Abstract
This pilot study aims to provide effect size confidence intervals, clinical trial and intervention feasibility data, and procedural materials for a full-scale randomized controlled trial that will determine the efficacy of Dietary Approaches to Stop Hypertension (DASH) as adjunct therapy to standard care for adults with uncontrolled asthma. The DASH diet encompasses foods (e.g., fresh fruit, vegetables, and nuts) and antioxidant nutrients (e.g., vitamins A, C, E, and zinc) with potential benefits for persons with asthma, but it is unknown whether the whole diet is beneficial. Participants (n = 90) will be randomized to receive usual care alone or combined with a DASH intervention consisting of 8 group and 3 individual sessions during the first 3 months, followed by at least monthly phone consultations for another 3 months. Follow-up assessments will occur at 3 and 6 months. The primary outcome measure is the 7-item Juniper Asthma Control Questionnaire, a validated composite measure of daytime and nocturnal symptoms, activity limitations, rescue medication use, and percentage predicted forced expiratory volume in 1 second. We will explore changes in inflammatory markers important to asthma pathophysiology (e.g., fractional exhaled nitric oxide) and their potential to mediate the intervention effect on disease control. We will also conduct pre-specified subgroup analyses by genotype (e.g., polymorphisms on the glutathione S transferase gene) and phenotype (e.g., atopy, obesity). By evaluating a dietary pattern approach to improving asthma control, this study could advance the evidence base for refining clinical guidelines and public health recommendations regarding the role of dietary modifications in asthma management.
Keywords: DASH, Dietary pattern, Asthma, Lung function, Asthma Control Questionnaire
1. Introduction
Asthma is a chronic inflammatory, obstructive disorder of the airways, involving a complex interplay of several inflammatory cells and multiple inflammatory mediators [1,2]. Asthma prevalence has increased in the U.S. over recent decades, affecting more than 20 million Americans currently [3]. Dietary change is one in a host of environmental exposures implicated in this trend [1,2]. As a hallmark of westernization, rapid changes in diet, including adoption of a more processed and “convenience-oriented” diet, have resulted in a chronic metabolic surplus and a relative reduction in the intake of complex carbohydrates and micronutrients [4]. The available literature on the relationship between diet and asthma has largely focused on individual nutrients. Epidemiologic and mechanistic studies have shown encouraging evidence of a plausible therapeutic or protective effect in asthma of a wide range of nutrients, such as increased intake of antioxidant nutrients (e.g., vitamins A, C, E, and zinc) and ω-3 fatty acids and decreased intake of saturated fat and sodium [5,6]. Numerous hypotheses and emerging data, albeit no definitive conclusions, underscore that the underlying mechanisms are likely multifactorial, involving changes in redox status, inflammatory and immune response [5,7-11]. However, many of these nutrients have not been tested in intervention studies with asthma patients, and in the few that have been studied by supplementation (e.g., vitamin C, fish oil/ω-3 fatty acid, and selenium) or dietary modification (e.g., sodium reduction), the results have been conflicting [12-16]. Moreover, a conceptual disadvantage of the single-nutrient approach is that people do not eat individual nutrients, but rather a combination of foods from various groups that form a dietary pattern.
Studying dietary patterns, rather than specific foods or nutrients, is a relatively new approach in nutritional epidemiology [17,18], which has been used to investigate the effect of overall diet in several non-respiratory chronic diseases [19-21]. Only more recently has this approach been applied in epidemiologic studies of chronic obstructive pulmonary disease [22-24] and asthma [25-35]. However, very limited intervention studies [11,36] have investigated whether any dietary pattern affects the clinical course of asthma.
Dietary Approaches to Stop Hypertension (DASH) is a recommended diet in the U.S. Dietary Guidelines for Americans [37] because of its proven cardiovascular benefits, particularly for the prevention and control of high blood pressure [38-40]. The DASH diet also may be associated with improved bio-markers of oxidative stress, bone turnover, and calcium metabolism [41,42], and lower all-cause mortality [43]. The DASH diet encompasses foods (e.g., fresh fruit, vegetables, and nuts) and antioxidant nutrients (e.g., vitamins A, C, E, and zinc) that have shown potential benefits for people with asthma [5,11,29,44], but it is unknown whether the whole diet is beneficial. To date, no firm recommendations can be made for (or against) dietary change, let alone a specific dietary pattern, as an evidence-based approach to asthma treatment or prevention.
The “DASH for Asthma” pilot study aims to provide effect size confidence intervals for the change in asthma control. It will also deliver clinical trial and intervention feasibility data and procedural materials for a full-scale randomized controlled trial that will determine the efficacy and mechanisms of action of the DASH diet as adjunct therapy to standard care for adults with uncontrolled asthma. This paper describes the study design and methodology.
2. Methods
2.1. Study design
This pilot study is a small, short-term randomized controlled trial in which patients ages 18–70 years with uncontrolled, persistent asthma (n = 90) will be equally randomized to receive usual care alone or combined with a DASH diet intervention. All procedures and materials are approved by the Kaiser Foundation Research Institute's Institutional Review Board. The study's specific aims are as follows:
| Aim 1. | Determine the 95% confidence interval of the effect of the DASH diet on asthma control measured by change in the 7-item Juniper Asthma Control Questionnaire (ACQ) [45,46] at 6 months. |
| Aim 2. | Estimate the confidence intervals of the effects of the DASH diet on specific manifestations of asthma that are indicative of current impairment (e.g., lung function, symptoms, and rescue medication use) and future risk (e.g, asthma exacerbations). |
| Aim 3. | Explore changes in inflammatory markers important to asthma pathophysiology and their potential mediating effects on treatment response: total and differential leukocyte counts, fractional exhaled nitric oxide (FeNO), exhaled breath condensate pH, and serum cytokines (e.g., IL-5, IL-6, IL-13, IL-8, IL-17, TNF-α, and IFN-γ). |
| Aim 4. | Conduct pre-specified subgroup analyses to examine genotypes ([e.g., polymorphisms on glutathione S transferase and microsomal epoxide hydrolase genes), phenotypes (e.g., age of onset, atopy, and obesity), and sociodemographic factors (sex, race/ethnicity, education, and income) that may modify the therapeutic effect of the DASH diet. |
2.2. Eligibility criteria
Participants will be recruited from the Kaiser Permanente (KP) medical centers in San Francisco and Hayward. Patients ages 18–70 years who have uncontrolled, persistent asthma will be eligible to participate. Table 1 enumerates the inclusion and exclusion criteria. Exclusion criteria are applied to ensure each participant's ability and safety to complete the study requirements, minimize error associated with the primary outcome, and prevent possible missing data. Individuals of both genders and any racial or ethnic background who speak English, meet the inclusion criteria, and have no exclusion criteria will be enrolled. Based on the demographics of asthma patients at the study recruitment sites, the gender and ethnic composition of the target enrollment population is expected to include 69% female, 15% non-Hispanic black, 23% Hispanic/Latino, and 6% Asian/Pacific Islander.
Table 1.
Participant inclusion and exclusion criteria.
| Inclusion criteria (patients will be included if meeting all of the following) |
|
| Exclusion criteria (patients will be excluded if meeting any of the following) |
| Medical history exclusions |
|
| Medication exclusions |
|
| Other exclusions |
|
DASH concordance index is calculated based on combining nine nutrient targets (i.e., total fat, saturated fat, protein, cholesterol, fiber, magnesium, calcium, sodium, and potassium) [119–121]. The intermediate target of each nutrient is half-way between the DASH target and population mean (based on the National Health and Nutrition Examination Surveys 2007–2008, most recent data available at the inception of this study). The table including the nine nutrient DASH targets, intermediate targets, and population means is included in the Appendix. For a nutrient, participants reaching the DASH target are assigned one point, those reaching the intermediate target are assigned a half-point, and those not meeting the intermediate target are given 0 point. The DASH concordance index is the sum of points for all nine nutrients.
