Abstract
A practical impediment in adaptive clinical trials is that outcomes must be observed soon enough to apply decision rules to choose treatments for new patients. For example, if outcomes take up to six weeks to evaluate and the accrual rate is one patient per week, on average three new patients will be accrued while waiting to evaluate the outcomes of the previous three patients. The question is how to treat the new patients. This logistical problem persists throughout the trial. Various ad hoc practical solutions are used, none entirely satisfactory. We focus on this problem in phase I–II clinical trials that use binary toxicity and efficacy, defined in terms of event times, to choose doses adaptively for successive cohorts. We propose a general approach to this problem that treats late-onset outcomes as missing data, uses data augmentation to impute missing outcomes from posterior predictive distributions computed from partial follow-up times and complete outcome data, and applies the design’s decision rules using the completed data. We illustrate the method with two cancer trials conducted using a phase I–II design based on efficacy-toxicity trade-offs, including a computer stimulation study.
Keywords: Bayesian Adaptive Clinical Design, Dose-Finding, Phase I-II Clinical Trial Design, Missing Data, Data Augmentation Algorithm, Piecewise Exponential Model
1 Introduction
Phase I–II clinical trial designs combine conventional phase I and phase II trials by determining a dose of a new agent based on both toxicity and efficacy (Gooley et al., 1994; Thall and Russell, 1998; O’Quigley et al., 2001; Braun, 2002; Thall and Cook, 2004; Bekele and Shen, 2005; Zhang et al., 2006; Yin et al., 2006; Yuan and Yin, 2011a). Most commonly, a small phase I trial based on toxicity (Storer, 1989; O’Quigley et al., 1990; Babb et al., 1998; Conaway et al., 2004) first is conducted to choose a putatively safe dose, the “maximum tolerated dose” (MTD), and a phase II trial then is conducted (Gehan, 1969; Fleming, 1982; Simon, 1989; Thall and Simon, 1994; Thall et al., 1995; Bryant and Day, 1995), using the MTD, with efficacy the primary outcome. This conventional approach may lead to several problems. (1) Informal dose adjustments often are made in phase II if excessive toxicity is observed, which invalidates assumed properties of any efficacy-based design. (2) Operating characteristics of the entire phase I – phase II process are seldom computed. (3) Separate designs ignore the trade-off between efficacy and toxicity that often underlies therapeutic decision making. Since phase I–II designs address these problems explicitly, a natural question is why such designs are used infrequently for actual trials. While these are complex issues, the following logistical problem may play a prominent role.
In outcome-adaptive clinical trials, a major practical impediment arises if patient outcomes are not observed quickly enough to apply decision rules that choose treatments or doses for newly accrued patients. Because it is undesirable, and often impossible, to delay a new patient’s treatment while waiting for previous patients’ outcomes to be scored so that an adaptive statistical rule can be applied, outcome-adaptive rules may be at odds with clinical practice. In phase I–II, this problem arises if either toxicity or efficacy is not scored quickly, relative to the accrual rate. One solution is to turn away new patients and treat them off protocol. This may be less desirable than giving the experimental regimen, or impossible if no alternative treatment exists. Another solution is to give all new patients the dose or treatment that is optimal based on the most recent data. This may have very undesirable consequences if the most recent optimal dose later turns out to be overly toxic, and it is the main reason that dose-finding usually is done sequentially with small cohorts of 1, 2, or 3 patients. In Phase I–II, the opposite effect can occur if the most recent optimal dose is safe but inefficacious. A third solution when some patients’ outcomes have not yet been evaluated fully is to treat all new patients immediately, but use a dose one level below the design’s current optimal dose. In Phase I, the problem of dealing with delayed (“late onset”) toxicity was first addressed by Cheung and Chappell (2000), who introduced the time-to-event continual reassessment method (TiTE-CRM), and later by Braun (2006), Bekele et al. (2008), and Yuan and Yin (2011b).
As an illustration, consider a phase I–II clinical trial of chemotherapy for acute leukemia, both toxicity and efficacy events may occur at any time during a common 6-week evaluation period, doses chosen adaptively for cohorts of size 3, and accrual rate 1 patient per week. On average, the first cohort will be accrued in 3 weeks with all of their outcomes scored by week 9, when adaptive rules are applied using their data to choose the second cohort’s dose. Since one also can expect to accrue 6 new patients between weeks 3 and 9, the question is how to deal therapeutically with these new patients. A second example is an autologous stem cell transplantation trial for multiple myeloma (MM) where toxicity may occur at any time during the first 30 days, but efficacy is evaluated only at 90 days post-transplant, and is defined as no detectable MM protein in the urine or blood serum and < 5% plasma cells in the bone marrow. If the accrual rate is 3 patients per month, one may expect to accrue 9 patients before efficacy is scored for any patients, so applying an adaptive rule to choose a dose for patients 4, 5, and 6, (cohort 2), using the data from patients 1, 2, and 3, (cohort 1), is not possible without delaying the second cohort’s therapy. The severity of this problem increases with accrual rate and persists throughout the trial.
In this paper, we consider phase I–II designs where both efficacy (E) and toxicity (T) are characterized as binary variables, YE and YT, evaluated either during or at the end of specified time intervals, [0, UE] for YE and [0, UT] for YT. We refer to YE and YT as “delayed outcomes” because they are not observed immediately. Denote accrual rate by α. We quantify the severity of the problem using the logistical difficulty indexes ζj = Ujα for each outcome j = E, T, and overall index ζ = max{ζE, ζT}. For example, if α = 1 patient/month and UE = UT = 3 months, then ζ = 3. Doubling the accrual rate to α = 2 gives the problematic value ζ = 6.
We propose a methodology that handles the problem of delayed outcomes in phase I–II by accounting for each patient’s follow up time prior to evaluation of YE and YT, and treating all Yj’s that have not yet been observed as missing values. We use data augmentation (Tanner and Wong, 1987) to impute each missing Yj using partial follow-up times and complete outcome data. Combining observed and imputed (YE, YT) data for all patients who have been treated, we then apply the phase I–II design’s decision rules. Our approach of treating delayed outcomes as missing data is similar to that of Yuan and Yin (2011b), who deal with the phase I setting with YT but not YE. Key differences are that the bivariate distribution and missingship patterns of (YE, YT) are much more complicated than those of YT alone. In addition, Yuan and Yin (2011b) use the EM algorithm under a frequentist framework to estimate toxicity probabilities, whereas we rely on predictive probabilities and imputatation to obtain a completed data set under a Bayesian formulation.
