Abstract
CD4+CD25hiCD127lo/− regulatory T cells (Treg) have been implicated in the resolution of asthma-associated inflammation while the opposite role of CD4+ invariant NKT (iNKT) cells has been the subject of recent investigations. Studies here focused on mechanisms of interaction between CD4+ iNKT cells and Treg to further explore their roles in allergic asthma (AA). Flow cytometry analysis revealed a significant increase in the expression of the natural cytotoxicity receptors NKp30 and NKp46 by CD4+ iNKT cells in AA subjects compared to healthy controls (HC) and non-allergic asthmatics (NA). Subsequent intracellular staining showed that CD4+ iNKT cells also expressed higher levels of granzyme B and perforin in AA than HC. In in vitro killing assays, AA CD4+ iNKT cells selectively killed autologous Treg, but not CD4+CD25− T cells, more potently than HC and NA counterparts. This increased cytotoxicity positively correlated with asthma severity and granzyme B/perforin expression of CD4+ iNKT cells. Furthermore, it could be abrogated by either inhibition of the granzyme B-/perforin-dependent cell death pathway or oral corticosteroid administration. Altogether, these findings suggest that increased cytotoxicity of CD4+ iNKT cells against Treg might contribute to dysfunctional cellular interactions in AA.
Keywords: Granzyme B, Invariant NKT cells, Natural cytotoxicity receptors, Perforin, Regulatory T cells
Introduction
Allergic asthma (AA) is a chronic inflammatory disease of the airways [1], generally associated with hyperactive responses of CD4+ T cells [2]. However, recent findings have suggested that dysfunctional regulation of CD4+ T cell activities by naturally occurring regulatory T cells (Treg) [3,4] also plays a key role in AA pathobiology. Treg are CD4+CD25hiCD127lo/− T cells that chiefly involve in immune suppression in the periphery. They express FoxP3, a transcription factor associated with their function and development [5, 6]. In human AA, Treg numbers decrease in bronchial alveolar lavage fluid [7]. They also do not suppress the proliferation and cytokine production of allergen-stimulated CD4+ T cells in AA subjects as well as in healthy individuals [7–9]. Furthermore, several murine studies have demonstrated that induction of Treg function reverses airway hyper-responsiveness [10,11] and protects against experimentally induced asthma [12, 13]. Collectively, these findings suggest a role of Treg in controlling Th2 inflammation in AA.
In contrast to the accepted immunosuppressive role of Treg in AA, the involvement of CD4+ invariant NKT (iNKT) cells in the disease has been a subject of controversy. Human iNKT cell repertoire consists of CD4+, CD8+, and CD4−CD8− cells, among which CD4+ iNKT cells account for approximately 50% of total iNKT cells [14]. Despite the phenotypic and functional heterogeneity of these subsets [14, 15], they all express the highly restricted Vα24 region of the TCR and respond to glycolipid antigens presented by the non-polymorphic MHC-like molecule CD1d [16, 17].
In murine asthmatic models, earlier studies demonstrated that glycolipid activation of iNKT cells is essential for the initiation of airway hyper-responsiveness [18]. iNKT cell-deficient mice do not develop airway hyper-responsiveness and the restoration of this cardinal feature of asthma could be induced by adoptive transfer of iNKT cells [19]. However, a more recent report showed that iNKT cells play only a minimal role in orchestrating allergic airway inflammation [20]. In human, selective ex vivo activation of CD4+CD161+ T cells, which include CD4+ iNKT cells, via IL-4/STAT6 signal transduction pathway occurs in peripheral blood of AA subjects but not in healthy individuals [21]. Frequency of CD4+ iNKT cells in bronchoalveolar lavage fluid of AA subjects also increased compared to healthy individuals [22]. However, there have been discrepancies on the enumeration of CD4+ iNKT cells in asthmatic airways [23–25]. These discordant results not only call into question the role of CD4+ iNKT cells in AA, but also implicate the complexity of their mechanisms of action in the modulation of inflammatory responses.
Synergistic and antagonistic interactions between iNKT cells and Treg have recently been reported [26]. IL-2 produced by activated iNKT cells affects the proliferation of Treg [27]. On the other hand, Treg have been shown to possibly suppress proliferation and cytokine production of iNKT cells [28]. No evidence of interaction between Treg and iNKT cells has been shown in AA to date. However, these cells both express high levels of CCR4 and CCR8, two Th2-associated chemokine receptors critical for lung migration [29, 30], suggesting the possibility of their co-localization and interaction during airway inflammation. Moreover, we have observed an increase in expression of granzyme B, a cytotoxic enzyme, in CD4+ iNKT cells compared to Treg (Supporting Information Fig. 1), suggesting that iNKT cells could possibly kill Treg upon their direct interaction. Studies here report a potentially important dysfunctional interaction between these two subsets in which Treg serve as targets for CD4+ iNKT cell-mediated cytotoxicity in AA subjects.
Results
Increased NKp30 and NKp46 expressions in allergic asthmatic CD4+ iNKT cells
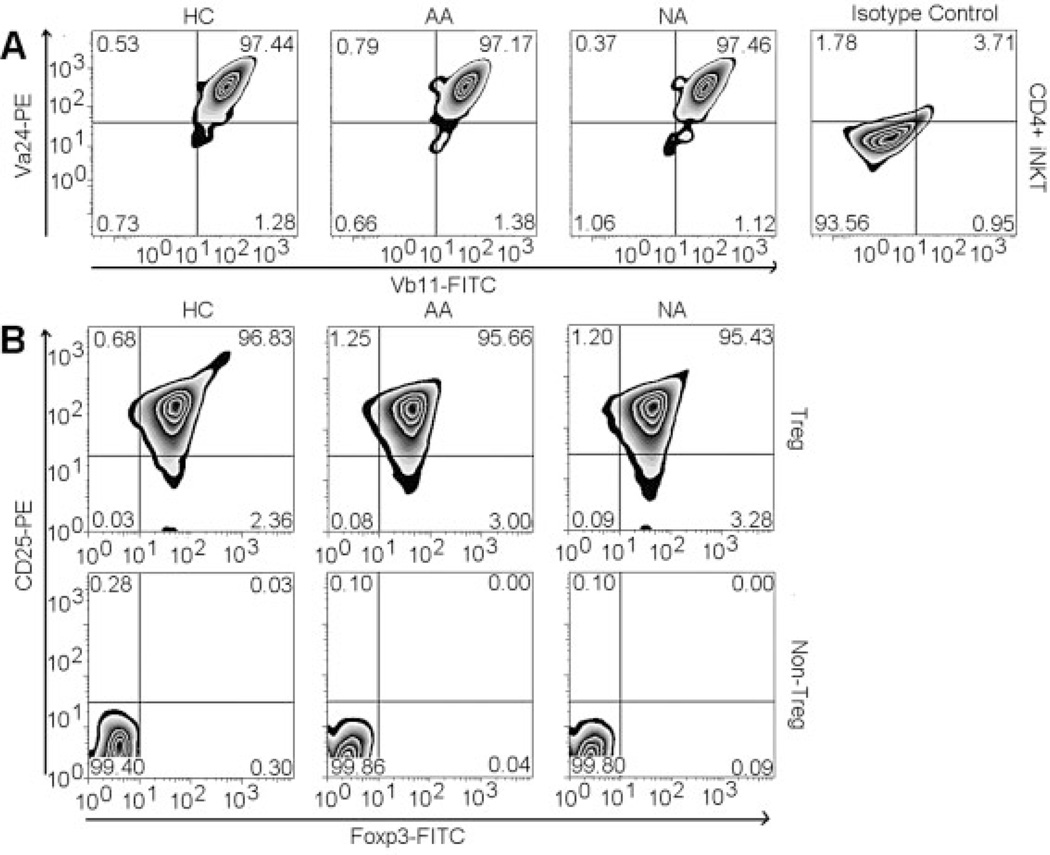
Subjects included 18 healthy controls (HC), 21 mild-to-severe AA, and seven non-allergic asthmatics (NA) (Table 1). CD4+ T cells were purified with a CD4+ Rosette kit. From this CD4+ T cell fraction, CD4+ iNKT cells were isolated with antibody recognizing the CDR3 region of the invariant (Vα24JαQ) TCR chain (clone 6B11) of iNKT cells by magnetic isolation. The purity of CD4+ iNKT cell fraction was confirmed to be higher than 95% with Vα24 and Vβ11 antibodies recognizing a TCR region distinct from the binding site of 6B11 antibody [15] (Fig. 1A).
