Abstract
Background
Understanding variations in the incidence of schizophrenia is a crucial step in unravelling the aetiology of this group of disorders. The aims of this review are to systematically identify studies related to the incidence of schizophrenia, to describe the key features of these studies, and to explore the distribution of rates derived from these studies.
Methods
Studies with original data related to the incidence of schizophrenia (published 1965–2001) were identified via searching electronic databases, reviewing citations and writing to authors. These studies were divided into core studies, migrant studies, cohort studies and studies based on Other Special Groups. Between- and within-study filters were applied in order to identify discrete rates. Cumulative plots of these rates were made and these distributions were compared when the underlying rates were sorted according to sex, urbanicity, migrant status and various methodological features.
Results
We identified 100 core studies, 24 migrant studies, 23 cohort studies and 14 studies based on Other Special Groups. These studies, which were drawn from 33 countries, generated a total of 1,458 rates. Based on discrete core data for persons (55 studies and 170 rates), the distribution of rates was asymmetric and had a median value (10%–90% quantile) of 15.2 (7.7–43.0) per 100,000. The distribution of rates was significantly higher in males compared to females; the male/female rate ratio median (10%–90% quantile) was 1.40 (0.9–2.4). Those studies conducted in urban versus mixed urban-rural catchment areas generated significantly higher rate distributions. The distribution of rates in migrants was significantly higher compared to native-born; the migrant/native-born rate ratio median (10%–90% quantile) was 4.6 (1.0–12.8). Apart from the finding that older studies reported higher rates, other study features were not associated with significantly different rate distributions (e.g. overall quality, methods related to case finding, diagnostic confirmation and criteria, the use of age-standardization and age range).
Conclusions
There is a wealth of data available on the incidence of schizophrenia. The width and skew of the rate distribution, and the significant impact of sex, urbanicity and migrant status on these distributions, indicate substantial variations in the incidence of schizophrenia.
Background
Why is a systematic review needed?
Gradients in the incidence of a disorder across time and across place can provide powerful clues to help unravel the aetiology of that disorder [1]. Numerous studies over the last few decades have presented original data on the incidence of schizophrenia, and several scholarly reviews have collated these studies [2-5]. Until recently, reviews of observational and experimental studies have used standard narrative approaches. However, over the last decade there has been a growing appreciation that reviews should be based on data that are as complete and as free of bias as possible [6]. Data from treatment or intervention studies have often been pooled using meta-analysis [7]. Even without pooling of data, the orderly sorting of data with meta-analytic techniques can provide useful insights into the structure of the relevant literature [8]. The need for such an exercise with respect to the incidence of schizophrenia was recognized by Jablensky [5] in an influential review: "Ideally, a meta-analysis involving a standardized recalculation of the rates from many previous studies should generate a distribution allowing one to estimate with some probability the extent to which populations differ" (p 219). We believe that a systematic review of the incidence of schizophrenia will help inform the research community, and thus contribute to the generation of hypotheses about the etiology of schizophrenia.
Ways to measure the incidence of schizophrenia
The incidence of a disease is a measure of the number of new cases that occur in a population over a given period of observation (see Additional File 1 for a glossary of definitions of terms and abbreviations used in this review). Traditional incidence studies (henceforth referred to as core studies) establish a rate based on the general population residing within a defined catchment area. National or health district catchment areas have often provided convenient boundaries regarding access to services and minimal out-of-area leakage. However, it should be noted that the boundaries chosen for incidence studies may not be optimal for the detection of variations of the disorder within or between various populations. In other words, there is no reason to believe that the genetic or environmental factors associated with the incidence of schizophrenia respect political or health-district boundaries. Nor should we expect that these risk factors are uniformly distributed within such boundaries.
Apart from general population-based studies, there are other studies that provide insights into the incidence of schizophrenia in subgroups of the general population. One type of population subgroup is the cohort defined by the year of birth. Subjects born in a particular time period (for example, 1966), can be identified and then tracked prospectively in order to identify the number of individuals who subsequently develop schizophrenia by a certain age. Cohorts can also be defined retrospectively. For example, a researcher may use a population register to identify all adults within a defined catchment area (for example, national boundary) who were born within a certain time period. Cross linking the population register with a mental health register can be used to identify cohort members who received treatment for schizophrenia up until a certain date. Cohort studies can provide information of the incidence of a disorder up to various ages within that cohort.
In addition to cohorts, the incidence of schizophrenia can also be examined in subgroups of the population defined by other criteria. Reporting the incidence of schizophrenia in subgroups of the population is standard practice in core incidence studies. For example, most studies report incidence rates for persons (that is, the general population) and then males and females separately (that is, subgroups of the population). In recent years there has been interest in subgroups defined by migrant status and risk of schizophrenia. Some studies report incidence rates in certain subgroups only (for example, twins, the deaf, those who belong to a certain religion or ethnic group, those aged 65 years or older). We have labelled these studies as Other Special Groups. Rates from migrant and Other Special Groups will be assessed separately in this review.
Studies from countries with comprehensive cross-linked national registers have been able to present incidence data based on person-years. Rather than present the number of new cases per 100,000 per year, these studies present the number of new cases that have accumulated over several years against a person-years denominator. This denominator takes into account the size of the underlying population sample, its age structure and mortality over the duration of observation. These studies will be considered separately to the core studies.
Finally, a systematic review such as this has to decide where to 'draw the line' between studies primarily designed to assess the incidence of schizophrenia, versus those studies primarily designed to compare incidence data with respect to the presence or absence of a candidate risk factor. For example, a study may compare the incidence of schizophrenia in a cohort exposed to different levels of urbanicity at birth, season of birth and family history [9]. Data related to the incidence of schizophrenia can often be found in studies exploring candidate nongenetic risk factors such as prenatal famine [10], influenza [11] and obstetric complications [12]. Such studies can provide additional data related to the incidence of schizophrenia but will not be covered in this review.
Key research questions related to the incidence of schizophrenia
What is the range of incidence rates?
The most influential study of the incidence of schizophrenia has been the WHO 10 Nation study [13]. This landmark study, which employed uniform methodology across sites, provided incidence data from eight sites (seven nations). When narrow criteria for schizophrenia were used (CATEGO S+), the incidence ranged from seven to 14 per 100,000 (Aarhus, Denmark to Nottingham, UK, respectively), while the range for ICD9 schizophrenia was 16 to 42 per 100,000 (Honolulu, Hawaii to the urban Chandigarh, India, respectively). Both definitions found at least a two-fold difference between the highest and lowest sites, and this difference for the broad (but not narrow) definition was statistically significant. Regardless of whether the incidence rates for the eight sites were significantly different, the authors of the study drew attention to the relatively narrow range of incidence rates identified in this study [13].
The results of the WHO 10 Nation study have often been misinterpreted as providing strong proof that the incidence of schizophrenia does not vary between sites. For example, Crow has stated, "The evidence points to the singular conclusion that, contrary to almost any other common condition, the incidence of schizophrenia is independent of the environment and a characteristic of human populations." (p119) [14]. In a recent narrative review, Jablenksy [15] restates the issue more concisely: "The general conclusion is that according to the great majority of studies, the prevalence and incidence rates of schizophrenia are similar across populations. However, a small number of populations have been identified that clearly deviate from this central tendency. The magnitude of these deviations is modest compared with the difference observed across populations with regard to other multifactorial diseases such as diabetes, ischaemic heart disease or cancer, where 10- to 30-fold differences in prevalence across populations are not uncommon." (p212). This systematic review will examine the central tendency and spread of data related to the incidence of schizophrenia.
Sex differences
Several reviews have drawn attention to sex differences in key epidemiological features of schizophrenia [16-18]. Recently, a systematic review and meta-analysis reported a higher incidence of schizophrenia in men versus women [17]. This systematic review will compare the distributions of rates for males versus females, and examine the central tendency and distribution of the male to female risk ratio.
Urban versus rural place of residence
There is evidence that urban settings are associated with higher rates of schizophrenia. While there are post-onset factors such as selective migration that may contribute to a higher prevalence of schizophrenia in cities, it is not clear if urban living is associated with a higher risk of developing schizophrenia. Building on the classic studies of Faris and Dunham [19], several recent studies have reported that being born in an urban versus rural region was associated with an increased risk of developing schizophrenia [9,20-23]. In addition, there is evidence from a Danish study that the number of years spent in an urban area during childhood increased the risk of developing schizophrenia [24]. However, because of the general urbanization of most nations over recent decades, it is difficult to disentangle the critical window of exposure for urbanicity [25]. A study from the Netherlands [26] looked at urbanicity of residence around the time of first admission as well as urbanicity at time of birth. This study reported that for those born in rural regions, urban residence around time of onset was not associated with an increased risk of schizophrenia. However, urban birth with or without urban place of residence around the onset of illness was associated with an increased risk. This systematic review will separate out incidence studies from urban, rural and mixed urban/rural setting and compare the distributions of these rates.
Migrant status
Since the pioneering studies by Ödegård in 1932 [27], the association between migrant status and increased risk of schizophrenia has stimulated a great deal of research and a wealth of creative explanatory hypotheses [28]. More recently the issue has been put back on the agenda by research from the United Kingdom showing higher rates in both Afro-Caribbean migrants [29] and other migrant groups [30]. While studies from the Netherlands [31], Denmark [32] and Sweden [33] have supported an increased risk of schizophrenia in migrants, not all studies have found this association [34,35]. This systematic review will compare the distributions of rates for native-born individuals versus migrant groups, and examine the central tendency and distribution of the migrant to native-born risk ratio.
The influence of methodology on incidence rates
Systematic reviews can explore possible sources of heterogeneity in data sets by sorting the data according to various rules and comparing the resulting distributions. Design features clearly need to be taken into account when comparing rates derived from different types of incidence studies.
When comparing rates derived from different catchment areas, it is important to note the population age structure within each site. The age of onset of schizophrenia is not randomly dispersed across the lifespan (peak onset in the second and third decades of life), thus sites with a younger population will yield higher incidence rates. In order to deal with this issue, some studies use techniques to adjust the raw incidence rate against a standard or reference population age structure, that is, age standardization. This provides a more valid way to compare the incidence of a disorder in two or more populations with different age distributions.
Apart from corrections related to age standardization, completeness of case identification is a critical feature in incidence studies. Thus one would predict that studies that cover a wider spectrum of potential recruitment sites (for example, inpatient and outpatient settings, general practitioners, other health care providers) would be able to identify more new cases. Method of diagnostic confirmation (for example, face-to-face interview versus chart diagnosis), diagnostic criteria (CATEGO S+ versus ICD9), age-range (all ages versus 15 to 54 years) can also influence the rate of schizophrenia [13].
In the field of systematic reviews, scores are often allocated to reflect desirable features related to the validity of the study [36]. In the field of descriptive epidemiology, the use of quality scores to rank order studies is relatively untested [37-39]. There are several features of Quality Scores that need to be kept in mind. The criteria used need to be easily operationalized and reliable, thus they tend to involve simple, categorical judgments (criteria met versus not met). Unfortunately, it has been shown that important items such as "Is the sample size adequate?" cannot be reliably operationalized [38]. It is self-evident that the ability to judge the quality of the study is contingent on the quality of the reporting of that study.
Finally, some commentators have noted that more recent studies have reported lower rates, suggesting that the incidence of schizophrenia is changing over time [40-42]. We explored this issue in the data set, and predicted that more recent studies would report rates that were lower compared to older studies.
Aims and hypotheses of this study
As part of a wider systematic review of the incidence and prevalence of schizophrenia, here we present those data and analyses related to the incidence of schizophrenia. We will present the methods of the analyses and the key characteristics of the included studies (divided into core studies, cohort and Other Special Groups). We will describe the distribution of rates and then sort these rates in order to examine several main hypotheses. Based the literature described above, we predict that: (a) males will have higher rates compared to females; (b) studies based on urban sites will have higher incidence compared to studies from rural or mixed urban-rural sites; and (c) migrant groups will have higher rates compared to native born. In addition, the influence of selected methodological features (quality score, year of study, scope of coverage, method of case finding, diagnostic criteria, age range, the use of age-standardization) will also be explored.
The over-riding aim of this research is to ensure that the highest quality evidence informs the scientific discourse about the incidence of schizophrenia. Therefore, we have made the data used in this systematic review freely available to the research community. The data used in the analyses are available in both Access and Excel files attached to the electronic version of this paper.
Methods
Identification of studies
As part of the wider study of the incidence and prevalence of schizophrenia, a broad search string ((schizo* OR psych*) AND (incidence OR prevalence)) was used in MEDLINE, PsychINFO, EMBASE and LILACS. Title and abstracts, if available, were reviewed in order to exclude irrelevant studies. Potentially relevant papers were accessed in order to review the full text. The references cited by each potentially relevant paper, as well as the citations in major review papers and book chapters, were scrutinized in order to locate additional potentially relevant papers. Posters were presented at two international schizophrenia conferences [43,44] in order to encourage researchers to contribute studies, especially studies from the 'grey literature' (for example, conference reports, theses, government reports, unpublished studies). Subsequently, letters or emails were sent to the senior authors of papers that met the inclusion criteria. In these letters, which included the most-recent list of included studies, authors were asked to inform us of missing papers and unpublished data.