In Section 2, we propose a general approach to delayed outcomes in phase I–II trials. In Section 3, we present imputation and Bayesian data augmentation methods. Section 4 illustrates the method with two trials designed using an extended version of the efficacy-toxicity (Eff-Tox) trade-off based design of Thall and Cook (2004). In Section 5, we present simulations of the proposed method and comparison with alternative methods. We conclude with a discussion in Section 6.
2 Observed Outcomes and Missing Values
2.1 Data Structures
The data structure assumed by most phase I–II dose-finding method consists of assigned doses and two binary outcomes. We denote this by 𝒟(Y) = {(d[1], Y1,E, Y1,T), …, (d[n], Yn,E, Yn,T)}, where d[i] is the dose given to the ith patient and interim sample size n = 1, ⋯, Nmax. To account for the fact that, at any interim decision, one or both of YE and YT may not yet be observed for a given patient, we denote the data including only the observed Yj’s by 𝒟obs(Y) and the unobserved (“missing”) Yj’s by 𝒟mis(Y). Our strategy will be to use 𝒟obs(Y) and partial follow up time data to impute any missing Yj’s in 𝒟mis(Y) and thus construct a completed version of 𝒟obs(Y) that has the form of 𝒟(Y), and then apply the phase I–II method.
Let Xj denote time to outcome j = E, T. Corresponding to the acute leukemia trial and the stem cell transplantation trial described previously, we distinguish between two data structures, depending on how YE is observed. In Case 1, YE is observed in real time, and YE = I(XE ≤ UE). In Case 2, YE is evaluated only at UE, and there is no XE. In both cases, YT = I(XT ≤ UT). To simplify exposition, we focus primarily on Case 1 since it is more complex, and later explain how to deal with Case 2. For trials where efficacy is evaluated periodically XE is interval censored. We include this by smoothing the interval censored data, placing XE at the midpoint or a randomly chosen value of the interval where efficacy was known to occur. We accommodate patient death during therapy by defining XT as the time to either non-fatal toxicity or death. For efficacy, if the patient dies prior to UE we define YE = 0, which is implied by defining XE = ∞. Denote U = max {UT, UE} and V = follow up time, where by design V ≤ U.
2.2 Missingship Mechanism
Denote πj(d, θ) = Pr(Yj = 1 | d, θ) for j = E, T, where θ is the model parameter vector. In the sequel, we often will suppress d and θ for brevity. The observed follow up time of Xj during the evaluation interval [0, Uj] is . We assume that V is independent of Xj. The key to our method is that provides useful information about Yj, because
must decrease as increases from 0 to Uj. This fact also underlies the TiTE-CRM (Cheung and Chappell, 2000). To account for missing values, we use V to extend the previous definitions of YE and YT, as follows. We define
That is, Yj = missing if the patient has not yet experienced the event and has not been fully followed to Uj, while Yj is observed if the patient either has experienced the event (Yj = 1) or has completed the defined follow-up time without the event (V = Uj and Yj = 0). Denote the missingship indicators Mj = I(Yj = missing) for j = E, T, and M = (ME, MT). The ith patient’s data are , and 𝒟i determines Mi. Our methodology uses the actual interim data from n patients, by 𝒟= (𝒟1, ⋯, 𝒟n), to impute missing Yj’s and construct a completed binary data set 𝒟(Y) for implementing the phase I–II method.
2.3 Event Time Distributions
To construct flexible survival functions, we assume piecewise exponential marginals for [Xj|d, Yj = 1], j = E, T, by partitioning [0, Uj] into Kj intervals, [0, hj,1), [hj,1, hj,2), ⋯, [hj,Kj−1, hj,Kj], and assuming hazard λj,k on [hj,k−1, hj,k). The marginal survival function for Xj is
denoting λj = (λj,1, ⋯, λj,Kj), and weights wj,k(x) = hj,k − hj,k−1 if x > hj,k, wj,k(x) = x − hj,k−1 if x ∈ [hj,k−1, hj,k), and wj,k(x) = 0 otherwise. Initially, we considered a more elaborate form of Sj, in which each λj,k was replaced by where γj > 1, so that the event time distributions varied with dose in a proportional hazard model with piecewise exponential baseline hazard. However, we found that fixing γE = γT = 1 did not change the method’s operating characteristics, due to the fact that there is little or no information to estimate the γj’s. To determine whether a more parsimonious survival model might give a design with similar performance, we replaced the piecewise exponential with a Weibull. However, simulations across eight dose-outcome scenarios (Supplementary Table S9) showed that the Weibull gave larger probabilities of incorrectly stopping early, and either similar or lower probabilities of selecting desirable doses.
Our imputation method requires the joint conditional survival probabilities S(xE, xT |a, b) = Pr(XE > xE, XT > xT |YE = a, YT = b) for (a, b) = (0,1), (1,0), (1,1). Assuming conditional independence, Sj(xj |YE, YT) = Sj(xj |Yj) for j = E, T, implies that S(xE, xT |1, 0) is determined by the marginal of XE and S(xE, xT |0, 1) is determined by the marginal of XT. Determining S(xE, xT |1, 1) requires accounting for association between XE and XT. We do this by defining a joint distribution using the Clayton copula (Clayton, 1978), given for ϕ ≥ 0 by
The likelihood of 𝒟 depends on YE, YT, and the censoring patterns of XE and XT. Denote , the pdf of Xj by fj(·), and
When YE = YT = 1, there are four possible likelihoods,
For the cases (YE, YT) = (1,0) or (0,1), the likelihoods are given by
Denoting the likelihood for n patients by , the posterior is f(λ, ϕ|𝒟) ∝ f(λ)f(ϕ)L(𝒟|λ, ϕ), for priors f(λ) and f(ϕ) of λ and ϕ.
3 Imputation Method
Let πa,b(d, θ) = Pr(YE = a, YT = b|d, θ) for a, b ∈ {0, 1} denote the joint distribution of [YE, YT | d], so πE(d, θ) = π1,1(d, θ) + π1,0(d, θ) and πT(d, θ) = π1,1(d, θ) + π0,1(d, θ). If no outcomes are missing the likelihood is the usual product
| (1) |
with posterior f(θ|𝒟(Y)) ∝ L(𝒟(Y)|θ)f(θ); for prior f(θ). Since L(𝒟(Y)|θ) cannot be computed if some Yi,j’s are missing, we obtain a completed version of the likelihood, of the form (1), by using Bayesian data augmentation (Tanner and Wong, 1987). We iterate between an imputation step sampling missing Yj’s from their full conditionals, and a step computing the posterior using the completed data. Importantly, the imputation depends on the dose-outcome model.
Missing Yj’s are nonignorable because, at follow up time V, a patient who will not experience outcome j by Uj is more likely to have Yj = missing (Mj=1) than a patient for whom outcome j will occur (Yuan and Yin, 2011b). By Bayes’ Law, Pr(Mj = 1|Yj = 0) > Pr(Mj = 1|Yj = 1) implies that
This says that the odds that Yj = 1 decreases if Yj is missing, so the missingship indicator Mj contains information about the future value of Yj.