Table 1.
Subject demographics
| Patient | Age (years) |
Asthma Duration (years) |
Severity | Medication | IgE (IU/ml) | Allergic Status | FEV1 |
|---|---|---|---|---|---|---|---|
| AA1 | 56 | 15 | Severe | ICS, Albuterol | 245 | Tree, Mold | 52 |
| AA2 | 35 | 32 | Severe | ICS, Albuterol | 173 | Cat | 53 |
| AA3 | 37 | 34 | Severe | ICS, Albuterol | 2342 | Tree, Dust Mite | 54 |
| AA4 | 21 | 15 | Severe | ICS, Salmeterol | 1274 | Tree | 57 |
| AA5 | 18 | 17 | Severe | ICS, Salmeterol | 1274 | Tree | 57 |
| AA6 | 21 | 8 | Moderate | ICS, Albuterol | 207 | Grass, Mold | 71 |
| AA7 | 23 | 22 | Moderate | ICS, Salmeterol | 2361 | Weed, Dust mite | 73 |
| AA8 | 13 | 10 | Moderate | ICS, Salmeterol | 186 | Weed, Grass | 73 |
| AA9 | 20 | 16 | Moderate | ICS, Salmeterol | 1493 | Weed, Dust mite | 73 |
| AA10 | 32 | 27 | Moderate | ICS, Albuterol | 103 | Cat | 80 |
| AA11 | 15 | 5 | Mild | ICS, Salmeterol | 275 | Grass | 82 |
| AA12 | 53 | 1 | Mild | Albuterol | 179 | Cat | 82 |
| AA13 | 21 | 6 | Mild | Albuterol | 373 | Mold | 85 |
| AA14 | 45 | 6 | Mild | Albuterol | 84 | Dust mite, Grass | 87 |
| AA15 | 29 | 25 | Mild | Albuterol | 205 | Grass, Mold | 90 |
| AA16 | 18 | 2 | Mild | Albuterol | 455 | Dust mite | 90 |
| AA17 | 29 | 2 | Mild | Albuterol | 158 | Grass | 91 |
| AA18 | 19 | 5 | Mild | Albuterol | 508 | Weed | 92 |
| AA19 | 38 | 36 | Mild | Albuterol | 292 | Tree, Grass | 93 |
| AA20 | 17 | 11 | Mild | Albuterol | 141 | Weed, Dog | 95 |
| AA21 | 28 | 5 | Mild | Albuterol | 210 | Dust mite, Grass | 96 |
| 27.9 ± 2.7 | 14.4 ± 2.4 | 597 ± 155.5 | 77.4 ± 3.3 | ||||
| NA1 | 42 | 21 | Moderate | ICS, Albuterol | less than 50 | n/a | 69 |
| NA2 | 44 | n/a | Moderate | Albuterol | less than 50 | n/a | 85 |
| NA3 | 45 | 25 | Mild | Albuterol | less than 50 | n/a | 85 |
| NA4 | 55 | n/a | Mild | Albuterol | less than 50 | n/a | 88 |
| NA5 | 25 | 5 | Mild | Albuterol | less than 50 | n/a | 95 |
| NA6 | 38 | n/a | Mild | Albuterol | less than 50 | n/a | 92 |
| NA7 | 19 | n/a | Mild | Albuterol | less than 50 | n/a | 91 |
| 38.3 ± 4.7 | 17.0 ± 6.1 | less than 50 | 86.4 ± 3.2 |
Figure 1.
Purity of Treg and CD4+ iNKT cells. (A) Expression of Vα24 and vβ11 by purified CD4+ iNKT cells in representative HC, AA, and NA samples. (B) Expression of CD25 and FoxP3 by purified Treg and non-Treg in representative HC, AA, and NA samples.
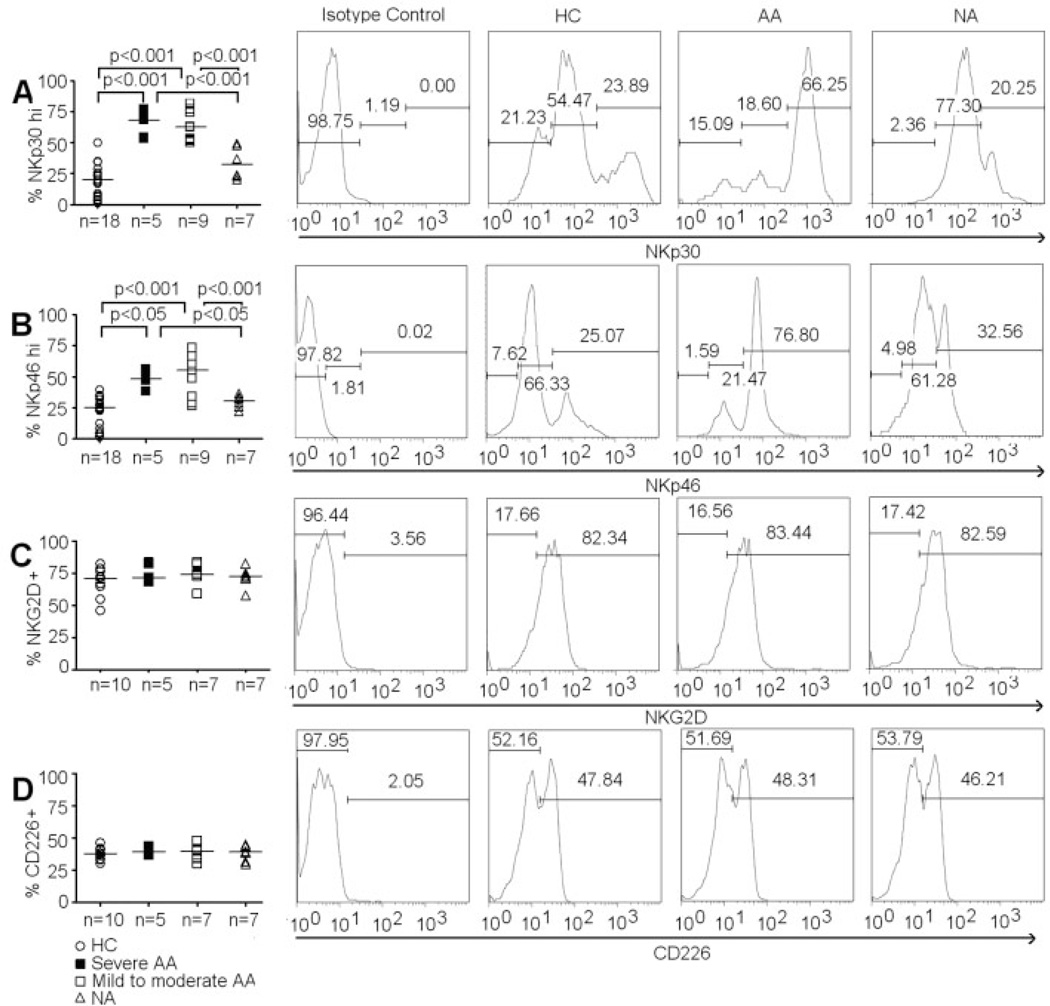
Phenotype of CD4+ iNKT cells was first examined among subject groups with respect to NKp30 and NKp46 expression. Negative thresholds were derived from samples of CD4+ iNKT cells stained with specific isotype antibodies as recommended by the manufacturers. CD4+ iNKT cells appeared to be a heterogeneous population with differential expression of NKp30 and NKp46 (Fig. 2A and B). Thresholds to identify cell subsets with respect to high and low expression of NKp30 and NKp46 were applied consistently to all samples. Significantly higher frequencies of CD4+NKp30hi iNKT cells and CD4+NKp46hi iNKT cells were found in both severe and mild-to-moderate AA compared to HC (Fig. 2A and B). Interestingly, we did not observe an increased expression of these natural cytotoxicity receptors in NA subjects compared to HC (Fig. 2A and B).