Included studies
We included studies first published between January 1965 and December 2001 that reported primary data on the incidence of schizophrenia (according to any diagnostic criteria), drawn from either a general population sample or a subgroup of the population. Where multiple publications presented identical data, the most informative version of the study was included and the other related papers were excluded (full list available on request). At the time of submission, papers that had not yet been located were allocated to the 'Awaiting Assessment' category.
Data extraction
Once a study was included, data were extracted related to study-level variables (for example, authors, year of publication, site, urban/rural status, recruitment duration and years covered, case finding method, method of confirming diagnosis, diagnostic criteria), and rate-level variables (for example, rates for persons, males, females, rate of different diagnostic criteria, different age-specific rates). Full rate-level data for these variables can be found in the Additional File 6 (Excel format) and Additional File 7 (Zipped Access format). Two or more of the authors checked all data used in the analysis. When disagreements arose, these were resolved by consensus. If required, we contacted the authors for clarification of issues.
Studies were given quality points for certain features (for example, greater coverage, use of diagnostic criteria, quality of diagnostic method, thoroughness of reporting, etc). Details of the quality score used in this review are provided in Additional File 2.
It is important to remain mindful that one study may generate many items of information on the incidence of schizophrenia. Some of the rates are discrete (non-overlapping) such as those related to sex, epoch (for example, 1976 versus 1977) or location (Chandigarh urban versus Chandigrah rural).
Some of the rates overlap, such as those for age range (all age versus age-specific age bands), diagnostic criteria (ICD criteria and DSM criteria), and epoch (1976 alone versus average for 1975–1980).
Sorting the rates by the application of sequential filters
In order to deal with the range of study level and rate level variables identified by the systematic review, the data were categorized and then filtered by a series of criteria. For example, the first filter parsed rates from the included studies into four categories: (a) core studies, (b) migrant studies, (c) cohort studies, and (d) Other Special Groups. Next, a study-level filter was applied in order to isolate discrete data from multiple studies that overlapped in both time and place. This was required in order to eliminate several papers counting the same subjects more than once. We selected one paper from overlapping papers according to rules that prioritized the most 'informative' paper. Priority was given to studies with: (a) larger catchment areas, (b) larger general populations, and (c) longer duration of recruitment.
For studies that presented rate level data for the same individuals according to various criteria, a third filter was designed in order to select one representative rate for inclusion in the cumulative distribution. Once again, we selected one rate according to rules that prioritized the most 'informative' rates. Priority was given to rates that covered (a) the widest age range, (b) crude incidence rates rather than age-standardized rates (because more papers presented only raw rates and those that did present age-standardized rates rarely provided information about the method of standardization), (c) diagnostic criteria that were more prevalent in the included studies (for example, as ICD criteria were most often used, for studies that presented rates according to ICD and any other criteria, we chose the ICD rate; for studies that presented multiple CATEGO diagnoses, we selected the broadest SPO+ clinical). Details of the study-level and rate-level selections are provided in the Additional File 6 and 7. These rules were applied by two of more of the authors, and when disagreements arose, the decision was made by consensus.
Presentation and analyses of the data
For practical purposes, it is not possible to show every numerator, denominator and rate for the studies in printed tables, thus we will provide the total number of rates per study, and, where available, show the largest rate level numerator and its associated denominator for persons (or, if this is not available, the range for males and females).
The distribution of incidence rates is presented in cumulative plots. The distribution of the data is shown in rank order for incidence rate (lowest to highest ranks) with the cumulative percent of rates shown on the vertical axis. The plots show the 50% (median), and 25% and 75% quantiles (within which lies the inter-quartile range). We wish to draw the reader's attention to several features of these graphs. Firstly, the central, near-linear segment of the cumulative distributions may extend beyond the inter-quartile range (for example, from the 10%–80% quantiles), thus shape features (where the tails start, the range of the linear central segment) can be more informative than traditional inter-quartile ranges. Secondly, steeper segments of the cumulative plots are underpinned by rates that have a narrow distribution, while flatter (that is, more horizontal) segments of the distribution are underpinned by rates that are relatively more dispersed. Finally, some distributions are derived from more data than others. Regardless of slope, that is, steep or flat, if many rates underpin segments of the distributions, these segments warrant closer investigation, especially in analytic contexts, compared to sparsely plotted segments of the distribution.
The data will be presented for persons, as well as for males and females separately. The main analyses will be based on discrete (non-overlapping) core rates. Readers familiar with meta-analyses may expect to see figures displaying confidence intervals and pooled data, as well as read information related to formal tests exploring heterogeneity in the data. For several reasons, the data in this review do not lend themselves to these types of analyses. Among the 68 discrete core studies (see below), only four [31,45-47] supply confidence limits, and no other measures of spread (for example, standard errors) are reported. We were able to derive the standard error of the crude annual incidence rate from studies that reported the corresponding numerator, denominator, and duration of recruitment. Based on these calculations, we were only able to generate standard errors for 117 of the 373 (31.4%) rates, drawn from only 45 (45.0%) of the discrete core studies. Faced with such a restricted pool of standard errors, the ability to assess the heterogeneity of rates in a manner generalizable across all core studies is compromised. In addition, the issues that underlie the decision to combine data from randomized controlled trials or risk-factor epidemiological studies are of less relevance to incidence rates. For example, should incidence studies from very large populations (for example, one study from former East Pakistan has a population denominator of 51 million) [48] be allowed to exert several hundred times more influence on analyses than 'smaller' studies?
However, merely presenting the graphical displays of rate distributions does not allow a formal test of the hypotheses outlined above. No matter how appealing the descriptive approach, we are inevitably drawn into inferential statistics, and are forced to choose the least biased and most transparent method to compare the distributions. The analysis of the data in this systematic review poses two particular issues. Firstly, rates drawn from the same study tend to be more alike than those from different studies. Thus any comparison between distributions needs to control for the fact that one study may generate more than one rate (that is, the within-study variance). Secondly, because the distribution of the rates was often positively-skewed, the data required log-transformation. In order to aid interpretation of the distribution of the rates, Tables 123 present three measures of central tendency: the median, the arithmetic mean and the harmonic mean (which is a better indicator of the central tendency of the log-transformed data). As a consequence of these two issues, the statistical comparisons between distributions may not always concur with the visual interpretation of the raw distributions.
Table 1.
Characteristics (quantiles and moments) of incidence rates per 100,000, for rates for persons, males and females, and male to female rate ratio
| ns (nr) | 10% | 25% | Median | 75% | 90% | Mean | s | m h | |
| Persons | 55 (170) | 7.7 | 10.2 | 15.2 | 22.0 | 43.0 | 23.7 | 30.3 | 15.9 |
| Males | 31 (100) | 6.6 | 11.4 | 15.0 | 24.8 | 34.1 | 21.8 | 27.4 | 16.2 |
| Females | 31 (100) | 3.0 | 6.3 | 10.0 | 21.8 | 30.2 | 21.3 | 45.1 | 11.3 |
| Male to female rate ratio | 31 (100) | 0.9 | 1.1 | 1.4 | 1.8 | 2.4 | 1.5 | 0.6 | 1.4 |
ns, number of studies; nr, number of rates; s, standard deviation; mh, harmonic mean of the rates.
Table 2.
Characteristics (quantiles and moments) of incidence rates per 100,000 by sex and urbanicity
| ns (nr) | 10% | 5% | Median | 75% | 90% | Mean | s | m h | |
| Persons | |||||||||
| Urban | 23 (67) | 7.5 | 11.2 | 19.0 | 39.9 | 67.0 | 30.8 | 38.5 | 20.4 |
| Rural | 5 (7) | 0.0 | 11.0 | 20.0 | 23.0 | 222.6 | 45.4 | 78.6 | 27.9 |
| Mixed | 29 (96) | 7.9 | 10.0 | 13.3 | 19.4 | 26.0 | 15.3 | 8.6 | 12.9 |
| Males | |||||||||
| Urban | 10 (20) | 10.7 | 13.5 | 24.6 | 45.4 | 64.5 | 39.6 | 57.8 | 24.8 |
| Rural | 2 (2) | 19.0 | 19.0 | 69.7 | 120.3 | 120.3 | 69.7 | 71.7 | 47.8 |
| Mixed | 20 (80) | 6.5 | 9.7 | 13.9 | 22.8 | 27.4 | 16.1 | 7.9 | 14.2 |
| Females | |||||||||
| Urban | 10 (20) | 7.5 | 11.5 | 22.7 | 49.3 | 74.5 | 43.3 | 67.4 | 23.4 |
| Rural | 2 (2) | 23.0 | 23.0 | 174.9 | 326.8 | 326.8 | 174.9 | 214.8 | 86.7 |
| Mixed | 20 (79) | 3.0 | 4.6 | 8.0 | 20.0 | 24.0 | 11.6 | 8.1 | 8.9 |
ns, number of studies, nr, number of rates, s, standard deviation, mh, harmonic mean of the rates.
Table 3.
Characteristics (quantiles and moments) of incidence rates per 100,000, by Migrant Status, and Migrant to Native-born rate ratio by sex
| ns (nr) | 10% | 25% | Median | 75% | 90% | Mean | s | m h | |
| Persons | |||||||||
| Migrant | 17 (48) | 13.5 | 40.0 | 60.0 | 109.5 | 615.4 | 140.9 | 208.2 | 77.3 |
| Native | 15 (20) | 6.9 | 8.4 | 16.9 | 32.5 | 111.0 | 35.8 | 45.1 | 19.9 |
| Males | |||||||||
| Migrant | 10 (51) | 10.2 | 18.0 | 39.0 | 131.0 | 161.0 | 72.5 | 66.1 | 46.6 |
| Native | 10 (14) | 9.0 | 18.6 | 22.5 | 27.0 | 41.9 | 26.4 | 21.0 | 20.1 |
| Females | |||||||||
| Migrant | 10 (51) | 6.0 | 8.0 | 22.0 | 51.0 | 66.9 | 41.5 | 61.5 | 24.4 |
| Native | 10 (14) | 4.9 | 9.0 | 15.5 | 19.1 | 30.0 | 20.3 | 23.8 | 13.4 |
| Migrant to Native born rate ratio | |||||||||
| Persons | 15 (43) | 1.0 | 2.7 | 4.6 | 6.8 | 12.8 | 7.4 | 12.0 | 4.7 |
| Males | 10 (47) | 0.8 | 1.2 | 2.3 | 4.8 | 6.5 | 3.1 | 2.2 | 2.5 |
| Females | 10 (47) | 0.4 | 0.9 | 1.7 | 3.1 | 3.9 | 2.5 | 2.9 | 1.8 |
ns, number of studies, nr, number of rates, s, standard deviation, mh, harmonic mean of the rates.
Results
The electronic search identified 834 papers, while manual reference checking identified an additional 249 references. We received responses from 52 authors (see Acknowledgements for full list), who provided an additional 41 references (Figure 1). At the level of potentially relevant papers, 74% were identified from electronic sources. We identified 98 studies in Languages Other Than English (LOTE). After translation, 10 were included in the study. The subsequent culling and final distribution of the papers is shown in Figure 1.
Figure 1.
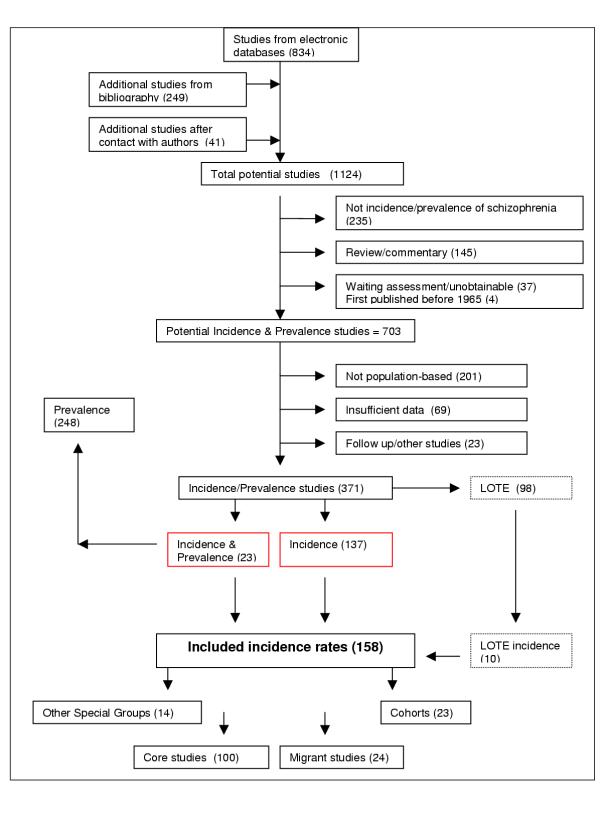
Flow diagram (selection strategy) of included studies. LOTE, Languages other than English.