2.3. Recruitment and screening process
Recruitment will begin by querying the KP electronic asthma registry to identify adult patients who are likely to have uncontrolled, persistent asthma based on their past ER visits, hospitalizations, and/or pharmacy dispensing records. We will obtain approval from primary care providers (PCPs) to further screen patients in their practices. Patients will receive recruitment letters briefly explaining the study and inviting them to complete initial eligibility screening, online or by phone, that will include the Asthma Control Test [47], Alcohol Use Disorders Identification Test [48], and questions pertaining to other eligibility criteria that patients can reliably assess themselves (e.g., age, pregnancy).
Patients eligible on initial screening will be scheduled to attend a 1-hour group orientation. The sessions will begin with height and weight measurements in private. Participants with body mass index (calculated as weight in kilograms divided by height in meters squared) between 18.5 and 39.9 can stay for the remainder of the session. After a trained study staff person presents the informed consent in detail, participants will discuss in small groups (3 or 4 people each) the pros and cons of being assigned to the intervention and control conditions, respectively, which they will then share with the entire group. The sharing segment of the session will provide an opportunity for the study staff to ask open-ended questions and engage in reflective listening, which is consistent with motivational interviewing principles [49], and for participants to hear a variety of pros and cons (from their fellow participants rather than from the researcher), which is consistent with active learning principles [50]. As others have shown [51], using these techniques to explicitly address ambivalence about joining a randomized controlled trial prior to enrollment can increase motivation, decrease potential for disappointment, and consequently, improve retention. The last portion of the orientation will be one-on-one participant and staff meetings during which participants can ask further questions and sign the informed consent if they decide to join the study. A staff person will call to follow up with any undecided participants in a week.
Consenting participants will be tentatively scheduled for their baseline clinic visit and receive written instructions and the forms for completing a fasting blood draw, 2-week asthma diary, and self-administered questionnaire before their visit. Also, the study staff will call participants to conduct unannounced 24-hour dietary recalls on 3 nonconsecutive days (2 weekdays and 1 weekend day within a 7-day period).
Participants with an eligible DASH concordance index (Table 1) according to the dietary recalls will be confirmed for their baseline clinic visit. At the visit, a trained staff person will review the participant's 2-week asthma diary and self-administered questionnaire for completeness, measure weight, waist circumference, and blood pressure, measure FeNO [52], collect EBC [53], and perform spirometry before and after administration of a short-acting beta agonist (typically albuterol) [54–56]. Patients with very poor lung function (i.e., pre-bronchodilator forced expiratory volume in one second [FEV1] less than 40% predicted or post-bronchodilator FEV1 less than 50% predicted), or who fail to demonstrate reversible obstruction of the airways may only participate if approved by the study physician based on a review of the patient's full medical record. Patients with severe airway obstruction (i.e., patients who may need more intense and immediate step-up in their asthma care, which would perhaps remove all “room” for intervention benefit) and fixed airway obstruction (i.e., patients who probably have COPD) are to be excluded. We include physician review to confirm asthma diagnosis, ensure patient safety, and allow exceptions that do not fit the crude exclusions and that can still make a valuable contribution in the study.
2.4. Randomization and blinding
Participants will be randomly assigned in a 1:1 ratio to receive usual care alone or usual care plus the DASH intervention (n = 45/arm). Using Pocock and Simon's “minimization” procedure [57], randomization will assure better-than-chance group balance at baseline in terms of age, sex, race/ethnicity, smoking status, DASH concordance index, and the 7-item ACQ score. We will use a Web-based random allocation system that we developed and successfully implemented in a recent trial [58]. The system's computational algorithm automatically adjusts the randomization probability on each occasion based on the selected baseline characteristics of all participants previously randomized to each arm, thus minimizing the total covariate imbalance between study arms after each new patient is randomized. Following Efron's recommendations [59,60], the algorithm uses biased, non-extreme randomization probabilities to protect allocation unpredictability. Specifically, for each patient about to be randomized, the system will automatically calculate an imbalance score for each of the above-mentioned baseline covariates, as the excess or deficit of previously randomized patients in either arm matching the current patient on that covariate. Next, these scores will be summed over covariates to form the total imbalance score, S. If S = 0, the randomization probability for receiving the intervention for that patient will be set to ½, and if S < 0 (S > 0) the randomization probability will be set to 2/3 (1/3) following Efron's recommendation [59].
Randomization will be performed by a designated study staff person who does not have the ability to influence its execution. By design, group assignments will be identifiable to participants and the interventionist, but blinding of outcome assessment and adjudication, data and safety monitoring, and data analysis will be enforced. Further, the interventionist will be masked to participants' official study measurements, but not to their self-measurements tracked as part of the intervention.
2.5. Continuation of usual care
We will recruit from patients who have used Kaiser Permanente Northern California for routine care for at least 1 year and thus have a higher likelihood of establishing a relationship with their PCP. During informed consent, patients will be clearly instructed that they continue to receive medical care as usual—no medical care is provided by this research study. For patient safety and generalizability, no standard care will be withheld at any time after enrollment; participants in either arm can use all primary care or specialty care services available to them in routine practice.
Participants in the control group will receive no intervention from the study. In theory, the control group could receive a generic health education intervention so as to separate the active intervention's therapeutic effects from potential nonspecific, e.g., attention, effects. However, randomized controlled trials are most useful in testing interventions as they would be delivered in real world settings. Also importantly, research has shown that use of attention control in behavioral randomized trials does not always have a positive effect on outcome but may be unnecessary and possibly even detrimental [61]. It is therefore recommended that attention control be considered only when prior data have demonstrated an effect of attention on the outcome of interest or an important mediator of that outcome [61]. Guidelines for the treatment of asthma do not call for nonspecific intervention. No empirical evidence suggests that attention alone results in improved asthma control or dietary changes, which are known to be difficult to initiate and even harder to maintain. The attention accompanying the DASH diet intervention under study is intrinsic to behavioral interventions of similar nature and intensity.
2.6. Intervention
In addition to usual care, participants in the intervention arm will complete a 6-month DASH diet intervention.
2.6.1. Theoretical basis
The theoretical bases of the intervention are Social Cognitive Theory and the Transtheoretical Model of Behavior Change [62]. The former emphasizes a triadic, reciprocally deterministic relationship between the individual, environment, and behavior, whereas the latter recognizes that behavior change is a dynamic process that moves, at variable speed, through stages of readiness to change. Positive outcome expectancies through realistic goal setting and guided action planning are associated with initiation of behavior change, and self-efficacy developed for specific behaviors (e.g., eating more fruit and vegetables) predict establishment and maintenance of behavior change. Social Cognitive Theory suggests that self-efficacy is enhanced through social support and gradual mastery of self-regulation skills (e.g., self-monitoring, problem solving) [63]. The interventionist will work closely with each participant to elicit outcome expectations, perform realistic, individualized goal-setting, and develop a customized action plan in the form of a mutually agreed-upon contract. To improve adherence, throughout the intervention the interventionist will attend to the presence of any disincentives and relapse warning signs and will apply motivational interviewing techniques to help participants explore and resolve ambivalence about making dietary changes.
2.6.2. Evidence-based intervention goals
A goal-based approach will be used to promote DASH dietary targets. Three primary daily dietary goals are (1) 9– 12 servings of fruits and vegetables, (2) 2–3 servings of low-fat dairy products, and (3) total fat grams at 27% of estimated caloric needs for weight maintenance. Reduction of saturated fat also will be emphasized by focusing on reduced consumption of red meat and regular-fat dairy products. These goals are critical because each represents a key aspect of the DASH dietary pattern [37,64,65]. We also will promote ingestion of no more than 2300 mg of sodium a day, increased intake of whole grains, nuts, seeds, and legumes, decreased intake of sweets and added sugars, and moderate alcohol intake for those who drink.