Figure 2.
Expression of activating receptors on CD4+ iNKT cells. (A) Percentage of CD4+ iNKT cells that are NKp30hi. (B) Percentage of CD4+ iNKT that are NKp46hi. (C) Percentage of CD4+ iNKT cells that express NKG2D. (D) Percentage of CD4+ iNKT cells that express CD226. Histograms of NKp30, NKp46, NKG2D, and CD226 expressions on CD4+ iNKT cells in representative HC, AA and NA samples. ANOVA was used for statistical analysis. p values represent results from post-ANOVA comparison. Horizontal bars represent median values.
We also examined the expression of other NK receptors such as NKG2D and CD226 in CD4+ iNKT cells. Unlike NKp30 and NKp46, there was an absence of the low- and high-density subsets with respect to expression of NKG2D and CD226. We did not find any significant differences in the percentages of CD4+NKG2D+ iNKT cells and CD4+CD226+ iNKT cells among HC, AA, and NA (Fig. 2C and D). Additionally, we did not observe any differences in expression of NKp30, NKp46, NKG2D, and CD226 between severe and mild-to-moderate AA subjects.
Increased granzyme B and perforin expression in allergic asthmatic CD4+ iNKT cells
Concurrent up-regulation of NKp30 and NKp46 has been associated with increased cellular activation [31]. Conversely, reduced expression of these natural cytotoxicity receptors has also been associated with decreased lysis of targets by effector cells [32, 33]. Therefore, the specific distributional increase in CD4+NKp30hi iNKT cells and CD4+NKp46hi iNKT cells in AA subjects raised the possibility that CD4+ iNKT cells have increased cytotoxicity in these subjects.
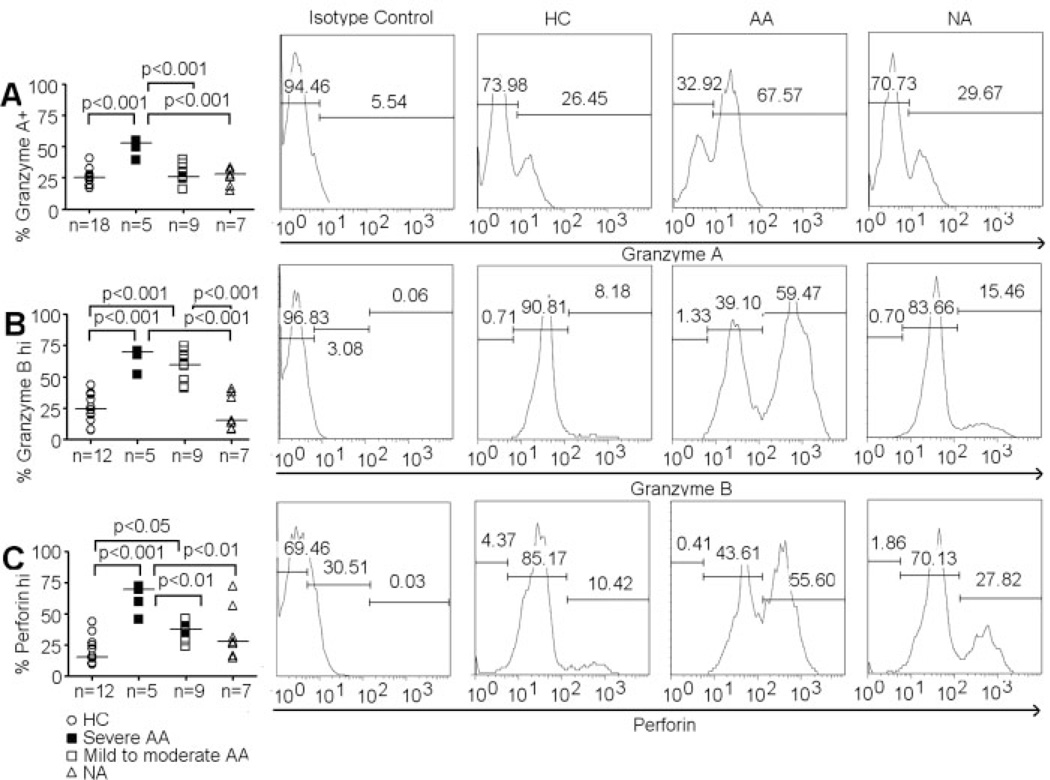
We first examined the expression of several cytotoxic molecules in CD4+ iNKT cells. Expressions of granzyme A, granzyme B, and perforin were evaluated via intracellular staining. We found two subsets of low and high expression for granzyme B and perforin, but not granzyme A, in CD4+ iNKT cells (Fig. 3A – C). Severe AA subjects had significantly higher CD4+granzyme A+ iNKT cells and CD4+perforin+ iNKT cells than mild-to-moderate AA subjects as well as HC and NA subjects (Fig. 3A and C). In addition, mild-to-moderate AA subjects had significantly higher percentages of CD4+perforinhi iNKT cells than HC (Fig. 3C). Percentages of CD4+granzyme Bhi iNKT cells also significantly increased in both severe and mild-to-moderate AA subjects compared to HC and NA subjects (Fig. 3B). However, no differences in CD4+granzyme Bhi iNKT cell distribution were observed between severe and mild-to-moderate AA subjects.
Figure 3.