The rates were based on an estimated 176,056 potentially overlapping incident cases. Key details of the included studies are shown in Tables S1–S4, which can be found in Additional File 4. The systematic review identified 100 core studies [13,31,33,40,41,45-61,63,65-127,148,180-192]. The 158 included studies were drawn from 32 countries. One study [13] provided rates for seven countries, and another provided rates for two countries [74]. Details of these studies are shown in Table S1 in Additional File 4.
There were 24 migrant studies [31,33,35,121,128-147], 23 cohort studies [9,10,21,23,42,149-165,193] and 14 studies that reported the incidence of schizophrenia in Other Special Groups [166-179]. Key features of these studies can be found in Tables S2, S3 and S4 in Additional File 4.
The review identified four studies that presented standardized incidence rates (rate ratio of specific group versus a reference group) [40,174,180,181], however rates from several of these studies also contributed to the core rates [40,180,181] and one study contributed to Other Special Groups [174]. Of the included studies, 13 included at least one rate with person years as the denominator [9,21,31,42,74,93,121,130,133,152,163,167,174]. Person-year incidence rates have been used in descriptive and model-based analyses alongside crude annual rates. While this has the potential to distort results (an annualised person-year rate will almost never agree with a notionally corresponding crude annual rate), it is hoped that the distortion is minimal.
Of the 1,457 rates in the included studies, 501 (34.4%), from 42 studies, employed age-standardization: [13,31,35,45,46,50,56,68,74,76,80,88,91,102,107,109,110,115,116,118,124,127,128,130,133,135-138,141,142,144-150,168,181,187,188]. As with person-year incidence rates, age-standardized rates have been used alongside crude rates. The impact of age-standardization versus raw rates will be addressed below.
Core studies
Out of 100 core studies, 39 studies report rates only for persons [33,40,45,47,48,55-57,59,61,73,75,79,86,87,116,91,93-96,99,103,104,107-109,112-114,118,120-122,124-127,183,192], 16 report rates only for males and females [41,63,68,74,82,102,110,111,116,117,119,123,187-190], and 45 studies report rates for persons, males, and females [13,31,46,49-54,58,60,65-67,69-72,76-78,80,81,83-85,88-90,92,97,98,100,101,105,106,115,148,180-182,184-186,191].
The studies provided between one to 96 rates per study; the WHO 10 nation study was the most informative study [13]. Of the 509 rates derived from the core studies, 79% relied on chart or register-based diagnosis, a further 7% relied on the application of diagnostic criteria applied to written case note material, and only 11% relied on face-to-face interview (the remaining rates used other methods or this information was not specified). The most commonly used diagnostic criterion for the rates in the core studies was one of the ICD classifications (60%), while 6% used a CATEGO-derived classification, 4% used DSM, and 4% used RDC. The remaining 28% used two criteria serially (for example, ICD9 chart diagnosis followed by DSM-III-R diagnoses) or did not specify the diagnostic criteria.
Discrete-core studies
Out of 100 core studies 68 studies provided at least one discrete rate [13,31,33,41,45-61,65,67,69-80,82-86,88,91-104,106,107,111,112,114,116,121,123-126,188,189]. The remaining 32 studies included rates that overlapped, by year and place, completely or partially with discrete core rates. The 68 discrete-core studies were drawn from 27 countries.
Thirty seven discrete-core studies report rates only for persons [31,33,45-49,55-57,59,61,65,71,73,75,76,78,79,85,86,88,91,93-96,99,103,104,107,112,114,121,124-126,180,190], 13 report rates only for males and females [41,50,52,53,67,74,82,102,111,116,123,188,189], and 18 studies report rates for persons, males, and females [13,51,54,58,60,69,70,72,77,80,83,84,92,97,98,100,101,106].
Of the 68 discrete-core studies, 41 (60.3%) relied on chart or register-based diagnosis, a further nine (13.2%) relied on the application of diagnostic criteria applied to written case note material, and 15 (22.1%) relied on face-to-face interview (the remaining core studies used other methods or this information was not specified). In terms of the 373 associated rates, 276 (74.0%) relied on chart or register-based diagnosis, 28 (7.5%) relied on the application of diagnostic criteria applied to written case note material, and 45 (12.1%) relied on face-to-face interview.
The most commonly used diagnostic criterion for the 68 discrete-core studies was one of the ICD classifications (51.5%), while 5.9% used a CATEGO-derived classification, 11.8% used DSM, and 2.9% used RDC. The remaining 27.9% either used another criterion, or used multiple criteria serially (for example, ICD 9 chart diagnosis followed by DSM IIIR diagnoses), or did not specify the diagnostic criteria.
Of the 373 discrete-core rates, 25.2% (n = 94), coming from 13 studies, were age-standardized [31,45,46,50,56,74,80,91,102,107,116,124,188].
Figure 2 shows the distribution of rates for persons, males and females, based on discrete-core data. In order to aid visual representation, the figure has been truncated at 100 per 100,000, however the quantiles and moments that characterize the distribution are based on all rates. Table 1 shows the quantiles and moments of these distributions. For persons, based on 170 rates, the median incidence rate was 15.2 per 100,000, and the 10% and 90% quantiles ranged from 7.7 to 43.0 per 100,000 (a 5.6-fold difference).
Figure 2.
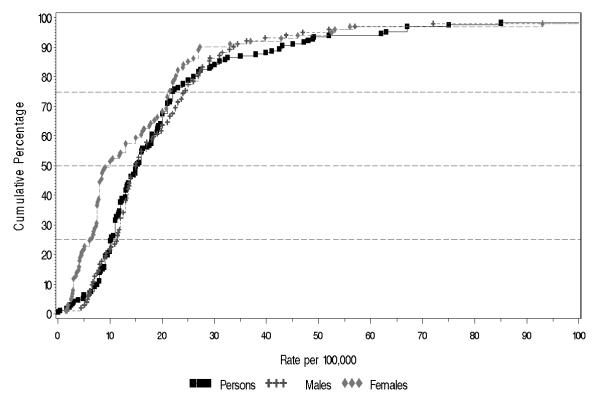
Cumulative plots of the incidence rates per 100,000, by sex (plot truncated at 100).
Sex ratio
Unexpectedly, we noted that studies that only report incidence rates for persons appeared to report higher rates than studies that reported rates for males and females only or studies reporting males, females and persons (for figures and tables related to these analyses, see Additional File 3 and 5 respectively). This feature needs to be kept in mind in analyses that split by persons, males and females.
Figure 3 shows the distribution of the rate ratio for males versus females. These ratios are matched, and derived from within-study male to female ratios. Values greater than one indicate a higher incidence of schizophrenia in males compared to females. The rate ratio curve is normally distributed. While there were nine studies with rate ratios less than one [13,41,51,60,69,70,72,83,106,111], over 84% of rates lie above one, with a median value (10% to 90% quantiles) of 1.40 (0.9, 2.4). In a random-effects model for the logarithm of the rates, the sex-difference is significant (F1,30 = 76.8, p < 0.001).
Figure 3.
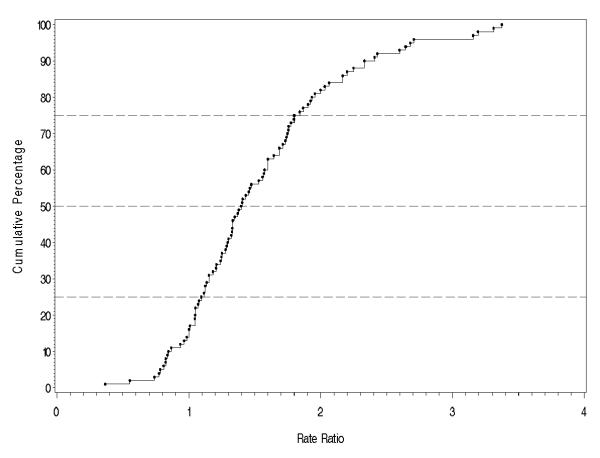
The cumulative percentage of the rate ratio of the incidence of schizophrenia in males versus females.
Urbanicity
We identified 21 discrete-core studies with rates from urban sites [31,33,54,61,69-71,75,78,80,84,91,93,97,98,100,103,106,121,124,125], three studies from rural sites [57,59,60], and 42 studies with mixed urban-rural catchment areas [41,45-49],230,[50-53,55,56,58,65,67,72-74,76,77,79,82,83,85,86,88,92,94-96,99,101,102,104,107,111,112,114,116,123,188,189]. Two further studies provide rates for both urban and rural categories [13,126].
Table 2 and Figure 4 summarize the characteristics of the rates by sex and urbanicity. Because of the within-study configuration of both sex and urbanicity, and the paucity of studies on the rural sector, significance tests can only be constructed for differences between the urban and mixed categorizations.
Figure 4.
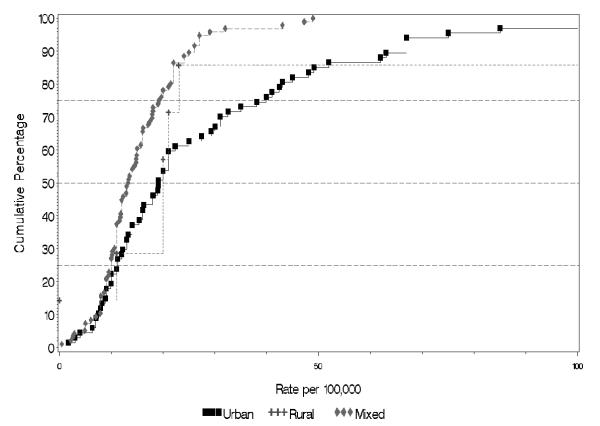
Cumulative plots of the incidence rates per 100,000, and urbanicity of site (plot truncated at 200).
The difference in harmonic means is significant for persons (F1,50 = 6.06, p = 0.02); non-significant for males (F1,28 = 2.7, p = 0.11); and of borderline significance for females (F1,28 = 4.2, p = 0.05).
Migrant status
We identified 24 migrant studies from five countries [128], Germany (n = 1) [35], Sweden (n = 1) [33], The Netherlands (n = 3) [31,129,130], and the United Kingdom (n = 18) [121,131-147]. One study [144] was excluded from the analysis because of overlapping by time and place. Table S2 (see Additional File 4) presents a list of migrant studies with key descriptive variables, incidence rates and within-study rate ratios. The table highlights the variable definitions of first and second generation migrants used in these studies – an issue that could be more closely scrutinized in the future.
Overall migrant groups displayed elevated incidence of schizophrenia compared to their native-born populations (Table 3 and Figure 5). Note that there are more rates for migrant groups than for native-born populations, reflecting the fact that many studies reported rates for several different migrant groups. The migrant to native-born rate ratio median (10% to 90% quantiles) was 4.6 (1.0 to 12.8) (Table 3 and Figure 6). In particular, the migrant groups had higher rates for persons in the upper half of the distribution. The difference in harmonic means between migrants versus native-born is significant for persons (F1,13 = 51.8, p < 0.001); for males (F1,8 = 27.1, p < 0.001); and for females (F1,8 = 10.4, p = 0.01).
Figure 5.
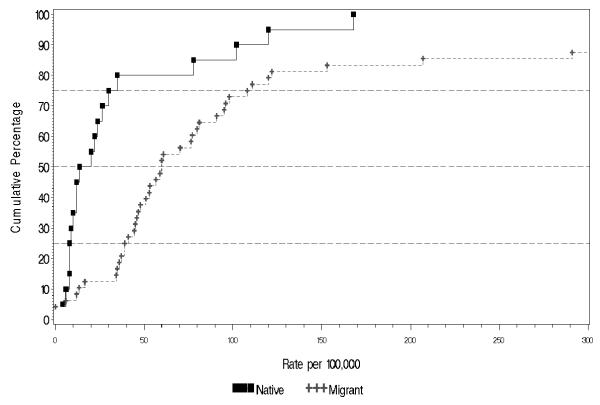
Cumulative percentage of incidence of schizophrenia per 100,000 in persons by migrant status.
Figure 6.
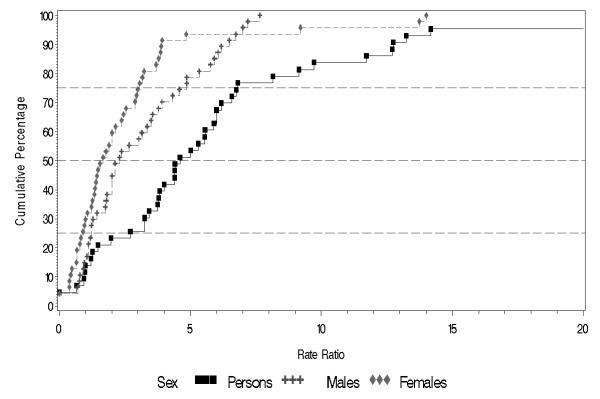
Cumulative percentage of rate ratio between migrants and natives in persons, males and females.