The intervention will employ clear criteria (branch points) and appropriate strategies to tailor the dietary change goals to each individual participant while maintaining the same essential goals and delivering common core information to all. The recommended food servings will be tailored to individual calorie needs for maintaining stable weight during the 6-month intervention period. We will base caloric requirements on the Harris Benedict formula, using baseline weights and physical activity levels estimated from the 7-Day Physical Activity Recall survey [66,67]. Intervention participants will be weighed in private before each individual and group session and their food servings will be adjusted, if necessary. The same approach was used in previous studies of the effects of the DASH diet, independent of weight change, on cardiovascular risk factors in free-living individuals [40,68]. Without intervention, significant weight change among usual care participants over 6 months is unlikely; the general U.S. adult population gains ∼1% weight each year [69]. In previous DASH intervention trials, caloric intake did not differ between the DASH diet group (without a concurrent weight loss intervention) and the usual care control group. We will exclude underweight (body mass index [BMI] in kg/m2 < 18.5) and severely obese patients (BMI ≥ 40) and patients who have cardiovascular and/or metabolic conditions (Table 1) that would make it inappropriate clinically to ask patients to maintain their weight, even for just 6 months, if they are overweight or obese.
2.6.3. Intervention format, structure, and content
The 6-month intervention will consist of an intensive phase and a maintenance phase.
2.6.3.1. Format
The intervention will employ a series of small group and individual sessions, followed by periodic telephone contacts, to help participants achieve and maintain appropriate dietary changes. The initial 3 months of intensive intervention will involve 8 group and 3 individual sessions for 45–60 min each. During the next 3 months, participants will receive counseling phone calls once per month for 20– 30 minutes each call. Group session sizes may range from 8 to 15 participants, and each session will take place on 2 or 3 evenings during the week at the participating KP sites. Participants will be assigned a primary group, but may attend another one in the same week if they miss a session. They will be encouraged to bring a family member or significant other, especially if this guest is primarily in charge of meal planning and preparation at home.
2.6.3.2. Structure
The intervention will begin with an individual session within 3 weeks of the baseline clinic visit. Group sessions will start with 4 weekly meetings, then a 2-week break in which a second individual session will take place, then another 4 weekly meetings, and end with the third and final individual session. This will be followed by 3 monthly counseling phone calls (Table 3). Based on indices of ongoing participation in intervention activities, interventionists also may schedule additional visits or calls with participants who have difficulties adhering to the intervention protocol (e.g., frequently missed sessions and/or self-monitoring records, lack of progress to DASH diet goals).
Table 3.
Intervention contact schedule.
| Intensive phase | Maintenance phasea | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1/2 | W3 | W4 | W5 | W6 | W7/8 | W9 | W10 | W11 | W12 | W13/14 | M1 | M2 | M3 |
| I1 | G | G | G | G | I2 | G | G | G | G | I3 | P | P | P |
G, group session; I, individual session; M, month; P, phone call; W, week.
At the interventionist's discretion, additional individual visits and/or phone calls may be held during the maintenance phase depending on participant needs and preferences.
2.6.3.3. Content and curriculum
The weekly group sessions will teach participants how to buy and prepare foods consistent with the DASH meal plans, reinforce their motivation to follow the diet, give feedback on their adherence, and provide training on problem-solving and relapse prevention skills. Each group session will include 6 main curriculum components: (1) food tasting and group sharing for social support, (2) review of progress since last session, (3) main content area (e.g., meal patterns, DASH dietary goals, fruit and vegetables and grains, low-fat dairy products, calories and dietary fat, and sodium), (4) behavioral skills training, (5) self-monitoring activity, and (6) goal setting and action planning for the next session. The sessions will be instructional, but not didactic, and will provide ample opportunities for participant input and smaller group activities that foster problem solving, support, and program ownership. The individual sessions and follow-up phone calls are designed to specifically tailor intervention strategies to individual participants' cultural, social, and environmental contexts.
2.7. Participant safety
Participants will be carefully screened, and individuals for whom the intervention is deemed medically inappropriate or unsafe will be excluded. PCP approval will be required before potentially eligible patients are contacted for study screening. During screening, women who are pregnant, lactating, or planning to become pregnant during the study period will be excluded. If a participant becomes pregnant during the study, she will be excluded from further participation in all study activities, and her PCP will be notified. Participants who are diagnosed with any other exclusionary condition (e.g., coronary heart disease, stroke, diabetes, and cancer) following randomization may continue in the trial with approval of the study physician. Established alert levels (e.g., for low lung function and high blood pressure) and alert conditions (e.g., cardiovascular events, and musculoskeletal injuries) (Appendix online) will help ensure that participants are referred for further evaluation and therapy when clinically indicated.
To ensure unbiased ascertainment between the intervention and control groups, participants will be systematically asked at baseline and 3- and 6-month clinic visits to report any possible adverse events occurring during baseline (from initial contact by mail to baseline clinic visit) and follow-up surveillance periods (from baseline clinic visit to 3-month visit and then to 6-month visit). Positive responses will be recorded and then reviewed by the study physician for seriousness, study relatedness, and expectedness. An adverse event is defined as any untoward medical or psychological event experienced by a patient during or as a result of his/her participation in the study that represents a new symptom or an exacerbation of an existing condition whether or not considered study-related based on appropriate medical judgment. Documentation in the electronic health records will be used to verify patient self-reports. Adverse events discovered outside these planned evaluations (e.g., during intervention encounters) will be duly noted and followed up with, as needed, to assure participant safety.
2.8. Retention
As we have done in our other studies [58,70,71], we will implement the following strategies to minimize loss to follow-up: (1) careful staff selection and standardized training in rapport building, motivational interviewing techniques, trial-specific protocols, and problem solving techniques as appropriate to their study roles; (2) legally adequate and effective informed consent; (3) careful eligibility screening, including assessment of willingness and motivation to adhere to data collection and treatment requirements; (4) education of participants about the importance of follow-up assessments regardless of treatment adherence; (5) prudent participant incentives and flexible scheduling; (6) promotion of study identity; (7) ongoing monitoring of recruitment and retention; (8) up-to-date contact information for the participant and two emergency contacts; (9) diligent efforts to re-engage inactive participants; and (10) prioritization of outcome measures when not all measures may be obtained from a participant.
2.9. Study measures and data collection schedule (Table 2)
Table 2.
List of measures and data collection schedule.
| Baseline | Follow-up month | ||
|---|---|---|---|
|
|
|||
| 3 | 6 | ||
| Questionnaires | |||
| Juniper Asthma Control Questionnaire (ACQ) | X | X | X |
| Juniper Mini Asthma-specific Quality of Life Questionnaire (mini-AQLQ) | X | X | X |
| Quality of Life Scales: Importance, Satisfaction, asthma impact | X | X | X |
| Two-week asthma symptom diary | X | X | X |
| Multiple-pass 24-hour diet recalls | X | X | X |
| Eating Habits Confidence Survey | X | X | X |
| Social Support and Eating Habits Survey | X | X | X |
| Stanford Seven-day Physical Activity Recall | X | X | X |
| Nine-item Patient Health Questionnaire | X | X | |
| 12-item Short Form Health Survey (SF-12) | X | X | |
| Berlin Questionnaire for Sleep Apnea | X | X | |
| Pittsburgh Sleep Quality Index | X | X | |
| Gastroesophageal Reflux Disease Symptom Assessment Scale (GSAS) | X | X | |
| Symptoms and adverse events | X | X | X |
| Care at non-Kaiser health care facilities | X | X | X |
| Demographics, age of asthma onset, and asthma triggers | X | ||
| Physical and laboratory measurements | |||
| Height | X | ||
| Weight, waist circumference, and blood pressure | X | X | X |
| Forced spirometry: FEV1, FVC, and FEV1/FVC (absolute and % predicted for all) | X | X | X |
| Exhaled breath condensate pH | X | X | |
| Fractional exhaled nitric oxide (FeNO) | X | X | X |
| Fasting blood specimens: complete blood count (total and differential leukocyte counts); blood lipids; serum carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene), folate, and vitamin B12; serum cytokines (e.g., IL-5, IL-6, IL-13, IL-17, TNF-α, and IFN-γ); | X | X | |
| Serum total and allergen-specific IgEs | X | X | X |
| Genotypes (e.g., glutathione S transferase and microsomal epoxide hydrolase genes) | X | ||
| Data from Patients' Electronic Health Recordsa | |||
| Diagnoses | X | ||
| Medications prescribed and dispensed | X | X | |
| Health care encounters (e.g., office, ER/hospital) | X | X | |
| Recruitment and intervention process measures (monitored continuously) | |||
| Safety of participants in the intervention arm (monitored continuously) | |||
Data will be extracted for 6 months before and after randomization.