Expression of cytotoxic molecules on CD4+ iNKT cells. (A) Percentage of CD4+ iNKT cells that express granzme A. (B) Percentage of CD4+ iNKT cells that are granzyme Bhi. (C) Percentage of CD4+ iNKT cells that are perforinhi. Histogram of granzyme A, granzyme B, and perforin expressions by CD4+ iNKT cells in representative HC, AA and NA samples. ANOVA was used for statistical analysis. p values represent results from post-ANOVA comparison. Horizontal bars represent median values.
Increased cytotoxicity of CD4+ iNKT cells against autologous Treg in allergic asthmatic subjects
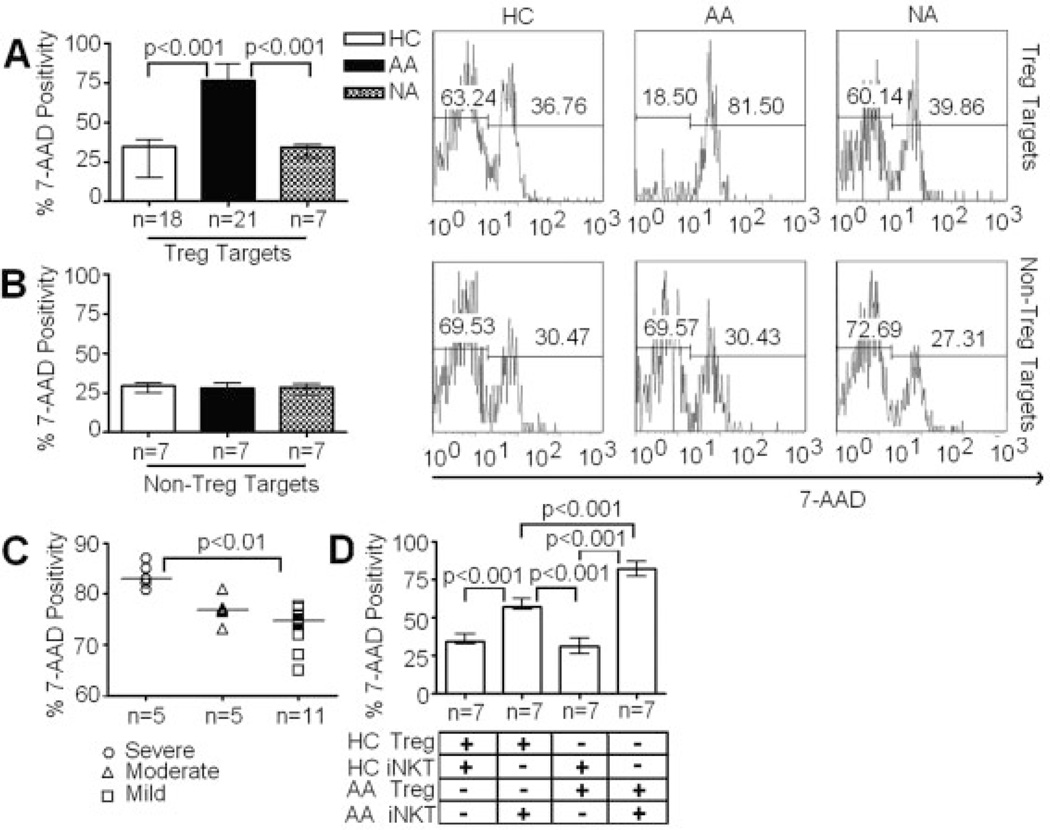
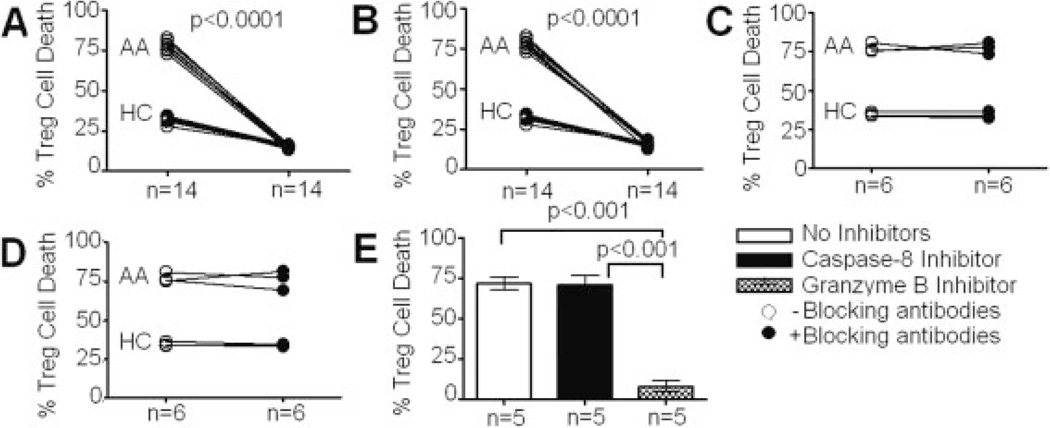
These increases in expression of activating receptors and cytotoxic enzymes in CD4+ iNKT cells from AA subjects prompted us to examine their in vitro cytotoxicity using Treg and CD4+CD25− T cells (non-Treg) as targets via a flow-based killing assay [34]. Treg and non-Treg were purified via flow sorting and their purities were confirmed to be more than 95% and 99%, respectively (Fig. 1B). Treg and non-Treg targets were activated with IL-2 at 10 IU/mL for 24 h before subjected to CFSE labeling for killing assays. Preliminary titration and kinetic experiments using 1:1, 5:1, and 10:1 ratios of CD4+ iNKT cells to Treg at 1-, 4-, and 8-h intervals showed that optimal killing activities occurred at 10:1 ratio in 4-h killing assays (data not shown). Thus, this experimental condition was used to evaluate killing activity of CD4+ iNKT cells. The analysis showed that CD4+ iNKT cells killed autologous Treg with significantly higher potency in AA than both HC and NA subjects (Fig. 4A).
Figure 4.
Cytotoxicity of CD4+ iNKT cells against autologous and allogeneic Treg and non-Treg targets. (A) Percentages of target cell death in killing assays of CD4+ iNKT cells against autologous Treg targets. (B) Percentages of target cell death in killing assays of CD4+ iNKT cells against autologous non-Treg targets. (C) Percentages of target cell death in killing assays of CD4+ iNKT cells against autologous Treg targets from different subsets of AA subjects with respect to disease severity. (D) Percentages of target cell death in killing assays of CD4+ iNKT cells against allogeneic Treg targets. Flow cytometric analysis of killing assays at 10:1 ratio of CD4+ iNKT cells to Treg or non-Treg targets. Cell populations represented in histograms were CSFE+ target cells. Cell death in each killing assay was determined by percentage of 7-aminoactinomycin D+CFSE+ cells out of total CSFE+ cells. ANOVA was used for statistical analysis. p values represent results from post-ANOVA comparison. Bar graph represents median values and ranges. Horizontal bars represent median values.
Killing assays of autologous non-Treg by CD4+ iNKT cells were also performed. Surprisingly, there were no significant differences in cytotoxicity of CD4+ iNKT cells against autologous non-Treg between HC, AA, and NA (Fig. 4B), suggesting that the increase in cytotoxicity of CD4+ iNKT cells in AA subjects is specific to Treg. Furthermore, cytotoxicity of CD4+ iNKT cells in severe AA subjects was significantly higher than in mild-to-moderate AA subjects (Fig. 4C).