Methodological features
Additional figures (Figures S1–S8) and detailed tables (Tables S6–S16) related to the following section can be found in the Additional File 3 and 5 respectively.
Eight of the 68 studies show variation in quality score within studies, but always by two points at most on the scale. Thus we used the average quality score per study as our measure of a study's quality. When divided into terciles for quality score, the distribution of rates did not differ significantly (F2,65 = 0.34, p = 0.72). Concerning methods of case identification, only eight studies used community-based surveys in order to ascertain cases [13,45,46,55,57,78,88,127]. When the rates were arranged by method of case identification, the rate distributions did not differ significantly (F3,64 = 0.14, p = 0.93). Fifteen of the 68 discrete-core studies relied on face-to-face interviews in order to confirm the diagnosis [13,31,45,56,57,59,65,73,76,78,82,88,101,104,125]. When the rates were arranged by method of diagnostic confirmation, they did not differ significantly (F3,62 = 0.17, p = 0.92). Curiously, the studies that went to the effort of using face-to-face diagnostic interview tended to yield similar incidence rates as studies using chart/case records (median 14.0). The studies with systematic review of case notes produced numerically higher incidence rates (median 21.0).
Twelve studies reported reliability data on their diagnostic techniques [13,33,45,54,65,71,78,83,96,102,121,124], and one study reported details of a 'leakage study' in order to identify potential missed cases [78]. Eight studies [46,53,54,61,72,74,101,117] provided more than one method of diagnostic assessment, however in this review we had to choose only one method in accordance with the rules outlined above. When the rates were arranged by diagnostic criteria, there were no significant differences amongst the distributions (F4,63 = 0.04, p = 0.99).
Surprisingly, when the rates were divided into those that were age-standardized versus raw rates, these distributions did not differ significantly (F1,48 = 0.24, p = 0.63), nor did age-range impact significantly on the rate distributions (F2,36 = 0.80, p = 0.42). Only one of the methodology variables revealed a significant difference – year of first intake, a measure related to possible secular change. The differences among harmonic means was significant (F2,18 = 13.3, p < 0.001), with significant differences among all three harmonic means (in particular 1949–1975 versus 1976–1983, p < 0.001 unadjusted; 1976–1983 versus 1984–1995, p = 0.005 unadjusted).
Cohort and Other Special Group studies
We identified 23 discrete cohorts that have reported either cumulative incidence proportion or other measures of the occurrence of schizophrenia [9,10,21,23,42,149-165,193]. Some of these cohorts have generated multiple publications reporting the incidence of schizophrenia over time. These cohorts came from nine sites: Australia (n = 2) [149,150], Denmark (n = 3) [9,151,193], Finland (n = 5) [42,152-155], Israel (n = 2) [156,157], Italy (n = 1) [158], Sweden (n = 2) [21,159], The Netherlands (n = 2) [10,23], USA (n = 3) [163-165], and the United Kingdom (n = 3) [160-162]. Because of differences in the age of the cohorts, the rates are not readily comparable; however the highest cumulative incidences have been reported from Finland and the United States.
In addition, we identified 14 studies that reported the incidence of schizophrenia in the following subgroups of the population: over age 65, twins, various ethnic and/or religious subgroups, the offspring of certain Jewish immigrants, students, deaf individuals, and workers in a radiation contamination zone [166-179]. These studies came from 11 countries: Denmark (n = 2) [166,167], Israel (n = 1) [168], Mauritius (n = 1) [169], New Zealand (n = 1) [170], Norway (n = 1) [171], Romania (n = 1) [172], Russian Federation (n = 1) [173], Sweden (n = 1) [174], USA (n = 1) [179], Ukraine (n = 1) [175], and the United Kingdom (n = 3) [176-178].
Key details of the Cohort and Other Special Groups can be found in the tables included in Additional File 4.
Discussion
A total of 1,457 rates from 158 studies were identified in this systematic review of the incidence of schizophrenia, thus it is understandable that the reader interested in this area may 'starve amidst plenty'. After the application of various filters, inspection of the distribution of the rates was informative. Most distributions were data-rich, and many had central segments densely underpinned by data.
The distribution of rates
For persons in discrete core studies, 55 different studies provided 170 rates. The median value for rates was 15.2 per 100,000. The distribution was positively skewed, consistent with previous comments on the distribution of the incidence of schizophrenia [5]. Excluding the top and lower 10% of the distribution, rates ranged from 7.7 to 43.0 per 100,000. Because schizophrenia has a relatively low incidence rate, zero provides an absolute 'floor' for the rate distribution. It is of interest to note that while the lower tails of the distributions were usually data-sparse, many of the distributions had upper tails that contained more than 25% of the rates. Several of the distributions had marked positive skewness. Thus, while Jablensky commented that "a small number of populations have been identified that clearly deviate from this central tendency" [5], the data suggest that the number of studies that 'deviate' may not be so small, and that this deviation is predominantly for high rates, rather than low rates.
Mindful that we are dealing with a disorder with a relatively low incidence rate, it is a moot point if the range is narrower or wider than other low incidence rate disorders. For example, Type I diabetes occurring before age 15 also has a relatively low incidence. However, a WHO-sponsored systematic review of studies that used a shared definition of Type I diabetes described the range of 10 to 40 per 100,000 as "prominent worldwide variation" [194]. So, comparable incidence ranges may be described as "lacking in variation" by some commentators, and "markedly variable" by others. Regardless of the adjectives used to describe the distribution of rates, researchers must strive to understand the factors that drive this variation.
Sex and schizophrenia
Based on studies identified in this systematic review, rates were generally higher in males compared to females. The rate distributions suggest that the magnitude of the sex difference is relatively constant across most studies, however for studies that report a higher incidence of schizophrenia (for example, greater than 32 per 100,000), the size of the male excess may be smaller. The median male/female rate ratio was 1.40 (inter-quartile range = 1.1 to 1.8), which is similar to a pooled risk ratio derived from core, migrant and cohort-derived incidence data [17]. We note that nine studies [13,41,51,60,69,70,72,83,106,111] reported a risk ratio of less than one (that is, higher incidence of schizophrenia in females versus males). We examined the major characteristics of these studies, but could not identify any consistent feature that distinguished these studies from those with rate ratios greater than one.
It should be noted that the studies that underpin the male to female rate distribution have been collected over several decades from many different nations, and have been based on many different design features. The fact that the distribution of the male to female rate ratio is a normal distribution is visually striking. While it is feasible that these studies share systematic biases that may have influenced the findings (for example, excluding older age groups), in order to 'wash out' this finding, a substantial number of new studies reporting a male to female ratio of less than one would need to be published.
Overall, the data indicate that the incidence of schizophrenia is higher in men than in women.
Urbanicity and the Incidence of schizophrenia
Based on the studies identified in this systematic review, the distribution of rates derived from urban catchment areas was higher compared with that based on mixed urban-rural catchment areas (p = 0.02 for persons, p = 0.11 for males, and p = 0.05 for females). The small number of rates available for rural catchment areas does not allow the more precise urban versus rural comparison.
This finding provides support for the hypothesis that the incidence of schizophrenia is higher in cities compared to mixed urban/rural catchment areas. Our findings are in keeping with recent cohort and case control studies based on place of birth [9,23]. However, within the mixed urban-rural studies, we were not able to infer exactly how 'rural' the mixed sites were. Indeed, we allocated studies to the mixed category if there was any possibility that rural sectors were included. This bias would have made any true difference between urban versus mixed urban/rural more difficult to detect.
Migrant status and the incidence of schizophrenia
Compared to native-born populations, rate distributions based on migrant groups had a relatively consistent pattern of higher rates compared to the distributions based on rates for native-born individuals. This association was also found when rates for males and females were examined separately (p < 0.001 for males, p = 0.01 for females). Based on migrant versus native-born rate ratios, the median rate ratio for persons was 4.6 (10% to 90% quantile = 1.0, 12.8).
It is important to note that migrant studies are prone to a range of methodological issues. These include factors related to differential pathways to care, diagnostic inaccuracies (language and cultural practices may hinder accurate diagnosis), potential confounding due to socioeconomic factors and problems in determining the numerator and denominator for the calculation of rates. Harrison and colleagues have argued that because of these potential biases, confidence in the link between migrant status and schizophrenia requires consistent findings based on different methods and from different sites [146]. While the studies analyzed in this systematic review would share common biases, they are drawn from many different sites, include many different migrant groups and differ on a range of methodological features. Thus the increased relative risk for migrants warrants added weight.
Design features of the studies
While the analyses related to the quality score and methodology are interesting, we urge caution in the interpretation of these results. Researchers interested in comparing the psychometric properties of face-to-face interviews versus chart diagnoses, or the DSM-III-R versus ICD-9 diagnostic criteria are best directed to studies that were primarily designed to address these issues. These methodological issues are important, but are more efficiently addressed by studies based on prevalent rather than incident cases. However, mindful of the above issues, we found that the distributions of rates did not significantly differ when categorized by (a) quality of the study, (b) different methods of diagnostic confirmation, (c) different diagnostic criteria, (d) the presence or absence of age-standardization, and (e) different age ranges. The distributions of rates seem relatively impervious to these design features. However, we found that rates from more recent studies generated distributions that encompassed lower rates compared to those distributions from older studies.
Cohort and Other Special Groups
In recent years several studies have reported the incidence of schizophrenia in well-described birth cohorts [161,163-165,195]. While the incidence rates from these studies are not readily comparable to core incidence studies, the cohort studies are making important contributions to risk factor research [196]. It is of interest to note that several of the authors of cohort studies have drawn attention to the relatively high cumulative incidence proportions being found in their studies [42,154,163,164]. For example, the Finish birth cohort at age 31 estimated the cumulative incidence of DSM-III-R schizophrenia between 0.73% and 1.08% [195].
The Other Special Group studies share few, if any, features. Perhaps the common feature was that the authors felt that the incidence of schizophrenia may have differentiated the particular groups under observation from the general population. Interestingly, several of these studies suggest that certain minority ethnic/cultural groups may have an elevated incidence of schizophrenia [168-170,179], which may complement the findings from migrant studies.
Caveats
Systematic reviews are secondary research ('research on research'). The object of scrutiny is not the incidence of schizophrenia per se, but the literature on this topic. As such, this type of research cannot be used to 'prove' a hypothesis about the underlying category of observation. However, the compiled data can encourage the generation of new hypotheses that can then be tested prospectively, with new data. In particular, systematic reviews can draw attention to factors that may underlie heterogeneity of the data.
Despite all of our efforts to ensure a complete dataset, reviews such as this are bound to miss studies, and/or to have data entry errors. We encourage readers to inform us of missing studies or errors in the data. Updated lists of relevant studies and raw data will be available from the authors. In the absence of clear guidelines, many of the rules we used to filter studies and extract data were ad hoc. In the future, researchers may wish to re-analyze the data set using different criteria, and explore sensitivity analyses related to these choices.
It is clear from the broad range of studies identified in this systematic review that there is no one 'perfect' design for measuring the incidence of schizophrenia – different studies have different strengths. Most of the studies provide rates that are in reality the 'treated incidence' of schizophrenia (that is, the count of individuals who are identified by services and treated). Factors related to service availability and the pathways to care vary considerably. Apart from the obvious issue of availability of services, recent research has shown that community education about psychosis can lead to sudden increases in the number of 'new' cases presenting to services [197]. However, we can reasonably infer that the studies included in this systematic review probably underestimate the true (underlying) incidence of schizophrenia.
There are many additional analyses that could be undertaken with these data. In future papers we plan to explore the relationship between incidence rates and ecological features of the catchment area (for example, population age structure, social and financial indices). The impact of age adjustment on the rates (which has been addressed in previous narrative reviews [198]), will also be examined more closely. However, in a commentary, Berlin [199] cautions the scientist as follows: "Meta-analysis is like any other form of data analysis in that it requires strict adherence to methodological guidelines, careful planning, the use of a priori definitions and analytic strategies, and extremely careful interpretation that does not go beyond the limits of the data" (p387). We endorse Berlin's recommendations.
Conclusions
This systematic review of the incidence of schizophrenia reveals a complex and varied epidemiological landscape [5]. While the median incidence rate for persons was 15.2 per 100,000, the 10% to 90% quantiles cover over a five-fold range of rates (7.7 to 43.0 per 100,000). The distribution is positively skewed, with many studies reporting rates in the upper range. The incidence of schizophrenia is higher in men compared to women, higher in urban sites compared to mixed urban/rural sites, and higher in migrants compared to native-born individuals.
Traditional Linnean taxonomy classifies living creatures into various hierarchical categories. By way of analogy, this systematic review has approached the broad genus of studies related to the incidence of schizophrenia, and classified rates from these studies to the epidemiological equivalents of species and subspecies. We believe that the orderly array of these data has been informative and, in some instances, visually appealing (like butterfly collections!). We encourage researchers to further explore these data in order to help generate testable hypotheses.
