Abstract
This study investigates whether the uncompetitive NMDA receptor antagonist, memantine, is able to protect dissociated cortical neurons from glutamate-induced excitotoxicity (GIE). Treatment with glutamate resulted in a significant loss of synchronization of neuronal activity as well as a significant increase in the duration of synchronized bursting events (SBEs). By administering memantine at the same time as glutamate, we were able to completely prevent these changes to the neuronal activity. Pretreatment with memantine was somewhat effective in preventing changes to the culture synchronization but was unable to fully protect the synchronization of electrical activity between neurons that showed high levels of synchronization prior to injury. Additionally, memantine pretreatment was unable to prevent the increase in the duration of SBEs caused by GIE. Thus, the timing of memantine treatment is important for conferring neuroprotection against glutamate-induced neurotoxicity. Finally, we found that GIE leads to a significant increase in the burst duration. Our data suggest that this may be due to an alteration in the inhibitory function of the neurons.
Key terms: Microelectrode array, memantine, NMDA receptor antagonist, traumatic brain injury, glutamate, excitotoxicity
Introduction
The excessive influx of calcium into cells is believed to be the key early step in glutamate-induced excitotoxicity (GIE) that occurs in many neurological disease states, including brain injury, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, HIV-associated neurocognitive disorders, and amyotrophic lateral sclerosis11,12,27,30. Entry of calcium through N-Methyl-D-aspartic acid receptors (NMDARs) is of particular interest because of the unique ability of these receptors to gate high levels of calcium influx12,41. Consequently, the focus of numerous laboratory and clinical studies of a variety of diseases in which GIE is believed to play a role has been on NMDAR antagonists. Unfortunately, the majority of NMDAR antagonists have failed in clinical trials because they are not tolerated by humans at neuroprotective doses27 due to the fact that at these doses, NMDA antagonists indiscriminately block normal synaptic function in addition to blocking the effects of excitotoxicity18,30. Physiological NMDAR activity is essential for many aspects of normal synaptic function, and hence, brain function26. In addition, there are several types of neurons with survival dependent on synaptic NMDAR activity2. As a result, NMDAR antagonists that block all receptor activity can cause widespread apoptosis and neuronal loss2, leading to intolerable side effects, including hallucinations, sleepiness, and coma26. Thus, while blocking NMDAR activity due to overstimulation may be an effective mechanism to prevent against excitotoxicity, this must be accomplished without significant interference in normal synaptic function.
Memantine is an uncompetitive NMDAR antagonist which has been approved by the FDA for the treatment of moderate to severe Alzheimer’s disease and has been used clinically for years outside of the United States for the treatment of Parkinson’s disease, spasticity, and dementia26. In Alzheimer’s disease studies, treatment with memantine slowed cognitive decline within just two weeks of treatment19 and significantly reduced clinical deterioration46. Various clinical trials of memantine have found that it is well-tolerated by humans at therapeutic concentrations5,35,38,45. Multiple in vitro and in vivo animals studies have shown that memantine blocks excessive NMDAR activity without disrupting physiological synaptic activity7,23,35,36. In addition, memantine had minimal effects on neuronal ultrastructure, learning, long-term potentiation (LTP), and synaptic transmission in an in vivo animal study of ischemia8. A different animal study found that treatment with memantine after experimental traumatic brain injury (TBI) in rats significantly prevented neuronal loss in both the CA2 and CA3 regions of the hippocampus37.
Memantine is able to block the excitotoxic effects of NMDAR over activation without altering normal synaptic transmission because it is a low-affinity, uncompetitive, open-channel blocker and has a relatively rapid off-rate from the channel6,27,28. The fast off-rate prevents memantine from accumulating in NMDAR-operated channels, thus avoiding interference with physiological neurotransmission27. At resting membrane potentials under normal conditions, NMDARs are blocked by extracellular magnesium that occupies the channel5,13,32,34. In excitotoxic conditions, the cells depolarize and the magnesium is repelled, opening the channel to calcium influx5,51. Memantine has been shown to be less voltage-dependent than magnesium, which allows memantine to continue to block the channels under relatively depolarized conditions48. In addition, studies have found that memantine effectively blocks NMDAR activity in the presence of prolonged elevations of glutamate concentrations, but it is not as active when glutamate levels increase for shorter periods of time, as in synaptic transmission6. Furthermore, recent studies have found that the activation of NMDARs may have opposing outcomes depending on where the NMDARs are located17. The stimulation of synaptic NMDARs trigger pro-survival events while the stimulation of extrasynaptic NMDARs may override these normal pathways, triggering the many deleterious pathways that all ultimately result in cell death17. Memantine has been shown to prefentially block extrasynaptic NMDAR channels while sparing normal synaptic activity1,2,23,49, which may explain the limited side effects found in patients treated with memantine.
In this paper, we investigated the ability of memantine to prevent against GIE in cortical cultures grown on microelectrode arrays (MEAs). We have previously determined parameters that are both stable over short periods of time and show both substantial and modest changes to the network resulting from glutamate treatment22. Using these parameters, we analyzed the ability of memantine to protect the function of the neuronal network from GIE. Using MEAs to investigate the neuroprotective ability of memantine allows one to measure the interaction between neurons in different areas of the network to determine if memantine cannot only prevent neuronal death but also whether memantine treatment can protect the ability of the neurons to send and receive signals as they did prior to GIE. Using MEAs offers some benefits over other techniques of measuring neuronal activity. MEAs are non-invasive, which allows for long-term sterile recordings from the same neurons10. In addition, MEAs have been shown to have better temporal resolution than intracellular calcium transient imaging, as a single action potential can cause a transient change in calcium signaling that lasts for 100s of milliseconds42. Our results indicate that memantine prevents against the effects of GIE when co-administered with glutamate. We found that pretreatment with memantine is somewhat effective in preventing against GIE. Pretreatment with memantine was unable to protect specific neuronal subsets that we have previously suggested may be more susceptible to glutamate-induced injury. In addition, we found that GIE dramatically changes the bursting behavior of the network. We observed an increase in the burst duration that may be caused by an alteration in the inhibitory behavior of the neurons.