A complication in carrying out the imputation is that there are three possible missingship patterns: (1) YE = missing and YT is observed, (2) YT = missing and YE is observed, and (3) both YE = missing and YT = missing. Since we impute missing values by sampling from their full conditional posteriors, these distributions must be specified for each missingship pattern. These posteriors are defined in terms of the following conditionals for XE and XT, given each of the four possible future outcome pairs, which for brevity we denote by
| (2) |
In any case, S00 = 1 due to the fact that Pr(Xj > V |Yj = 0) = 1 for 0 ≤ V ≤ Uj, which also implies that S10 = Pr(XE > V |YE = 1, YT = 0) and S01 = Pr(XT > V |YE = 0, YT = 1). Thus, only S11 involves a joint distribution for (XE, XT) given YE = YT = 1.
To obtain consistent estimates when imputing the missing Yj’s, we condition on the actual data 𝒟. The following posterior conditional distributions are derived in the Appendix. When YE = missing and YT is observed, we impute YE from its conditional posterior
When YT = missing and YE is observed, we impute YT from its conditional posterior
When both YE and YT are missing, we impute (YE, YT) from the joint conditional posterior
At the posterior step using the completed data, we sample parameters from their full conditional posteriors in two steps: (1) Sample θ from f(θ|𝒟(Y)) and (2) sample ϕ and λj,k for each k = 1, ⋯, Kj and j = (E, T) from f(λ, θ|𝒟). This is iterated until the Markov chain converges, with posteriors computed using adaptive rejection Metropolis sampling (Gilks et al., 1995).
We now turn to Case 2, in which YE is evaluated only at UE. Analytically, Case 2 is much simpler than Case 1 because in Case 2 efficacy is YE with no random event time involved, and YE is missing completely at random (MCAR) at all V < UE. For the same reason, trial conduct in Case 2 is much harder logistically than in Case 1. This is because there is no XE in Case 2 and hence no partial information about YE when V < UE. Inference for πE relies entirely on observed YE values from previously treated patients, while YT may be imputed by exploiting the event time data using the marginal of XT.
Our proposed method can be applied to any phase I–II combination of probability model and decision rules based on [YE, YT | d] with delayed outcomes. To make things concrete, we apply the method to the Efficacy-Toxicity (EffTox) trade-off method of Thall and Cook (2004). Case 1 is illustrated by the trial of chemotherapy for acute leukemia where UT = UE = 42 days, and Case 2 by the stem cell transplantation trial with UT = 30 days and UE = 90 days.
4 EffTox Designs
4.1 The Design Assuming Complete Data
We first briefly review the EffTox phase I–II design (Thall and Cook, 2004) assuming complete data 𝒟(Y). The model for [YE, YT | d] assumes marginal probabilities πE = logit−1{μE + βE,1d + βE,2d2} and πT = logit−1{μT + βT,1d + βT,2d2} and uses a Gumbel copula (Murtaugh and Fisher, 1990) to obtain a joint distribution,
| (3) |
where ψ parameterizes association between YE and YT. To prevent mis-estimation with small sample sizes, we require that βE,1 + 2βE,2d > 0 and βT,1 + 2βT,2d > 0, corresponding to agents where both πT and πE increase with dose.
The desirability of a dose is defined by first defining the desirability δ(πE, πT) of each probability pair π = (πE, πT) in [0, 1]2; with δ(πE, πT) increasing in πE and decreasing in πT. Denoting the posterior means for j = E, T, the desirability of d is , for use as a decision criterion. The function δ may be obtained from a target efficacy-toxicity tradeoff contour, 𝒞, in [0, 1]2 in several ways (cf. Thall, Cook and Estey, 2006). To avoid doses that are too toxic or inefficacious, two admissibility criteria are imposed. Given elicited fixed lower limit π̲E on πE and upper limit π̄T on πT, a dose d is acceptable if
| (4) |
for prespecified cutoffs pE and pT. The trial starts at a dose chosen by the physician and each new cohort is treated with the acceptable dose having largest δ(d). An untried dose may not be skipped when escalating, and when escalating to an untried dose only the toxicity admissibility rule is imposed. If no dose is acceptable the trial is stopped with no dose selected.
We refer to the new version of the EffTox design that incorporates our proposed imputation methodology as the late onset (LO)-EffTox design. It differs from the EffTox design in one fundamental way, namely that event time data are exploited to compute posterior decision criteria using the Bayesian data augmentation methodology described in Section 3.
4.2 Prior Specification
In any model-based Bayesian adaptive clinical trial design, the prior must be sufficiently vague that the accumulating data dominates the posterior distribution, and thus the adaptive decisions. Thall and Cook (2004) provided a framework for establishing priors from elicited mean values of πE(d, θ) and πT (d, θ) that solves for prior hyperparameters using nonlinear least squares. We derive a prior based on the weakly informative prior for logistic regression proposed by Gelman et al. (2008). Following Gelman et al. (2008), for priors we assume μT, μE, βT,1, βE,1, βT,2, βE,2 ~ iid Cauchy(0, 2.5), and then shift the six Cauchy prior location parameters from 0 to μ̃ = (μ̃μT, μ̃βT,1, μ̃βT,2, μ̃μE, μ̃βE,1, μ̃βE,2), to reflect prior opinion. To obtain μ̃, similarly to Thall and Cook (2004), we first elicit the means m̃E,dr and m̃T,dr of πE(dr, θ) and πT(dr, θ) from the physician for each dose dr, r = 1, …, R. For each j = E, T, we use least squares to solve for (μ̃μj, μ̃βj,1, μ̃βj,2) by assuming .
To obtain a vague prior on λ, we assume [Xj |Yj = 1] ~ Unif(0, Uj), which implies that the hazard at the midpoint of the subinterval [hj,k−1, hj,k) of the partition is λ̃j,k = Kj/{Uj(Kj − k+0.5)}. We assume that λj,k ~ Gam(λ̃j,k/C, 1/C), where Gam(a, b) denotes the gamma distribution with mean a/b and variance a/b2. Thus, λj,k has prior mean λ̃j,k and variance Cλ̃j,k, so C is a tuning parameter that determines var(λj,k) and that can be calibrated by simulation. In preliminary simulations, we found that C = 2 yields a reasonably vague prior and a design with good operating characteristics. Finally, we assume ψ ~ Normal(0, 1) and ϕ ~ Gam(0.2, 0.2).