Goethe noted that data are the natural enemy of hypotheses. In a Darwinian sense, the provision of data alters the landscape such that 'unfit' hypotheses should be less prosperous. In other words, the addition of new data should assist in the culling of less heuristic hypotheses. By assembling and sorting rates related to the incidence of schizophrenia, we hope that we have enriched the epidemiological environment and that these data will facilitate the survival of the most heuristic hypotheses.
Competing interests
None declared.
Authors' contributions
JM conceived and supervised the study. All authors assisted with design of the study, data extraction, and writing up. DC supervised and carried out the statistical analysis. All authors were involved in data management and the preparation of the manuscript.
Additional files
(1) Glossary: Word document
(2) Quality Reporting Scale: Word document
(3) Additional figures: Word document
• Figure S1. Cumulative plots of the incidence rates per 100,000, by features of reporting by sex
• Figure S2. Cumulative plots of the incidence rates per 100,000, by tercile rank of the quality score
• Figure S3. Cumulative plots of the incidence rates per 100,000, by Case-Finding Method
• Figure S4. Cumulative plots of the incidence rates per 100,000, by Confirmation of Diagnosis
• Figure S5. Cumulative plots of the incidence rates per 100,000, by Diagnostic Assessment Method
• Figure S6. Cumulative plots of the incidence rates per 100,000, by Sex and Age Standardization
• Figure S7. Cumulative plots of the incidence rates per 100,000, by Age Range
• Figure S8. Cumulative plots of the incidence rates per 100,000, by Years of Intake
(4) Description of studies: Word document
a. Table S1: Summary table of incidence of schizophrenia: Core studies
b. Table S2: Summary table of incidence of schizophrenia: Migrant studies
c. Table S3: Birth cohort studies providing incidence in person years, cumulative incidence or cumulative percent
d. Table S4: The incidence of schizophrenia in Other Special Groups
(5) Additional tables and detailed results: Word document
• Table S5: Characteristics (quantiles and moments) of incidence rates per 100,000, for rates for persons, males and females, and male to female rate ratio
• Table S6: Characteristics (quantiles and moments) of incidence rates per 100,000, by Sex and Urbanicity
• Table S7: Characteristics (quantiles and moments) of incidence rates per 100,000, by Migrant Status, and Migrant to Native-born rate ratio by sex
• Table S8: Characteristics (quantiles and moments) of incidence rates per 100,000, by Sex, and by features of reporting by sex.
• Table S9. Characteristics (quantiles and moments) of incidence rates per 100,000, by terciles of the Quality Score
• Table S10: Characteristics (quantiles and moments) of incidence rates per 100,000, by Method of Finding Cases
• Table S11: Characteristics (quantiles and moments) of incidence rates per 100,000, by Confirmation of Diagnosis
• Table S12: Characteristics (quantiles and moments) of incidence rates per 100,000, by Diagnostic Assessment Method
• Table S13: Characteristics (quantiles and moments) of incidence rates per 100,000, by Sex and Age Standardization
• Table S14: Characteristics (quantiles and moments) of incidence rates per 100,000, by Age Range
• Table S15: Characteristics (quantiles and moments) of incidence rates per 100,000, by year of first Intake
(6) Complete data set in Excel format.
(7) Complete data set in Access format. (zipped).
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
http://www.biomedcentral.com/imedia/1862872583417819/sup1.doc
http://www.biomedcentral.com/imedia/8656447533417738/sup2.doc
http://www.biomedcentral.com/imedia/6800396953417826/sup3.doc
http://www.biomedcentral.com/imedia/1144262997341774/sup4.doc
http://www.biomedcentral.com/imedia/1480669390341774/sup5.doc
http://www.biomedcentral.com/imedia/1396785452341783/sup6.xls
http://www.biomedcentral.com/imedia/1705991844341783/sup7.zip
Acknowledgments
Acknowledgements
The authors wish to express their gratitude to the following colleagues who assisted in the search for data and translation of the studies: J Allardyce, P Allebeck, G Andrews, M Balestrieri, N Beer, D Bhugra, M Biggs, J Boydell, M Bresnahan, S Caleo, R Cochrane, T Crow, J Culberg, C D'Arcy, D Downham, J Eagles, W Eaton, RW Grawe, V Folnegovic, Z Folnegovic, H Hafner, G Harrison, C Harvey, QK Hii, F Hickling, W Iacono, M Isohanni, E Jacko, A Jablensky, P Jorgensen, P Joyce, M Kawai, E Klaning, A Lesage, I Lynge, W Mallett, O Mors, PB Mortensen, P Munk-Jorgensen, RM Murray, T Oldehinkel, A Preti, M Prince, A Rybak, R Scheurer, JP Selten, I Shvetson, E Susser, J Suvisaari, N Takei, D Tantam, R Thara, EF Torrey, J van Os, D Walsh, and Z Welham. The Stanley Medical Research Institute supported this project.
Contributor Information
John McGrath, Email: john_mcgrath@qcsr.uq.edu.au.
Sukanta Saha, Email: sukanta_saha@qcsr.uq.edu.au.
Joy Welham, Email: joy_welham@qcsr.uq.edu.au.
Ossama El Saadi, Email: ossama_elsaadi@qcsr.uq.edu.au.
Clare MacCauley, Email: clare_maccauley@qcsr.uq.edu.au.
David Chant, Email: david_chant@qcsr.uq.edu.au.
References
- McGrath JJ. Gaining traction on the epidemiological landscape of schizophrenia. Am J Epidemiol. 2003;158:291–300. doi: 10.1093/aje/kwg119. [DOI] [PubMed] [Google Scholar]
- Eaton WW. Epidemiology of schizophrenia. Epidemiol Rev. 1985;7:105–126. doi: 10.1093/oxfordjournals.epirev.a036278. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Tien AY, Poeschla BD. Epidemiology of schizophrenia. In: Den Boer JA, Westenberg HGM, van Praag HM, editor. In Advances in the Neurobiology of Schizophrenia. John Wiley & Sons; Oxford, UK; 1995. [Google Scholar]
- Warner R, de Girolamo G. Schizophrenia. Geneva: World Health Organization; 1995. [Google Scholar]
- Jablensky A. Schizophrenia: the epidemiological horizon. In: Hirsch SR, Weinberger DR, editor. In Schizophrenia. Oxford: Blackwell Science; 2003. pp. 203–231. [Google Scholar]
- Breslow RA, Ross SA, Weed DL. Quality of reviews in epidemiology. Am J Public Health. 1998;88:475–477. doi: 10.2105/ajph.88.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers I, Haynes B. Reporting, updating, and correcting systematic reviews of the effects of health care. BMJ. 1994;309:862–865. doi: 10.1136/bmj.309.6958.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD. Meta-Analysis. Potentials and promise. BMJ. 1997;315:1371–1374. doi: 10.1136/bmj.315.7119.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, Andersen PK, Melbye M. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603–608. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, Gorman JM. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- Selten JP, Brown AS, Moons KG, Slaets JP, Susser ES, Kahn RS. Prenatal exposure to the 1957 influenza pandemic and non-affective psychosis in The Netherlands. Schizophr Res. 1999;38:85–91. doi: 10.1016/S0920-9964(99)00005-5. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, Day R, Bertelsen A. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as the price that homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Brain Res Rev. 2000;31:118–129. doi: 10.1016/S0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Jablensky AV, Kalaydjieva LV. Genetic epidemiology of schizophrenia: phenotypes, risk factors, and reproductive behavior. Am J Psychiatry. 2003;160:425–429. doi: 10.1176/appi.ajp.160.3.425. [DOI] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Castle DJ. Women and schizophrenia: an epidemiological perspective. In: Castle DJ, McGrath J, Kulkarni J, editor. In Women and schizophrenia. Cambridge: Cambridge University Press; 2000. pp. 19–33. [Google Scholar]
- Faris REL, Dunham HW. Mental disorders in urban areas. Chicago: Chicago University Press; 1939. [Google Scholar]
- Takei N, O'Callaghan E, Sham P, Glover G, Murray RM. Winter birth excess in schizophrenia: its relationship to place of birth. Schizophr Res. 1992;6:102. doi: 10.1016/0920-9964(92)90114-K. [DOI] [Google Scholar]
- Lewis G, David A, Andreasson S, Allebeck P. Schizophrenia and city life. Lancet. 1992;340:137–140. doi: 10.1016/0140-6736(92)93213-7. [DOI] [PubMed] [Google Scholar]
- O'Callaghan E, Colgan K, Cotter D, Larkin C, Walsh D, Waddington JL. Evidence for confinement of winter birth excess in schizophrenia to those born in cities. Schizophr Res. 1992;6:102. doi: 10.1016/0920-9964(92)90113-J. [DOI] [PubMed] [Google Scholar]
- Marcelis M, Navarro-Mateu F, Murray R, Selten JP, Van Os J. Urbanization and psychosis: a study of 1942–1978 birth cohorts in The Netherlands. Psychol Med. 1998;28:871–879. doi: 10.1017/S0033291798006898. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 2001;58:1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- McMichael AJ. Human frontiers, environments and disease. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Marcelis M, Takei N, Van Os J. Urbanization and risk of schizophrenia: does the effect operate before or around the time of illness onset? Psychol Med. 1999;29:1197–1203. doi: 10.1017/S0033291799008983. [DOI] [PubMed] [Google Scholar]
- Odegard O. Emigration and insanity: a study of mental disease among Norweigian-born population in Minnesota. Acta Psychiatrica et Neurologica Scandinavica. 1932;7:1–206. [Google Scholar]
- Eaton W, Harrison G. Ethnic disadvantage and schizophrenia. Acta Psychiatr Scand Suppl. 2000;102:38–43. doi: 10.1034/j.1600-0447.2000.102001038.x. [DOI] [PubMed] [Google Scholar]
- Harrison G. Searching for the causes of of schizophrenia: The role of migrant studies. Schizophr Bull. 1990;16:663–671. doi: 10.1093/schbul/16.4.663. [DOI] [PubMed] [Google Scholar]
- Aesop Study Team Raised incidence of all psychosis in UK migrant populations. Schizophr Res. 2002;53:33. [Google Scholar]
- Selten JP, Veen N, Feller W, Blom JD, Schols D, Camoenie W, Oolders J, van der Velden M, Hoek HW, Rivero VM, van der Graaf Y, Kahn R. Incidence of psychotic disorders in immigrant groups to The Netherlands. Br J Psychiatry. 2001;178:367–372. doi: 10.1192/bjp.178.4.367. [DOI] [PubMed] [Google Scholar]
- Cantor-Graae E, Pedersen CB, McNeil TF, Mortensen PB. Migration as a risk factor for schizophrenia: a Danish population-based cohort study. Br J Psychiatry. 2003;182:117–122. doi: 10.1192/bjp.182.2.117. [DOI] [PubMed] [Google Scholar]
- Zolkowska K, Cantor-Graae E, McNeil TF. Increased rates of psychosis among immigrants to Sweden: is migration a risk factor for psychosis? Psychol Med. 2001;31:669–678. doi: 10.1017/S0033291701003786. [DOI] [PubMed] [Google Scholar]
- McGrath J, El-Saadi O, Cardy S, Chapple B, Chant D, Mowry B. Urban birth and migrant status as risk factors for psychosis: an Australian case-control study. Soc Psychiatry Psychiatr Epidemiol. 2001;36:533–536. doi: 10.1007/s001270170003. [DOI] [PubMed] [Google Scholar]
- Weyerer S, Hafner H. The high incidence of psychiatrically treated disorders in the inner city of Mannheim. Susceptibility of German and foreign residents. Soc Psychiatry Psychiatr Epidemiol. 1992;27:142–146. doi: 10.1007/BF00788760. [DOI] [PubMed] [Google Scholar]
- Moher D, Olkin I. Meta-analysis of randomised controlled trials. A concern for standards. JAMA. 1995;274:1962–1964. doi: 10.1001/jama.274.24.1962. [DOI] [PubMed] [Google Scholar]
- Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: Prevalence or incidence of a health problem. Chronic Dis Can. 1998;19:170–176. [PubMed] [Google Scholar]
- Al-Jader LN, Newcombe RG, Hayes S, Murray A, Layzell J, Harper PS. Developing a quality scoring system for epidemiological surveys of genetic disorders. Clin Genet. 2002;62:230–234. doi: 10.1034/j.1399-0004.2002.620308.x. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Allardyce J, Morrison G, Van Os J, Kelly J, Murray RM, McCreadie RG. Schizophrenia is not disappearing in south-west Scotland. Br J Psychiatry. 2000;177:38–41. doi: 10.1192/bjp.177.1.38. [DOI] [PubMed] [Google Scholar]
- Al Mousawi AH, Dunstan FD. Changes in the risk of schizophrenia in Scotland: is there an environmental factor? Schizophr Bull. 1998;24:529–535. doi: 10.1093/oxfordjournals.schbul.a033347. [DOI] [PubMed] [Google Scholar]
- Suvisaari JM, Haukka JK, Tanskanen AJ, Lonnqvist JK. Decline in the incidence of schizophrenia in Finnish cohorts born from 1954 to 1965. Arch Gen Psychiatry. 1999;56:733–740. doi: 10.1001/archpsyc.56.8.733. [DOI] [PubMed] [Google Scholar]
- El-Saadi O, Welham J, Saha S, MaCaulay C, Collingwood L, Chant D, McGrath JJ. The incidence and prevalence of schizophrenia: preliminary results from a systematic review. Schizophr Res. 2001;S49 [Google Scholar]
- El-Saadi O, Welham J, Saha S, MaCaulay C, Collingwood L, Chant D, Torrey EF, McGrath JJ. The incidence and prevalence of schizophrenia: preliminary results from a systematic review. Schizophr Res. 2002;S53:S132–32. [Google Scholar]
- Bhugra D, Hilwig M, Hossein B, Marceau H, Neehall J, Leff J, Mallett R, Der G. First-contact incidence rates of schizophrenia in Trinidad and one-year follow-up. Br J Psychiatry. 1996;169:587–592. doi: 10.1192/bjp.169.5.587. [DOI] [PubMed] [Google Scholar]
- Mahy GE, Mallett R, Leff J, Bhugra D. First-contact incidence rate of schizophrenia on Barbados. Br J Psychiatry. 1999;175:28–33. doi: 10.1192/bjp.175.1.28. [DOI] [PubMed] [Google Scholar]
- D'Arcy C, Rawson NSB, Lydick E, Epstein R. The epidemiology of treated schizophrenia Saskatchewan 1976–1990. In World Psychiatric Association, Section of Epidemiology and Community Psychiatry The Netherlands. 1993.