In addition to CD4+ iNKT cells, we have examined function and phenotype of CD4− iNKT cells. We found no significant differences in cytotoxicity against Treg as well as the expressions of NKp30, NKp46, granzyme B, and perforin in CD4− iNKT cells between AA and HC (Supporting Information Fig. 2). Thus, increased cytotoxicity in AA subjects appears to be associated with the CD4+ iNKT cell population.
We next performed allogeneic killing assays to examine whether the increase in killing of CD4+ iNKT cells against Treg in AA resulted from the increased cytotoxicity of CD4+ iNKT cells alone, or other factors such as an increased susceptibility of Treg to CD4+ iNKT cell-mediated killing. We paired CD4+ iNKT cells from five severe and two moderate AA subjects with allogeneic Treg from seven HC and vice versa in these killing assays. Allogeneic killing assays showed that both AA Treg and HC Treg were killed more potently by AA CD4+ iNKT cells than by their HC counterparts (Fig. 4D). Furthermore, AA CD4+ iNKT cells killed autologous (AA) Treg more potently than they killed HC Treg. Altogether, these findings not only confirmed that AA CD4+ iNKT cells were more cytotoxic than those from HC, but also suggested that AA Treg were more vulnerable to CD4+ iNKT cell-mediated killing than HC Treg.
Correlation among cytotoxic potentials of CD4+ iNKT cells and clinical parameters of asthma
Using Spearman tests, we found significant positive correlation between expression of granzyme B and perforin in CD4+ iNKT cells and Treg cell death percentage in killing assays (Table 2). Expression of NKp30 and NKp46 did not significantly correlate with Treg cell death percentages. We also examined possible correlations of CD4+ iNKT cell-mediated killing of Treg with clinical parameters of asthma such as patient age, disease duration, age at onset, IgE levels, and forced expiratory volume in 1 s (FEV1). We did not find any significant correlations of IgE levels and patient age with Treg cell death. However, FEV1, and age at onset negatively correlated with Treg cell death; while disease duration positively correlated with Treg cell death (Table 2).
Table 2.
Correlation of cytotoxic potentials of CD4+ iNKT cells with clinical parameters of asthmaa)
| Treg Cell Death vs. | R values | P values | Summary |
|---|---|---|---|
| Granzyme A Expression | 0,4505 | 0,1059 | |
| Granzyme B Expression | 0,6308 | 0,0156 | * |
| Perforin Expression | 0,6615 | 0,01 | * |
| NKp30 Expression | 0,3714 | 0,191 | |
| NKp46 Expression | 0,03297 | 0,9109 | |
| Age | 0,1386 | 0,5491 | |
| Age at Onset | −0,4963 | 0,0221 | * |
| Asthma Duration | 0,5473 | 0,0102 | * |
| IgE Levels | 0,2735 | 0,2303 | |
| FEV1 | −0,6979 | 0,0004 | *** |
*p<0.05; ***p<0.001
Blocking granzyme B and perforin abrogated killing of Treg by CD4+ iNKT cells
CD4+ iNKT cell-mediated cytotoxicity is a complex phenomenon consisting of several effector molecules directly involved in triggering cell death. Two main pathways of iNKT cell-mediated cell death have been characterized: the granule exocytosis pathway mediated by perforin and granzymes, and the death receptor pathway mediated by FasL [35]. Anti-cytotoxic enzyme and anti-cell death receptor blocking antibodies were successful in elucidating mechanisms of cell death in previous reports [36, 37]. Thus, we utilized these reagents to investigate CD4+ iNKT cell-mediated killing of Treg.
Since cytotoxic enzymes are released directly into a tight synapse between target and effector cells, blocking with low concentrations of antibodies is often inefficient. Indeed, by increasing antibody concentrations, inhibition of cytotoxicity of CD4+ iNKT cells showed significant improvement (Supporting Information Fig. 3A). Killing assays with granzyme B-blocking antibody at 1 µg/mL showed more than 70% and 50% reduction of Treg cell death in AA and HC, respectively (Fig. 5A). Others have shown that antibodies to perforin (clone dG9) were able to block cell lysis via inhibition of perforin binding to target cell surface [36]. Similarly, we performed killing assays with blocking antibody against perforin and also found a significant reduction in Treg cell death (Fig. 5B).
Figure 5.
Effects of inhibitors of cell death pathways on CD4+ iNKT cell cytotoxicity. (A) Effects of a granzyme B-blocking antibody in killing assays of CD4+ iNKT cells against Treg. (B) Effects of a perforin-blocking antibody in killing assays of CD4+ iNKT cells against Treg. (C) Effects of a FasL-blocking antibody in killing assays of CD4+ iNKT cells against Treg. (D) Effects of a low-endotoxin IgG1 antibody in killing assays of CD4+ iNKT cells against Treg. (E) Effects of a granzyme B inhibitor and a caspase-8 inhibitor in killing assays of CD4+ iNKT cells against Treg. Data were collected from equal numbers of HC and AA subjects for each of the first four panels. Paired t-tests were used for the first four panels. All AA subjects were used for the last panel. ANOVA was used for statistical analysis of the last panel, p values represent results from paired t-tests or post-ANOVA comparison. Bar graph represents median values and ranges.
To examine the possibility that CD4+ iNKT cell-mediated killing could occur via a FasL-dependent cell death pathway, we used blocking antibodies against FasL (clone 100409) in killing assays. In our laboratory, this antibody was confirmed to be able to neutralize serum-derived FasL-mediated apoptosis of neutrophils in juvenile idiopathic neutropenia (Supporting Information Fig. 3B). Anti-FasL antibodies used at the same concentration (1 µg/mL) showed no significant reduction in Treg killing (Fig. 5C). As a negative control for our killing assays, we used low-endotoxin IgG1 antibodies at the same concentration and did not find any reduction in Treg killing (Fig. 5D).
Further assays with small-molecule inhibitors of cell death pathways confirmed that CD4+ iNKT cell-mediated killing of Treg occurred via a granule exocytosis pathway. The results obtained with a potent granzyme B inhibitor (Z-AAD-CMK) showed a significant reduction of cell death of Treg by CD4+ iNKT cells (Fig. 5E). To confirm that the FasL pathway was not associated with CD4+ iNKT cell-mediated killing, a caspase-8 inhibitor (Z-IETD) was also introduced to killing assays [38], which indeed showed no significant decrease in Treg cell death (Fig. 5E). These findings confirmed that CD4+ iNKT cells killed Treg via a granzyme B-/perforin-dependent, not FasL-dependent, pathway.
Since NKT cytotoxicity is also regulated by natural cytotoxicity receptors [39], we performed killing assays with blocking antibodies against these molecules. Due to the lack of commercially available blocking antibodies against NKp46, only blocking experiments with neutralizing antibodies against NKp30 (clone P30-15) were performed. However, we did not find a significant reduction in the percentage of Treg cell death in either HC or AA subjects (Supporting Information Fig. 3C).
Oral corticosteroids abrogated the increased cytotoxicity of CD4+ iNKT cells in allergic asthmatic subjects
Corticosteroids have been shown to down-regulate NKp30 and NKp46 expression as well as the cytotoxicity of NK cells [40–42]. In our studies, several of 21 AA subjects, whose CD4+ iNKT cells were used in flow cytometric analysis of NKp30, NKp46, granzyme B, and perforin expression and killing assays, were receiving intermittent inhaled corticosteroids with little to no systemic absorption (Table 1). Thus, the indicated medication status might not best represent the effects of inhaled corticosteroids on immune cells in peripheral blood.