5 Computer Simulations
5.1 Clinical Trial Designs
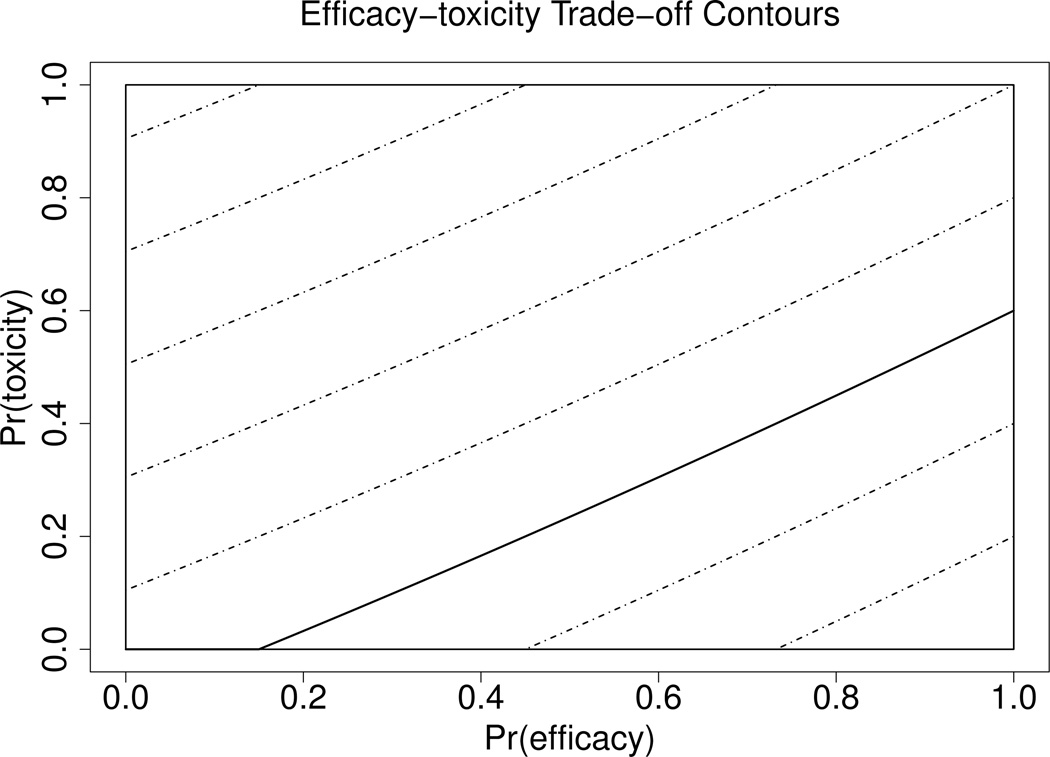
We simulated our proposed methodology to study its behavior when applied to the EffTox design for phase I–II trials in each of Cases 1 and 2. To assess robustness, we conducted a second set of simulations in which we varied several model, design, and simulation scenario parameters. In all simulations, we considered hypothetical phase I–II trials with five raw doses (2.5, 5.0, 7.5, 10.0, 12.5). In the model, we replaced each raw dose by the standardized dose , where s is the standard deviation of the centered log doses, so d1, ⋯, d5 are centered around 0 and have standard deviation .5. The trade-off contour, 𝒞, was determined by fitting a quadratic curve to the trade-off target probability pairs (πE, πT) = (0.15, 0), (0.45, 0.20), (1, 0.60), which gives target contour function . Figure 1 illustrates the target contour by a solid line, with contours on which all (πE, πT) have the same desirability θ(πE, πT) shown as dashed lines. Dose acceptability was determined by π̲E = 0.25 for efficacy and π̄T = 0.35 for toxicity, with decision cutoffs pE = pT = 0.10. In Case 2, a dose could be declared inefficacious due to the first inequality in (4) being violated only after at least one cohort was fully evaluated at that dose. In the first set of simulations, we considered trials with 16 cohorts of size three, so Nmax = 48. In practice, Nmax should be chosen via simulation by doing a sensitivity analysis that evaluates a design’s properties over a range of practically feasible Nmax values.
Figure 1.
Desirability Contours: The target contour is represented by a solid line. Other contours on which all (πE, πT) have the same desirability are shown as dashed lines.
The following designs are constructed to mimic dose-finding trials of either chemotherapy for acute leukemia in Case 1, or an agent that is part of a preparative regimen in the stem cell transplantation trial for multiple myeloma in Case 2. Recall that, in Case 1, XE may occur at any time during [0, UE], and, in Case 2, YE is observed at UE. For Case 1, we assumed that UE = UT = 6 weeks with accrual rate α = 1.5 patients per week. For Case 2, we assumed UE = 90 days, (12.85 weeks), toxicity evaluation interval UT = 30 days (4.3 weeks), and accrual rate α = 2.1 patients per week. The logistical difficulty indices are ζ = 1.5 × 6 = 9 for Case 1 and ζ = 2.1 × 12.85 = 27 for Case 2. We kept all other parameters for Case 2 the same as those in Case 1.
We assumed prior means 0.15, 0.20, 0.25, 0.30, 0.35 for πE(d1, θ), …, πE(d5, θ) and 0.15, 0.20, 0.27, 0.35, 0.45 for πT (d1, θ), …, πT(d5, θ). Applying the method described in Section 4.2, this gave location parameters (μ̃μE, μ̃βE,1, μ̃βE,2) = (−1.21, 0.96, 0.35) and (μ̃μT, μ̃βT,1, μ̃βT,2) = (−1.16, 1.39, 0.85) for the shifted Cauchy priors. For the Gamma piecewise exponential event rate priors, we assumed K = 6. The formula in Section 4.2 gives (λ̃E,1, ⋯, λ̃E,6) = (λ̃T,1, ⋯, λ̃T,6) = (0.182, 0.222, 0.286, 0.400, 0.667, 2.000) in Case 1, and (λ̃T,1, ⋯, λ̃T,6) = (0.364, 0.444, 0.571, 0.800, 1.333, 4.000) in Case 2. We used tuning parameter C = 2. Thus, for example, λE,1 ~ Gam(0.093, 0.5) in Case 1.
