Abstract
Polymer networks are critically important for numerous applications including soft biomaterials, adhesives, coatings, elastomers, and gel-based materials for energy storage. One long-standing challenge these materials present lies in understanding the role of network defects, such as dangling ends and loops, developed during cross-linking. These defects can negatively impact the physical, mechanical, and transport properties of the gel. Here we report chemically cross-linked poly(ethylene glycol) (PEG) gels formed through a unique cross-linking scheme designed to minimize defects in the network. The highly resilient mechanical properties of these systems (discussed in a previous publication1), suggests that this cross-linking technique yields more homogeneous network structures. Four series of gels were formed based on chains of 35,000 g/mol, (35K), 12,000 g/mol (12K) g/mol, 8,000 g/mol (8K) and 4,000 g/mol (4K) PEG. Gels were synthesized at five initial polymer concentrations ranging from 0.077 g/mL to 0.50 g/mL. Small-angle neutron scattering (SANS) was utilized to investigate the network structures of gels in both D2O and d-DMF. SANS results show the resulting network structure is dependent on PEG length, transitioning from a more homogeneous network structure at high molecular weight PEG to a two phase structure at the lowest molecular weight PEG. Further investigation of the transport properties inherent to these systems, such as diffusion, will aid to further confirm the network structures.
Introduction
Polymer networks, in their many forms, remain critically important materials from both a fundamental and technological viewpoint. Industrially important adhesives, high temperature epoxides2, and soft hydrogels3, 4 found in biomaterials and consumer products demonstrate the wide application and importance of networked materials. Many biological materials, both naturally-occurring (e.g., tissues)5–7 and synthetic8 are composed of soft material networks. Despite significant progress in understanding the basic structure-property relationships of networks, much remains to be learned about how the foundational macromolecular building blocks transmit properties across the length-scales to the macroscopic sample. Fundamental grand challenges include understanding the relationship between network structure, dynamics, and mechanical properties.
The ability to manipulate and predict the structure and resulting physical properties of a polymer network by changing specific variables (i.e. polymer molecular weight, polymer concentration, cross-linking time), is advantageous for industrial and academic applications of a given material. One key step to developing structure/property relationships of polymer networks is the reduction of network defects (i.e. highly cross-linked junctions, looping chains, dangling ends). These defects typically form in an unpredictable manner and can impact the resulting physical properties of the network. For example, highly cross-linked network junctions found in some hydrogels developed for in vivo applications result in difficulty when predicting physical properties such as the degradation rate or drug release profiles.9 Looping chains and dangling ends detract from the elastic properties and resilience of a network. Polymer networks with minimal defects are also of interest for applications in energy storage. For example, poly(ethylene glycol) (PEG)-based networks are currently being investigated for energy storage application due to their ability to conduct lithium ions. PEG achieves lithium ion conductance through chain relaxation, however, energy storage applications require materials with robust mechanical properties. Therefore, the optimization of ion transport in PEG-based networks is achieved by balancing the mechanical properties with ion conductivity.10, 11 As network defects detract from the mechanical properties of the hydrogel, efficient cross-linking techniques designed to reduce defect formation are highly desired.12, 13
The need for more homogeneous polymer networks has lead to the development of cross-linking techniques that allow for greater control over the resulting network microstructure. One of the most basic chemical cross-linking techniques is the photopolymerization of end-functionalized, or telechelic, polymers. While this technique allows for some control over the cross-link density of the network,14 it does not define cross-link functionality and commonly results in the formation of cross-linked clusters in the network (i.e. high functionality cross-links).15, 16 A more recent approach utilizes click chemistry to control cross-linking in networks.17, 18 Click reactions are highly efficient, have high functional group tolerance, and are highly active in water making them ideal for use as a hydrogel cross-linking strategy.18, 19 Hydrogels formed through click chemistry have demonstrated high elastic moduli, suggesting that this cross-linking strategy can reduce the formation of defects in the network.17, 20 Greater control over the cross-link functionality was obtained through the development of multifunctional cross-linkers designed to react with a specific number of telechelic polymer chains. Small angle neutron scattering (SANS) studies have revealed that defects are still present in these networks upon swelling.21–27 A recent approach by Sakai and coworkers28 utilized 4-arm star-shaped polymers to reduce network defects and form highly elastic, remarkably homogeneous hydrogels.29 They achieved this through the use of tetra-arm PEG macromers that cross-link through activated-ester chemistry. The resulting gels, referred to as Tetra-PEG gels, were found to have a remarkably homogeneous network structure through small-angle neutron scattering (SANS)30 and static-light scattering (SLS) studies.31
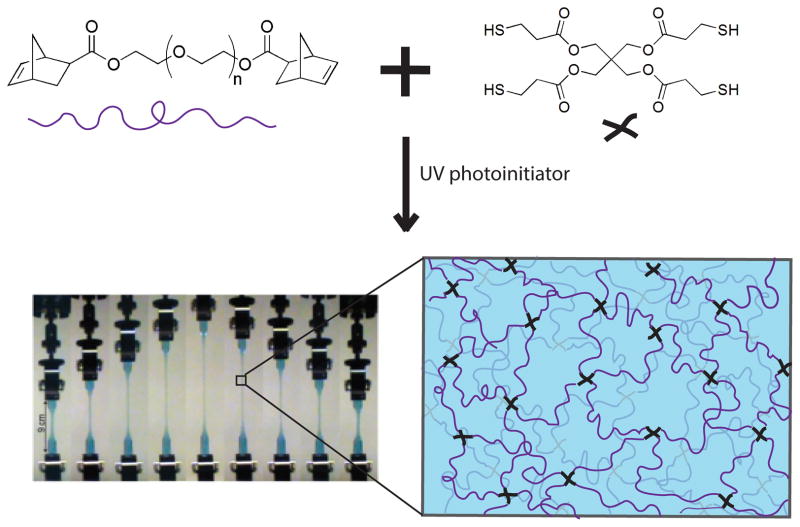
Tew and coworkers1 recently developed a novel cross-linking technique that utilizes thiol-norbornene chemistry to form PEG-based hydrogel networks with minimal defects, or inhomogeneities (Figure 1). Also referred to as a “click reaction”, thiolene reactions are simple, highly efficient, do not produce side products, and rapidly achieve high yield. Thiolene chemistry has been used to form several different types of materials, including nearly ideal, uniform polymer networks.32, 33 The synthesis technique developed by the Tew group utilizes norbonene functionalized PEG macromers and a tetra-functional thiol cross-linker to produce PEG-based networks with well-defined cross-link functionalities and minimal defects.. The resulting hydrogels are optically clear and have displayed high toughness and resilience. Resilience is a measure of a material’s ability to deform reversibly (elastically) without loss of energy. A recent publication demonstrates that tetra-functional PEG hydrogels with an equilibrium water content greater than 95% have a resilience ≥97% at strains of up to 300%.1 As network defects typically contribute to viscous losses in mechanical behavior, the high resilience values suggest that these materials may have a relatively low level of defects.
Figure 1.