Methods
Network preparation
Cortices were dissected from rat embryos at embryonic day 18 (E18) gestation. Meninges were removed, and the cells were dissociated with gentle trituration. Cells were plated on microelectrode arrays (MEA; Multi Channel Systems, Germany) in glutamate-depleted serum containing media (SCM; 84.4% MEM (Minimum Essential Medium; Gibco 42360, USA), 10% horse serum, 0.6% glucose, supplemented with 5% penicillin and streptomycin). Prior to use, the SCM was depleted of glutamate by incubating the media for at least 6 hours in tissue culture flasks that contained mature astrocytes and then filtered50. The MEAs consist of 60 electrodes (59 active electrodes and 1 reference electrode), each with a diameter of 30 µm, arranged in a square grid with a spacing of 200 µm between them. Prior to plating, the MEAs were prepared similar to the method by Hales et al.16 with some modifications. 100 µL of 0.3% polyethylene imine (PEI Sigma 03880, USA) dissolved in borate buffer was added to the center of each completely dry MEA for 1 hour at room temperature in order to cover all of the electrodes. The MEAs were then washed three times with deionized water and allowed to dry completely under a laminar flow hood. Immediately prior to plating, 10 µL of 1 mg/mL laminin (Sigma L2020, USA) was dissolved in 1 mL of SCM, and 20 µL of this solution was added to the center of the MEA (where the electrodes are located) and allowed to adhere for 20 minutes. The laminin was aspirated, and 20 µL of the dissociated cells, diluted to a concentration of 5 million cells per mL in SCM, were added to the center of the MEAs (where the laminin coating was). The MEAs were placed in a 37°C with 5% CO2 incubator for 40 minutes. After 40 minutes, the MEAs were inspected under a light microscope to ensure that the cells had adhered to the electrodes after which 1 mL of glutamate-depleted SCM was gently added to the MEAs. This plating protocol differs from our previous studies22. We found that this method of cell plating resulted in better cell coverage on the electrodes, and at the termination of the experiments, the cellular debris was easier to remove from the MEAs. Medium was replaced every other day. All animals were cared for using ethical practices in accordance with IACUC standards.
Pharmacological treatments
Glutamate was dissolved in SCM at a concentration of 300 µM. Memantine (Sigma M9292, USA) was dissolved in SCM at a concentration of 50 µM. At day in vitro (DIV) 14, the cell growth medium was replaced with fresh SCM with or without glutamate or memantine. Cultures were either treated for 1 hour with 300 µM glutamate, treated for 1 hour with 300 µM glutamate + 50 µM memantine, or pretreated for 1 hour with 50 µM memantine followed by a 1 hour treatment of 300 µM glutamate. After the completion of glutamate and/or memantine treatment, the solution was replaced with fresh SCM. It should be noted that the concentration of glutamate used in these studies differs from our previous work22. This is a result of the change in our plating protocol as noted above. Due to better adherence and survival of cells using this protocol, the glutamate concentration was increased to observe a change in cell firing behavior. In order to investigate the mechanism by which bursts change in duration, we applied 10 µM bicuculline, an γ-Aminobutyric acid (GABA) receptor antagonist, in recording media to the MEAs for 10 minutes and then recorded the electrical activity in the presence of bicuculline. The neuronal activity on the MEAs was recorded in the presence of 50 µM memantine for one hour alone to determine the effects of memantine on the normal synaptic signaling of the neuronal network.
Micro-electrode array recording
Micro-electrode array recordings were taken from each MEA immediately preceding drug treatment(s) and 24 after the removal of the drug(s). Prior to each recording, recording media (NaCl 144mM, KCl 10mM, MgCl2 1mM, CaCl2 2mM, HEPES 10mM, Na-pyruvate 2mM, glucose 10mM, pH 7.4) was added to the MEAs for 10 minutes to allow the cultures to reach equilibrium. The recording media formulation was chosen so as to regularize the bursting behavior between the electrodes. After each recording, the MEAs were washed three times with growth media.
Data analysis: Detection of electrical activity
The electrical signals were monitored, recorded, and analyzed as previously described22. Briefly, the data acquisition software, MCRack (Multi Channel Systems, Germany) was used to record the signals. The data were imported into Matlab using MEA-Tools, an open-source toolbox. The data were then filtered to remove any recording artifacts. Spikes were defined as any signal with voltage that surpassed a specified positive or negative threshold15,24. The threshold was chosen to be at least two standard deviations above the level of the background noise that the signals were embedded in. Electrodes often recorded signals from more than one neuron. This “whole channel analysis approach” was chosen in order to gather data from ‘micro’ networks within the area of each electrode10,15,33. Spontaneous bursting events (SBEs), or bursts, were defined as periods of high spike activity recorded simultaneously on multiple electrodes9. Spikes were identified as belonging to a specific SBE if they occurred within a specified period of time of a minimum number of other spikes9. The existence of each burst was verified by visual inspection. The burst duration was calculated by subtracting the time point at which the first spike occurred in the burst from the time point at which the last spike occurred in each burst.
Data analysis: Measurement of synchronous firing behavior
In order to measure changes in the synchronous behavior of the network, the synchronization index (SI) between all pairs of electrodes was calculated as previously described22. Given two electrodes, x and y, the synchrony of firing (SF) was defined as the number of times that the two electrodes recorded a spike within the same burst (Bxy) normalized to the total possible number of times either of the two electrodes recorded a spike within the same burst (Bx|y):
These values were then weighted by the frequency by which the two electrodes recorded a spike within the same burst (Nxy) compared with the number of bursts that occurred on that MEA during that recording period (NB) as a function of the maximum frequency of bursts that occurred on that MEA during either recording period (MB).
The SI values prior to drug treatment were subtracted from the SI values after drug treatment in order to find the change in synchronization (CS) of action potential firing that occurred as a result of drug treatment. The SI value was calculated between every pair of electrodes such that each electrode had 58 SI values. These 58 values were then averaged in order to obtain the average synchronization index (ASI) for each electrode.
In order to quantitatively compare the change in synchronization of action potentials within the neuronal network between treatment groups, the CS values between all electrode pairs of each experimental group were combined. Each CS value was normalized to the initial SI value of that electrode pair. The results were binned into three groups depending on the initial SI value, 0.1–0.4 (weak initial synchronization), 0.4–0.7 (moderate initial synchronization) and 0.7–1 (strong initial synchronization), to determine if the initial level of synchronization of action potential firing between two electrodes determined their susceptibility to GIE.