5.2 Simulation Study Design
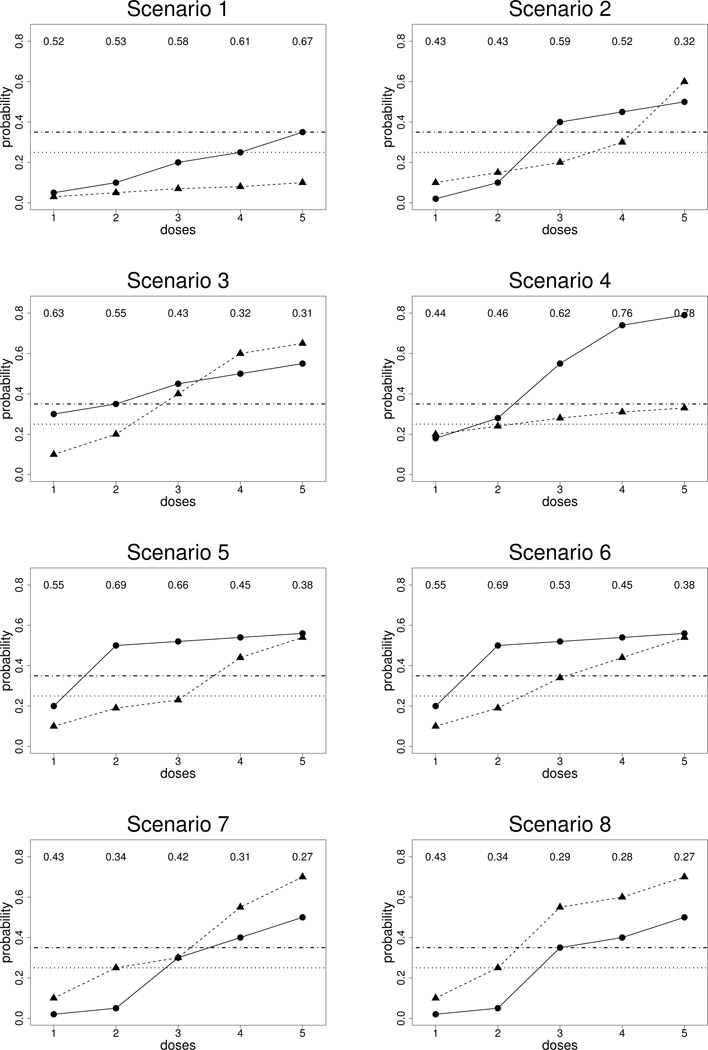
Each simulation scenario was specified in terms of assumed true efficacy and toxicity probabilities at each dose, πj(dr)true, for j = E, T and r = 1, …, 5. We modeled association between XE and XT by assuming a Clayton copula (Clayton, 1978) with ϕ = 1.0. We considered eight scenarios, illustrated in Figure 2. In Scenario 8, no dose is admissible, since d = 1,2 are inefficacious and d = 3,4,5 are too toxic. For the first simulation study, we generated XE, XT from Weibull distributions. For each j and dr, the Weibull scale and shape parameters were chosen so that (1) Pr(Xj ≤ Uj |dr)true = πj(dr)true and (2) πj,late(dr)true = Pr(Uj/2 ≤ Xj ≤ Uj |dr)true = 0.50, that is, 50% of the events occurred in the second half of the evaluation interval. Because πE(dr)true and πT (dr)true vary with dr in each scenario, the scale and shape parameters of the corresponding true Weibull distributions both vary with dr. Each scenario was simulated 1000 times.
Figure 2.
Illustration of scenarios: The solid lines with circles represent true probabilities of efficacy and the dashed lines with triangles represent true probabilities of toxicity. The horizontal lines represent π̄T and π̲E.
5.3 Simulation Results for Case 1
For Case 1, we compared the LO-EffTox design to three methods that are used in practice to deal with the late onset problem. The first method is the “One Level Down” rule. With this method, if some of the patients treated at the current optimal dose, dopt = dr have not yet been evaluated fully, i.e. Yi,E,dr = missing or Yi,T,dr = missing, then any new patient is treated at dr−1. The second method is the “Look Ahead” rule (Thall et al., 1999) which says that, for each possible value 𝒟̃mis(Y) that 𝒟mis(Y) may take on, use the completed data 𝒟obs(Y) ∪ 𝒟̃mis(Y) to compute dopt. If this dose is the same for all possible 𝒟̃mis(Y), then use that dose to treat the next patient immediately. Otherwise, the only two options for new patients are to make them wait to be treated, which usually is impossible in practice, or to turn them away and treat them off protocol. The third method uses all complete cases, where both YE and YT are observed, to compute dopt and treat the next patient immediately.
We will use two summary criteria to evaluate each method’s performance and compare the three methods. Denote the true desirability of dose dr by and the true set of acceptable doses by 𝒜true. The first criterion is the desirability-weighted selected percentage,
which quantifies dose selection reliability and thus potential benefit for future patients. The second criterion is the ratio NE/NT where NE and NT denote the number of patients who experienced efficacy and toxicity, respectively. This criterion quantifies benefit to the patients in the trial, hence may be considered an index of ethical desirability.
Table 1 gives the operating characteristics of the LO-EffTox design and three competing methods. The percentage of trials with no dose selected is denoted by “None,” with the numbers of patients turned away from the trial by the Look Ahead method given in parentheses. In general, LO-EffTox outperforms the One Level Down, Look Ahead rule, and Complete Case method. The One Level Down rule has much smaller correct selection rate and mean NE. The Look Ahead rule design performs roughly equivalently to LO-EffTox in terms of dose selection percentages and NE/NT, but the trial durations under the Look Ahead rule are dramatically larger compared to LO-EffTox. This is because the Look Ahead rule turns many patients away, while LO-EffTox treats all patients. Compared to the Complete Case method, LO-EffTox has either similar or higher correct selection percentages and more patients treated at doses with higher desirability. In Scenario 8, where all doses are inefficacious or too toxic, LO-EffTox has by far the largest probability of correctly stopping early and selecting no dose.
Table 1.
Simulation study comparing the four phase I–II designs in Case 1. The probability pairs given in parentheses for each dose level are (πE(dr)true, πT(dr)true).