Synthesis of tetra-functional PEG hydrogels. Image of hydrogel in tension was obtained from Cui et al.1
Here, we have employed small-angle neutron scattering (SANS) to investigate the network microstructure and relative homogeneity of these tetra-functional PEG networks. Four series of gels were created by varying the initial polymer concentration of 35,000 g/mol PEG, 12,000 g/mol PEG, 8,000 g/mol PEG and 4,000 g/mol PEG. These systems will be referred to as 35K, 12K, 8K and 4K tetra-functional PEG hydrogels, respectively. Analysis of the SANS data revealed that resulting network structure was dependent on the length of the PEG macromer. We find that the network structure in D2O transitions from a homogeneous network to a two-phase network as the length of the PEG macromer is decreased. This effect decreased significantly for gels swollen with deuterated N,N-dimethylformamide (d-DMF), suggesting that clustering of hydrophobic chain ends and crosslinker occurs in the lower molecular weight gels; however, it did not disappear completely in d-DMF, indicating that there may also be more network defects at lower molecular weights that become “locked in” to the network structure during cross-linking in d-DMF. We have validated this through fitting of empirical models to the data sets. Additionally, the model fits revealed that the mesh size of the networks were tunable within each molecular weight series, varying inversely with initial polymer concentration as expected.
Experimental Section
Poly(ethylene glycol) (PEG) (Mn = 35 kDa,12 kDa, 8 kDa, 4 kDa), exo-5-norbornenecarboxylic acid, triphenylphosphine, diisopropyl azodicarboxylate (DIAD), pentaerythritol tetrakis(3-mercaptopropionate) (PETMP), 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (PI), and N,N-dimethylformamide (DMF) and deuterated N,N-dimethylformamide (d-DMF) were purchased from Alfa Aesar, Sigma Aldrich, Acros Organics, or Fisher and used without further purification.
GPC Characterization
Gel permeation chromatography (GPC) was conducted with a Polymer Laboratories PC-GPC50 with two 5 μm mixed-D columns, a 5 μm guard column, and a RI detector (HP1047A), with polystyrene standards and THF as the eluent at a flow rate of 1.0 mL/min. The polydispersity index (PDI) of the resulting 35K, 12K, 8K and 4K PEG macromers were found to be 1.07, 1.07, 1.04, and 1.06 respectively.
Hydrogel Preparation
The norbornene end-functionalized PEG (n-PEG-n) precursors were prepared by the Mitsunobu reaction according to the procedure described previously.1 The desired amount of n-PEG-n (0.077 g, 0.10 g, 0.14 g, 0.25 g, 0.33 g, or 0.50 g) was dissolved in DMF (1 mL) to form a clear solution. The tetra-functional cross-linker, PETMP, and the PI (0.5 wt% with respect to the polymer) were added to form the precursor solution. The molar ratio of the polymer (n-PEG-n) to the cross-linker (PETMP) was 2 to 1, so the molar ratio of norbornene to thiol groups was 1 to 1. After thorough mixing, the precursor solution was transferred to the desired mold (a customized Teflon or syringe mold) and exposed to ultraviolet light with a wavelength of 365 nm for 45–60 min. The cross-linked gel was removed from the mold and washed with excess DMF, which was replaced three times, to remove unreacted materials. The gel then was immersed in deionized water, which was replaced daily until equilibrium swelling was reached.
Small-Angle Neutron Scattering
Samples for small-angle neutron scattering (SANS) were prepared as described above, except the immersion and equilibration steps were performed in D2O. SANS measurements were conducted on the 30 m small angle neutron scattering instrument on the NG-7 beamline at the National Institute of Standards and Technology (NIST) Center for Neutron Research, Gaithersburg, MD.34 Spectra were obtained at room temperature in quartz sample cells with a path length of 2 mm. Gels were synthesized prior to placement in the sample cells according to the procedure listed above. Great care was taken to produce gels with a thickness of 2 mm and diameter of 0.75” in order to fill the sample cell. Once the sample was in place, excess D2O was added to the cell to maintain equilibrium swollen conditions and prevent any solvent evaporation during scattering. Spectra were collected for 105 minutes per sample. The sample to detector distance varied from 1.0 to 13 m, resulting in q-range for these experiments of 0.003 Å−1 < q < 0.5 Å−1. Data reduction and normalization were performed using standard techniques.35 Model fitting to the first sample run for the 12K tetra-functional hydrogel at an initial polymer concentration of 0.14 g/mL gave non-physical results, so the values reported here are from a second, rehydrated sample.
Results and Discussion
Qualitative analysis
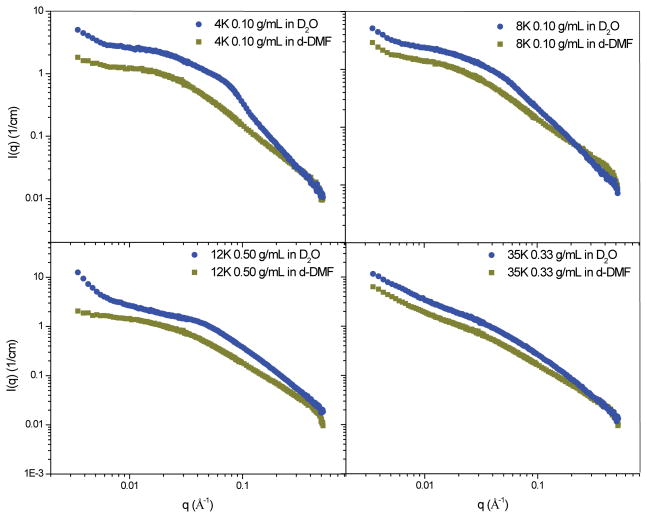
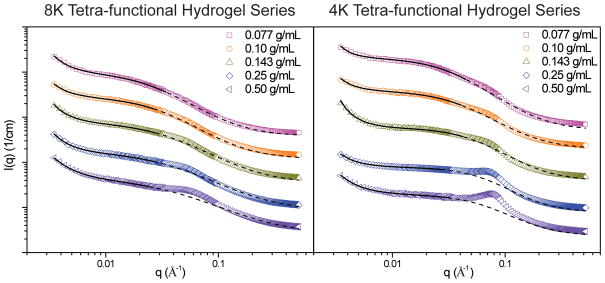
SANS experiments were carried out on tetra-functional PEG hydrogels formed from norbornene-functionalized 4K, 8K, 12K and 35K PEG. The initial polymer concentration was varied from 0.077 g/mL to 0.50 g/mL to form a series of hydrogels at each molecular weight. Due to its high molecular weight, cross-linking reactions done with 35K PEG had consistently lower yields than the other three PEG chain lengths. This is most likely due to the lower concentration of functional groups, especially at very low concentrations for this system.36 Therefore, the lowest initial polymer concentration used for that series was 0.10 g/mL. An additional hydrogel was formed at an initial polymer concentration of 0.33 g/mL for the 35K series. Figure 2 contains spectra from hydrogels with varying PEG length at initial polymer concentrations of 0.077 g/mL, 0.10 g/mL, 0.143 g/mL, 0.25 g/mL and 0.50 g/mL.
Figure 2.