2.9.1. Primary and secondary outcomes
As the primary outcome measure, the ACQ [45,46] is a reliable, validated 7-item instrument assessing the components of current asthma impairment as defined in the asthma treatment guidelines [1], i.e., daytime and nocturnal asthma symptoms, activity limitations, rescue medication use (excluding use to prevent exercise-induced bronchospasm), and lung function (FEV1). The ACQ is the only composite measure of asthma control that is recommended for research purposes that includes an objective lung function measure in its overall rating [72,73].
Secondary asthma outcomes include spirometric lung function measurements [56], asthma symptom-free and [β2-agonist-free days in 2 weeks [74], a global health-related [75] and an asthma-related quality of life measure [76]. The NIH Asthma Outcomes Workshop [77] recommended development of a new measure of patients' perceived impact of asthma on quality of life. As part of its psychometric evaluation, the Asthma-Impact on Quality of Life Scale, developed by Wilson, will be administered along with the Importance and Satisfaction scales of the Flanagan Quality of Life Scale [78]. The Asthma-Impact on Quality of Life Scale utilizes the 15 Flanagan dimensions of quality of life plus one additional dimension [79]. Asthma-related health care utilization will be assessed through data extraction from KP electronic databases for the 6-month periods pre- and post-randomization. These databases contain information on all outpatient and inpatient encounters at KP facilities and non-KP facilities with which KP contracts for emergency and hospital services, and on any reimbursable emergency hospital care at non-contract facilities. Data also will be extracted on all medications prescribed and dispensed for the same time periods. In KP, it is estimated that well over 95% of all prescriptions are filled at a KP pharmacy, and patients are very unlikely to have concurrent non-KP sources of asthma care or prescription medications. The dispensing records will allow calculation of reliable, unbiased indices of medication refill adherence and regimen potency, to be calculated as in Wilson et al. [80].
Additional secondary outcomes include diet adherence, psychosocial predictors of dietary change, and comorbidities. Participants' adherence to the DASH diet will be assessed by multiple-pass 24-hour diet recalls [81-83] for intake of fruits, vegetables, dairy products, whole grains, fats, and sodium; increases in serum carotenoids, folate and vitamin B12; and reductions in fasting plasma lipids. Self-efficacy and social support for dietary change will be assessed using previously validated instruments [84-86]. Sleep apnea, gastroesophageal reflux disease, and depression are common comorbidities of asthma and will be assessed by the Berlin Questionnaire for Sleep Apnea [87], Pittsburg Sleep Quality Index [88], Gastroesophageal Reflux Disease Symptom Assessment Scale [89], and 9-item Patient Health Questionnaire [90], respectively.
2.9.2. Potential mediators
Markers of inflammation will be examined as mechanistic outcomes and potential mediators of the intervention effect on asthma control, including FeNO (using a NIOX MINO portable hand-held NO analyzer [91-95]), EBC pH (by CO2 standardization method) [96,97], serum total and differential leukocyte counts, and serum markers of lymphocytic inflammation important to asthma pathophysiology [98,99] (e.g., IL-5, IL-6,IL-13,IL-17, TNF-α, and IFN-γ).
2.9.3. Potential effect modifiers
To explore potential differences in the intervention effect among subgroups, we will measure participants' socio-demographics (age, sex, race/ethnicity, education, marital status, and household income), phenotypes (e.g., age of asthma onset, atopy, and obesity), and genotypes (e.g., glutathione S transferase and microsomal epoxide hydrolase polymorphisms, which have been shown to contribute strongly to the susceptibility and occurrence of asthma [100–104]). Asthma is a complex syndrome comprised of distinct, yet overlapping phenotypes that can be recognized by characteristics such as age of onset, atopic status, and obesity [105]. Phenotypes of disease as well as genetic alterations may play a critical role in the response to therapy. Among studies examining the link between genes and oxidative stress and inflammation [100–103], results by Salam et al. [104] suggested that certain variants in the glutathione S transferase and microsomal epoxide hydrolase genes contribute strongly to the susceptibility and occurrence of asthma.
2.9.4. Process measures
We will collect data on the screening process, e.g., the proportion and representativeness of patients who are eligible at initial and subsequent screenings, reasons for exclusions, demographics of patients who screen ineligible or decline participation. We also will assess the proportion and representativeness of physicians willing to approve screening of their potentially eligible patients, retention rates, the representativeness of patients who complete follow-up assessments, and reasons for dropout.
Intervention exposure and adherence measures will include attendance at group and individual sessions, reasons for missed sessions, frequency and completeness of self-monitoring records, and completion of counseling phone calls. Self-monitoring data obtained during the intervention program will be used for adherence monitoring and for individual feedback. The 6-month assessment visit will include an exit interview to elicit participants' feedback regarding their overall satisfaction with this pilot study and with the intervention (for intervention participants only), perceived benefits, relevance, feasibility, problems encountered, and suggestions for improvement in a subsequent full trial.
2.10. Statistical analysis
2.10.1. Analytic plan
We will evaluate between-group differences in primary (Aim 1) and secondary outcomes (Aim 2) by intention-to-treat using tests of group by time interactions in repeated-measures mixed-effects linear (for continuous outcomes) or logistic models (for categorical outcomes). The fixed effects of each model will include the baseline value of the outcome of interest, randomization balancing factors, group, time point (3 or 6 months), and group-by-time interaction. The models will account for the non-independence of repeated measures using a covariance structure within participants to be determined by the least Bayesian information criterion. The primary analysis will use all available follow-up data, with missing data handled directly through maximum likelihood estimation in mixed modeling. We will document the extent and pattern of missing data and the reasons, and will conduct sensitivity analyses of the impact of missing data (e.g., with multiple imputation) on stability of the primary results. We will check residuals after fitting a model using residual plots, and the model will be adjusted accordingly if nonlinearity and/or unequal variances are detected. Polynomial terms may be included if indicated. Appropriate transformation of the outcome variable (e.g., logarithmic) will be considered as a remedy for unequal variances. Also, the model could be altered to a heterogeneous variance model if participants in different intervention arms are found to have different variances. We will verify that mixed model-based results are not sensitive to violations of model assumptions with permutation and bootstrap resampling tests [106,107]. Least-square means and CIs will be obtained from the models. Supportive analyses will include DASH concordance index as a covariate to examine the impact of intervention adherence on outcome and hence elucidate the primary intention-to-treat findings.
We will conduct mediation analyses to explore changes in inflammatory markers implicated in asthma pathophysiology and their potential mediating effects on treatment response (Aim 3). Longitudinal [i.e., changes in mediators from baseline to 3 months and change in an outcome (e.g., ACQ) from 3 to 6 months] and contemporaneous (i.e., changes in mediators and change in the outcome from baseline to 6 months) mediation will be examined separately by MacKinnon's product of coefficients test (αβ) [108]. Asymmetric confidence limits will be constructed based on the distribution of the product using the PRODCLIN program [109]. Because multicollinearity may be present in a multiple mediator model, we first will test each mediator separately in a single-mediator model. Next, multiple-mediator models will be used to test for independent and suppression effects when all variables found to be at least marginally significant in the single-mediator models are entered simultaneously. To determine the extent of mediated effect, the percentage of total effect mediated will be calculated for each significant mediator as αβ / (αβ + γ), where γ is the direct intervention effect on outcome.