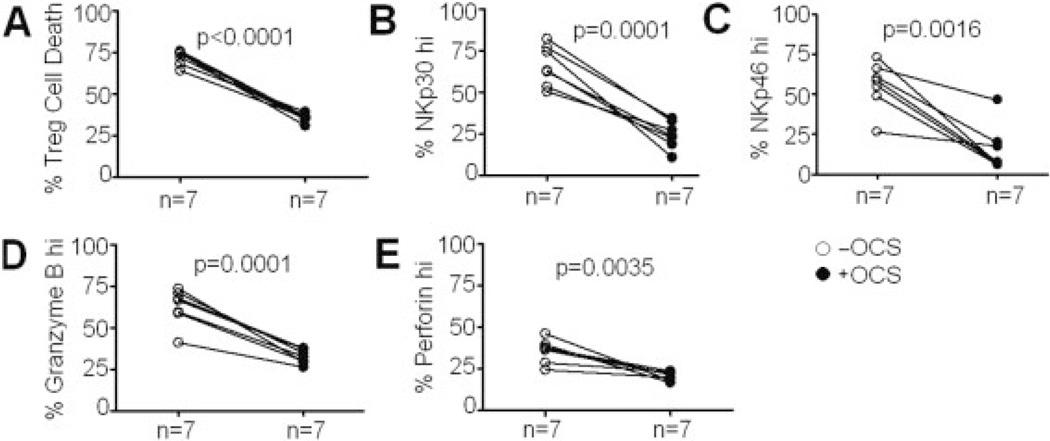
To address this issue, we followed up seven AA subjects – who experienced mild asthma symptoms and had previously been examined for CD4+ iNKT cell function in the absence of systemic oral corticosteroids (OCS) – with OCS during the longitudinal course of the study. Peripheral blood samples were drawn at day 2 of a 5-day OCS therapy course at 50 mg/day. Upon OCS administration, clinical symptoms of asthma were resolved by day 2 (data not shown). CD4+ iNKT cells and Treg were purified and co-cultured in killing assays as described above. CD4+ iNKT cells were then assessed for expression of NKp30, NKp46, granzyme B, and perforin. Compared to paired samples from the same subjects collected and analyzed prior to OCS administration, percentages of Treg death in killing assays as well as levels of CD4+NKp30hi, CD4+NKp46hi, CD4+granzyme Bhi, CD4+perforinhi iNKT cells were significantly reduced (Fig. 6). These findings confirmed the suppressive effects of corticosteroids on cytotoxic potentials of CD4+ iNKT cells.
Figure 6.
Modulation of cytotoxicity of CD4+ iNKT cells by OCS. (A) Effects of OCS on CD4+ iNKT cell-mediated cytotoxicity against autologous Treg in killing assays. (B) Effects of OCS on expression of NKp30 on CD4+ iNKT cells. (C) Effects of OCS on expression of NKp46 on CD4+ iNKT cells. (D) Effects of OCS on expression of granzyme B on CD4+ iNKT cells. (E) Effects of OCS on expression of perforin on CD4+ iNKT cells. Paired t-tests were used for statistical analysis, p values represent results from t-tests.
Discussion
Our analysis of surface receptor expression showed that AA CD4+ iNKT cells expressed higher levels of NKp30hi and NKp46hi cells than both HC and NA counterparts. Interestingly, an earlier study by Walzer et al. [43] investigating NKp46 expression in three HC found that only a minor fraction (less than 2%) of iNKT cells express NKp46 while this surface marker is expressed abundantly on NK cells. Our extensive studies of 18 HC also demonstrated that CD4+ iNKT cells from a subset of HC (n = 5) had low expression of NKp46 (from 1.82% to 4.71%). However, in general, our data showed that expression of NKp46 in CD4+ iNKT cells from 18 HC represents a broader spectrum (from 1.82% to 39.7%) (Fig. 2B). Since peripheral blood iNKT cells appear in low frequency, flow cytometric analysis of this rare subset, even after purification, could lead to varying results depending on the number of cells being analyzed. In our studies, we used purified iNKT cells and collected 20 000 iNKT cells during flow cytometric analysis to achieve statistical rigor. It is possible that a different flow cytometric acquisition threshold used in the study by Walzer et al. [43] led to this discrepancy.
To ensure that our purification protocol did not result in an in vitro up-regulation of these receptors on CD4+ iNKT cells, we analyzed expression of NKp30 and NKp46 on CD4+ iNKT cells in peripheral blood mononuclear cells before purification in 19 AA, HC, and NA. We did not find any significant differences in expression of NKp30 and NKp46 in CD4+ iNKT cells between pre-purification and post-purification samples (Supporting Information Fig. 4). Altogether, these experiments demonstrated that human CD4+ iNKT cells express NKp30 and NKp46 and there is an increased expression of NKp30 and NKp46 in CD4+ iNKT cells from AA subjects.
Besides NKp30 and NKp46, we looked at expression of NKG2D and CD226 but did not find any differences among HC, AA, and NA. In contrast, an earlier study by Sen et al. [44] showed that CD226 mRNA expression is increased in iNKT cells from AA subjects. Since mRNA expression of a protein does not always correlate with its actual expression, it would be difficult to make a comparison to our protein analysis. Sen et al. [44] did examine expression of CD226 on iNKT cells at the protein level but did not perform statistical analysis to confirm that the difference between AA and HC is significant. Data presented here showed that CD4+ iNKT cells do express CD226. However, there are no statistically significant differences among CD4+ iNKT cells from HC, AA, and NA in their expression of CD226 (Fig. 2D).
We also found that granzyme B was significantly increased in CD4+ iNKT cells from both severe and mild-to-moderate AA subjects compared to HC and NA, confirming a previous finding on elevated levels of this cytotoxic enzyme in lymphocytes from AA subjects [45]. Only severe AA subjects expressed higher levels of granzyme A and perforin than HC and NA subjects (Fig. 3A and C).The results suggested potentially distinct regulatory mechanisms of granzymes and perforin expression in CD4+ iNKT cells during the course of inflammation in AA.
Our killing assays revealed increased cytotoxicity of CD4+ iNKT cells against Treg in AA compared to HC and NA subjects. This increase in cytotoxicity positively correlated with disease severity, suggesting a possible involvement of this iNKT cell-Treg interaction in the inflammatory cascade of AA. In addition, OCS treatment, which resolved cardinal features of asthmatic inflammation in seven AA subjects in our studies, abrogated the increased cytotoxicity of CD4+ iNKT cells against Treg. It is likely that reduced AA symptoms in these subjects resulted from the OCS-mediated down-regulation of CD4+ iNKT cell cytotoxicity against Treg. Alternatively, it has been proposed that CD4+ iNKT cells modulate and sustain Th2-mediated inflammation by conventional CD4+ T cells [46]. Thus, OCS could also affect CD4+ iNKT cell interaction with CD4+ T cells, leading to diminished clinical symptoms in AA subjects receiving this treatment. Experiments to evaluate effects of ex vivo OCS treatment on CD4+ iNKT cell function during their interaction with Treg and CD4+ T cells are being conducted to investigate these hypotheses.