Results
The ability of memantine to protect the synchronized firing of neuronal networks
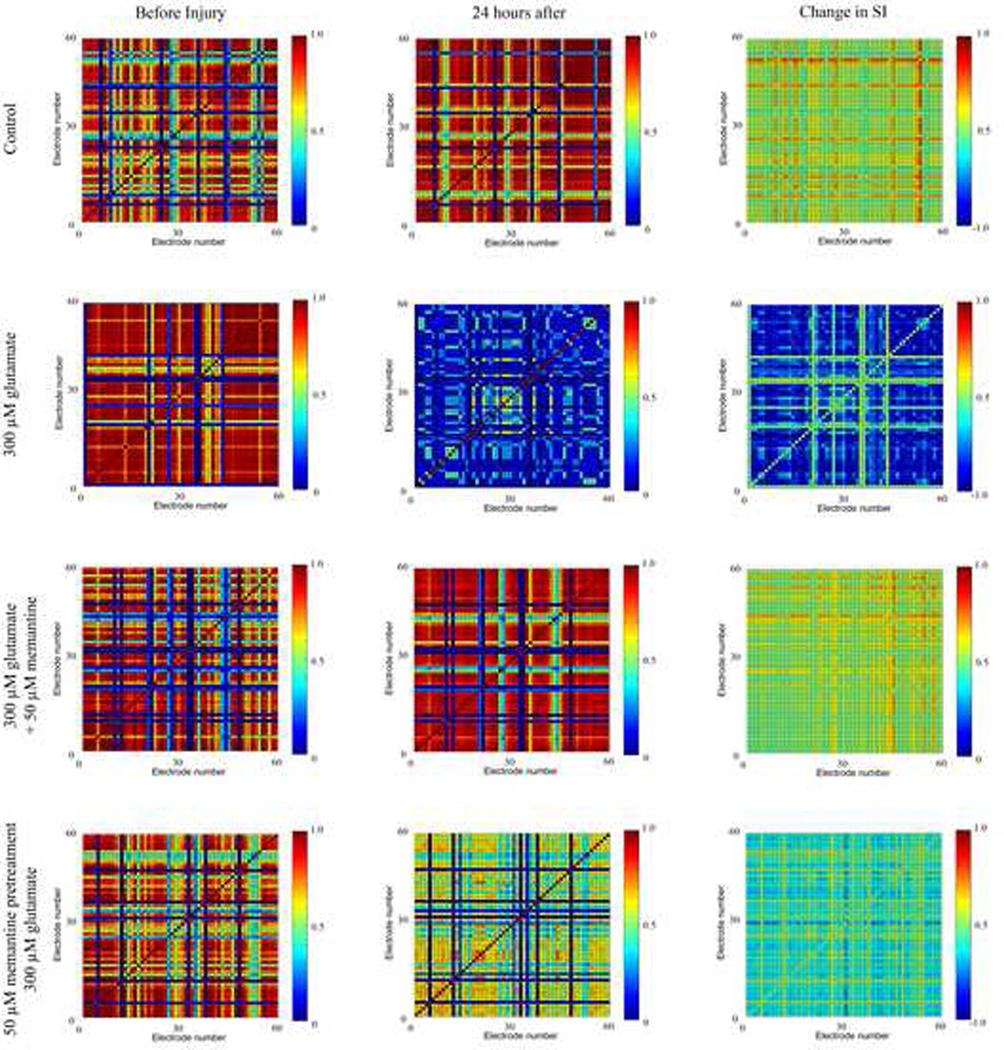
We analyzed the effect of glutamate treatment with or without memantine treatment on the pattern of action potential firing of the neuronal network. We performed this assessment 24 hours after treatment as this is the time point we previously used to assess the effects of glutamate treatment both cellularly14 and electro physiologically22. The synchronization index (SI) was calculated between all pairs of electrodes before and after glutamate and memantine treatments in order to measure changes in the synchronization of neuronal firing throughout the neuronal network. This resulted in a matrix in which each electrode is plotted against all other electrodes, yielding a 59 × 59 matrix (Figure 1). In control cultures and in cultures treated with glutamate and memantine simultaneously, a large percentage of the electrode pairs showed either no change in the SI or an increase in the SI (Figure 1, 1st and 3rd rows). In cultures treated with 300 µM glutamate, the majority of electrode pairs showed a large decrease in the SI (Figure 1, 2nd row). Treatment of cultures with 50 µM memantine prior to glutamate treatment also resulted in decreases in the SI of many electrode pairs (Figure 1, 4th row). However, these decreases were not as great as cultures treated with glutamate alone. In addition, in these cultures, there were a number of electrode pairs that did not show a change in the SI before and after drug treatments.
Figure 1. The ability of memantine to protect the synchronized firing between electrode pairs.
A representative MEA for each condition is shown. SI=synchronization index. Column 1 shows the synchronization grids before drug treatment, column 2 shows the synchronization grids 24 hours after drug treatment, and column 3 shows the change in synchronization (CS) as a result of drug treatment. In the left and middle columns, a red color designates that the two electrodes have a high SI, indicating that both electrodes frequently record a spike within the same burst. A dark blue color designates little to no synchronization between the neurons whose action potentials are recorded on those electrodes. In the third column, a red or yellow color indicates an increase in the SI (positive CS), a pale green color represents no change in the SI, and a blue color represents a decrease in the SI (negative CS).
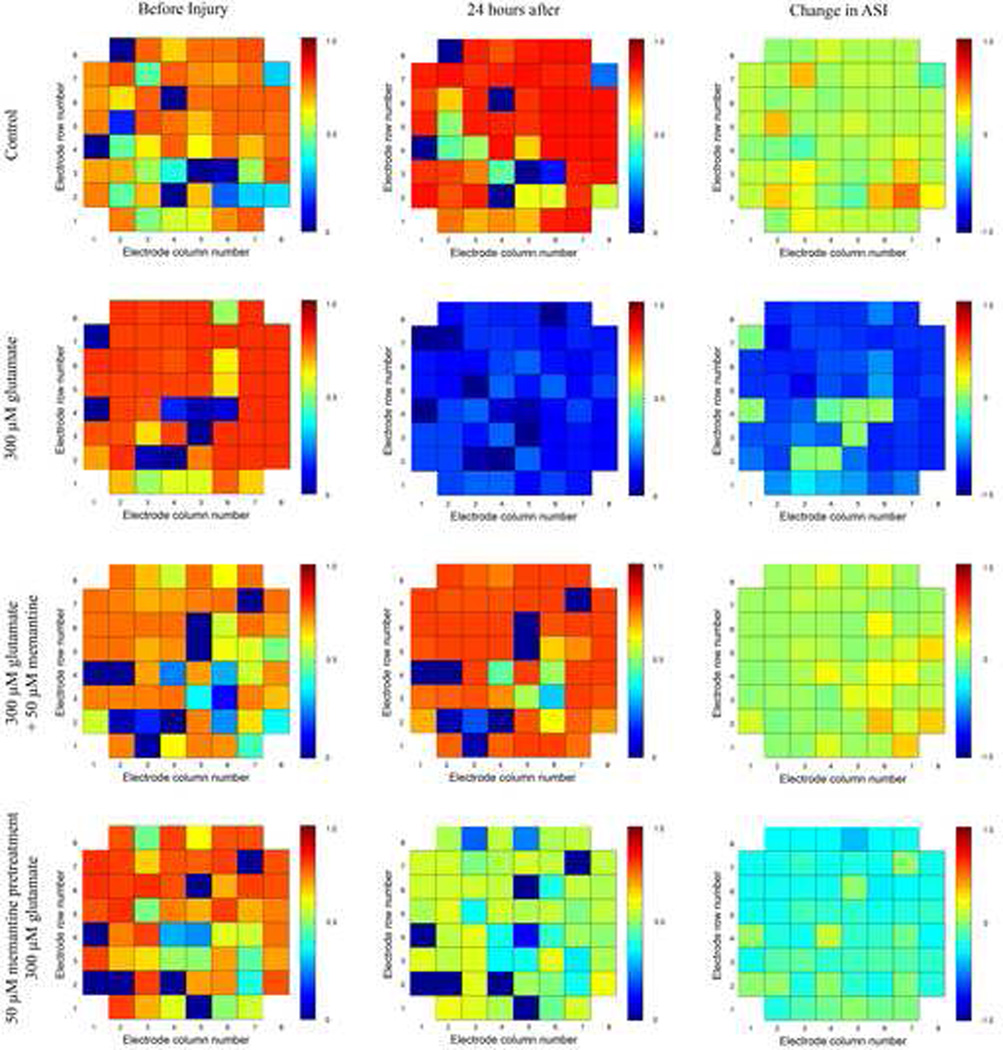
The 58 SI values for each electrode were averaged in order to calculate an average synchronization index (ASI) for each electrode. The SI value of the electrode with itself and with the reference electrode was excluded. This resulted in a matrix where the locations of the ASI values represent the actual physical location of each electrode (Figure 2). The change in ASI grids for each of the experimental groups is similar to those seen in the SI grids (Figure 1) except that increases and decreases in ASI values are reduced, because in this analysis, the individual SI values were averaged. In control cultures and in cultures simultaneously treated with glutamate and memantine, the ASI values of the electrodes either did not change or increased slightly. For cultures treated with 300 µM glutamate or pretreated with 50 µM memantine and then treated with 300 µM glutamate, the ASI value of a large number of electrodes decreased, with larger decreases occurring for the culture treated with only 300 µM glutamate.