- Chowdhury AK. Admissions to an East Pakistan mental hospital. Br J Psychiatry. 1966;112:65–68. doi: 10.1192/bjp.112.482.65. [DOI] [PubMed] [Google Scholar]
- Messias E, Sampaio JJ, Messias NC, Kirkpatrick B. Epidemiology of schizophrenia in Northeast Brazil. J Nerv Ment Dis. 2000;188:118–120. doi: 10.1097/00005053-200002000-00009. [DOI] [PubMed] [Google Scholar]
- Bland RC. Demographic aspects of functional psychoses in Canada. Acta Psychiatr Scand. 1977;55:369–80. doi: 10.1111/j.1600-0447.1977.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Bland RC, Orn H. 14-year outcome in early schizophrenia. Acta Psychiatr Scand. 1978;58:327–338. doi: 10.1111/j.1600-0447.1978.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Bland RC. Long term mental illness in Canada: an epidemiological perspective on schizophrenia and affective disorders. Can J Psychiatry. 1984;29:242–246. doi: 10.1177/070674378402900310. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Beiser M. Are males more likely than females to develop schizophrenia? Am J Psychiatry. 1992;149:1070–1074. doi: 10.1176/ajp.149.8.1070. [DOI] [PubMed] [Google Scholar]
- Nicole L, Lesage A, Lalonde P. Lower incidence and increased male:female ratio in schizophrenia. Br J Psychiatry. 1992;161:556–557. doi: 10.1192/bjp.161.4.556. [DOI] [PubMed] [Google Scholar]
- Ma SP. [An epidemiologic investigation of psychoses in Laoshan County of Qingdao] Zhonghua Shen Jing Jing Shen Ke Za Zhi. 1980;13:22–23. [PubMed] [Google Scholar]
- Chen CH. [Incidence and prevalence of schizophrenia in a community mental health service from 1975 to 1981] Zhonghua Shen Jing Jing Shen Ke Za Zhi. 1984;17:321–324. [PubMed] [Google Scholar]
- Yucun S, Weixi Z, Lian S, Xiaoling Y, Yuhua C, Dongfeng Z, Hengyao S, Entao S. A survey of mental disorders in a suburb of Beijing. Int J Ment Health. 1988;16:75–80. [Google Scholar]
- Folnegovic Z, Folnegovic-Smalc V, Kulcar Z. The incidence of schizophrenia in Croatia. Br J Psychiatry. 1990;156:363–365. doi: 10.1192/bjp.156.3.363. [DOI] [PubMed] [Google Scholar]
- J Nielsen J. The Samso project from 1957 to 1974. Acta Psychiatr Scand. 1976;54:198–222. doi: 10.1111/j.1600-0447.1976.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Nielsen JA. A census study of mental illness in Samso. Psychol Med. 1977;7:491–503. doi: 10.1017/s0033291700004475. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. Delusional psychosis. Hospital incidence, symptoms and classification. Acta Psychiatr Scand. 1986;74:18–23. doi: 10.1111/j.1600-0447.1986.tb06221.x. [DOI] [PubMed] [Google Scholar]
- Munk-Jorgensen P. Decreasing first-admission rates of schizophrenia among males in Denmark from 1970 to 1984. Changing diagnostic patterns? Acta Psychiatr Scand. 1986;73:645–650. doi: 10.1111/j.1600-0447.1986.tb02738.x. [DOI] [PubMed] [Google Scholar]
- Munk-Jorgensen P, Mortensen PB. Incidence and other aspects of the epidemiology of schizophrenia in Denmark, 1971–87. Br J Psychiatry. 1992;161:489–495. doi: 10.1192/bjp.161.4.489. [DOI] [PubMed] [Google Scholar]
- Munk-Jorgensen P, Lutzhoft JH, Jensen J, Stromgren E. Trends in psychiatric hospitalization in Denmark: a 10-year register-based investigation. Acta Psychiatr Scand. 1992;86:79–83. doi: 10.1111/j.1600-0447.1992.tb03231.x. [DOI] [PubMed] [Google Scholar]
- Mors O, Sorensen LV. Incidence and comorbidity of psychiatric disorders from a well-defined catchment area in Denmark. Eur Psychiatry. 1993;8:193–199. [Google Scholar]
- Lynge I, Jacobsen J. Schizophrenia in Greenland: a follow-up study. Acta Psychiatr Scand. 1995;91:414–422. doi: 10.1111/j.1600-0447.1995.tb09802.x. [DOI] [PubMed] [Google Scholar]
- Lynge I, Mortensen PB, Munk-Jorgensen P. Mental disorders in the Greenlandic population. A register study. Int J Circumpolar Health. 1999;58:188–197. [PubMed] [Google Scholar]
- Schelin EM, Munk-Jorgensen P, Olesen AV, Gerlach J. Regional differences in schizophrenia incidence in Denmark. Acta Psychiatr Scand. 2000;101:293–299. doi: 10.1034/j.1600-0447.2000.101004293.x. [DOI] [PubMed] [Google Scholar]
- Niskanen P, Achte KA. The course and prognosis of schizophrenic psychoses in helsinki, a comparative study of first admissions in 1960 and 1965. Helsinki; Helsinki University Central Hospital. 1972.
- Salokangas RK. First admissions for psychosis in Turku. A time trend study. Acta Psychiatr Scand. 1979;60:249–262. doi: 10.1111/j.1600-0447.1979.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Kuusi K. Prognosis of schizophrenia psychosis in Helsinki in 1975–1983. Monographs of Psychiatria Fennica. 1986;13 [Google Scholar]
- Salokangas RK. First-contact rate for schizophrenia in community psychiatric care. Consideration of the oestrogen hypothesis. Eur Arch Psychiatry Clin Neurosci. 1993;242:337–346. doi: 10.1007/BF02190246. [DOI] [PubMed] [Google Scholar]
- Lehtinen V, Veijola J, Lindholm T, Moring J, Puukka P, Vaisanen E. Incidence of mental disorders in the Finnish UKKI study. Br J Psychiatry. 1996;168:672–678. doi: 10.1192/bjp.168.6.672. [DOI] [PubMed] [Google Scholar]
- van Os J, Galdos P, Lewis G, Bourgeois M, Mann A. Schizophrenia sans frontieres: concepts of schizophrenia among French and British psychiatrists. BMJ. 1993;307:489–492. doi: 10.1136/bmj.307.6902.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner H, an der Heiden W. The contribution of European case registers to research on schizophrenia. Schizophr Bull. 1986;12:26–51. doi: 10.1093/schbul/12.1.26. [DOI] [PubMed] [Google Scholar]
- Loffler W, Hafner H. Ecological pattern of first admitted schizophrenics in two German cities over 25 years. Soc Sci Med. 1999;49:93–108. doi: 10.1016/S0277-9536(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Helgason L. Psychiatric services and mental illness in Iceland. Incidence study (1966–1967) with 6–7 year follow-up. Acta Psychiatr Scand Suppl. 1977:1–140. [PubMed] [Google Scholar]
- Rajkumar S, Padmavati R, Thara R, Sarada Menon M. Incidence of shizophrenia in an urban community in Madras. Indian J Psychiatry. 1993;35:18–21. [PMC free article] [PubMed] [Google Scholar]
- Walsh D. The ups and downs of schizophrenia in Ireland. Ir J Psychiatry. 1992;13:12–16. [Google Scholar]
- Walsh D. Mental illness in Dublin – first admissions. Br J Psychiatry. 1969;115:449–456. doi: 10.1192/bjp.115.521.449. [DOI] [PubMed] [Google Scholar]
- O'Hare A, Walsh D. Further data on activities of Irish psychiatric hospitals and units, 1965–1969. J Ir Med Assoc. 1974;67:57–63. [PubMed] [Google Scholar]
- ni Nuallain M, O'Hare A, Walsh D, Blake B, Halpenny JN, O'Brien PF. The incidence of mental illness in Ireland – patients contacting psychiatric services in three Irish counties. Ir J Psychiatry. 1984;5:23–29. [Google Scholar]
- Repetto F, Formigaro F, Ferrari P, Frascaroli G, Lora A, Magnani G, Mastroeni A, Travasso B, Morosini P. [Estimate of the hospital incidence of schizophrenia in Lombardy] Epidemiol Prev. 1988;10:20–25. [PubMed] [Google Scholar]
- Tansella M, Balestrieri M, Meneghelli M, Micciolo Rl. Trends in the provision of psychiatric care 1979–1988. Psychol Med. 1991;19:1–54. [Google Scholar]
- de Salvia D, Barbato A, Salvo P, Zadro F. Prevalence and incidence of schizophrenic disorders in Portogruaro. An Italian case register study. J Nerv Ment Dis. 1993;181:275–82. doi: 10.1097/00005053-199305000-00001. [DOI] [PubMed] [Google Scholar]
- Mata I, Beperet M, Madoz V, Psicost Y. Prevalence and incidence of schizophrenia in Navarra. Anales Sis San Navara. 2000;23:29–36. [Google Scholar]
- Preti A, Miotto P. Increase in first admissions for schizophrenia and other major psychoses in Italy. Psychiatry Res. 2000;94:139–152. doi: 10.1016/S0165-1781(00)00136-0. [DOI] [PubMed] [Google Scholar]
- Hickling FW, Rodgers-Johnson P. The incidence of first contact schizophrenia in Jamaica. Br J Psychiatry. 1995;167:193–196. doi: 10.1192/bjp.167.2.193. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Nakane Y, Nishihara J, Takemoto T. Ecological structure and incidence rates of schizophrenia in Nagasaki City. Acta Psychiatr Scand. 1992;86:113–120. doi: 10.1111/j.1600-0447.1992.tb03238.x. [DOI] [PubMed] [Google Scholar]
- Giel R, Sauer HC, Slooff CJ, Wiersma D. Over de epidemiologie van functionele psychosen en invaliditeit [Epidemiological observations on schizophrenia and disability in the Netherlands] Tijdschrift voor psychiatrie. 1980;22:710–722. [Google Scholar]
- Oldehinkel AJ, Giel R. Time trends in the care-based incidence of schizophrenia. Br J Psychiatry. 1995;167:777–782. doi: 10.1192/bjp.167.6.777. [DOI] [PubMed] [Google Scholar]
- Peen J, Dekker J. Admission rates for schizophrenia in The Netherlands: an urban/rural comparison. Acta Psychiatr Scand. 1997;96:301–305. doi: 10.1111/j.1600-0447.1997.tb10167.x. [DOI] [PubMed] [Google Scholar]
- van Os J, Driessen G, Gunther N, Delespaul P. Neighbourhood variation in incidence of schizophrenia. Evidence for person-environment interaction. Br J Psychiatry. 2000;176:243–248. doi: 10.1192/bjp.176.3.243. [DOI] [PubMed] [Google Scholar]
- Joyce PR. Changing trends in first admissions and readmissions for mania and schizophrenia in New Zealand, 1974 to 1984. Aust N Z J Psychiatry. 1987;21:82–86. doi: 10.3109/00048678709160903. [DOI] [PubMed] [Google Scholar]
- Johannessen JO. Schizofreni-forekomst i et norsk fylke, Rogaland. Nord Psykiatr Tidsskr. 1985;39:217–223. [Google Scholar]
- Grawe R, Levander S, Krueger M. Incidence, clinical characteristics, and short-term outcome of first-episode schizophrenia. Nordisk Psykiatrisk Tidsskrift. 1991;45:383–390. [Google Scholar]
- Lieberman YI. [The incidence of schizophrenia (materials from a clinico-epidemiologic study)] Zh Nevropatol Psikhiatr Im S S Korsakova. 1974;74:1224–1233. [PubMed] [Google Scholar]
- Rotshtein VG. [Incidence of paranoid schizophrenia] Zh Nevropatol Psikhiatr Im S S Korsakova. 1982;82:91–98. [PubMed] [Google Scholar]
- Vul FR, Mishkind AD, Gil'burd OA, Vorobeichik DA. Clinico-epidemiologic characteristics of mental diseases in one of the regions of western Siberia. Zh Nevropatol Psikhiatr Im S S Korsakova. 1988;88:111–116. [PubMed] [Google Scholar]
- Tsoi WF, Chen AJ. New admissions to Woodbridge Hospital 1975 with special reference to schizophrenia. Ann Acad Med Singapore. 1979;8:275–279. [PubMed] [Google Scholar]
- Vazquez-Barquero JL, Cuesta Nunez MJ, de la Varga M, Herrera Castanedo S, Gaite L, Arenal A. The Cantabria first episode schizophrenia study: a summary of general findings. Acta Psychiatr Scand. 1995;91:156–162. doi: 10.1111/j.1600-0447.1995.tb09759.x. [DOI] [PubMed] [Google Scholar]