In our studies, the 10:1 ratio of CD4+ iNKT cells to Treg in our killing assays does not reflect the physiological ratio of these cells in peripheral blood (please see Supporting Information Fig. 5 for enumeration data of iNKT cells and Treg in peripheral blood). However, such interaction could possibly occur in the actual sites of asthmatic inflammation such as the airway, where both decreased frequency of Treg and possible increased frequency of CD4+ iNKT cells have been documented [7, 22]. Studies are now underway to analyze function and phenotype of CD4+ iNKT cells in lung tissue and bronchoalveolar lavage fluid of AA subjects to examine this possibility.
Effective blockade of CD4+ iNKT cell-mediated killing of Treg was achieved by inhibition of only granzyme B and perforin, but not caspase-8 or FasL. Furthermore, expression of granzyme B and perforin positively correlated with Treg cell death in killing assays. These results suggested that killing of Treg by CD4+ iNKT cells occurred via a granzyme B-/perforin-dependent cell death pathway. Our data also showed that the increase in CD4+ iNKT cell-mediated cytotoxicity in AA is specific to Treg targets compared to non-Treg targets. In addition, AA Treg were more susceptible to the cytotoxicity of autologous CD4+ iNKT cells than their HC counterparts. These data suggested that abnormalities in AA Treg are also potentially involved in the dysfunctional iNKT cell-Treg interaction in AA. It is possible that increased expression of CD4+ iNKT cell-associated binding moieties on AA Treg makes them more susceptible to CD4+ iNKT cell killing. We are currently pursuing research to elucidate the precise recognition mechanism by which iNKT cells recognize Treg and distinguish Treg from non-Treg as targets.
In conclusion, we identify an important dysfunctional interaction between CD4+ iNKT cells and Treg that is specific to AA and correlated with disease severity. Based on evidence of cross-regulation between CD4+ iNKT cells and Treg, it is plausible to suggest a role of CD4+ iNKT cells in controlling Treg activity in AA. The enhanced CD4+ iNKT cell-mediated killing of Treg could be a potential pathological mechanism to weaken immune suppression, thus prolonging Th2 inflammation in AA.
Materials and methods
Human subjects
The study was approved by the Stanford Administrative Panel on Human Subjects in Medical Research. All subjects signed informed consent forms before participating in the study. Comprehensive clinical data were collected at each patient visit including history, disease severity, medication status, common allergens, IgE level, and FEV1. AA and NA subjects were classified based on physical exam, history, elevated blood IgE levels (above 50 IU/mL for AA) (ImmunoCap), and positive skin tests to allergens (for AA). Skin testing was performed with QuinTest applicators (Bayer) to allow for reproducibility; one nurse coordinator performed the skin tests to decrease variability in technique; interpretation was performed by the same, board-certified Allergy and Immunology specialist. The diagnosis of asthma was assessed via NHLBI guidelines (Expert Panel Report 3, 2007), using clinical symptoms, signs, and FEV1. Management of asthma occurred according to the NHLBI guidelines (Expert Panel Report 3, 2007).
HC were defined as non-smoking subjects greater than 13 years of age with a total serum IgE of less than 25 IU/mL, negative skin testing as compared to positive histamine control, and no evidence of lung disease. In addition, on spirometry testing, there was no evidence of obstructive or restrictive lung disease for HC. Patients with FEV1 below 60% were considered severe. Those in the range of 60–80% were considered moderate and those with FEV1 above 80% were considered mild.
Cell purification
CD4+ T cells were purified from buffy coats derived from up to 450 mL of whole blood using CD4+ Rosette kits (StemCell Technologies). CD4+ T cell fraction was first incubated with mouse anti-human Vα24JαQ antibodies (clone 6B11; BD) at 2 (µ/107 cells in the presence of FcR blocking solution (Miltenyi). Cells were washed and then incubated with rat anti-mouse IgG1 microbeads (Miltenyi) to positively select for CD4+ iNKT cells. The CD4+ iNKT cell fraction was then used to positively isolate CD4+CD25+ T cells with CD25 microbeads (Miltenyi), which was subsequently stained with anti-CD25 (Miltenyi) and anti-CD127 (Biolegend) antibodies and eventually sorted for CD4+CD25hi CD127lo/− Treg. The flow-through fraction after magnetic purification contained non-Treg. All procedures were performed with manufacturers' standard protocols.
Flow cytometry and antibodies
For surface staining, cells were stained with anti-CD4-FITC, anti-CD226-FITC, anti-IgG1-FITC, anti-Vα24JαQ-PE, anti-CD3-PerCP, anti-CD25-CyChrome (BD), anti-CD25-PE (Miltenyi), anti-Vβ11-FITC, anti-NKp30-PE, anti-NKp46-PE, anti-Vα24-PE, anti-IgG2a-PE (Beckman Coulter), anti-NKG2D-allophycocyanin, and anti-IgG1-allophycocyanin (Biolegend) antibodies per manufacturers' standard protocols. For intracellular staining, cells were fixed and permeabilized with Cytofix/Cytoperm solution (BD), then stained with anti-FoxP3-FITC (eBiosciences), anti-granzyme B-PE, anti-IgG1-PE (Caltag), anti-granzyme A-FITC, anti-IgG1-FITC, anti-perforin-allophycocyanin, and anti-IgG1-allophycocyanin (Biolegend) antibodies per manufacturers' standard protocols. Data were collected on a FACSCalibur (BD), then compensated and analyzed with FlowJo software (Treestar).
Flow-based killing assay
Treg and non-Treg targets were activated with IL-2 at 10 IU/mL for 24 h in RPMI/10% FBS/1% L-glutamine after purification. Target cells were then labeled with CFSE (Invitrogen) at the final concentration of 2 µM at 37°C in the dark for 10 min and washed with PBS. Effector CD4+ iNKT cells were co-cultured with CFSE-labeled targets for 4 h at a ratio of 5×105 effectors to 5×104 targets at 37°C. 7-Aminoactinomycin D (Sigma) was added after 4 h at 1 µg/mL in PBS/1% paraformaldehyde. For inhibition of cell death pathways, blocking antibodies for granzyme B (clone GB04; Caltag), perforin (clone dG9; BD), and FasL (clone 100409; R&D Systems) were used at 1 µg/mL. Low-endotoxin IgG1 antibody (BD) was also used at 1 µg/mL. Granzyme B inhibitor (CalBiochem) and caspase-8 inhibitor (R&D Systems) were used at 100 nM.
Statistical analysis
Data were tested for normality and variance equality before being subjected to appropriate statistical tests. All statistical procedures were performed with Prism software (GraphPad). Differences with p<0.05 were considered statistically significant.
Supplementary Material
Acknowledgements
The study was supported by grants from the Mary Hewitt Loveless Foundation (Kari Nadeau, MD, PhD), the Parker B. Francis Foundation (Kari Nadeau, MD, PhD), and the American Academy of Allergy, Asthma, and Immunology (Kari Nadeau, MD, PhD). We thank the asthmatic and healthy subjects who provided clinical samples for the study.