Figure 2. The effect of glutamate and memantine treatment on the average synchronization index value of electrodes.
A representative MEA for each condition is shown. ASI = average synchronization index. The location of the ASI value for each electrode represents the actual physical location of the electrode on the MEA. Column 1 shows the ASI values prior to drug treatment, column 2 shows the ASI values 24 hours after drug treatment, and column 3 represents the change in the average synchronization of each electrode as a result of drug treatment. In the left and middle columns, a red color designates that that electrode has a high ASI, indicating that the neurons on that electrode fire in synchrony with neurons on other electrodes. A dark blue color designates that the neurons whose action potentials are recorded on that electrode do not fire with a high degree of synchrony with many neurons on other electrodes. In the third column, a yellow color indicates an increase in the ASI, a pale green color represents no change in the ASI, and a blue color represents a decrease in the ASI.
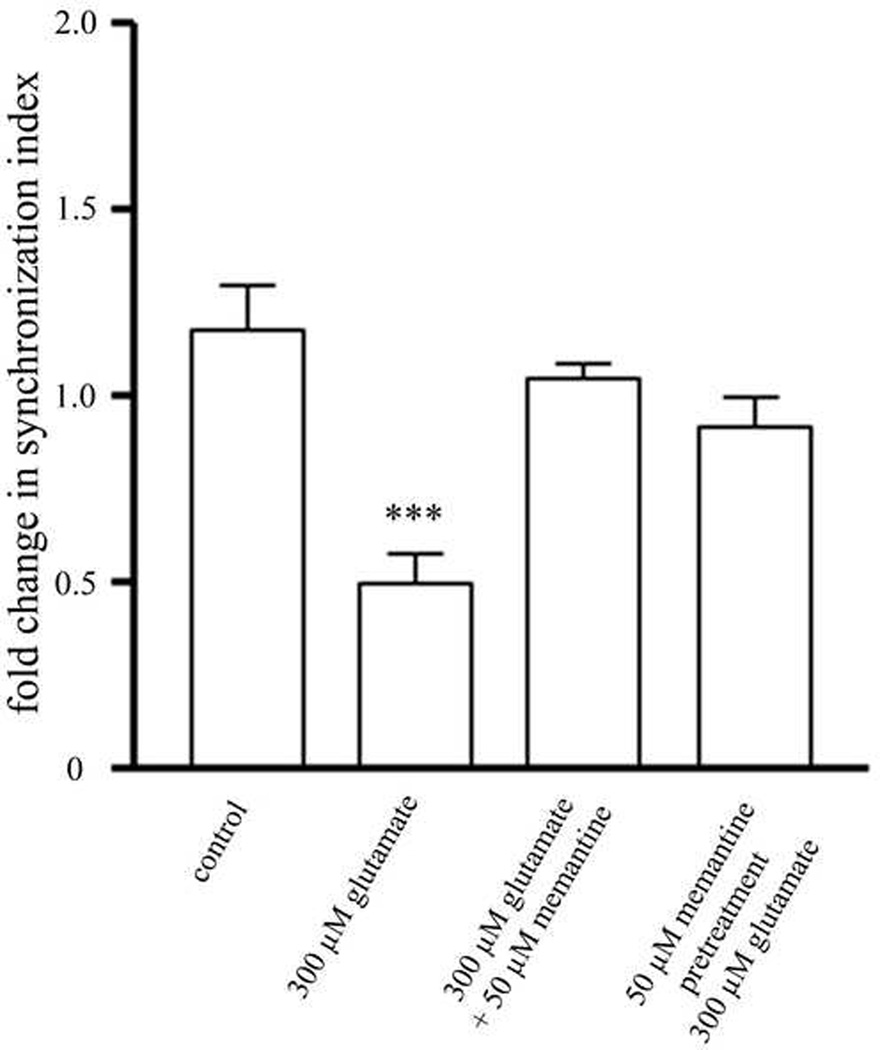
The SI values of all of the MEAs before and after drug treatments of each experimental group were combined in order to quantitatively measure changes in the synchronization of action potentials resulting from treatment with glutamate (Figure 3). The values were normalized to the SI before drug treatment. There was a significant decrease in synchronization in cultures treated with 300 µM glutamate. Cultures treated with memantine, either prior to glutamate treatment or at the same time as glutamate treatment, completely protected this overall loss in synchronization. There was a trend towards a decrease in synchronization in cultures treated with memantine prior to glutamate treatment, but the decrease was not significant.
Figure 3. Memantine fully protects neurons from an overall loss in SI.
Treatment with different memantine either during or prior to treatment with 300 µM glutamate results in complete protection from the overall loss of synchronization observed in cultures treated with glutamate alone. * = statistically different from control. ***p<0.001 determined by one-way ANOVA followed by Student-Newman-Keuls Multiple Comparison Test. Statistical analysis was performed on a per MEA basis (n=20 for control, n=21 for 300 µM glutamate, n=12 for concurrent 300 µM glutamate and 50 µM memantine treatment, n=12 for pretreatment with 50 µM memantine treatment followed by treatment with 300 µM glutamate).