We will conduct moderation analyses to explore differences in intervention effect by genotypes and phenotypes (Aim 4). These analyses will follow the same general analytic approach as described above for Aims 1 and 2, with the inclusion of appropriate moderator main effects and moderator-by-group interaction terms.
2.10.2. Sample size and data interpretation
An efficacy-based power analysis is beyond the scope of a pilot study. Instead, we will use a confidence interval (CI) approach to guide our interpretation of the pilot results by considering sampling variability of both the outcome measure and the CI width in reference to conventional effect size standards of clinical significance. A follow-up sample of 40 participants each arm (after a projected 10% loss to follow-up) at 6 months will achieve 90% power for the expected 2-sided 95% CI to have a standardized half-width no greater than 0.5 [110-112]. With this level of precision, we will be unlikely to miss a between-group mean ACQ score difference that has a Cohen's d = 0.5 (medium effect) or larger because the confidence intervals for these scenarios (A-B, Fig. 1) are to the right of the null; such findings will be convincing enough to justify a full trial. Scenarios C-E are less decided, demonstrating varying degrees of certainty depending on the location of the point estimate relative to null. If the pilot study demonstrates one of these scenarios, we will consider the ACQ results in the context of secondary outcomes (e.g., asthma symptoms, lung function, and quality of life) and markers of potential mechanisms (e.g., FeNO, EBC pH, and serum cytokines) and results from other studies to decide whether a full trial is warranted. If findings of likely favorable response to the DASH diet are consistent among these data, we will proceed to a full trial. But lack of consistent findings will prompt us to do the same as we would for Scenario F. Finally, if we encounter Scenario F, in which the upper confidence limit is less than d = 0.2 (considered a small effect), we will conclude that a full trial is not warranted without substantive modifications to the design or approach. We will assess findings on secondary outcomes, inflammatory markers, and subgroups as well as process evaluation data to determine if modifications to the design, intervention, or target population should be made prior to a larger trial and/or if additional pilot trials (e.g., with further intervention tailoring to particular subgroups) are indicated instead.
Fig. 1.
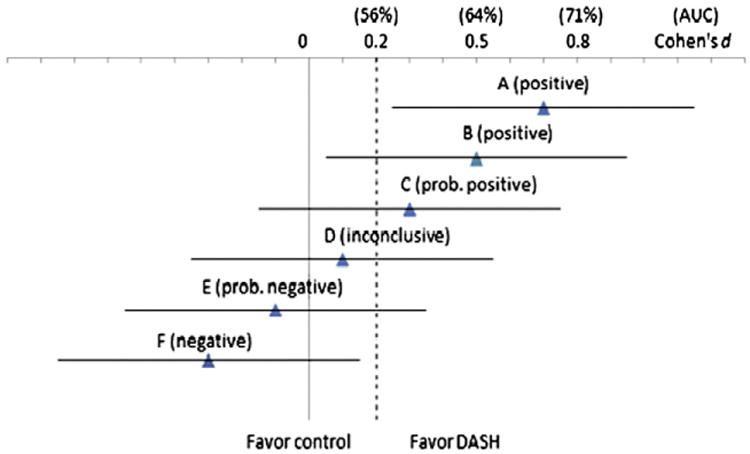
Illustrative scenarios (A–F) of pilot study results for the primary outcome — ACQ score: effect size estimates are expressed in Cohen's d together with the expected 2-sided 95% confidence intervals (standardized half-width = 0.45 at n = 45/arm with a projected 10% attrition over 6 months). AUC (area under the receiver operating characteristic curve) values are given to indicate standards for assessing clinical significance corresponding to those for d. AUC measures the probability that a randomly selected subject in the intervention has a better response than a randomly selected subject in the control [122–124]. Above each line in parentheses is the decision concerning whether a full trial is warranted.
2.11. Quality control
2.11.1. Data management
All study data will be entered into computerized data files utilizing the following: (1) Microsoft Access for enrollment, follow-up, and intervention process data entry and verification; (2) a secure web-based survey tool for entry and verification of self- and interviewer-administered questionnaire data and physical measurements; and (3) the Nutrition Data System for Research (Minneapolis, MN) for dietary data collection and analysis. Data sets will be cleaned, verified and archived, and then read into SAS data sets (Version 9.2, SAS Institute Inc., Cary, North Carolina), which also will be archived. One official copy of all the study data and a master data dictionary will be maintained and updated regularly. All analytic and tracking database files will be stored in a password-protected, encrypted network drive with continuous backups. For the protection of patient confidentiality, unique, anonymous study IDs will be used for data storing, tracking and reporting. Protected health information will be stored separately from all other study data and will be used and disclosed in accordance with the Health Insurance Portability and Accountability Act regulations. Reports will be produced regularly to track the following: (1) patient accrual and follow-up completion/retention in relation to goals and timeline; (2) the randomization process and group comparability on the balancing variables; (3) key baseline characteristics of the sample; (4) intervention exposure and adherence; and (5) protocol deviations and violations. Any observed delays in the recruitment and intervention processes or data irregularities will be followed up and resolved in a timely manner.
2.11.2. Intervention fidelity
Following recommendations for behavioral intervention studies [113], we will standardize intervention materials and provide rigorous interventionist training and oversight to ensure intervention fidelity. All group and individual sessions will be audiotaped and a random 10% sample from the “early,” “middle” and “late” sessions will be reviewed and rated for protocol adherence using a structured rating scale. Interventionists score below a priori performance standards (e.g., 90% of session objectives achieved) will receive more frequent audit and feedback and “booster” training (if necessary). Interventionists will complete a checklist of critical intervention behaviors and materials delivered in each session, and will document the frequency, duration, and content of phone contacts with participants. They also will report enabling factors and challenges to intervention delivery and participant adherence.
To monitor and support participants' receipt of and adherence to the intervention, interventionists will review and give feedback on homework and self-monitoring records and document participants' mastery of protocol-specific, achievement-based objectives. During in-person sessions and phone contacts, interventionists will routinely inquire about barriers to intervention receipt and adherence, recommend problem-solving strategies, and provide ongoing counseling using motivational interviewing techniques [49]. Participants will complete a brief survey after each in-person session that assesses their understanding and satisfaction with content and activities. Participant exit interviews will be conducted at 6 months to elicit feedback on the relevance and acceptability of knowledge and skills gained from the intervention, practice of program strategies, perceived benefits, problems encountered, overall satisfaction with program format and materials, and suggestions for changes. These intervention process data will be used to refine the intervention.
3. Discussion
There is a paucity of epidemiologic studies, and an absence of intervention studies, on the effect of overall diet on asthma control in adults. Two U.S.-based prospective studies found that a “prudent” pattern (high intake of fruit, vegetables, fish, and whole grains) was associated with a decreased risk of incident COPD among adults [22,23], whereas a “Western” pattern (high intake of refined grains, cured and red meats, desserts, and French fries) was associated with an increased risk. These studies also investigated incident asthma as a secondary outcome but reported no significant findings. But a positive association between a “Western” dietary pattern and the risk of frequent asthma exacerbations was reported in a large cohort of French females [30]. Several studies of the Mediterranean diet, which is characterized by high fruit and vegetable intake and low consumption of saturated fatty acids from animal sources, have shown an inverse association with asthma symptoms among children in Spain [25,27], Greece [26,35], and Mexico [28]. Also, in a study with 174 Portuguese adults (mean [SD] age, 40 [15] years; 82% female), Barros et al. showed that adherence to the Mediterranean diet was associated with a 78% lower risk of uncontrolled asthma (adjusted odds ratio, 0.22; 95% CI, 0.05-0.85; P for trend, 0.028) [29]. These results suggest a strong association between the Mediterranean diet and asthma control—the primary outcome of the current study. We chose the DASH diet because it is officially embraced in the U.S. Dietary Guidelines [37] and very similar to the Mediterranean diet with 2 notable differences: Mediterranean promotes high monounsaturated fatty acids (mainly from olive oil) and regular alcohol (red wine) consumption. Data regarding the role of individual oils and fatty acids in asthma are highly inconsistent [5,114], and the Barros study found that higher alcohol intake was associated with an increased risk of uncontrolled asthma. The DASH diet emphasizes fresh fruit, vegetables, and low-fat dairy products; includes whole grains, poultry, fish, and nuts; contains only small amounts of red meat, sweets, and sugar-containing beverages; and contains decreased amounts of total and saturated fat, cholesterol, and sodium [64,65]. The typical American diet differs markedly from the DASH diet and 90% of U.S. adults consume a diet with low concordance with the DASH [43].