- Osby U, Hammar N, Brandt L, Wicks S, Thinsz Z, Ekbom A, Sparen P. Time trends in first admissions for schizophrenia and paranoid psychosis in Stockholm County, Sweden. Schizophr Res. 2001;47:247–254. doi: 10.1016/S0920-9964(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Svedberg B, Mesterton A, Cullberg J. First-episode non-affective psychosis in a total urban population: a 5-year follow-up. Soc Psychiatry Psychiatr Epidemiol. 2001;36:332–337. doi: 10.1007/s001270170037. [DOI] [PubMed] [Google Scholar]
- Neehall J. An analysis of psychiatric inpatient admissions from a defined geographic catchment area over a one-year period. West Indian Med J. 1991;40:16–21. [PubMed] [Google Scholar]
- Adelstein AM, Downham DY, Stein Z, Susser MW. The epidemiology of mental illness in an English city: inceptions recognized by Salford Psychiatric services. Soc Psychiatry. 1968;3:47–59. [Google Scholar]
- Mezey AG, Evans E. Psychiatric in-patients and out-patients in a London borough. Br J Psychiatry. 1971;118:609–616. doi: 10.1192/bjp.118.547.609. [DOI] [PubMed] [Google Scholar]
- Eagles JM, Whalley LJ. Decline in the diagnosis of schizophrenia among first admissions to Scottish mental hospitals from 1969–78. Br J Psychiatry. 1985;146:151–154. doi: 10.1192/bjp.146.2.151. [DOI] [PubMed] [Google Scholar]
- Giggs JA. Mental disorders and ecological structure in Nottingham. Soc Sci Med. 1986;23:945–961. doi: 10.1016/0277-9536(86)90252-2. [DOI] [PubMed] [Google Scholar]
- Eagles JM, Hunter D, McCance C. Decline in the diagnosis of schizophrenia among first contacts with psychiatric services in north-east Scotland, 1969–1984. Br J Psychiatry. 1988;152:793–798. doi: 10.1192/bjp.152.6.793. [DOI] [PubMed] [Google Scholar]
- de Alarcon JG, Seagroatt V. Trends in schizophrenia. Lancet. 1990;335:852–853. [Google Scholar]
- Der G, Gupta S, Murray RM. Is schizophrenia disappearing? Lancet. 1990;335:513–516. doi: 10.1016/0140-6736(90)90745-Q. [DOI] [PubMed] [Google Scholar]
- Prince M, Phelan M. Trends in Schizophrenia. Lancet. 1990;335:851–852. doi: 10.1016/0140-6736(90)90963-6. [DOI] [Google Scholar]
- Bamrah JS, Freeman HL, Goldberg DP. Epidemiology of schizophrenia in Salford, 1974–84. Changes in an urban community over ten years. Br J Psychiatry. 1991;159:802–810. doi: 10.1192/bjp.159.6.802. [DOI] [PubMed] [Google Scholar]
- Harrison G, Cooper JE, Gancarczyk R. Changes in the administrative incidence of schizophrenia. Br J Psychiatry. 1991;159:811–816. doi: 10.1192/bjp.159.6.811. [DOI] [PubMed] [Google Scholar]
- Ring N, Tantam D, Montague L, Newby D, Black D, Morris J. Gender differences in the incidence of definite schizophrenia and atypical psychosis – focus on negative symptoms of schizophrenia. Acta Psychiatr Scand. 1991;84:489–496. doi: 10.1111/j.1600-0447.1991.tb03182.x. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Black RJ, Whalley LJ, Eagles JM. Persistence of the decline in the diagnosis of schizophrenia among first admissions to Scottish hospitals from 1969 to 1988. Br J Psychiatry. 1993;163:620–626. doi: 10.1192/bjp.163.5.620. [DOI] [PubMed] [Google Scholar]
- Castle DJ, Wessely S, Murray RM. Sex and schizophrenia: effects of diagnostic stringency, and association with premorbid variables. Br J Psychiatry. 1993;162:658–664. doi: 10.1192/bjp.162.5.658. [DOI] [PubMed] [Google Scholar]
- Kendell RE, Malcolm DE, Adams W. The problem of detecting changes in the incidence of schizophrenia. Br J Psychiatry. 1993;162:212–218. doi: 10.1192/bjp.162.2.212. [DOI] [PubMed] [Google Scholar]
- Goldacre M, Shiwach R, Yeates D. Estimating incidence and prevalence of treated psychiatric disorders from routine statistics: the example of schizophrenia in Oxfordshire. J Epidemiol Community Health. 1994;48:318–322. doi: 10.1136/jech.48.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Phelan M. Invisible schizophrenia: A postal survey of the incidence and management of new cases of schizophrenia in primary care. J Ment Health. 1994;3:91–98. [Google Scholar]
- van Os J, Castle DJ, Takei N, Der G, Murray RM. Psychotic illness in ethnic minorities: clarification from the 1991 census. Psychol Med. 1996;26:203–208. doi: 10.1017/s0033291700033845. [DOI] [PubMed] [Google Scholar]
- McNaught AS, Jeffreys SE, Harvey CA, Quayle AS, King MB, Bird AS. The Hampstead Schizophrenia Survey 1991. II: Incidence and migration in inner London. Br J Psychiatry. 1997;170:307–311. doi: 10.1192/bjp.170.4.307. [DOI] [PubMed] [Google Scholar]
- Cavanagh JT, Shajahan PM. Increasing rates of hospital admission for men with major mental illnesses: data from Scottish mental health units, 1980–1995. Acta Psychiatr Scand. 1999;99:353–359. doi: 10.1111/j.1600-0447.1999.tb07241.x. [DOI] [PubMed] [Google Scholar]
- Boydell J, van Os J, McKenzie K, Allardyce J, Goel R, McCreadie RG, Murray RM. Incidence of schizophrenia in ethnic minorities in London: ecological study into interactions with environment. BMJ. 2001;323:1336–1338. doi: 10.1136/bmj.323.7325.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham HW. Community and Schizophrenia: an epidemiological analysis. Detroit, Michigan: Wayne State University Press; 1965. [Google Scholar]
- Eaton WW. Residence, social class, and schizophrenia. J Health Soc Behav. 1974;15:289–299. [PubMed] [Google Scholar]
- Tien AY, Eaton WW. Psychopathologic precursors and sociodemographic risk factors for the schizophrenia syndrome. Arch Gen Psychiatry. 1992;49:37–46. doi: 10.1001/archpsyc.1992.01820010037005. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Cochrane R. Migration and mental health – a comparative study. J Int Studies. 1980;1:49–57. [Google Scholar]
- Selten JP, Sijben N. First admission rates for schizophrenia in immigrants to The Netherlands. The Dutch National Register. Soc Psychiatry Psychiatr Epidemiol. 1994;29:71–77. doi: 10.1007/BF00805625. [DOI] [PubMed] [Google Scholar]
- Selten JP, Slaets JP, Kahn RS. Schizophrenia in Surinamese and Dutch Antillean immigrants to The Netherlands: evidence of an increased incidence. Psychol Med. 1997;27:807–811. doi: 10.1017/S0033291797005199. [DOI] [PubMed] [Google Scholar]
- Hemsi LK. Psychiatric morbidity of West Indian immigrants. Soc Psychiatry. 1967;2:95–100. [Google Scholar]
- Giggs J. High rates of schizophrenia among immigrants in Nottingham. Nurs Times. 1973;69:1210–1212. [PubMed] [Google Scholar]
- Rwegellera GG. Psychiatric morbidity among West Africans and West Indians living in London. Psychol Med. 1977;7:317–329. doi: 10.1017/s0033291700029421. [DOI] [PubMed] [Google Scholar]
- Carpenter L, Brockington IF. A study of mental illness in Asians, West Indians and Africans living in Manchester. Br J Psychiatry. 1980;137:201–205. doi: 10.1192/bjp.137.3.201. [DOI] [PubMed] [Google Scholar]
- Hitch PJ, Rack PH. Mental illness among Polish and Russian refugees in Bradford. Br J Psychiatry. 1980;137:206–211. doi: 10.1192/bjp.137.3.206. [DOI] [PubMed] [Google Scholar]
- Dean G, Walsh D, Downing H, Shelley E. First admissions of native-born and immigrants to psychiatric hospitals in South-East England 1976. Br J Psychiatry. 1981;139:506–512. doi: 10.1192/bjp.139.6.506. [DOI] [PubMed] [Google Scholar]
- Dean G, Downing H, Shelley E. First admissions to psychiatric hospitals in south-east England in 1976 among immigrants from Ireland. Br Med J (Clin Res Ed) 1981;282:1831–1833. doi: 10.1136/bmj.282.6279.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane R, Bal SS. Migration and schizophrenia: an examination of five hypotheses. Soc Psychiatry. 1987;22:181–191. doi: 10.1007/BF00583553. [DOI] [PubMed] [Google Scholar]
- McGovern D, Cope RV. First psychiatric admission rates of first and second generation Afro Caribbeans. Soc Psychiatry. 1987;22:139–149. doi: 10.1007/BF00583848. [DOI] [PubMed] [Google Scholar]
- Harrison G, Owens D, Holton A, Neilson D, Boot D. A prospective study of severe mental disorder in Afro-Caribbean patients. Psychol Med. 1988;18:643–657. doi: 10.1017/s0033291700008321. [DOI] [PubMed] [Google Scholar]
- Cochrane R, Bal SS. Mental hospital admission rates of immigrants to England: a comparison of 1971 and 1981. Soc Psychiatry Psychiatr Epidemiol. 1989;24:2–11. doi: 10.1007/BF01788193. [DOI] [PubMed] [Google Scholar]
- Castle D, Wessely S, Der G, Murray RM. The incidence of operationally defined schizophrenia in Camberwell, 1965–84. Br J Psychiatry. 1991;159:790–794. doi: 10.1192/bjp.159.6.790. [DOI] [PubMed] [Google Scholar]
- Thomas CS, Stone K, Osborn M, Thomas PF, Fisher M. Psychiatric morbidity and compulsory admission among UK-born Europeans, Afro-Caribbeans and Asians in central Manchester. Br J Psychiatry. 1993;163:91–99. doi: 10.1192/bjp.163.1.91. [DOI] [PubMed] [Google Scholar]
- King M, E Coker M, Leavey G, Hoare A, Johnson-Sabine E. Incidence of psychotic illness in London: comparison of ethnic groups. BMJ. 1994;309:1115–1119. doi: 10.1136/bmj.309.6962.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhugra D, Leff J, Mallett R, Der G, Corridan B, Rudge S. Incidence and outcome of schizophrenia in whites, African-Caribbeans and Asians in London. Psychol Med. 1997;27:791–798. doi: 10.1017/S0033291797005369. [DOI] [PubMed] [Google Scholar]
- Harrison G, Glazebrook C, Brewin J, Cantwell R, Dalkin , Fox R, Jones P, Medley I. Increased incidence of psychotic disorders in migrants from the Caribbean to the United Kingdom. Psychol Med. 1997;27:799–806. doi: 10.1017/S0033291796004643. [DOI] [PubMed] [Google Scholar]
- Goater N, King M, Cole E, Leavey G, Johnson-Sabine E, Blizard R, Hoar A. Ethnicity and outcome of psychosis. Br J Psychiatry. 1999;175:34–42. doi: 10.1192/bjp.175.1.34. [DOI] [PubMed] [Google Scholar]
- Malzberg B. Internal migration and mental disease among the white population of New York state, 1960–1961. Int J Soc Psychiatry. 1967;13:184–191. doi: 10.1177/002076406701300302. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Hall W, Andrews G. The incidence of schizophrenia in New South Wales, Australia. A psychiatric register study. Acta Psychiatr Scand. 1984;70:220–227. doi: 10.1111/j.1600-0447.1984.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Jones IH, Kirkby KC, Hay DA, Daniels BA, Longmore LM. Decline in diagnoses of schizophrenia in Tasmania during the period 1965–1990. Acta Psychiatr Scand. 1997;95:13–18. doi: 10.1111/j.1600-0447.1997.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Mednick SA, Hodgins S. Major mental disorders and criminal violence in a Danish birth cohort. Arch Gen Psychiatry. 2000;57:494–500. doi: 10.1001/archpsyc.57.5.494. [DOI] [PubMed] [Google Scholar]