Scattering spectra from tetra-functional PEG hydrogels formed with 4K, 8K, 12K, and 35K MW PEG with varying initial polymer concentrations between 0.077 g/mL and 0.50 g/mL. Spectra have been shifted for clarity.
These SANS experiments probed network structures between 180 nm and 2 nm. As the size of polymer chains used to form these networks range from 4.9 nm to 14.5 nm, the size range probed by SANS would provide structural information on the conformation of single polymer chains as well as multiple cross-linking sites (i.e. nano-scale network structure). Network defects that occur on length scales larger than 180 nm would not be captured in this SANS experiment, however, an upturn in the spectra at low q would indicate their presence. The spectra plotted in Figure 2 qualitatively demonstrate that a change in network structure occurs with changes in the length of the PEG macromer. While all spectra contain a broad shoulder and upturn at low q, the shoulder becomes more pronounced as the length of the PEG is decreased. Structural differences are most striking at the highest initial polymer concentration, 0.50 g/mL, where a distinct peak forms in the spectra of the 4K hydrogel. The other three gels exhibit a broad shoulder that shifts towards lower q as the molecular weight of PEG is increased.
The presence of a peak is commonly found in scattering spectra from networks with high junction functionality.15, 16, 37 These types of networks have a higher density of polymer near the junctions, resulting in the junction points serving as scattering centers. Due to the cross-linking chemistry used to form the tetra-functional PEG networks, we would expect these systems to have a uniform junction functionality of 4 and would therefore not expect to see a peak in the scattering spectra. For the 35K and 12K gels, the absence of a maximum in the spectra confirm this low junction functionality. The presence of a broad shoulder indicates that the mesh size is fairly uniform. Similar behavior has been observed in SANS studies of poly(dimethylsiloxane) (PDMS) and polystyrene (PS) networks with low cross-link functionalities.22, 38 Spectra obtained from these studies lack a correlation peak as the cross-link junctions are small in comparison to the rest of the network, preventing them from acting as distinct scattering centers.15, 22, 38
While the SANS results for the 35K and 12K systems indicate a relatively homogeneous structure for the majority of the q range probed, the upturn at low q suggests the presence of structural heterogeneities on larger length scales. The shape of the scattering curves from the 35K and 12K tetra-functional PEG systems are notably similar to those obtained for PEG solutions.39 Scattering spectra from PEG solutions have also shown an upturn at low q which has been attributed to clustering of the chains in solution. Several groups have compared scattering from a semi-dilute polymer solution to that of the cross-linked gel in order to investigate network homogeneity.22, 30, 31, 40 For many of these systems, the scattering from the unswollen (or as-prepared) gel was similar to scattering from the semi-dilute precursor solution. However, at higher degrees of swelling, scattering from the gel began to deviate from that of the semi-dilute solution. In these instances, the high q scattering from both systems remained similar, while at low q, the gel exhibited scattering at a higher intensity than the solution scattering.22, 40 The excess in scattering was attributed to concentration fluctuations in the network that result in regions of inhomogeneity due to the formation of “hard-to-swell” zones.22, 41
We have qualitatively compared the scattering from the 35K and 12K tetra-functional PEG hydrogels to scattering from linear PEO chains in solution (Mw = 100K at 90°C, 10 wt%) reported by Hammouda and coworkers.39 For both systems, the upturn at low q is slight but still present. Matsunaga and coworkers31 observed a similar upturn in SANS from as-prepared Tetra-PEG hydrogels, which were also compared to scattering from PEG in solution (102K PEO at 10°C reported by Hammouda and coworkers42).31
The spectra obtained for the 8K and 4K series are noticeably different than those obtained for the higher MW series. Hydrogels formed at initial polymer concentrations of 0.077 g/mL and 0.10 g/mL have two broad shoulders in their spectra, one at low q and one at high q. As the initial polymer concentration increases from 0.10 g/mL to 0.14 g/mL, the high q shoulder becomes more pronounced, decreasing in broadness and increasing in intensity. At the highest initial polymer concentration (0.50 g/mL), the high q shoulder becomes more pronounced for the 8K series, and for the 4K tetra-functional hydrogel the shoulder becomes a peak. Similar spectra were obtained from SANS studies of randomly cross-linked PEG hydrogels.15, 16 The presence of a peak in the scattering spectra for these systems indicates the presence of scattering centers, and is a common feature in scattering from highly branched gels and end-cross-linked gels with high end group functionality.37 For these systems, a peak indicates clusters or highly cross-linked regions within the network. A study by Lin-Gibson and coworkers16 investigated the structure of randomly cross-linked 1K, 2K, 4K, and 8K PEG-dimethacrylate hydrogels through SANS. They concluded that it was reasonable to assume a network structure of cross-linked clusters in a solution like matrix. The absence of a defined peak occurred at low polymer concentrations when the polymer was too diffuse to form uniform clusters, resulting in large defects in the network. Waters and co-workers15 investigated the scattering from randomly cross-linked 3.4K, 4.6K, and 8K PEG-diacrylate and PEG-diacrylamide hydrogels. They also observed a defined peak in the scattering spectra of these systems, and related it to the average spacing between dense cross-linked junction regions in the network.
For the tetra-functional PEG systems discussed in this paper, it is unlikely that the correlation peak seen in the spectra is due solely to network defects, as is the case in the randomly cross-linked PEG networks discussed above. The cross-linking technique used to form the tetra-functional PEG systems should result in junctions of low functionality regardless of the length of PEG macromer used. Additionally, both the 4K and 8K systems demonstrate highly resilient mechanical properties similar to the 12K and 35K systems.36 This supports the idea that the structure seen in the 4K and 8K systems does not primarily arise from network defects, but rather from clustering or segregation of the hydrophobic endgroups and crosslinker within the gels, which we expect to be more significant as PEG length decreases. Therefore, we believe that these structural differences between different molecular weight tetra-functional PEG hydrogels are mainly due to the formation of domains rich in the hydrophobic components of the network (norbornene end-groups and tetra-thiol cross-linker) upon swelling in water. It is important to note here that all hydrogels are synthesized in DMF, and that both the hydrophilic PEG with the norbornene end-groups and the hydrophobic cross-linker are readily soluble in DMF. The cross-linked gels are then washed with DMF to remove any unreacted material that is not connected to the network.
As the length of the PEG macromer is decreased, the ratio of hydrophobic material (norborne end-groups and tetra-thiol cross-linker) to hydrophilic material (PEG chains) in the network increases. At the same polymer concentration, there will be more low molecular weight chains present in solution which will result in a higher cross-link density (i.e. more hydrophobic norbornene end-groups and tetra-thiol cross-linker are contained in the gel). The reduction in PEG length also results in less hydrophilic units between the hydrophobic norbornene end-groups which could result in clustering due to end effects43 when the network is swollen with water. As discussed further below, there is also evidence of a higher incidence of defects in lower molecular weight samples, which also contributes to the development of a shoulder and low q scattering in the spectra. However, the structural differences observed as molecular weight decreases appears to be mainly due to a microphase separation of chain ends and crosslinker.