The current pilot study aims to provide critical data on the feasibility and potential efficacy of the DASH diet among adults with uncontrolled asthma. We will collect data needed to (1) justify whether a full trial is warranted, (2) estimate accrual and attrition rates, (3) determine whether adequate adherence to the DASH diet is achievable in the target study population, (4) identify the need and potential for further improvement in the intervention and trial procedures, and (5) explore potential mechanisms and effect modifiers to guide further investigation. This pilot study will enable us to refine the design and approach of a full-scale trial that will be adequately powered to test the efficacy of the DASH diet in improving asthma control among adults. If ultimately proven efficacious, the DASH diet would add a nonpharmacological approach to the armamentarium of medical and environmental strategies for the treatment of asthma, and an approach with demonstrated health benefits beyond asthma control. Process measures from the pilot and (if warranted) subsequent efficacy trial also will inform the intervention's potential for implementation into routine practice. It is worth noting that this research is being conducted by researchers embedded within large delivery systems in partnership with leading academic institutions—a model that members of the team have successfully used for translation of other efficacious lifestyle interventions, for example, for weight management in primary care settings [115]. Even though translation is out of the scope for a pilot study, the current intervention design takes into account the delivery setting so that primary care patients with asthma can participate and, potentially, benefit.
Suboptimal asthma control is prevalent and it exerts a significant burden for patients and on the healthcare system [116–118]. Even with the proper use of controller medications and other conventional therapies, clinical responses among asthma patients are variable, and a stepwise approach requires add-on therapeutic options [1]. The DASH diet could provide a practical, safe, and acceptable public health intervention in the form of dietary modification to reduce the burden of asthma. This line of investigation addresses an important area of research due to the growing problem of asthma and the need for additional adjunct therapy. It will contribute to the evidence base for refining public health recommendations and clinical guidelines regarding the roles of diet and nutrition in asthma.
Supplementary Material
Acknowledgments
The project described is supported by Award Number R34 HL108753 from the National Heart, Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health. The authors wish to thank the study Data and Safety Monitoring Board members (William Haskell, PhD, Ware Kuschner, MD, and Manisha Desai, PhD).
Abbreviations
- ACQ
Asthma Control Questionnaire
- CI
confidence interval
- DASH
Dietary Approaches to Stop Hypertension
- FeNO
fractional exhaled nitric oxide
- FEV1
forced expiratory volume in one second
- KP
Kaiser Permanente
- PCP
primary care provider
Footnotes
Trial registration: NCT01725945.
Authors' contributions: JM conceived the study, obtained funding, has the overall responsibility for its design and conduct, and drafted the manuscript. LX contributed to planning the statistical analyses and drafting the manuscript. PS, PWL, ASB, CAC, KCN, and SRW participated in the design of the study and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Competing interests: Dr. Camargo has received financial support from a variety of groups for participation in conferences, consulting, and medical research. During 2005 to 2010, industry sponsors with an interest in asthma were AstraZeneca, Critical Therapeutics, Dey, Genentech, GSK, Merck, Novartis, Respironics, and Schering-Plough. Dr. Buist is, or has been recently, a member of advisory boards for AstraZeneca, Merck, GSK, Sepracor, Pfizer and Novartis.
All other authors declare that they have no financial, research, organizational, or other interests to disclose that are relevant to the execution of this research or this publication.
Appendix A. Supplementary data: Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cct.2013.04.008.
References
- 1.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR 3): guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 2007. [Google Scholar]
- 2.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention. 2007 [Google Scholar]
- 3.Vital signs: asthma prevalence, disease characteristics, and self-management education: United States,2001–2009. MMWR Morb Mortal Wkly Rep. 2011;60(17):547–52. [PubMed] [Google Scholar]
- 4.Kant AK, Schatzkin A. Consumption of energy-dense, nutrient-poor foods by the US population: effect on nutrient profiles. J Am Coll Nutr. 1994;13(3):285–91. doi: 10.1080/07315724.1994.10718410. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Ellwood PE, Asher MI. Diet and asthma: looking back, moving forward. Respir Res. 2009;10:49. doi: 10.1186/1465-9921-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127(3):724–33. doi: 10.1016/j.jaci.2010.11.001. e721-730. [DOI] [PubMed] [Google Scholar]
- 7.Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115(6):1109–17. doi: 10.1016/j.jaci.2004.12.1139. quiz 1118. [DOI] [PubMed] [Google Scholar]
- 8.Misso NL, Thompson PJ. Oxidative stress and antioxidant deficiencies in asthma: potential modification by diet. Redox Rep. 2005;10(5):247–55. doi: 10.1179/135100005X70233. [DOI] [PubMed] [Google Scholar]
- 9.Wood LG, Gibson PG. Dietary factors lead to innate immune activation in asthma. Pharmacol Ther. 2009;123(1):37–53. doi: 10.1016/j.pharmthera.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127(5):1133–40. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Wood LG, Garg ML, Smart JM, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr. 2012;96(3):534–43. doi: 10.3945/ajcn.111.032623. [DOI] [PubMed] [Google Scholar]
- 12.Ram FS, Ardern KD. Dietary salt reduction or exclusion for allergic asthma. Cochrane Database Syst Rev. 2004;(3):CD000436. doi: 10.1002/14651858.CD000436. [DOI] [PubMed] [Google Scholar]
- 13.Allam MF, Lucane RA. Selenium supplementation for asthma. Cochrane Database Syst Rev. 2004;(2):CD003538. doi: 10.1002/14651858.CD003538.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisman J, Schachter HM, Dales RE, Tran K, Kourad K, Barnes D, et al. Treating asthma with omega-3 fatty acids: where is the evidence? A systematic review. BMC Complement Altern Med. 2006;6:26. doi: 10.1186/1472-6882-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur B, Rowe BH, Arnold E. Vitamin C supplementation for asthma. Cochrane Database Syst Rev. 2009;(1):CD000993. doi: 10.1002/14651858.CD000993.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen S, Britton JR, Leonardi-Bee JA. Association between antioxidant vitamins and asthma outcome measures: systematic review and meta-analysis. Thorax. 2009;64(7):610–9. doi: 10.1136/thx.2008.101469. [DOI] [PubMed] [Google Scholar]
- 17.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs DR, Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78(3 Suppl):508S–13S. doi: 10.1093/ajcn/78.3.508S. [DOI] [PubMed] [Google Scholar]
- 19.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72(4):912–21. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 20.Terry P, Hu FB, Hansen H, Wolk A. Prospective study of major dietary patterns and colorectal cancer risk in women. Am J Epidemiol. 2001;154(12):1143–9. doi: 10.1093/aje/154.12.1143. [DOI] [PubMed] [Google Scholar]
- 21.van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med. 2002;136(3):201–9. doi: 10.7326/0003-4819-136-3-200202050-00008. [DOI] [PubMed] [Google Scholar]