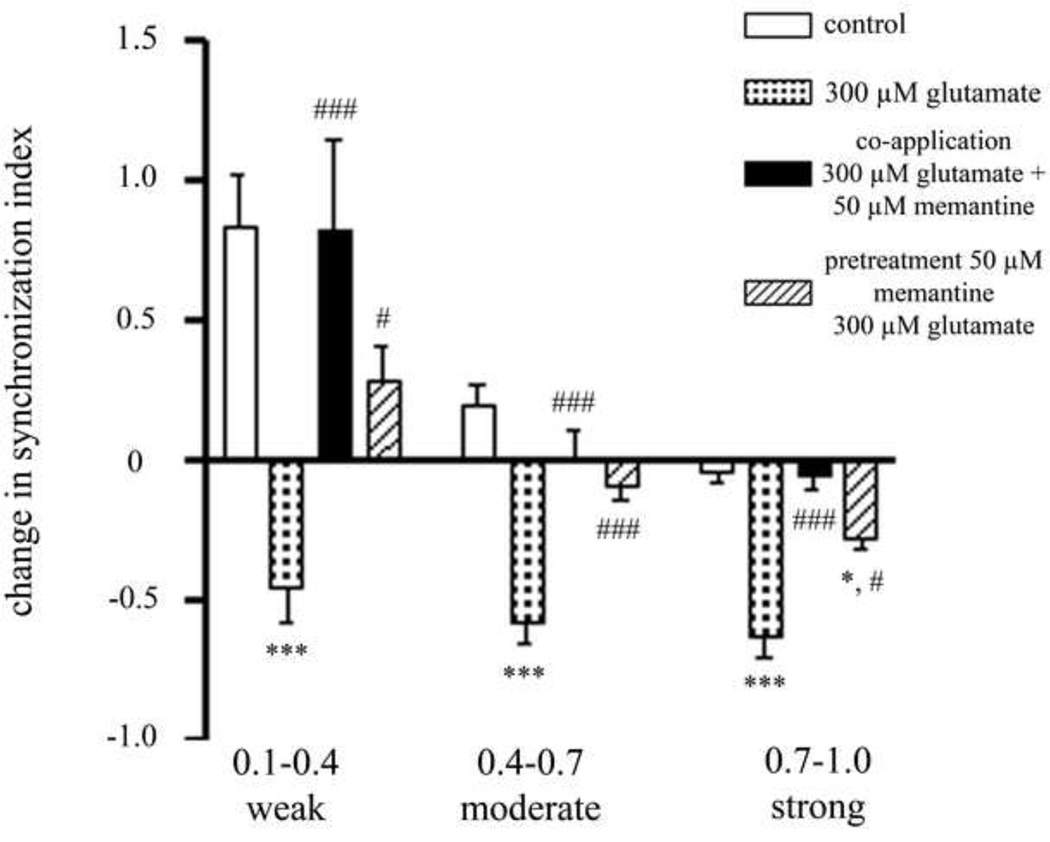
In order to determine if the initial level of synchronization of action potential firing between two electrodes determines the ability of memantine to protect their synchronization, the CS values between all electrode pairs of each treatment group were normalized to the initial SI value and binned into 3 groups depending on the initial SI value: 0.1–0.4 (weak initial synchronization), 0.4–0.7 (moderate initial synchronization) and 0.7–1 (strong initial synchronization) (Figure 4). Cultures treated with 300 µM glutamate resulted in a loss of synchronization of action potential firing in all groups. Treatment with memantine at the same time as glutamate treatment completely protected all losses in synchronization resulting from glutamate treatment. Treating cultures with 50 µM memantine before glutamate treatment completely protected the loss of synchronization of firing of electrode pairs that were initially weakly and moderately synchronized. Pretreatment with memantine was able to partially protect the synchronization of activity of electrode pairs that were initially strongly synchronized, as the loss in synchronization was significantly greater than the control but significantly less than cultures treated with only glutamate.
Figure 4. The initial synchronization between electrode pairs determines the ability of memantine to protect.
Treatment with memantine at the same time as glutamate treatment is able to fully prevent against the loss of action potential synchrony observed in glutamate treated cultures. Treatment with memantine prior to glutamate treatment blocks the effects of glutamate treatment on the synchronization of electrode pairs that are initially weakly and moderately synchronized, but is unable to fully protect electrode pairs that are initially strongly synchronized. *= statistically different from control. # = statistically different from 300 µM glutamate. *** p<0.001, *p<0.05 determined by one-way ANOVA followed by Student-Newman-Keuls Multiple Comparison Test. Statistical analysis was performed on a per MEA basis (n=20 for control, n=21 for 300 µM glutamate, n=12 for concurrent 300 µM glutamate and 50 µM memantine treatment, n=12 for pretreatment with 50 µM memantine treatment followed by treatment with 300 µM glutamate).
The ability of memantine to protect against changes in burst duration
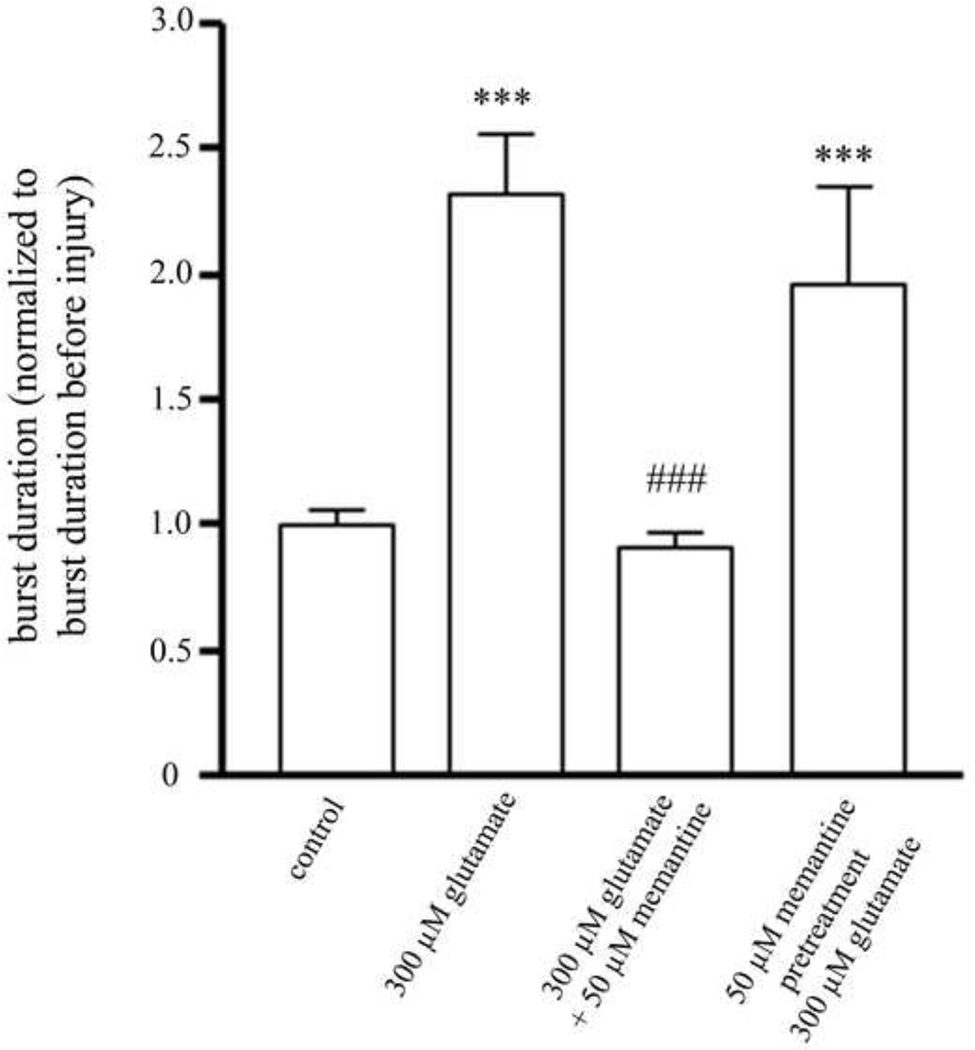
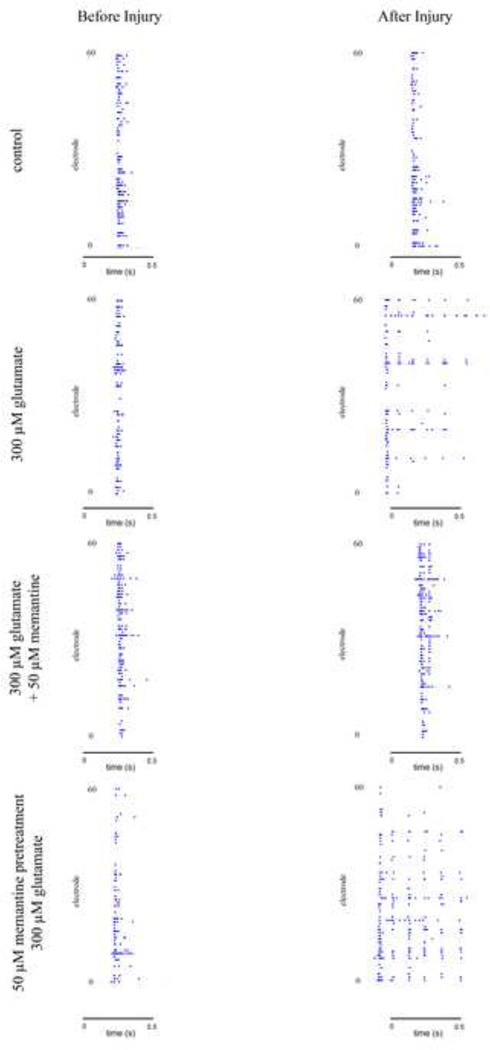
We assessed whether treatment with glutamate affects the shape of the SBEs, specifically whether it affects the length or duration of the bursts, and whether memantine treatment can prevent these effects. The average burst duration of all the bursts on a single MEA was calculated and combined with the averages of all of the other MEAs treated with that glutamate concentration (Figure 5). We found a significant increase in the burst duration of cultures treated with 300 µM glutamate as compared to control cultures. When memantine was applied at the same time as glutamate, the increase in burst duration was completely blocked. However, pretreatment of cultures with memantine did not prevent the increase in burst duration resulting from glutamate treatment. Rastor plots of example SBEs for each of the treatment groups are shown in Figure 6.