Effect of Solvent on Hydrogel Structure
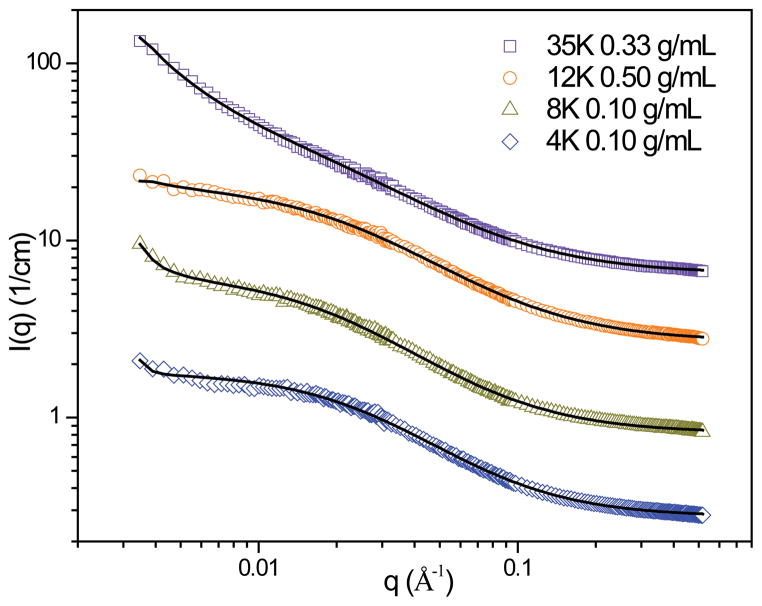
In order to investigate these structural differences further, we conducted a SANS experiment on tetra-functional PEG networks swollen in deuterated N,N-dimethylformamide (d-DMF). Results were obtained for the following hydrogels: 35K at 0.33 g/mL, 12K at 0.50 g/mL, 8K at 0.10 g/mL, and 4K at 0.10 g/mL (Figure 3). The intensity of background scattering was determined with a high q scaling approximation (Equation 1), where n is a high-q Porod exponent and B is incoherent background.
Figure 3.
Spectra from hydrogels swollen in D2O and d-DMF with background scattering subtracted.
| (1) |
A plot of qnI(q) vs qn at high q yields a linear plot that has a slope, B, and intercept A.42 The value for background determined from these plots was subtracted from the original scattering spectra.. Spectra for hydrogels swollen in D2O have been shifted up so both spectra can be seen clearly as they would otherwise overlap.
The effect of solvent on network structure is most clearly seen for the 4K, 8K, and 12K hydrogels. Swelling these systems in d-DMF appears to result in a more homogeneous network structure for all systems, which is indicated by the reduced presence of high q features that are prominent in spectra from their D2O-swollen counterparts. Additionally, the slope of the upturn at low q that is present for the 12K system is greatly reduced when swollen in d-DMF. These results support the theory that the networks undergo phase separation when swollen in D2O. The only system that remains relatively unaffected by the change in solvent is the 35K system, which would have the highest ratio of hydrophilic to hydrophobic components due to the high MW of the PEG macromer used. Therefore, this system would be less likely to undergo a large degree of phase separation in D2O even at the highest initial polymer concentration as the hydrophobic content of the network is much less than the hydrophilic content. However, both D2O and d-DMF swollen networks display an upturn at low q, suggesting that the large scale inhomogeneities in these systems form independently of solvent and could possibly be the result of chain entanglements that become locked into place after cross-linking.
It is interesting to note that, while spectra from all d-DMF gels show a more homogenous structure than is obtained in D2O, the 8K and 4K samples in d-DMF still exhibit a small upturn at low q and development of a small shoulder. These indicate that there is some presence of clusters or inhomogeneity in the polymer segment density for these lower molecular weights, even in d-DMF. Thus, the inhomogeneity in network structure that begins to appear for shorter length PEG cannot completely be attributed to phase separation. We believe there may be some structural defects in the network, such as loops or dangling ends, that become locked into place as the gels are cross-linked in d-DMF, and that these are more significant for the lower molecular weight samples. Results from swelling studies confirm that there is some deviation from ideal network behavior for lower molecular weight hydrogels in d-DMF.36
These differences can be seen more clearly by replotting the data in a Kratky plot. A Kratky plot is used to highlight scattering at high q, and has been used to investigate the structure of hydrogel networks.44–48 The shape of the Kratky plot indicates the conformation of the scattering unit. For a rod at high q, I(q) ≈ 1/q. Therefore, a Kratky plot of scattering from a rodlike object would become linear at high q as q2I(q) = A + Bq. Scattering from Gaussian chains at high q approximates to I(q) ≈ 1/q2, while scattering from a three-dimensional object at high q approximates to I(q) ≈ 1/q4. Therefore, Kratky plots of scattering from Gaussian chains would increase monotonically with q and would reach a plateau at high q, while that for a three dimensional object should have a peak and would then decrease as 1/q2 at high q.
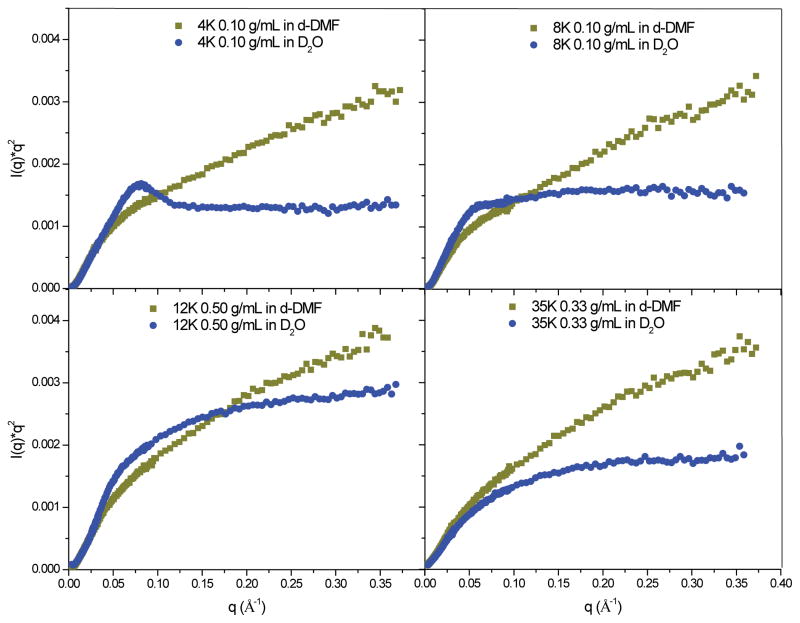
A large peak at low q is commonly observed in the Kratky plots of polymer gels and indicates the presence of frozen inhomogeneities in the gel network.45 Shinohara and coworkers44 and Karino and coworkers45 recently used the Kratky plot to provide further evidence that their cross-linking technique, which forms cross-links that can move position by sliding along polymer chains in the network, could be used to reduce inhomogeneities in the networks of their hydrogels. Kratky plots of the SANS data from tetra-functional gels swollen in D2O and d-DMF are shown in Figure 4.
Figure 4.
Kratky plots for 35K, 12K, 8K and 4K tetra-functional hydrogels in D2O and d-DMF. Background has been subtracted through the same method discussed in a previous section.