- 22.Varraso R, Fung TT, Hu FB, Willett W, Camargo CA. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62(9):786–91. doi: 10.1136/thx.2006.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varraso R, Fung TT, Barr RG, Hu FB, Willett W, Camargo CA., Jr Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86(2):488–95. doi: 10.1093/ajcn/86.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKeever TM, Lewis SA, Cassano PA, Ocke M, Burney P, Britton J, et al. Patterns of dietary intake and relation to respiratory disease, forced expiratory volume in 1 s, and decline in 5-y forced expiratory volume. Am J Clin Nutr. 2010;92(2):408–15. doi: 10.3945/ajcn.2009.29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Marcos L, Canflanca IM, Garrido JB, Varela AL, Garcia-Hernandez G, Guillen Grima F, et al. Relationship of asthma and rhinoconjunctivitis with obesity, exercise and Mediterranean diet in Spanish schoolchildren. Thorax. 2007;62(6):503–8. doi: 10.1136/thx.2006.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatzi L, Apostolaki G, Bibakis I, Skypala I, Bibaki-Liakou V, Tzanakis N, et al. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax. 2007;62(8):677–83. doi: 10.1136/thx.2006.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro-Rodriguez JA, Garcia-Marcos L, Alfonseda Rojas JD, Valverde-Molina J, Sanchez-Solis M. Mediterranean diet as a protective factor for wheezing in preschool children. J Pediatr. 2008;152(6):823–8. doi: 10.1016/j.jpeds.2008.01.003. 828 e821-822. [DOI] [PubMed] [Google Scholar]
- 28.de Batlle J, Garcia-Aymerich J, Barraza-Villarreal A, Anto JM, Romieu I. Mediterranean diet is associated with reduced asthma and rhinitis in Mexican children. Allergy. 2008;63(10):1310–6. doi: 10.1111/j.1398-9995.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 29.Barros R, Moreira A, Fonseca J, de Oliveira JF, Delgado L, Castel-Branco MG, et al. Adherence to the Mediterranean diet and fresh fruit intake are associated with improved asthma control. Allergy. 2008;63(7):917–23. doi: 10.1111/j.1398-9995.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- 30.Varraso R, Kauffmann F, Leynaert B, Le Moual N, Boutron-Ruault MC, Clavel-Chapelon F, et al. Dietary patterns and asthma in the E3N study. Eur Respir J. 2009;33(1):33–41. doi: 10.1183/09031936.00130807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romieu I, Barraza-Villarreal A, Escamilla-Nunez C, Texcalac-Sangrador JL, Hernandez-Cadena L, Diaz-Sanchez D, et al. Dietary intake, lung function and airway inflammation in Mexico City school children exposed to air pollutants. Respir Res. 2009;10:122. doi: 10.1186/1465-9921-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagel G, Weinmayr G, Kleiner A, Garcia-Marcos L, Strachan DP. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax. 2010;65(6):516–22. doi: 10.1136/thx.2009.128256. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez Barcala FJ, Pertega S, Bamonde L, Garnelo L, Perez Castro T, Sampedro M, et al. Mediterranean diet and asthma in Spanish schoolchildren. Pediatr Allergy Immunol. 2010;21(7):1021–7. doi: 10.1111/j.1399-3038.2010.01080.x. [DOI] [PubMed] [Google Scholar]
- 34.Bakolis I, Hooper R, Thompson RL, Shaheen SO. Dietary patterns and adult asthma: population-based case–control study. Allergy. 2010;65(5):606–15. doi: 10.1111/j.1398-9995.2009.02215.x. [DOI] [PubMed] [Google Scholar]
- 35.Arvaniti F, Priftis KN, Papadimitriou A, Papadopoulos M, Roma E, Kapsokefalou M, et al. Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10–12 years old children: the PANACEA study. Pediatr Allergy Immunol. 2011;22(3):283–9. doi: 10.1111/j.1399-3038.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 36.Wood LG, Garg ML, Powell H, Gibson PG. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free Radic Res. 2008;42(1):94–102. doi: 10.1080/10715760701767307. [DOI] [PubMed] [Google Scholar]
- 37.Dietary guidelines for Americans. 2010 http://www.cnpp.usda.gov/dietaryguidelines.htm.
- 38.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 39.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170(2):126–35. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension. 2003;41(3):422–30. doi: 10.1161/01.HYP.0000053450.19998.11. [DOI] [PubMed] [Google Scholar]
- 42.Lin PH, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133(10):3130–6. doi: 10.1093/jn/133.10.3130. [DOI] [PubMed] [Google Scholar]
- 43.Parikh A, Lipsitz SR, Natarajan S. Association between a DASH-like diet and mortality in adults with hypertension: findings from a population-based follow-up study. Am J Hypertens. 2009;22(4):409–16. doi: 10.1038/ajh.2009.10. [DOI] [PubMed] [Google Scholar]
- 44.Uddenfeldt M, Janson C, Lampa E, Leander M, Norback D, Larsson L, et al. High BMI is related to higher incidence of asthma, while a fish and fruit diet is related to a lower — results from a long-term follow-up study of three age groups in Sweden. Respir Med. 2010;104(7):972–80. doi: 10.1016/j.rmed.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 46.Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–21. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption — II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 49.Miller WR, Rollnick S. Motivational interviewing: preparing people for change. 2nd. New York: Guilford Press; 2002. [Google Scholar]
- 50.Meyers C, Jones TB. Promoting active learning: strategies for the college classroom. San Francisco, CA: Jossey-Bass; 1993. [Google Scholar]
- 51.Goldberg JH, Kiernan M. Innovative techniques to address retention in a behavioral weight-loss trial. Health Educ Res. 2005;20(4):439–47. doi: 10.1093/her/cyg139. [DOI] [PubMed] [Google Scholar]
- 52.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 53.Kostikas K, Koutsokera A, Papiris S, Gourgoulianis KI, Loukides S. Exhaled breath condensate in patients with asthma: implications for application in clinical practice. Clin Exp Allergy. 2008;38(4):557–65. doi: 10.1111/j.1365-2222.2008.02940.x. [DOI] [PubMed] [Google Scholar]
- 54.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 55.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 56.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 57.Pocock SJ. Clinical trials, a practical approach. Chichester: John Wiley and Sons; 1991. pp. 84–6. [Google Scholar]
- 58.Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–21. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Efron B. Forcing sequential experiment to be balanced. Biometrika. 1971;58:403–17. [Google Scholar]
- 60.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15. [PubMed] [Google Scholar]
- 61.Pagoto SL, McDermott MM, Reed G, Greenland P, Mazor KM, Ockene JK, et al. Can attention control conditions have detrimental effects on behavioral medicine randomized trials? Psychosom Med. 2013;75(2):137–43. doi: 10.1097/PSY.0b013e3182765dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 63.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, N.J.: Prentice Hall; 1986. [Google Scholar]
- 64.Karanja NM, Obarzanek E, Lin PH, McCullough ML, Phillips KM, Swain JF, et al. Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension Trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99(8 Suppl):S19–27. doi: 10.1016/s0002-8223(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 65.United States Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Facts about the DASH eating plan vol NIH Publication No 03-4082. 1999;8:S19–27. http://www.nhlbi.nih.gov/health/public/heart/hbp/dash/ [Google Scholar]
- 66.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 67.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 68.Blumenthal JA, Babyak MA, Sherwood A, Craighead L, Lin PH, Johnson J, et al. Effects of the dietary approaches to stop hypertension diet alone and in combination with exercise and caloric restriction on insulin sensitivity and lipids. Hypertension. 2010;55(5):1199–205. doi: 10.1161/HYPERTENSIONAHA.109.149153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–5. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 70.Ma J, King AC, Wilson SR, Xiao L, Stafford RS. Evaluation of lifestyle interventions to treat elevated cardiometabolic risk in primary care (E-LITE): a randomized controlled trial. BMC Fam Pract. 2009;10:71. doi: 10.1186/1471-2296-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma J, Strub P, Camargo CA, Jr, Xiao L, Ayala E, Gardner CD, et al. The breathe easier through weight loss lifestyle (BE WELL) intervention: a randomized controlled trial. BMC Pulm Med. 2010;10:16. doi: 10.1186/1471-2466-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 73.Cloutier MM, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, et al. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol. 2012;129(3 Suppl):S24–33. doi: 10.1016/j.jaci.2011.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santanello NC, Barber BL, Reiss TF, Friedman BS, Juniper EF, Zhang J. Measurement characteristics of two asthma symptom diary scales for use in clinical trials. Eur Respir J. 1997;10(3):646–51. [PubMed] [Google Scholar]