- Haukka J, Suvisaari J, Varilo T, Lonnqvist J. Regional variation in the incidence of schizophrenia in Finland: a study of birth cohorts born from 1950 to 1969. Psychol Med. 2001;31:1045–1053. doi: 10.1017/S0033291701004299. [DOI] [PubMed] [Google Scholar]
- Makikyro T, Isohanni M. Is a child's risk of early onset schizophrenia increased in the highest social class? Schizophr Res. 1997;23:245–252. doi: 10.1016/S0920-9964(96)00119-3. [DOI] [PubMed] [Google Scholar]
- Rasanen S, Veijola J, Hakko H, Joukamaa M, Isohanni M. Gender differences in incidence and age at onset of DSM-III-R schizophrenia: Preliminary results of the Northern Finland 1966 birth cohort study. Schizophr Res. 1999;37:197–198. doi: 10.1016/S0920-9964(98)00152-2. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K, Forsen T, Osmond C, Barker DJ, Eriksson JG. Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Arch Gen Psychiatry. 2001;58:48–52. doi: 10.1001/archpsyc.58.1.48. [DOI] [PubMed] [Google Scholar]
- Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry. 1999;156:1328–1335. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, Susser ES. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- Balestrieri M, Rucci P, Nicolaou S. Gender-specific decline and seasonality of births in operationally defined schizophrenics in Italy. Schizophr Res. 1997;27:73–81. doi: 10.1016/S0920-9964(97)00071-6. [DOI] [PubMed] [Google Scholar]
- Malmberg A, Lewis G, David A, Allebeck P. Premorbid adjustment and personality in people with schizophrenia. Br J Psychiatry. 1998;172:308–313. doi: 10.1192/bjp.172.4.308. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Done DJ. Prenatal exposure to influenza does not cause schizophrenia. Br J Psychiatry. 1992;161:390–393. doi: 10.1192/bjp.161.3.390. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/S0140-6736(94)90569-X. [DOI] [PubMed] [Google Scholar]
- Takei N, Lewis G, Sham PC, Murray RM. Age-period-cohort analysis of the incidence of schizophrenia in Scotland. Psychol Med. 1996;26:963–973. doi: 10.1017/s0033291700035297. [DOI] [PubMed] [Google Scholar]
- Bresnahan MA, Brown AS, Schaefer CA, Begg MD, Wyatt RJ, Susser ES. Incidence and cumulative risk of treated schizophrenia in the prenatal determinants of schizophrenia study. Schizophr Bull. 2000;26:297–308. doi: 10.1093/oxfordjournals.schbul.a033454. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Rosso IM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of neurodevelopmental processes in the genesis and epigenesis of schizophrenia. Dev Psychopathol. 1999;11:467–485. doi: 10.1017/S0954579499002163. [DOI] [PubMed] [Google Scholar]
- Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry. 2000;157:196–202. doi: 10.1176/appi.ajp.157.2.196. [DOI] [PubMed] [Google Scholar]
- Alberdi F. Psychiatric admissions among the deaf population in Denmark. Nord J Psychiatry. 1996;50:387–394. [Google Scholar]
- Klaning U, Mortensen PB, Kyvik KO. Increased occurrence of schizophrenia and other psychiatric illnesses among twins. Br J Psychiatry. 1996;168:688–692. doi: 10.1192/bjp.168.6.688. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Levav I. Schizophrenia, social class and ethnic disadvantage. A study of first hospitalization among Israeli-born Jews. Isr J Psychiatry Relat Sci. 1982;19:289–302. [PubMed] [Google Scholar]
- Raman AC, Murphy HB. Failure of traditional prognostic indications in Afro-Asian psychotics: results of a long-term follow-up survey. J Nerv Ment Dis. 1972;154:238–247. [PubMed] [Google Scholar]
- Kelly R. Mental illness in the Maori population of New Zealand. Acta Psychiatr Scand. 1973;49:722–734. doi: 10.1111/j.1600-0447.1973.tb04461.x. [DOI] [PubMed] [Google Scholar]
- Noreik K, Odegard O. Psychoses in Norwegians with a background of higher education. Br J Psychiatry. 1966;112:43–55. doi: 10.1192/bjp.112.482.43. [DOI] [PubMed] [Google Scholar]
- Nica-Udangiu S. Epidemiologic aspects of schizophrenias, schizotypal disorders and schizoaffective psychoses in the student population. Neurol Psychiatr (Bucur) 1983;21:115–120. [PubMed] [Google Scholar]
- Gavrilov SI, Kirzhanova VV. [Incidence of mental disorders among the late middle-aged and elderly population (according to the primary registration data of the psychoneurologic institutions of Moscow)] Zh Nevropatol Psikhiatr Im S S Korsakova. 1983;83:1338–1345. [PubMed] [Google Scholar]
- Kendler KS, Pedersen NL, Farahmand BY, Persson PG. The treated incidence of psychotic and affective illness in twins compared with population expectation: a study in the Swedish Twin and Psychiatric Registries. Psychol Med. 1996;26:1135–1144. doi: 10.1017/s0033291700035856. [DOI] [PubMed] [Google Scholar]
- Loganovsky KN, Loganovskaja TK. Schizophrenia spectrum disorders in persons exposed to ionizing radiation as a result of the Chernobyl accident. Schizophr Bull. 2000;26:751–773. doi: 10.1093/oxfordjournals.schbul.a033492. [DOI] [PubMed] [Google Scholar]
- Copeland JR, Dewey ME, Scott A, Gilmore C, Larkin BA, Cleave N, McCracken CF, McKibbin PE. Schizophrenia and delusional disorder in older age: community prevalence, incidence, comorbidity, and outcome. Schizophr Bull. 1998;24:153–161. doi: 10.1093/oxfordjournals.schbul.a033307. [DOI] [PubMed] [Google Scholar]
- Reeves SJ, Sauer J, Stewart R, Granger A, Howard RJ. Increased first-contact rates for very-late-onset schizophrenia-like psychosis in African- and Caribbean-born elders. Br J Psychiatry. 2001;179:172–174. doi: 10.1192/bjp.179.2.172. [DOI] [PubMed] [Google Scholar]
- Castle DJ, Murray RM. The epidemiology of late-onset schizophrenia. Schizophr Bull. 1993;19:691–700. doi: 10.1093/schbul/19.4.691. [DOI] [PubMed] [Google Scholar]
- Babigian HM. Schizophrenia: epidemiology. In: Freedman AM, Kaplan HI, Sadock BJ, editor. In Comprehensive textbook of psychiatry. 3. Vol. 2. Baltimore/London: Williams & Wilkins; 1980. pp. 860–866. [Google Scholar]
- Allardyce J, Boydell J, Van Os J, Morrison G, Castle D, Murray RM, McCreadie RG. Comparison of the incidence of schizophrenia in rural Dumfries and Galloway and urban Camberwell. Br J Psychiatry. 2001;179:335–339. doi: 10.1192/bjp.179.4.335. [DOI] [PubMed] [Google Scholar]
- Brewin J, Cantwell R, Dalkin T, Fox R, Medley I, Glazebrook C, Kwiecinski R, Harrison G. Incidence of schizophrenia in Nottingham. A comparison of two cohorts, 1978–80 and 1992–94. Br J Psychiatry. 1997;171:140–144. doi: 10.1192/bjp.171.2.140. [DOI] [PubMed] [Google Scholar]
- Cooper JE. The incidence of schizophrenia in Nottingham. Br J Psychiatry. 1987;151:619–626. doi: 10.1192/bjp.151.5.619. [DOI] [PubMed] [Google Scholar]
- Dauncey K, Giggs J, Baker K, Harrison G. Schizophrenia in Nottingham: lifelong residential mobility of a cohort. Br J Psychiatry. 1993;163:613–619. doi: 10.1192/bjp.163.5.613. [DOI] [PubMed] [Google Scholar]
- Joensen S, Wang AG. First admissions for psychiatric disorders. A comparison between the Faroe Islands and Denmark. Acta Psychiatr Scand. 1983;68:66–71. doi: 10.1111/j.1600-0447.1983.tb06982.x. [DOI] [PubMed] [Google Scholar]
- McCabe MS. Demographic differences in functional psychoses. Br J Psychiatry. 1975;127:320–323. doi: 10.1192/bjp.127.4.320. [DOI] [PubMed] [Google Scholar]
- Munk-Jorgensen P. Schizophrenia in Denmark. Incidence and utilization of psychiatric institutions. Acta Psychiatr Scand. 1986;73:172–180. doi: 10.1111/j.1600-0447.1986.tb10583.x. [DOI] [PubMed] [Google Scholar]
- Munk-Jorgensen P. First-admission rates and marital status of schizophrenics. Acta Psychiatr Scand. 1987;76:210–216. doi: 10.1111/j.1600-0447.1987.tb02886.x. [DOI] [PubMed] [Google Scholar]
- Munk-Jorgensen P, Jorgensen P. Decreasing rates of first-admission diagnoses of schizophrenia among females in Denmark 1970–84. Acta Psychiatr Scand. 1986;74:379–383. doi: 10.1111/j.1600-0447.1986.tb06257.x. [DOI] [PubMed] [Google Scholar]
- Munk-Jorgensen P, Mortensen PB. Is schizophrenia really on the decrease? Eur Arch Psychiatry Clin Neurosci. 1993;242:244–247. doi: 10.1007/BF02189970. [DOI] [PubMed] [Google Scholar]
- Palmquist J, Wang AG. Schizophrenia in the Faroe Islands. Nord J Psychiatry. 1997;51:351–357. [Google Scholar]
- Wig NN, Varma VK, Mattoo SK, Behere PB, Phookan HR, Misra AK, Srinivasa MR, Tripathi BM, Menon DK. An Incidence Study of Schizophrenia in India. Indian J Psychiatry. 1993;35:11–17. [PMC free article] [PubMed] [Google Scholar]
- de Alarcon JG, Sainsbury P, Costain WR. Incidence of referred mental illness in Chichester and Salisbury. Psychol Med. 1975;5:32–54. doi: 10.1017/s0033291700007200. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Mortensen PB, Frydenberg M. Obstetric factors, urbanization and psychosis. Schizophr Res. 2000;43:117–123. doi: 10.1016/S0920-9964(99)00152-8. [DOI] [PubMed] [Google Scholar]
- Karvonen M, Tuomilehto J, Libman I, LaPorte R. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia. 1993;36:883–892. doi: 10.1007/BF02374468. [DOI] [PubMed] [Google Scholar]
- Isohanni M, Jones PB, Moilanen K, Rantakallio P, Veijola J, Oja H, Koiranen M, Jokelainen J, Croudace T, Jarvelin M. Early developmental milestones in adult schizophrenia and other psychoses. A 31-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophr Res. 2001;52:1–19. doi: 10.1016/S0920-9964(00)00179-1. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ, Susser ES. U.S. birth cohort studies of schizophrenia: a sea change. Schizophr Bull. 2000;26:255–256. doi: 10.1093/oxfordjournals.schbul.a033450. [DOI] [PubMed] [Google Scholar]
- Friis S, Larsen TK, Melle I, Opjordsmoen S, Johannessen JO, Haahr U, Simonsen E, Rund BR, Vaglum P, McGlashan T. Methodological pitfalls in early detection studies – the NAPE Lecture 2002. Nordic Association for Psychiatric Epidemiology. Acta Psychiatr Scand. 2003;107:3–9. doi: 10.1034/j.1600-0447.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- Yolles SF, Kramer M. Vital Statistics. In: Bellak L, editor. In The Schizophrenic Syndrome. New York: Grune and Stratton; 1969. pp. 66–113. [Google Scholar]
- Berlin JA. Invited commentary: benefits of heterogeneity in meta-analysis of data from epidemiologic studies. Am J Epidemiol. 1995;142:383–387. doi: 10.1093/oxfordjournals.aje.a117645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
http://www.biomedcentral.com/imedia/1862872583417819/sup1.doc
http://www.biomedcentral.com/imedia/8656447533417738/sup2.doc
http://www.biomedcentral.com/imedia/6800396953417826/sup3.doc
http://www.biomedcentral.com/imedia/1144262997341774/sup4.doc
http://www.biomedcentral.com/imedia/1480669390341774/sup5.doc
http://www.biomedcentral.com/imedia/1396785452341783/sup6.xls
http://www.biomedcentral.com/imedia/1705991844341783/sup7.zip