Kratky plots of scattering from networks swollen in d-DMF vs D2O provide further insight into solvent-based structural changes (Figure 4). For all systems, the high q behavior of the Kratky plots indicate that the polymer chains in the network become more swollen in d-DMF. The most drastic change occurs for the 4K system, and is seen in the elimination of the peak when the network is swollen in d-DMF.
Model Fitting
Results for 35K and 12K Tetra-functional Hydrogels
More insight into the nano-scale structure of these networks can be obtained by fitting the SANS data with an empirical model. The scattering spectra for the 35K and 12K PEG tetra-functional hydrogel series were successfully fit with the correlation length model through a nonlinear, least squares fit (Figure 5).35
Figure 5.
Scattering spectra for 35K and 12K tetra-functional hydrogel series. Symbols indicate scattering data, while solid lines indicate the fit of the correlation length model to the data is indicated by the solid line. Spectra have been shifted for clarity.
This model was developed by Hammouda and coworkers39 and has been used to analyze the scattering spectra of polymer solutions as well as scattering from other hydrogel systems.31, 49–51 Scattering intensity is modeled by Equation 2:
| (2) |
where I(q) is the scattering intensity, q is the scattering vector, and Bkg is scattering from background. Parameters n, m, and ξL are the Porod exponent, the Lorentzian exponent, and the Lorentzian screening length, respectively. The Porod exponent characterizes the fractal structure of the gel, while the Lorenztian exponent characterizes the polymer/solvent interactions and therefore describes the thermodynamics of the system. The Lorenztian screening length, ξL, is the correlation length for polymer chains39 and in the case of a gel network gives an indication of the gel mesh size. Results of the fit of this model to the 35K and 12K SANS spectra are shown in Table 1.
Table 1.
Results from correlation length model fit to 35K and 12K PEG hydrogels.
| Parameters | 35K PEG | ||||
|---|---|---|---|---|---|
|
| |||||
| C0 (g/mL) | 0.10 | 0.143 | 0.25 | 0.33 | 0.50 |
| n | 2.5 ± 0.08 | 2.1 ± 0.05 | 1.9 ± 0.04 | 1.4 ± 0.03 | 2.2 ± 0.02 |
| ξL (nm) | 13.0 ± 0.8 | 7.3 ± 0.2 | 4.8 ± 0.1 | 2.9 ± 0.1 | 3.7 ± 0.8 |
| m | 1.7 ± 0.01 | 1.7 ± 0.01 | 1.8 ± 0.01 | 1.8 ± 0.02 | 1.7 ± 0.01 |
| Bkg (1/cm) | 0.07 ± 8e-5 | 0.08 ± 1e-4 | 0.07 ± 1e-4 | 0.09 ± 2e-4 | 0.09 ± 2e-4 |
| Parameters | 12K PEG | ||||
|---|---|---|---|---|---|
|
| |||||
| C0 (g/mL) | 0.077 | 0.10 | 0.143 | 0.25 | 0.50 |
| n | 2.4 ± 0.09 | 3.0 ± 0.10 | 1.1 ± 0.004 | 2.2 ± 0.05 | 2.3 ± 0.02 |
| ξL (nm) | 6.3 ± 0.1 | 5.8 ± 0.1 | 2.8 ± 0.4 | 2.9 ± 0.02 | 2.1 ± 0.01 |
| m | 1.8 ± 0.004 | 1.7 ± 0.01 | 2.4 ± 0.04 | 1.9 ± 0.02 | 2.0 ± 0.005 |
| Bkg (1/cm) | 0.08 ± 9e-5 | 0.13 ± 2e-4 | 0.09 ± 5e-4 | 0.09 ± 4e-4 | 0.11 ± 2e-4 |
The reduced χ2 values for all the fits were equal to or less than 2.6, indicating a good agreement between the model and the data. All of the gels are mass fractal, indicated by a Porod exponent, n, of 2 or greater, with the exception of the 35K and 12K tetra-functional gels with an initial polymer concentrations of 0.33 g/mL and 0.143 g/mL respectively. A Porod exponent, n, of 2 or greater suggests that the 35K and 12K tetra-functional PEG hydrogels are a one-phase system.31 The correlation length model was also used to fit master curves of SANS from as-prepared Tetra-PEG hydrogel systems by Matsunaga et al.,31 Similar to our results, they obtained a Porod exponent of 2, which they argued suggested homogeneity of their hydrogel network structure.31
The Lorentzian exponent, m, for all of 35K and 12K tetra-functional PEG hydrogels was less than or equal to 2, indicating that the polymer chains in the system are behaving as though in a good solvent. The 12K hydrogel with an initial polymer concentration of 0.143 g/mL is again an outlier, with a Lorentzian exponent slightly greater than 2 indicating a state between theta and bad solvent. The outlying values obtained for both the 12K and 35K systems at a single concentration are difficult to understand. Qualitatively, the spectra from these gels do not appear significantly different than the rest of the gels in the series. Also, mechanical and swelling data support the fact that these systems are very reproducible.36 However, these fitting results could indicate slight variations between samples on a level that would not be detected in a study of the macroscopic properties of these systems.
The Lorentzian screening length, or gel mesh size, decreased with increasing initial polymer concentration as expected, and with values of 3.7 nm ≤ ξL ≤ 13.0 nm and 2.1 nm ≤ ξL ≤ 6.3 nm respectively for 35K and 12K. We can compare this mesh size to the end-to-end distance of 35K and 12K PEG chains. Assuming a random walk Gaussian chain confirmation, the end-to-end distance can be calculated as:
| (3) |
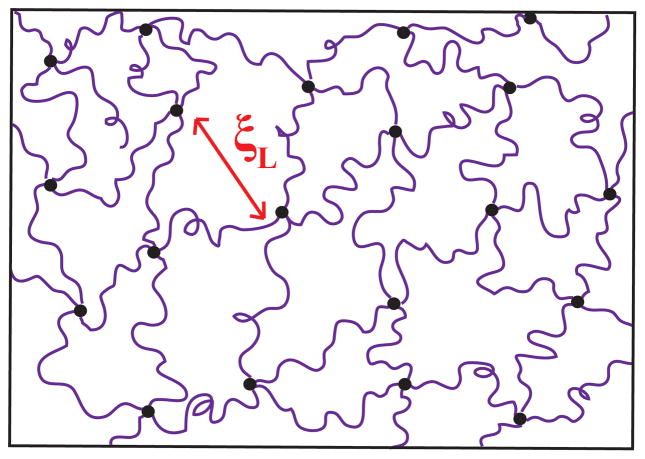
where ro is the end-to-end distance, b is the Kuhn length for PEG (0.76 nm), N is the number of Kuhn segments,52 and v1 is the scaling exponent (equal to 0.5 for an ideal Gaussian chain modeled as a random walk).53 Using eq. 2, we estimate ro = 14.5 nm and ro = 8.5 nm for a 35K and 12K PEG chain in an ideal Gaussian chain conformation. The correlation length found through the model fit is similar to the estimated ro at low concentrations and decreases with increasing polymer concentration, as we would expect. We speculate that the chains are in an environment similar to a semi-dilute solution. Classic work by de Gennes54 supports the idea that the correlation length, ξL, in homogeneous gels should be equal to or less than size of the average mesh, and should not differ significantly from the correlation length of a polymer solution at the same concentration.40 A schematic of the 35K and 12K tetra-functional PEG network structure is shown in Figure 6.