Figure 5. The change in burst duration after glutamate and memantine treatments.
Cultures treated with 300 µM glutamate had a significant increase in average burst duration. Cultures treated with 50 µM memantine at the same time as 300 µM glutamate had no change in the average burst duration. Cultures pretreated with 50 µM memantine prior to glutamate treatment had a significant increase in burst duration. *= statistically different from control. # = statistically different from 300 µM glutamate. ***p<0.001, **p<0.01, *p<0.05 determined by one-way ANOVA followed by Student-Newman-Keuls Multiple Comparison Test. Statistical analysis was performed on a per MEA basis (n=20 for control, n=21 for 300 µM glutamate, n=12 for concurrent 300 µM glutamate and 50 µM memantine treatment, n=12 for pretreatment with 50 µM memantine treatment followed by treatment with 300 µM glutamate).
Figure 6. The effect of glutamate and memantine treatment on burst duration.
Example SBEs before and after drug treatment for control cultures, cultures treated with 300 µM glutamate, cultures treated with 50 µM memantine at the same time as 300 µM glutamate, and cultures pretreated with 50 µM memantine followed by 300 µM memantine treatment. Each picture encompasses a single SBE. Each dot in the graphs represents a recorded spike.
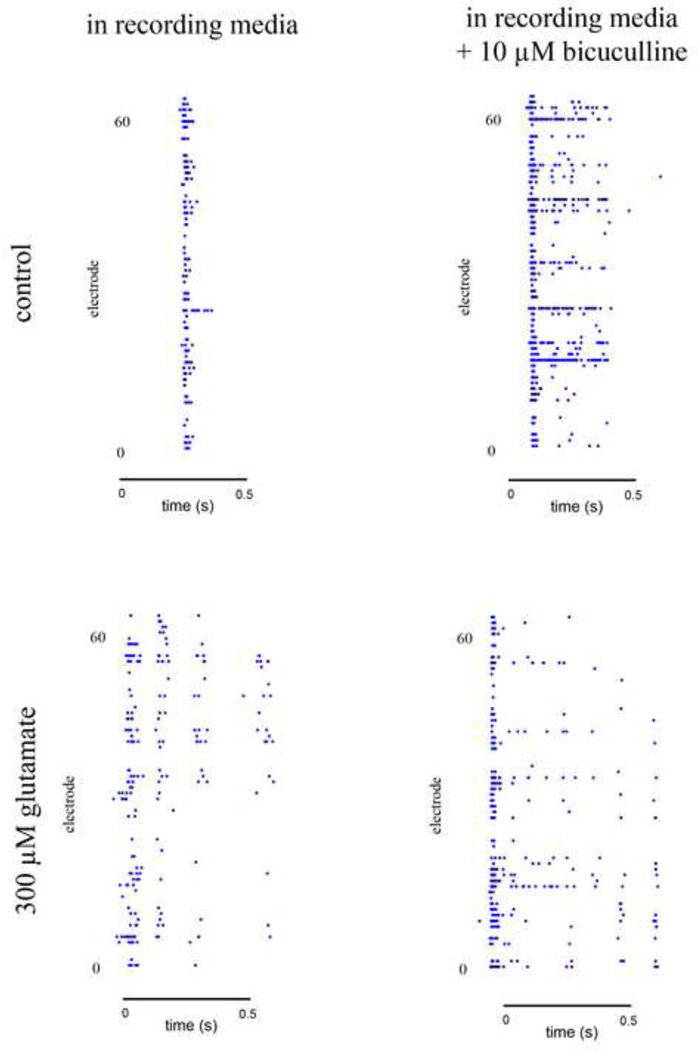
In order to further investigate the mechanism by which the burst duration is increased in injured cultures, we recorded the activity of both untreated control cultures and cultures treated with 300 µM glutamate while in a solution of 10 µM bicuculline diluted in recording media (Figure 7). After allowing the cultures to equilibrate in this media for ten minutes, we recorded the culture activity and compared the duration of bursts of each MEA in regular recording media and in the presence of bicuculline. The application of bicuculline to cultures injured with 300 µM glutamate did not change the width of the bursts. In control cultures, bicuculline consistently resulted in a dramatic increase in burst duration, similar to what is seen after GIE.
Figure 7. Bicuculline increases burst width in uninjured cultures.
Example SBEs of MEA recordings in recording media with and without 10 µM bicuculline. The addition of bicuculline dramatically increases the burst duration of control cultures but not does significantly impact the burst duration of injured cultures. Each picture encompasses a single SBE. Each dot in the graph represents a recorded spike.
Discussion
In this paper, we used the tools we developed in our previous work to determine the ability of memantine, an uncompetitive NMDAR antagonist, to protect the neuronal network from injury due to glutamate treatment. Previous studies have indicated that unlike competitive NMDAR antagonists, memantine is able to block the effects of NMDAR overactivation without altering normal synaptic function6,27,28. Investigating the efficacy of memantine using MEA recordings allows us to measure activity before and after glutamate-induced excitotoxicity and protective treatments in order to detect changes in the way the network in functioning.