| Method | Dose Level | NE/NT | Duration (Weeks) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | None | ||||
| Scenario 1 | (0.05, 0.03) | (0.10, 0.05) | (0.20, 0.07) | (0.25, 0.08) | (0.35, 0.10) | ||||
| δtrue | 0.52 | 0.53 | 0.58 | 0.61 | 0.67 | ||||
| LO-EffTox | % selected | 0.0 | 0.1 | 2.6 | 2.5 | 91.7 | 3.1 | 11.8/3.8 | 36.9 |
| # patients | 6.15 | 6.80 | 6.94 | 4.96 | 22.80 | ||||
| One Down | % selected | 0.0 | 0.7 | 6.0 | 16.1 | 76.6 | 0.6 | 5.7/2.4 | 37.0 |
| # patients | 18.89 | 13.69 | 10.42 | 4.46 | 0.50 | ||||
| Look Ahead | % selected | 0.0 | 0.2 | 2.4 | 2.5 | 88.7 | 6.2 | 13.1/4.0 | 69.7 |
| # patients | 3.40 | 3.84 | 4.30 | 3.97 | 30.90 | (52.07) | |||
| Complete Case | % selected | 0.0 | 0.2 | 2.4 | 3.4 | 91.8 | 2.2 | 9.8/3.3 | 37.6 |
| # patients | 11.18 | 8.11 | 6.76 | 5.43 | 16.40 | ||||
| Scenario 2 | (0.02, 0.10) | (0.10, 0.15) | (0.40, 0.20) | (0.45, 0.30) | (0.50, 0.60) | ||||
| δtrue | 0.43 | 0.43 | 0.59 | 0.52 | 0.32 | ||||
| LO-EffTox | % selected | 0.0 | 11.9 | 61.6 | 20.5 | 5.0 | 1.0 | 15.5/13.1 | 36.9 |
| # patients | 5.90 | 9.31 | 13.79 | 9.60 | 9.14 | ||||
| One Down | % selected | 0.0 | 1.0 | 5.1 | 24.3 | 69.1 | 0.5 | 8.3/7.6 | 37.2 |
| # patients | 18.73 | 13.51 | 10.49 | 4.67 | 0.51 | ||||
| Look Ahead | % selected | 0.0 | 8.0 | 67.5 | 19.0 | 4.6 | 0.9 | 16.7/12.5 | 74.2 |
| # patients | 3.39 | 7.14 | 20.58 | 9.95 | 6.67 | (57.54) | |||
| Complete Case | % selected | 0.0 | 8.9 | 44.3 | 27.4 | 18.5 | 0.9 | 13.1/11.9 | 37.0 |
| # patients | 11.27 | 10.19 | 9.86 | 8.56 | 7.95 | ||||
| Scenario 3 | (0.30, 0.10) | (0.35, 0.20) | (0.45, 0.40) | (0.50, 0.60) | (0.55, 0.65) | ||||
| δtrue | 0.63 | 0.55 | 0.43 | 0.32 | 0.31 | ||||
| LO-EffTox | % selected | 75.0 | 22.5 | 2.3 | 0.2 | 0.0 | 0.0 | 16.2/8.9 | 37.4 |
| # patients | 26.21 | 14.21 | 5.11 | 1.88 | 0.59 | ||||
| One Down | % selected | 77.6 | 14.4 | 4.7 | 2.6 | 0.0 | 0.7 | 15.5/7.3 | 37.1 |
| # patients | 31.30 | 12.79 | 3.34 | 0.33 | 0.00 | ||||
| Look Ahead | % selected | 63.8 | 32.6 | 3.3 | 0.1 | 0.0 | 0.2 | 16.4/8.9 | 62.4 |
| # patients | 24.79 | 16.39 | 5.32 | 1.23 | 0.23 | (39.65) | |||
| Complete Case | % selected | 78.5 | 17.2 | 2.0 | 1.2 | 0.0 | 1.1 | 15.7/8.1 | 37.0 |
| # patients | 28.99 | 12.44 | 4.61 | 1.27 | 0.30 | ||||
| Scenario 4 | (0.18, 0.20) | (0.28, 0.24) | (0.55, 0.28) | (0.74, 0.31) | (0.79, 0.33) | ||||
| δtrue | 0.44 | 0.46 | 0.62 | 0.76 | 0.78 | ||||
| LO-EffTox | % selected | 3.4 | 3.0 | 7.1 | 19.5 | 65.2 | 1.8 | 25.5/13.1 | 36.8 |
| # patients | 9.63 | 6.87 | 7.77 | 9.98 | 13.08 | ||||
| One Down | % selected | 9.1 | 5.8 | 9.2 | 29.3 | 40.4 | 6.2 | 13.9/10.6 | 35.7 |
| # patients | 23.22 | 12.06 | 7.57 | 2.76 | 0.23 | ||||
| Look Ahead | % selected | 8.5 | 6.1 | 11.1 | 21.0 | 51.8 | 1.5 | 26.5/13.2 | 70.8 |
| # patients | 9.45 | 5.48 | 6.77 | 9.85 | 15.83 | (52.81) | |||
| Complete Case | % selected | 6.9 | 2.8 | 8.2 | 18.5 | 57.6 | 6.0 | 20.3/11.8 | 35.7 |
| # patients | 15.70 | 7.79 | 8.02 | 7.67 | 6.73 | ||||
| Scenario 5 | (0.20, 0.10) | (0.50, 0.19) | (0.52, 0.23) | (0.54, 0.44) | (0.56, 0.54) | ||||
| δtrue | 0.55 | 0.69 | 0.66 | 0.45 | 0.38 | ||||
| LO-EffTox | % selected | 13.9 | 46.8 | 31.4 | 6.8 | 0.6 | 0.5 | 21.1/10.2 | 37.3 |
| # patients | 11.35 | 16.31 | 14.22 | 4.63 | 1.33 | ||||
| One Down | % selected | 10.8 | 22.6 | 39.3 | 22.1 | 4.0 | 1.2 | 17.5/7.7 | 37.2 |
| # patients | 21.61 | 16.20 | 8.29 | 1.42 | 0.03 | ||||
| Look Ahead | % selected | 10.7 | 48.3 | 37.7 | 3.1 | 0.1 | 0.1 | 21.9/10.4 | 76.1 |
| # patients | 8.23 | 18.61 | 15.77 | 4.36 | 0.98 | (60.26) | |||
| Complete Case | % selected | 13.2 | 40.7 | 34.4 | 9.7 | 1.1 | 0.9 | 19.6/9.4 | 37.2 |