Figure 6.
Representation of 12K tetra-functional PEG hydrogel network, net-like mesh structure with minimal inhomogeneities. ξL indicates the Lorentzian screening length.
Results for 8K and 4K Tetra-functional Hydrogels
Scattering spectra from both the 8K and 4K tetra-functional hydrogels contain an additional broad shoulder that becomes more well-defined at higher concentrations, and therefore the correlation length model could not be used to fit the entire scattering specta. We hypothesize that at low concentrations the presence of two shoulders indicates two correlation lengths; the first corresponding to the formation of relatively hydrophobic domains of cross-linker and chain ends dispersed in a hydrophilic gel matrix. As the polymer concentration is increased, the second soulder becomes more pronounced. In the case of the 4K system, this shoulder transitions into a well-defined peak. This indicates the presence of a sharp boundary between domains that corresponds to a specific d-spacing.
To elicit more information about these structural changes, we chose to fit the shoulder that appears at low q for all spectra with the correlation length model (Figure 7). The background (calculated from Eq. 1), was held constant during the fitting process for consistency. In spectra that contain a well defined peak, the position of the peak maxima was determined and related to a d-spacing according to the relation d = 2π/q. The values obtained from the model fit and d-spacing analysis can be seen in Table 2.
Figure 7.
8K and 4K tetra-functional PEG hydrogels at varying initial polymer concentrations. Symbols indicate scattering data, while solid lines indicate the fit of the correlation length model the data. The dashed lines indicate the portion of the model that was not fit to the data. Spectra have been shifted for clarity.
Table 2.
Results of the fit of the correlation length model to 8K and 4K PEG tetra-functional hydrogels.
| Parameters | 8K PEG | ||||
|---|---|---|---|---|---|
|
| |||||
| C0 (g/mL) | 0.077 | 0.10 | 0.143 | 0.25 | 0.50 |
| n | 2.6 ± 0.08 | 2.5 ± 0.13 | 2.6 ± 0.09 | 2.6 ± 0.10 | 2.3 ± 0.10 |
| ξL (nm) | 4.0 ± 0.10 | 3.4 ± 0.09 | 3.0 ± 0.08 | 2.8 ± 0.11 | 2.6 ± 0.20 |
| m | 1.9 ± 0.05 | 1.9 ± 0.12 | 1.7 ± 0.12 | 1.6 ± 0.17 | 1.5 ± 0.33 |
| Bkg (1/cm)* | 0.08 | 0.08 | 0.08 | 0.08 | 0.10 |
| Peak Position (Å−1) | - | - | - | 0.051 | 0.055 |
| d-spacing (nm) | - | - | - | 12.3 | 11.4 |
| Parameters | 4K PEG | ||||
|---|---|---|---|---|---|
|
| |||||
| C0 (g/mL) | 0.077 | 0.10 | 0.143 | 0.25 | 0.50 |
| n | 2.9 ± 0.1 | 2.6 ± 0.12 | 2.9 ± 0.05 | 2.4 ± 0.18 | 2.2 ± 0.10 |
| ξL (nm) | 3.2 ± 0.03 | 2.6 ± 0.03 | 1.9 ± 0.01 | 1.4 ± 0.34 | 1.4 ± 0.18 |
| m | 2.0 ± 0.03 | 2.0 ± 0.05 | 2.1 ± 0.08 | 2.1 ± 0.75 | 2.2 ± 0.52 |
| Bkg (1/cm)* | 0.08 | 0.07 | 0.08 | 0.08 | 0.09 |
| Peak Position (Å−1) | - | - | 0.068 | 0.070 | 0.074 |
| d-spacing (nm) | - | - | 9.2 | 9.0 | 8.5 |
As expected, the Lorentzian screening length, ξL, decreased with increasing initial polymer concentration and ranged from 2.6 nm ≤ ξL ≤ 4.0 nm for the 8K series and 1.4 nm ≤ ξL ≤ 3.2 nm for the 4K series. These values were also consistently smaller the end-to-end distance of 8K and 4K PEG chains assuming a random walk Gaussian chain confirmation (4K PEG = 4.9 nm, 8K PEG = 7.0 nm). The Lorentzian exponents indicate good solvent for the 8K system and theta to poor solvent for the 4K system. This further supports our hypothesis that the features seen at low q describe the structure of the swollen gel network of these systems.
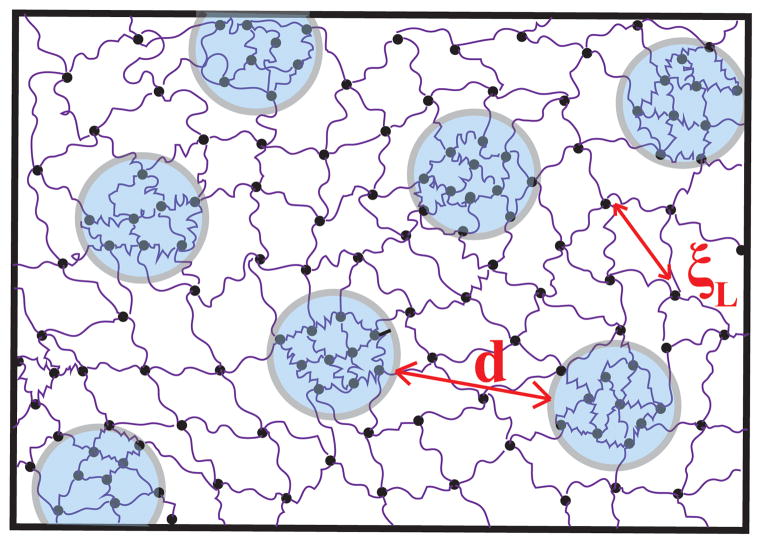
The d-spacing found for both systems decreased as the initial polymer concentration increased, ranging from 12.3 to 11.4 nm in the 8K system and 9.2 to 8.5 nm in the 4K system. This supports our theory that these structural changes are due to phase separation. The increase in polymer concentration corresponds to an increase in the hydrophobic content (e.g. norbornene end-groups and cross-linker). Therefore, it is expected that these hydrophobic domains would grow in size and the distance between them would decrease as the concentration of polymer in the system is increased. The d-spacings are smallest in the 4K systems, likely due to the higher ratio of hydrophobic to hydrophilic contant in these networks. This is also supported by the well-defined peak present in these spectra, which suggests a sharp transition between hydrophobic domains and hydrophilic gel matrix. A depiction of the resulting network structures of these systems is shown in Figure 8.
Figure 8.
Representation of the 4K and 8K tetra-functional hydrogel network in D2O. The two-phase, net-like mesh structure that occurs in the water swollen network contains phase-separated regions (indicated by the circles)separated by a characteristic length scale, d. The mesh size in these networks is denoted by ξL.