- 75.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 76.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14(1):32–8. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 77.Wilson SR, Rand CS, Cabana MD, Foggs MB, Halterman JS, Olson L, et al. Asthma outcomes: quality of life. J Allergy Clin Immunol. 2012;129(3 Suppl):S88–S123. doi: 10.1016/j.jaci.2011.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flanagan JC. A research approach to Improving our quality of life. Am Psychol. 1978;33:138–47. [Google Scholar]
- 79.Burckhardt CS, Woods SL, Schultz AA, Ziebarth DM. Quality of life of adults with chronic illness: a psychometric study. Res Nurs Health. 1989;12(6):347–54. doi: 10.1002/nur.4770120604. [DOI] [PubMed] [Google Scholar]
- 80.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566–77. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moshfegh AJ, Goldman J, Lacomb R, Perloff B, Cleveland L. Research results using the new USDA Automated Multiple-Pass Method. FASEB J. 2001;15(4):A278. abstr. [Google Scholar]
- 82.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77(5):1171–8. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 83.Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104(4):595–603. doi: 10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 84.Sallis JF, Pinski RB, Grossman RM, Patterson TL, Nader PR. The development of self-efficacy scales for health-related diet and exercise behaviors. Health Educ Res. 1988;3:283–92. [Google Scholar]
- 85.Ball K, Crawford D. An investigation of psychological, social and environmental correlates of obesity and weight gain in young women. Int J Obes (Lond) 2006;30(8):1240–9. doi: 10.1038/sj.ijo.0803267. [DOI] [PubMed] [Google Scholar]
- 86.Kiernan M, Moore SD, Schoffman DE, Lee K, King AC, Taylor CB, et al. Social support for healthy behaviors: scale psychometrics and prediction of weight loss among women in a behavioral program. Obesity (Silver Spring) 2012;20(4):756–64. doi: 10.1038/oby.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 88.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 89.Rothman M, Farup C, Stewart W, Helbers L, Zeldis J. Symptoms associated with gastroesophageal reflux disease: development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46(7):1540–9. doi: 10.1023/a:1010660425522. [DOI] [PubMed] [Google Scholar]
- 90.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 91.Alving K, Janson C, Nordvall L. Performance of a new hand-held device for exhaled nitric oxide measurement in adults and children. Respir Res. 2006;7:67. doi: 10.1186/1465-9921-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boot JD, de Ridder L, de Kam ML, Calderon C, Mascelli MA, Diamant Z. Comparison of exhaled nitric oxide measurements between NIOX MINO((R)) electrochemical and Ecomedics chemiluminescence analyzer. Respir Med. 2008;102(11):1667–71. doi: 10.1016/j.rmed.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 93.Gill M, Graff GR, Adler AJ, Dweik RA. Validation study of fractional exhaled nitric oxide measurements using a handheld monitoring device. J Asthma. 2006;43(10):731–4. doi: 10.1080/02770900601031045. [DOI] [PubMed] [Google Scholar]
- 94.Khalili B, Boggs PB, Bahna SL. Reliability of a new hand-held device for the measurement of exhaled nitric oxide. Allergy. 2007;62(10):1171–4. doi: 10.1111/j.1398-9995.2007.01475.x. [DOI] [PubMed] [Google Scholar]
- 95.Menzies D, Nair A, Lipworth BJ. Portable exhaled nitric oxide measurement: comparison with the “gold standard” technique. Chest. 2007;131(2):410–4. doi: 10.1378/chest.06-1335. [DOI] [PubMed] [Google Scholar]
- 96.Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26(3):523–48. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 97.Koczulla R, Dragonieri S, Schot R, Bals R, Gauw SA, Vogelmeier C, et al. Comparison of exhaled breath condensate pH using two commercially available devices in healthy controls, asthma and COPD patients. Respir Res. 2009;10:78. doi: 10.1186/1465-9921-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–54. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holt PG, Strickland DH, Bosco A, Jahnsen FL. Pathogenic mechanisms of allergic inflammation: atopic asthma as a paradigm. Adv Immunol. 2009;104:51–113. doi: 10.1016/S0065-2776(08)04003-0. [DOI] [PubMed] [Google Scholar]
- 100.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8(3):169–82. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 101.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122(3):456–68. doi: 10.1016/j.jaci.2008.08.004. quiz 469-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pinto LA, Stein RT, Kabesch M. Impact of genetics in childhood asthma. J Pediatr (Rio J) 2008;84(4 Suppl):S68–75. doi: 10.2223/JPED.1781. [DOI] [PubMed] [Google Scholar]
- 103.Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J. 2008;31(1):179–97. doi: 10.1183/09031936.00128106. [DOI] [PubMed] [Google Scholar]
- 104.Salam MT, Lin PC, Avol EL, Gauderman WJ, Gilliland FD. Microsomal epoxide hydrolase, glutathione S-transferase P1, traffic and childhood asthma. Thorax. 2007;62(12):1050–7. doi: 10.1136/thx.2007.080127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–13. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 106.Tang L, Duan N, Klap R, Asarnow JR, Belin TR. Applying permutation tests with adjustment for covariates and attrition weights to randomized trials of health-services interventions. Stat Med. 2009;28(1):65–74. doi: 10.1002/sim.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DiCiccio TJ, Efron B. Bootstrap confidence intervals. Stat Sci. 1996;11(3):189–228. [Google Scholar]
- 108.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav Res Methods. 2007;39(3):384–9. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bristol DR. Sample sizes for constructing confidence intervals and testing hypotheses. Stat Med. 1989;8(7):803–11. doi: 10.1002/sim.4780080705. [DOI] [PubMed] [Google Scholar]
- 111.Beal SL. Sample size determination for confidence intervals on the population mean and on the difference between two population means. Biometrics. 1989;45(3):969–77. [PubMed] [Google Scholar]
- 112.Julious SA, Patterson SD. Sample sizes for estimation in clinical research. Pharm Stat. 2004;3:213–5. [Google Scholar]
- 113.Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443–51. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 114.McKeever TM, Britton J. Diet and asthma. Am J Respir Crit Care Med. 2004;170(7):725–9. doi: 10.1164/rccm.200405-611PP. [DOI] [PubMed] [Google Scholar]
- 115.Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–21. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 117.Accordini S, Bugiani M, Arossa W, Gerzeli S, Marinoni A, Olivieri M, et al. Poor control increases the economic cost of asthma. A multicentre population-based study. Int Arch Allergy Immunol. 2006;141(2):189–98. doi: 10.1159/000094898. [DOI] [PubMed] [Google Scholar]
- 118.Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. doi: 10.1186/1471-2466-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.United States Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Your guide to lowering your blood pressure with DASH. 2006 [Google Scholar]
- 120.Mellen PB, Gao SK, Vitolins MZ, Goff DC., Jr Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168(3):308–14. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 121.Lin PH, Appel LJ, Funk K, Craddick S, Chen C, Elmer P, et al. The PREMIER intervention helps participants follow the Dietary Approaches to Stop Hypertension dietary pattern and the current Dietary Reference Intakes recommendations. J Am Diet Assoc. 2007;107(9):1541–51. doi: 10.1016/j.jada.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 122.Grissom RJ. Probability of the superior outcome of one treatment over another. J Appl Psychol. 1994;79:314–6. [Google Scholar]
- 123.McGraw KO, Wong SP. A common language effect size statistic. Psychol Bull. 1992;111:361–5. [Google Scholar]
- 124.Kraemer HC, Morgan GA, Leech NL, Gliner JA, Vaske JJ, Harmon RJ. Measures of clinical significance. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1524–9. doi: 10.1097/00004583-200312000-00022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