| # patients | 15.67 | 15.23 | 11.96 | 3.97 | 0.85 | ||||
| Scenario 6 | (0.20, 0.10) | (0.50, 0.19) | (0.52, 0.34) | (0.54, 0.44) | (0.56, 0.54) | ||||
| δtrue | 0.55 | 0.69 | 0.53 | 0.45 | 0.38 | ||||
| LO-EffTox | % selected | 20.0 | 60.6 | 17.5 | 1.6 | 0.2 | 0.1 | 20.4/10.8 | 37.5 |
| # patients | 13.32 | 19.09 | 11.05 | 3.38 | 1.13 | ||||
| One Down | % selected | 14.9 | 37.5 | 35.1 | 9.7 | 1.6 | 1.2 | 17.3/8.4 | 36.9 |
| # patients | 21.78 | 17.25 | 7.68 | 0.83 | 0.01 | ||||
| Look Ahead | % selected | 16.1 | 65.4 | 17.1 | 1.2 | 0.1 | 0.1 | 21.2/10.8 | 73.8 |
| # patients | 10.18 | 23.41 | 10.76 | 2.87 | 0.74 | (56.73) | |||
| Complete Case | % selected | 21.7 | 50.3 | 21.5 | 5.3 | 0.4 | 0.8 | 18.9/9.7 | 37.3 |
| # patients | 17.69 | 17.31 | 9.65 | 2.50 | 0.56 | ||||
| Scenario 7 | (0.02, 0.10) | (0.05, 0.25) | (0.30, 0.30) | (0.40, 0.55) | (0.50, 0.70) | ||||
| δtrue | 0.43 | 0.34 | 0.42 | 0.31 | 0.27 | ||||
| LO-EffTox | % selected | 0.0 | 3.5 | 60.4 | 8.3 | 1.7 | 26.1 | 11.0/15.5 | 33.4 |
| # patients | 6.27 | 9.08 | 16.71 | 6.28 | 5.91 | ||||
| One Down | % selected | 10.9 | 4.3 | 10.9 | 29.7 | 37.4 | 6.8 | 13.9/10.7 | 35.5 |
| # patients | 23.48 | 11.90 | 7.48 | 2.62 | 0.19 | ||||
| Look Ahead | % selected | 9.6 | 8.3 | 22.1 | 47.2 | 11.8 | 1.0 | 25.4/14.9 | 74.3 |
| # patients | 10.00 | 6.26 | 9.31 | 15.72 | 6.38 | (57.84) | |||
| Complete Case | % selected | 0.0 | 3.0 | 49.3 | 11.4 | 6.6 | 29.7 | 8.8/13.8 | 34.1 |
| # patients | 11.27 | 12.21 | 11.94 | 5.28 | 4.44 | ||||
| Scenario 8 | (0.02, 0.10) | (0.05, 0.25) | (0.35, 0.55) | (0.40, 0.60) | (0.50, 0.70) | ||||
| δtrue | 0.43 | 0.34 | 0.29 | 0.28 | 0.27 | ||||
| LO-EffTox | % selected | 0.0 | 1.8 | 17.0 | 0.8 | 0.1 | 80.3 | 8.0/14.5 | 25.7 |
| # patients | 6.09 | 10.12 | 13.10 | 4.13 | 2.40 | ||||
| One Down | % selected | 0.0 | 1.8 | 33.9 | 14.8 | 7.2 | 42.3 | 5.4/11.9 | 34.3 |
| # patients | 18.71 | 14.62 | 9.86 | 2.28 | 0.07 | ||||
| Look Ahead | % selected | 0.0 | 1.8 | 18.7 | 0.5 | 0.1 | 78.9 | 8.3/13.6 | 59.7 |
| # patients | 3.33 | 7.93 | 16.40 | 3.73 | 1.26 | (50.76) | |||
| Complete Case | % selected | 0.0 | 2.3 | 22.1 | 2.9 | 1.2 | 71.5 | 7.3/13.9 | 29.6 |
| # patients | 11.25 | 12.78 | 10.99 | 4.07 | 1.91 | ||||
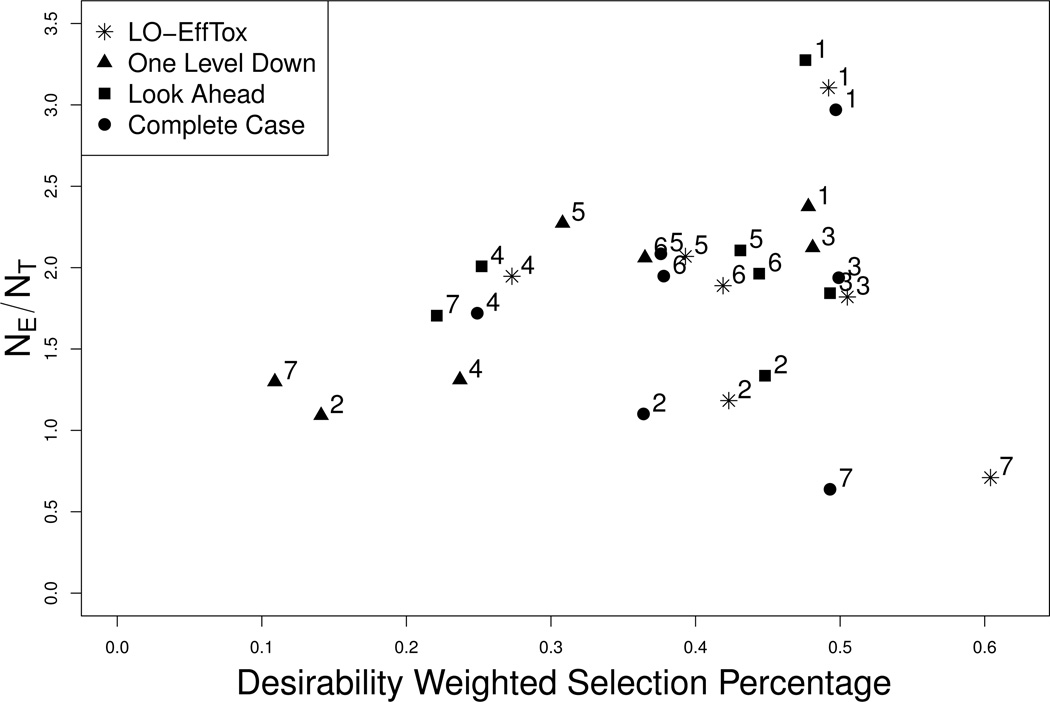
Figure 3 illustrates the results in Table 1 in terms of δ̄ plotted on the vertical axis and NE/NT on the horizontal axis, under each of Scenarios 1 – 7. Scenario 8 is not included in Figure 3 since in this case no dose is acceptable, so δ̄ is not relevant. Values in the upper right portion of the figure are more desirable, while values in the lower left are less desirable. Figure 3 shows that the One Level Down rule produces designs with very poor properties, in terms of both δ̄ and NE/NT. These two criteria are roughly equivalent for LO-EffTox and the Look Ahead version of EffTox for each of Scenarios 1 – 6, In Scenario 7, which has true desirability not monotone in dose, LO-EffTox has much greater δ̄ and much smaller NE/NT compared to the “Look Ahead” version of EffTox, so in this case there is no clear winner. However, since the Look Ahead rule turns away many patients and produces a very long trial, the apparent equivalence in terms of the two criteria in Figure 3 only tells part of the story. Compared to the Complete Case method, LO-EffTox has either similar or much larger δ̄ values and similar NE/NT.
Figure 3.
Comparison of the Late Onset Eff-Tox, One Level Down, Look Ahead, and Complete Case methods. The X-axis is δ̄, the desirability weighted selection percentage, and the Y-axis is NE/NT. Simulation scenarios are identified by integers.
