keep it moist with saline solution and make sure it stays flat throughout the suturing.
Once the graft has healed (3-7 days) the well containing the dosing solution can be attached to the skull above the graft. It can be fixed temporarily to the skull with adhesive glue and permanently with bone cement. Once the bone cement is applied, it will not be possible to make any corrections to the well position therefore all optimization must be done prior to its application. Once the well is in position and held in place with cyanoacrylate adhesive, it should be filled with sterile saline. If the procedure was done successfully the liquid level will not change and the solution will be clear. If there is a leak, the level will drop and the location of where the water escaped should be visible. After applying more adhesive on the leak site, more saline should be added to verify the leak was sealed. If the adhesive contaminates the well solution, a translucent film will be visible at the top of the fluid level. If this occurs the well should be washed several times with saline until the solution is clear. Additionally, a cotton swap can be used to remove the film by touching it to the liquid surface. Only when the well solution is stable and clear should the cement be applied. When the cement is dry, the saline from the well can be removed and replaced with the dosing solution. Care should be taken to not damage the membrane when pipetting into and out of the well. Once filled, the top of the well should be sealed with a piece of nitrile using cyanoacrylate adhesive. It is important that space is left between the surface of the solution and the top of the well. This will ensure that the adhesive does not touch the solution when putting on the nitrile barrier.
Once it is time to analyze the effect of the dosing procedure, the brain must be removed and imaged. If immunohistochemistry is to be performed, standard perfusion and antibody staining can be done. If the location of the compound dosed is to be determined, it is recommended to flash freeze the brain in a dry ice/isobutane solution. This will ensure that the location of the compound in the brain slices is the same as it was immediately prior to euthanasia.
The protocol described herein describes a method to investigate drug delivery to the mouse brain through a surgically grafted semipermeable mucosal membrane18. This is analogous to the outcome of transnasal tumor removal surgery in humans in which skull base defects are repaired with a vascularized nasal mucosal membrane19,20. Using this mouse model, it is now possible to study how these mucosal grafts are capable of bypassing the BBB and allowing for direct high molecular weight drug delivery to the brain. We have now performed this procedure over 100 times. Crucial to this procedure is the successful harvesting of a nasal septal graft from a donor mouse and the transfer of the mucosal membrane from the graft onto the test mouse. This procedure, for the first time, enables preclinical testing of high molecular weight therapeutics for a variety of neurological and psychiatric disorders.
Disclosures
Benjamin S. Bleier MD is lead inventor of provisional patent covering methods of drug delivery to the central nervous system.
Acknowledgments
This study was funded by the Mcihael J. Fox Foundation for Parkinson's Research 2011 Rapid Response Innovations Awards Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Cardoso FL, et al. Looking at the blood-brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012;64:640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Cheng F-C, et al. Glial cell line-derived neurotrophic factor protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in C57BL/6 mice. Neurosci. Lett. 1998;252:87–90. doi: 10.1016/s0304-3940(98)00554-0. [DOI] [PubMed] [Google Scholar]
- Grondin R, et al. controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–2201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- Kirik D, et al. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat. Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug and gene targeting to the brain with molecular trojan horses. Nat. Rev. Drug Discov. 2002;1:131–139. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier delivery of protein and non-viral gene therapeutics with molecular Trojan horses. J. Control. Release. 2007;122:345–348. doi: 10.1016/j.jconrel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance M-A, et al. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008;10:166–177. doi: 10.1208/s12248-008-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkus FHM, Berg M. Can nasal drug delivery bypass the blood-brain barrier. Drugs R. D. 2007;8:133–144. doi: 10.2165/00126839-200708030-00001. [DOI] [PubMed] [Google Scholar]
- Dhuria SV, et al. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- Illum L. Nasal drug delivery-possibilities, problems and solutions. J. Control. Release. 2003;87:187–198. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. Intranasal insulin therapy for alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiherr J, et al. Intranasal insulin as a treatment for Alzheimer's Disease: A review of basic research and clinical evidence. CNS Drugs. 2013;27:505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Love S, et al. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat. Med. 2005;11:703–704. doi: 10.1038/nm0705-703. [DOI] [PubMed] [Google Scholar]
- Antunes MB, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. BioTechniques. 2007;43:195–204. doi: 10.2144/000112531. [DOI] [PubMed] [Google Scholar]
- Bleier BS, et al. Permeabilization of the blood-brain barrier via mucosal engrafting: implications for drug delivery to the brain. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0061694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Sprekelsen M, et al. Closure of cerebrospinal fluid leaks prevents ascending bacterial meningitis. Rhinology. 2005;43:277–281. [PubMed] [Google Scholar]
- Bleier BS, et al. Laser-assisted cerebrospinal fluid leak repair: An animal model to test feasibility. Otolaryngol. Head Neck Surg. 2007;137:810–814. doi: 10.1016/j.otohns.2007.05.060. [DOI] [PubMed] [Google Scholar]