We chose two protocols for memantine treatment based on previous studies that used similar treatment protocols4,21: pretreatment for one hour followed by one hour glutamate treatment and treatment at the same time as glutamate treatment. Because glutamate is released during the secondary phase of TBI, it is possible that patients could be protected with memantine after the initial mechanical injury has occurred but before excess glutamate begins to accumulate. Using these two treatment protocols allows us to investigate the appropriate time point at which memantine should be applied following injury. Our results indicate that when applied at the same time as glutamate, memantine is able to completely prevent the effects of GIE in our culture system. Memantine treatment, according to this protocol, completely preserved the pattern of electrical activity of the network. In addition, memantine prevented the increase in burst duration that we observed when cultures were treated with 300 µM glutamate. Importantly, in our preliminary experiments we found that memantine did not interfere with normal synaptic signaling as activity observed in the presence of memantine did not differ from the activity observed prior to the application of memantine. This agrees with previous studies that found that memantine does not alter normal synaptic function8,49. This property of memantine has proven extremely important as long-term treatment with NMDAR antagonists that do alter normal synaptic function have been found to produce intolerable side effects27,29,39,40 while long-term treatment with memantine has not resulted in the same side effects8,49. Chen and colleagues found that memantine treatment had no adverse effects on learning, even when the memantine was administered to rats over a period of several days. In contrast, MK-801, administered just twice, resulted in a complete disruption of spatial learning8.
Pretreatment with 50 µM memantine prior to treatment with 300 µM glutamate resulted in a partial protection from the effects of GIE. The overall level of culture synchrony was maintained in these cultures. However, a closer inspection of the changes in synchronization found that memantine pretreatment was unable to fully protect the neuronal subgroups that we have previously found to be more susceptible to glutamate-induced injury. Specifically, memantine pretreatment only partially protected the synchronization of action potentials of electrode pairs that were highly synchronized prior to GIE. Our previous data indicate that this subgroup of electrode pairs may be more susceptible to GIE22 as neuronal susceptibility has been shown to be activity-dependent25. As a result, it is possible that drug treatments may be less effective at preventing GIE. Memantine pretreatment was also unable to prevent the increase we observed in burst duration due to glutamate treatment. This result suggests that glutamate treatment may alter inhibitory activity. Thus, while memantine pretreatment is able to prevent some of the effects of GIE, it is unable to protect the most susceptible neuron groups. Memantine pretreatment appears to be less effective than concurrent application of memantine with glutamate. This is not surprising as memantine acts as an NMDAR blocker but has a relatively fast off-rate6,26. Thus, it is possible that in cultures that are only pretreated with memantine, the blocked NMDARs become unblocked at some point during the glutamate treatment, allowing for overstimulation of these receptors.
Treatment with 300 µM glutamate resulted in a significant increase in burst duration. In the example bursts shown in Figure 6, it appears that the same electrodes record repeated action potentials. Our previous experiments have determined that each electrode may be recording the activity of 1–3 neurons. Thus, as there are many more than 3 action potentials being detected on a single electrode within these post-injury bursts, a single neuron must be producing multiple action potentials within a short period of time, producing an epileptic type activity pattern. We believe this increase in burst duration may be due to a loss of inhibitory function in our cultures. In our studies, we found that we could produce a similar increase in burst duration that we observed in glutamate-injured cultures by treating uninjured control cultures with the GABA receptor antagonist bicuculline. Thus, by eliminating the inhibitory activity chemically we were able to mimic the behavior of the neurons that is observed after GIE. Furthermore, treatment of injured cultures with bicuculline did not result in a change in burst duration, suggesting that the inhibitory activity after GIE is already suppressed. These data suggest that the increase in burst duration that we see in our injured cultures may result from a loss of GABA receptor-mediated inhibitory activity, as was observed in previous studies43.
Similarly, studies have found that a loss of inhibitory function may be a cause of post traumatic epilepsy (PTE) following TBI43,44. It is not uncommon for adults to develop PTE following TBI47. Studies of US veterans of WWI, WWII, and the Korean War found that 50% of military personnel that experienced head injuries developed PTE31. In the civilian population, approximately 35–50% of individuals with penetrating head injuries develop PTE3. While the link between TBI and PTE is well established, the mechanisms behind the development of PTE is not yet fully understood20. It is generally believed that epileptic activity is caused by an imbalance in excitatory and inhibitory synaptic function44. One study found a reduction in GABA receptor binding in both the rat hippocampus and cortex after fluid percussion injury, resulting in a decrease in inhibitory function43. This impairment of GABA receptor-mediated inhibition may result from the loss of interneurons.
In this paper, we investigated the efficacy of the drug memantine to protect against GIE. Our results provide further evidence that memantine may be able to block the effects of secondary injury that result from GIE that occurs in TBI. Importantly, our analysis of the electrical activity of neurons found that memantine fully protects the function of neurons as not a single parameter we measured differed significantly from the control recordings in cultures treated with memantine at the same time as glutamate treatment. Previous studies6–8,23,35,36, as well as our preliminary experiments, suggest that memantine does not disrupt normal synaptic function, supporting its use in long-term treatment following TBI. Finally, our results of cultures pretreated with memantine further support our theory that specific subgroups of neurons are more susceptible to glutamate-induced injury, and therefore, may be more difficult to protect.
Acknowledgments
This work was funded by a grant from the New Jersey Commission on Brain Injury Research, #09-3209-BIR-E-2 to BLF. MLK was supported by Biotechnology Fellowship, Grant 5T32GM008339 from National Institute of General Medical Sciences.