Results for Tetra-functional PEG Hydrogels Swollen with Deuterated N,N-dimethylformamide
The spectra obtained for hydrogels swollen with deuterated N,N-dimethylformamide (d-DMF) were all fit with the correlation length model, which yields a mesh size and information about chain conformation. Results of these fits are shown in Figure 9 and Table 3. There was a good agreement between the model fit and the data for all d-DMF networks, which was indicated by reduced χ2 values of 2.2 or less. However, the model fit to the 4K system does not capture the shoulder in the low q region even though the reduced χ2 value of 2.0 indicates a good agreement.
Figure 9.
Correlation length model fits to tetra-functional PEG networks swollen with d-DMF. Open symbols represent the scattering spectra, while the model fit is indicated by the solid line. Spectra have been shifted for clarity.
Table 3.
Results of correlation length model fits to tetra-functional PEG networks swollen in D2O and d-DMF.
| 35K Tetra-functional PEG Gel, C0 = 0.33 g/mL
| ||
|---|---|---|
| Parameter | D2O | d-DMF |
| n | 1.4 ± 0.03 | 1.7 ± 0.04 |
| ξL (nm) | 2.9 ± 0.1 | 4.5 ± 0.2 |
| m | 1.8 ± 0.02 | 1.5 ± 0.01 |
| Bkg (1/cm) | 0.09 ± 2e-4 | 0.32 ± 4e-4 |
| 12K Tetra-functional PEG Gel, C0 = 0.50 g/mL
| ||
|---|---|---|
| Parameter | D2O | d-DMF |
| n | 2.3 ± 0.02 | 2.2 ± 0.5 |
| ξL (nm) | 2.1 ± 0.01 | 4.0 ± 0.06 |
| m | 2.0 ± 0.005 | 1.6 ± 0.01 |
| Bkg (1/cm) | 0.11 ± 2e-4 | 0.27 ± 3e-4 |
| 8K Tetra-functional PEG Gel, C0 = 0.10 g/mL
| ||
|---|---|---|
| Parameter | D2O | d-DMF |
| n | 2.5 ± 0.13 | 3.3 ± 0.2 |
| ξL (nm) | 3.4 ± 0.09 | 4.7 ± 0.05 |
| m | 1.9 ± 0.12 | 1.6 ± 0.01 |
| Bkg (1/cm) | 0.08 | 0.27 ± 2e-4 |
| 4K Tetra-functional PEG Gel, C0 = 0.10 g/mL
| ||
|---|---|---|
| Parameter | D2O | d-DMF |
| n | 2.6 ± 0.12 | 4.5 ± 0.8 |
| ξL (nm) | 2.6 ± 0.03 | 3.7 ± 0.03 |
| m | 2.0 ± 0.05 | 1.7 ± 0.01 |
| Bkg (1/cm) | 0.07 | 0.28 ± 2e-4 |
A comparison of the model fit results for networks swollen in D2O and d-DMF is shown in Table 3. The effect of solvent quality on network structure can most clearly be seen in the change in mesh size, ξL. As expected, all systems demonstrated an increase in mesh size in d-DMF when compared to D2O. The Lorentzian exponent for gels swollen in d-DMF is less than for gels swollen in D2O, indicating that d-DMF is a better solvent for the network.
However, the resulting d-DMF mesh sizes are still less than the predicted length of the same length PEG polymer in a random walk configuration (Table 4) even though they are thermodynamically behaving as though in a good solvent (indicated by a Lorentzian exponent, m, less than 2). This, in conjunction with the persistence of the upturn at low q for all spectra, indicates that there are still some inhomogeneities present in these networks.
Table 4.
Comparison of mesh size (lorentzian screening length) for tetra-functional PEG hydrogels in D2O and d-DMF, and comparison to calculated length of PEG macromer assuming a random walk confirmation.
| Mesh size (nm)
| ||||
|---|---|---|---|---|
| Solvent | 4K | 8K | 12K | 35K |
| C0 = 0.10 g/mL | C0 = 0.10 g/mL | C0 = 0.50 g/mL | C0 = 0.33 g/mL | |
| D2O | 2.9 | 3.6 | 2.1 | 2.9 |
| d-DMF | 3.7 | 4.7 | 4.0 | 4.5 |
|
| ||||
| Random Walk Length (nm) | 4.9 | 7.0 | 8.5 | 14.5 |
Conclusions
Tetra-functional chemically cross-linked 35K, 12K, 8K and 4K poly(ethylene glycol) (PEG) hydrogels with well-defined network structures were synthesized via photo-initiated thio-norbornene chemistry. A previous publication has discussed the highly resilient mechanical properties of these hydrogels, which suggest that they have more homogeneous network structures.1 Through qualitative analysis and model-fitting of SANS data, we have shown that the 35K and 12K tetra-functional PEG hydrogels have a remarkably homogeneous network structure with low junction functionality. SANS results from the 8K and 4K tetra-functional PEG hydrogels suggests a deviation from the homogeneous network structure seen in the 35K and 12K systems that we believe is primarily due to some segregation of the hydrophobic chain ends and cross-linker upon swelling the network in water. This effect becomes more significant as PEG chain length decreases and is supported by spectra of gels swollen in d-DMF, which show a much more homogeneous structure than the gels in D2O. However, there are still some small indications of inhomogeneity for the 8K and 4K networks even in d-DMF, suggesting a higher level of defect formation during cross-linking for these systems. Future investigations of the physical properties inherent to systems, such as diffusion, will aid to further confirm the network structures.
Acknowledgments
This work utilized facilities partially supported by the National Science Foundation under agreement no. DMR-0944772. We acknowledge the support of the National Institute of Standards and Technology, U.S. Department of Commerce, in providing the neutron research facilities used in this work. Support for EMS was provided by the NSF-funded IGERT in Cellular Engineering (DGE-0654128), support for DMG was provided by the NSF-funded IGERT program in Nanotechnology Innovation (DGE-0504485) and an NIH-sponsored Chemistry-Biology Interface Training Grant (National Research Service Award T32 GM08515), and support for SK was provided by NSF CBET 0853551.