Abbreviations
- AA
allergic asthma/allergic asthmatic
- FEV1
forced expiratory volume in 1 s
- HC
healthy controls
- iNKT cells
invariant NKT cells
- NA
non-allergic asthmatic
- non-Treg
CD4+CD25− T cells
- OCS
oral corticosteroids
- Treg
CD4+CD25hiCD127lo/− regulatory T cells
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
Supporting Information for this article is available at www.wiley-vch.de/contents/jc_2040/2008/38082_s.pdf
References
- 1.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N. Engl. J. Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 2.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 3.Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Curr. Opin. Immunol. 2006;18:727–732. doi: 10.1016/j.coi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Robinson DS. The role of regulatory T lymphocytes in asthma pathogenesis. Curr. Allergy Asthma Rep. 2005;5:136–141. doi: 10.1007/s11882-005-0087-8. [DOI] [PubMed] [Google Scholar]
- 5.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin. Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Kim JM, Rudensky A. The role of the transcription factor FoxP3 in the development of regulatory T cells. Immunol. Rev. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 7.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, Griese M, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J. Allergy Clin. Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Grindebacke H, Wing K, Andersson AC, Suri-Payer E, Rak S, Rudin A. Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clin. Exp. Allergy. 2004;34:1364–1372. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 9.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 10.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, et al. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J. Exp. Med. 2006;203:2649–2660. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 13.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, Rugeles MT, et al. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology. 2007;122:1–14. doi: 10.1111/j.1365-2567.2007.02647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfrey DI, Kronenberg M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan BA, Kronenberg M. Activation or anergy: NKT cells are stunned by alpha-galactosylceramide. J. Clin. Invest. 2005;115:2328–2329. doi: 10.1172/JCI26297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 19.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, Fourneau JM, et al. Cutting edge: Invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 20.Das J, Eynott P, Jupp R, Bothwell A, Van Kaer L, Shi Y, Das G. Natural killer T cells and CD8+ T cells are dispensable for T cell-dependent allergic airway inflammation. Nat. Med. 2006;12:1345–1346. doi: 10.1038/nm1206-1345. [DOI] [PubMed] [Google Scholar]
- 21.Gernez Y, Tirouvanziam R, Nguyen KD, Herzenberg LA, Krensky AM, Nadeau KC. Altered phosphorylated signal transducer and activator of transcription profile of CD4+CD161+ T cells in asthma: Modulation by allergic status and oral corticosteroids. J. Allergy Clin. Immunol. 2007;120:1441–1448. doi: 10.1016/j.jaci.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N. Engl. J. Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 23.Bratke K, Julius P, Virchow JC. Invariant natural killer T cells in obstructive pulmonary diseases. N. Engl. J. Med. 2007;357:194. [PubMed] [Google Scholar]
- 24.Thomas SY, Lilly CM, Luster AD. Invariant natural killer T cells in bronchial asthma. N. Engl. J. Med. 2006;354:2613–2616. doi: 10.1056/NEJMc066189. [DOI] [PubMed] [Google Scholar]
- 25.Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D, Gadola SD, et al. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N. Engl. J. Med. 2007;356:1410–1422. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 26.La Cava A, Van Kaer L, Fu Dong S. CD4+CD25+ Tregs and NKT cells: Regulators regulating regulators. Trends Immunol. 2006;27:322–327. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Jiang S, Game DS, Davies D, Lombardi G, Lechler RI. Activated CD1d-restricted natural killer T cells secrete IL-2: Innate help for CD4+CD25+ regulatory T cells? Eur. J. Immunol. 2005;35:1193–1200. doi: 10.1002/eji.200425899. [DOI] [PubMed] [Google Scholar]
- 28.Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63:4516–4520. [PubMed] [Google Scholar]
- 29.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D'Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inngjerdingen M, Damaj B, Maghazachi AA. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and 1–309. J. Immunol. 2000;164:4048–4054. doi: 10.4049/jimmunol.164.8.4048. [DOI] [PubMed] [Google Scholar]
- 31.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur. J. Immunol. 2007;37:445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 32.Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, Zou J, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109:4816–4824. doi: 10.1182/blood-2006-07-035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lecoeur H, de Oliveira-Pinto LM, Gougeon ML. Multiparametric flow cytometric analysis of biochemical and functional events associated with apoptosis and oncosis using the 7-aminoactinomycin D assay. J. Immunol. Methods. 2002;265:81–96. doi: 10.1016/s0022-1759(02)00072-8. [DOI] [PubMed] [Google Scholar]
- 35.Shi L, Kraut RP, Aebersold R, Greenberg AH. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J. Exp. Med. 1992;175:553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkinson EA, Barry M, Darmon AJ, Shostak I, Turner PC, Moyer RW, Bleackley RC. Cytotoxic T lymphocyte-assisted suicide. Caspase 3 activation is primarily the result of the direct action of granzyme B. J. Biol. Chem. 1998;273:21261–21266. doi: 10.1074/jbc.273.33.21261. [DOI] [PubMed] [Google Scholar]
- 37.Grullich C, McGoldrick S, Zeiser R, Finke J. The death receptor pathway is not involved in alloreactive T-cell induced mitochondrial membrane permeability. Leuk. Lymphoma. 2005;46:1207–1216. doi: 10.1080/10428190500138435. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann C, Zeis M, Schmitz N, Uharek L. Impaired binding of perforin on the surface of tumor cells is a cause of target cell resistance against cytotoxic effector cells. Blood. 2000;96:594–600. [PubMed] [Google Scholar]
- 39.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 40.Chiossone L, Vitale C, Cottalasso F, Moretti S, Azzarone B, Moretta L, Mingari MC. Molecular analysis of the methylprednisolone-mediated inhibition of NK-cell function: Evidence for different susceptibility of IL-2- versus IL-15-activated NK cells. Blood. 2007;109:3767–3775. doi: 10.1182/blood-2006-07-037846. [DOI] [PubMed] [Google Scholar]
- 41.Mavoungou E, Bouyou-Akotet MK, Kremsner PG. Effects of prolactin and Cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30) Clin. Exp. Immunol. 2005;139:287–296. doi: 10.1111/j.1365-2249.2004.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitale C, Chiossone L, Cantoni C, Morreale G, Cottalasso F, Moretti S, Pistorio A, et al. The corticosteroid-induced inhibitory effect on NK cell function reflects down-regulation and/or dysfunction of triggering receptors involved in natural cytotoxicity. Eur. J. Immunol. 2004;34:3028–3038. doi: 10.1002/eji.200425418. [DOI] [PubMed] [Google Scholar]
- 43.Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, Jaeger S, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sen Y, Yongyi B, Yuling H, Luokun X, Li H, Jie X, Tao D, et al. V alpha 24-invariant NKT cells from patients with allergic asthma express CCR9 at high frequency and induce Th2 bias of CD3+ T cells upon CD226 engagement. J. Immunol. 2005;175:4914–4926. doi: 10.4049/jimmunol.175.8.4914. [DOI] [PubMed] [Google Scholar]
- 45.Bratke K, Bottcher B, Leeder K, Schmidt S, Kupper M, Virchow JC, Jr., Luttmann W. Increase in granzyme B+ lymphocytes and soluble granzyme B in bronchoalveolar lavage of allergen challenged patients with atopic asthma. Clin. Exp. Immunol. 2004;136:542–548. doi: 10.1111/j.1365-2249.2004.02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer EH, DeKruyff RH, Umetsu DT. iNKT cells in allergic disease. Curr. Top. Microbiol. Immunol. 2007;314:269–291. doi: 10.1007/978-3-540-69511-0_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.