References
- 1.Anitha M, Nandhu MS, Anju TR, Jes P, Paulose CS. Targeting glutamate mediated excitotoxicity in Huntington's disease: neural progenitors and partial glutamate antagonist--memantine. Med Hypotheses. 2011;76:138–140. doi: 10.1016/j.mehy.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Bordji K, Becerril-Ortega J, Buisson A. Synapses, NMDA receptor activity and neuronal Abeta production in Alzheimer's disease. Rev Neurosci. 2011;22:285–294. doi: 10.1515/RNS.2011.029. [DOI] [PubMed] [Google Scholar]
- 3.Caveness WF. Epilepsy, a product of trauma in our time. Epilepsia. 1976;17:207–215. doi: 10.1111/j.1528-1157.1976.tb03398.x. [DOI] [PubMed] [Google Scholar]
- 4.Cente M, Mandakova S, Filipcik P. Memantine prevents sensitivity to excitotoxic cell death of rat cortical neurons expressing human truncated tau protein. Cell Mol Neurobiol. 2009;29:945–949. doi: 10.1007/s10571-009-9379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen HS, Lipton SA. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol. 1997;499(Pt 1):27–46. doi: 10.1113/jphysiol.1997.sp021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, Lipton SA. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HS, Wang YF, Rayudu PV, Edgecomb P, Neill JC, Segal MM, Lipton SA, Jensen FE. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience. 1998;86:1121–1132. doi: 10.1016/s0306-4522(98)00163-8. [DOI] [PubMed] [Google Scholar]
- 9.Chiappalone M, Vato A, Berdondini L, Koudelka-Hep M, Martinoia S. Network dynamics and synchronous activity in cultured cortical neurons. Int J Neural Syst. 2007;17:87–103. doi: 10.1142/S0129065707000968. [DOI] [PubMed] [Google Scholar]
- 10.Chiappalone M, Vato A, Tedesco MB, Marcoli M, Davide F, Martinoia S. Networks of neurons coupled to microelectrode arrays: a neuronal sensory system for pharmacological applications. Biosens Bioelectron. 2003;18:627–634. doi: 10.1016/s0956-5663(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 11.Choi DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 12.Choi DW, Koh JY, Peters S, et al. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 14.Du Y, Chen CP, Tseng CY, Eisenberg Y, Firestein BL. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. 2007;55:463–472. doi: 10.1002/glia.20472. [DOI] [PubMed] [Google Scholar]
- 15.Gopal KV. Neurotoxic effects of mercury on auditory cortex networks growing on microelectrode arrays: a preliminary analysis. Neurotoxicol Teratol. 2003;25:69–76. doi: 10.1016/s0892-0362(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 16.Hales CM, Rolston JD, Potter SM. How to culture, record and stimulate neuronal networks on micro-electrode arrays (MEAs) J Vis Exp. 2010 doi: 10.3791/2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 18.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6:61–67. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Kharlamov EA, Lepsveridze E, Meparishvili M, Solomonia RO, Lu B, Miller ER, Kelly KM, Mtchedlishvili Z. Alterations of GABA(A) and glutamate receptor subunits and heat shock protein in rat hippocampus following traumatic brain injury and in posttraumatic epilepsy. Epilepsy Res. 2011;95:20–34. doi: 10.1016/j.eplepsyres.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Kuszczyk M, Slomka M, Antkiewicz-Michaluk L, Salinska E, Lazarewicz JW. 1-Methyl-1,2,3,4-tetrahydroisoquinoline and established uncompetitive NMDA receptor antagonists induce tolerance to excitotoxicity. Pharmacol Rep. 2010;62:1041–1050. doi: 10.1016/s1734-1140(10)70366-2. [DOI] [PubMed] [Google Scholar]
- 22.Kutzing MK, Luo V, Firestein BL. Measurement of synchronous activity by microelectrode arrays uncovers differential effects of sublethal and lethal glutamate concentrations on cortical neurons. Ann Biomed Eng. 2011;39:2252–2262. doi: 10.1007/s10439-011-0319-0. [DOI] [PubMed] [Google Scholar]
- 23.Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22:4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Zhou W, Li X, Zeng S, Liu M, Luo Q. Characterization of synchronized bursts in cultured hippocampal neuronal networks with learning training on microelectrode arrays. Biosens Bioelectron. 2007;22:2976–2982. doi: 10.1016/j.bios.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Lin CH, Juan SH, Wang CY, Sun YY, Chou CM, Chang SF, Hu SY, Lee WS, Lee YH. Neuronal activity enhances aryl hydrocarbon receptor-mediated gene expression and dioxin neurotoxicity in cortical neurons. J Neurochem. 2008;104:1415–1429. doi: 10.1111/j.1471-4159.2007.05098.x. [DOI] [PubMed] [Google Scholar]
- 26.Lipton SA. NMDA receptors, glial cells, and clinical medicine. Neuron. 2006;50:9–11. doi: 10.1016/j.neuron.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 28.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 29.Lipton SA. Prospects for clinically tolerated NMDA antagonists: open-channel blockers and alternative redox states of nitric oxide. Trends Neurosci. 1993;16:527–532. doi: 10.1016/0166-2236(93)90198-u. [DOI] [PubMed] [Google Scholar]
- 30.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 31.Lowenstein DH. Epilepsy after head injury: an overview. Epilepsia. 2009;50(Suppl 2):4–9. doi: 10.1111/j.1528-1167.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 32.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 33.Morefield SI, Keefer EW, Chapman KD, Gross GW. Drug evaluations using neuronal networks cultured on microelectrode arrays. Biosens Bioelectron. 2000;15:383–396. doi: 10.1016/s0956-5663(00)00095-6. [DOI] [PubMed] [Google Scholar]
- 34.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer DE, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Vincent Chen HS, Tong G, Hayden MR, Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15:1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao VL, Dogan A, Todd KG, Bowen KK, Dempsey RJ. Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res. 2001;911:96–100. doi: 10.1016/s0006-8993(01)02617-8. [DOI] [PubMed] [Google Scholar]
- 38.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 39.Rogawski MA. Therapeutic potential of excitatory amino acid antagonists: channel blockers and 2,3-benzodiazepines. Trends Pharmacol Sci. 1993;14:325–331. doi: 10.1016/0165-6147(93)90005-5. [DOI] [PubMed] [Google Scholar]
- 40.Rogawski MA, Wenk GL. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease. CNS Drug Rev. 2003;9:275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sattler R, Charlton MP, Hafner M, Tymianski M. Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. J Neurochem. 1998;71:2349–2364. doi: 10.1046/j.1471-4159.1998.71062349.x. [DOI] [PubMed] [Google Scholar]
- 42.Shew WL, Bellay T, Plenz D. Simultaneous multi-electrode array recording and two-photon calcium imaging of neural activity. J Neurosci Methods. 2010;192:75–82. doi: 10.1016/j.jneumeth.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sihver S, Marklund N, Hillered L, Langstrom B, Watanabe Y, Bergstrom M. Changes in mACh, NMDA and GABA(A) receptor binding after lateral fluid-percussion injury: in vitro autoradiography of rat brain frozen sections. J Neurochem. 2001;78:417–423. doi: 10.1046/j.1471-4159.2001.00428.x. [DOI] [PubMed] [Google Scholar]
- 44.Tani H, Bandrowski AE, Parada I, Wynn M, Huguenard JR, Prince DA, Reimer RJ. Modulation of epileptiform activity by glutamine and system A transport in a model of post-traumatic epilepsy. Neurobiol Dis. 2007;25:230–238. doi: 10.1016/j.nbd.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson D, Andersen HF. Analysis of the effect of memantine in reducing the worsening of clinical symptoms in patients with moderate to severe Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:138–145. doi: 10.1159/000105162. [DOI] [PubMed] [Google Scholar]
- 47.Willmore LJ, Lowenstein DH. Epilepsy and trauma: a persistent challenge. Neurology. 2010;75:202–203. doi: 10.1212/WNL.0b013e3181e8e94f. [DOI] [PubMed] [Google Scholar]
- 48.Wrighton DC, Baker EJ, Chen PE, Wyllie DJ. Mg2+ and memantine block of rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J Physiol. 2008;586:211–225. doi: 10.1113/jphysiol.2007.143164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye ZC, Sontheimer H. Astrocytes protect neurons from neurotoxic injury by serum glutamate. Glia. 1998;22:237–248. doi: 10.1002/(sici)1098-1136(199803)22:3<237::aid-glia3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 51.Zeevalk GD, Nicklas WJ. Evidence that the loss of the voltage-dependent Mg2+ block at the N-methyl-D-aspartate receptor underlies receptor activation during inhibition of neuronal metabolism. J Neurochem. 1992;59:1211–1220. doi: 10.1111/j.1471-4159.1992.tb08430.x. [DOI] [PubMed] [Google Scholar]