References
- 1.Cui J, Lackey MA, Madkour AE, Saffer EM, Griffin DM, Bhatia SR, Crosby AJ, Tew GN. Biomacromolecules. 2012;13:584–588. doi: 10.1021/bm300015s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman D, Banu D, Natansohn A, Wang J. J App Polym Sci. 1991;42:1537–1550. [Google Scholar]
- 3.Agrawal SK, Sanabria-DeLong N, Jemian PR, Tew GN, Bhatia SR. Langmuir. 2007;23:5039–5044. doi: 10.1021/la063390x. [DOI] [PubMed] [Google Scholar]
- 4.Prestwich GD. J Control Release. 2011;155:193–199. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott GF, Huxley AF, Weisfogh T. J Mol Bio. 1965;13:791. [Google Scholar]
- 6.Weisfogh T. J Mol Bio. 1961;3:648. [Google Scholar]
- 7.Weber KT. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Alemany A, Compan V, Refojo MF. J Biomed Mater Res. 2002;63:319–325. doi: 10.1002/jbm.10186. [DOI] [PubMed] [Google Scholar]
- 9.Metters AT, Anseth KS, Bowman CN. J Phys Chem B. 2001;105:8069–8076. [Google Scholar]
- 10.Meyer WH. Advanced Materials. 1998:10. doi: 10.1002/(SICI)1521-4095(199804)10:6<439::AID-ADMA439>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Mullerplathe F, Vangunsteren WF. J Chem Phys. 1995:103. [Google Scholar]
- 12.Walker CN, Versek C, Touminen M, Tew GN. Acs Macro Lett. 2012;1:737–741. doi: 10.1021/mz300090m. [DOI] [PubMed] [Google Scholar]
- 13.Tarascon JM, Armand M. Nature. 2001;414:359–367. doi: 10.1038/35104644. [DOI] [PubMed] [Google Scholar]
- 14.Flory P. Principles of Polymer Chemistry. Cornell University Press; Ithaca, NY: 1953. [Google Scholar]
- 15.Waters DJ, Engberg K, Parke-Houben R, Hartmann L, Ta CN, Toney MF, Frank CW. Macromolecules. 2010;43:6861–6870. doi: 10.1021/ma101070s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin-Gibson S, Jones RL, Washburn NR, Horkay F. Macromolecules. 2005;38:2897–2902. [Google Scholar]
- 17.Malkoch M, Vestberg R, Gupta N, Mespouille L, Dubois P, Mason AF, Hedrick JL, Liao Q, Frank CW, Kingsbury K, Hawker CJ. Chem Commun. 2006:2774–2776. doi: 10.1039/b603438a. [DOI] [PubMed] [Google Scholar]
- 18.Nimmo CM, Shoichet MS. Bioconjugate Chemistry. 2011;22:2199–2209. doi: 10.1021/bc200281k. [DOI] [PubMed] [Google Scholar]
- 19.Binder WH, Sachsenhofer R. Macromol Rapid Comm. 2008;29:952–981. [Google Scholar]
- 20.Ossipov DA, Hilborn J. Macromolecules. 2006:39. [Google Scholar]
- 21.Shibayama M, Takahashi H, Nomura S. Macromolecules. 1995;28:6860–6864. [Google Scholar]
- 22.Mendes E, Hakiki A, Herz J, Boue F, Bastide J. Macromolecules. 2004;37:2643–2649. [Google Scholar]
- 23.Mark JE, Sullivan JL. J Chem Phys. 1977;66:1006–1011. [Google Scholar]
- 24.Sullivan JL, Mark JE, Hampton PG, Cohen RE. J Chem Phys. 1978;68:2010–2012. [Google Scholar]
- 25.Mark JE, Rahalkar RR, Sullivan JL. J Chem Phys. 1979;70:1794–1797. [Google Scholar]
- 26.Llorente MA, Mark JE. J Chem Phys. 1979;71:682–689. [Google Scholar]
- 27.Jong L, Stein RS. Macromolecules. 1991;24:2323–2329. [Google Scholar]
- 28.Sakai T, Matsunaga T, Yamamoto Y, Ito C, Yoshida R, Suzuki S, Sasaki N, Shibayama M, Chung UI. Macromolecules. 2008;41:5379–5384. [Google Scholar]
- 29.Sakai T, Akagi Y, Matsunaga T, Kurakazu M, Chung U, Shibayama M. Macromol Rapid Comm. 2010;31:1954–1959. doi: 10.1002/marc.201000286. [DOI] [PubMed] [Google Scholar]
- 30.Matsunaga T, Sakai T, Akagi Y, Chung U, Shibayama M. Macromolecules. 2009;42:1344–1351. [Google Scholar]
- 31.Matsunaga T, Sakai T, Akagi Y, Chung UI, Shibayama M. Macromolecules. 2009;42:6245–6252. [Google Scholar]
- 32.Lu H, Carioscia JA, Stansbury JW, Bowman CN. Dent Mater. 2005;21:1129–1136. doi: 10.1016/j.dental.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Hoyle CE, Bowman CN. Angew Chem Int Edit. 2010;49:1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 34.Glinka CJ, Barker JG, Hammouda B, Krueger S, Moyer JJ, Orts WJ. J Appl Crystallogr. 1998;31:430–445. [Google Scholar]
- 35.Kline SR. J App Crystallogr. 2006;39:895–900. [Google Scholar]
- 36.Lackey MA. University of Massachusetts; Amherst: 2013. [Google Scholar]
- 37.Mendes E, Lutz P, Bastide J, Boue F. Macromolecules. 1995;28:174–179. [Google Scholar]
- 38.Sukumaran SK, Beaucage G, Mark JE, Viers B. Eur Phys J E. 2005;18:29–36. doi: 10.1140/epje/i2005-10030-x. [DOI] [PubMed] [Google Scholar]
- 39.Hammouda B, Ho DL, Kline S. Macromolecules. 2004;37:6932–6937. [Google Scholar]
- 40.Mendes E, Lindner P, Buzier M, Boue F, Bastide J. Phys Rev Lett. 1991;66:1595–1598. doi: 10.1103/PhysRevLett.66.1595. [DOI] [PubMed] [Google Scholar]
- 41.Bastide J, Leibler L. Macromolecules. 1988;21:2647–2649. [Google Scholar]
- 42.Hammouda B, Ho D, Kline S. Macromolecules. 2002;35:8578–8585. [Google Scholar]
- 43.Dudowicz J, Freed KF, Douglas JF. ACS Macro Lett. 2012;1:88–91. doi: 10.1021/mz200101p. [DOI] [PubMed] [Google Scholar]
- 44.Shinohara Y, Kayashima K, Okumura Y, Zhao CM, Ito K, Amemiya Y. Macromolecules. 2006;39:7386–7391. [Google Scholar]
- 45.Karino T, Shibayama M, Okumura Y, Ito K. Physica B. 2006;385:807–809. [Google Scholar]
- 46.Kobayashi M, Yoshioka T, Kozasa T, Tashiro K, Suzuki J, Funahashi S, Izumi Y. Macromolecules. 1994;27:1349–1354. [Google Scholar]
- 47.Tada T, Matsumoto T, Masuda T. Carbohyd Polym. 1999;39:53–59. [Google Scholar]
- 48.Kanaya T, Ohkura M, Takeshita H, Kaji K, Furusaka M, Yamaoka H, Wignall GD. Macromolecules. 1995;28:3168–3174. [Google Scholar]
- 49.Hule RA, Nagarkar RP, Altunbas A, Ramay HR, Branco MC, Schneider JP, Pochan DJ. Faraday Discuss. 2008;139:251–264. doi: 10.1039/b717616c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hule RA, Nagarkar RP, Hammouda B, Schneider JP, Pochan DJ. Macromolecules. 2009;42:7137–7145. doi: 10.1021/ma9003242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammouda B, Horkay F, Becker ML. Macromolecules. 2005;38:2019–2021. [Google Scholar]
- 52.Mark JE, Flory PJ. J Am Chem Soc. 1965;87:1415. [Google Scholar]
- 53.Rubinstein M, Colby RH. Polymer Physics. Oxford University Press; New York: 2003. [Google Scholar]
- 54.de Gennes PG. Scaling Concepts in Polymer Physics. Cornell University Press; Ithaca, NY: 1979. [Google Scholar]