5.4 Simulation Results for Case 2
For Case 2, we compared the LO-EffTox design to the One Level Down, Look Ahead, and Complete Case rules, and also the TiTE-CRM (Cheung and Chappell, 2000). We included the TiTE-CRM because, when the efficacy indicator YE is observed at UE, the only time-to-event variables are XT and V, which are the basis for the TiTE-CRM, so the TiTE-CRM is a reasonable alternative in Case 2. To implement the TiTE-CRM, we assumed the dose-toxicity model with fixed skeleton (p1, ⋯, p5) = (0.15, 0.20, 0.27, 0.35, 0.45), parameter α having N(0, σ2 = 2) prior, and target toxicity probability 0.35.
The simulation results for the Case 2 under six of the eight scenarios are summarized in Table 2. Results for the other two scenarios are summarized in Table S1. Table 2 shows that, across all scenarios, LO-EffTox greatly outperforms the One Level Down, Look Ahead, and Complete Case methods, in terms of both selection percentages and NE/NT. The “One Level Down” rule is most likely to select d3 or lower doses, and almost never selects d4 or d5, regardless of their δ values. It thus greatly sacrifices efficacy in many cases, as shown by the extremely small NE values. As in Case 1, the “Look Ahead” rule has selection percentages similar to those of the LO-EffTox design, but the price is a much longer trial with many patients turned away. Because the TiTE-CRM design completely ignores efficacy, it is unlikely to select doses having acceptable toxicity and high efficacy, which is the case in nearly all scenarios. As in Case 1, for Scenario 8 LO-EffTox has by far the largest probability of correctly stopping early and selecting no dose.
Table 2.
Simulation study comparing the five phase I–II designs in Case 2. The probability pairs given in parentheses for each dose level are (πE(dr)true, πT(dr)true).
| Method | Dose Level | NE/NT | Duration (Days) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | None | ||||
| Scenario 1 | (0.05, 0.03) | (0.10, 0.05) | (0.20, 0.07) | (0.25, 0.08) | (0.35, 0.10) | ||||
| δtrue | 0.52 | 0.53 | 0.58 | 0.61 | 0.67 | ||||
| LO-EffTox | % selected | 0.0 | 0.4 | 1.1 | 2.2 | 90.9 | 5.4 | 12.7/3.9 | 237 |
| # patients | 3.58 | 4.25 | 5.99 | 4.91 | 27.75 | ||||
| One Down | % selected | 0.0 | 4.8 | 94.2 | 1.0 | 0.0 | 0.0 | 2.9/1.6 | 246 |
| # patients | 38.74 | 9.23 | 0.03 | 0.00 | 0.00 | ||||
| Look Ahead | % selected | 0.0 | 0.0 | 1.6 | 1.9 | 91.0 | 5.5 | 13.3/4.0 | 399 |
| # patients | 3.25 | 3.49 | 4.54 | 6.11 | 29.26 | (49.2) | |||
| Complete Case | % selected | 0.0 | 2.7 | 7.3 | 6.3 | 80.7 | 3.0 | 6.2/2.4 | 244 |
| # patients | 28.96 | 3.64 | 3.91 | 3.35 | 7.97 | ||||
| TiTE-CRM | % selected | 0.0 | 0.0 | 0.0 | 0.0 | 99.9 | 0.1 | 14.2/4.3 | 240 |
| # patients | 3.22 | 3.23 | 3.18 | 3.40 | 34.92 | ||||
| Scenario 2 | (0.02, 0.10) | (0.10, 0.15) | (0.40, 0.20) | (0.45, 0.30) | (0.50, 0.60) | ||||
| δtrue | 0.43 | 0.43 | 0.59 | 0.52 | 0.32 | ||||
| LO-EffTox | % selected | 0.0 | 9.4 | 62.5 | 21.4 | 5.4 | 1.3 | 16.4/12.6 | 244 |
| # patients | 3.35 | 8.30 | 18.77 | 10.09 | 7.03 | ||||
| One Down | % selected | 0.0 | 1.8 | 96.3 | 1.4 | 0.0 | 0.5 | 1.6/5.2 | 246 |
| # patients | 38.72 | 9.17 | 0.04 | 0.00 | 0.00 | ||||
| Look Ahead | % selected | 0.1 | 8.9 | 56.9 | 27.9 | 5.7 | 0.5 | 17.3/13.2 | 436 |
| # patients | 3.27 | 6.46 | 17.54 | 13.38 | 7.24 | (58.8) | |||
| Complete Case | % selected | 0.0 | 5.3 | 29.2 | 32.0 | 33.2 | 0.3 | 7.9/9.1 | 246 |
| # patients | 29.11 | 3.65 | 5.10 | 5.09 | 5.04 | ||||
| TiTE-CRM | % selected | 0.0 | 0.7 | 14.0 | 70.8 | 13.3 | 1.2 | 18.3/15.7 | 238 |
| # patients | 3.99 | 4.39 | 8.27 | 18.97 | 11.89 | ||||
| Scenario 3 | (0.30, 0.10) | (0.35, 0.20) | (0.45, 0.40) | (0.50, 0.60) | (0.55, 0.65) | ||||
| δtrue | 0.63 | 0.55 | 0.43 | 0.32 | 0.31 | ||||
| LO-EffTox | % selected | 68.1 | 27.9 | 3.4 | 0.4 | 0.0 | 0.2 | 16.4/9.2 | 247 |
| # patients | 26.19 | 13.53 | 5.82 | 1.99 | 0.46 | ||||
| One Down | % selected | 44.9 | 8.0 | 47.0 | 0.1 | 0.0 | 0.0 | 14.8/5.6 | 246 |
| # patients | 38.98 | 8.99 | 0.02 | 0.00 | 0.00 | ||||
| Look Ahead | % selected | 58.3 | 32.2 | 8.7 | 0.7 | 0.0 | 0.1 | 16.7/9.7 | 420 |
| # patients | 22.21 | 16.28 | 7.54 | 1.69 | 0.23 | (54.0) | |||
| Complete Case | % selected | 85.1 | 11.3 | 2.0 | 1.5 | 0.1 | 0.0 | 14.9/6.2 | 247 |
| # patients | 39.77 | 5.24 | 2.39 | 0.57 | 0.03 | ||||
| TiTE-CRM | % selected | 1.1 | 30.1 | 62.3 | 5.1 | 0.0 | 1.4 | 20.1/17.4 | 238 |
| # patients | 5.32 | 12.13 | 18.08 | 8.98 | 2.90 | ||||
| Scenario 4 | (0.18, 0.20) | (0.28, 0.24) | (0.55, 0.28) | (0.74, 0.31) | (0.79, 0.33) | ||||
| δtrue | 0.44 | 0.46 | 0.62 | 0.76 | 0.78 | ||||
| LO-EffTox | % selected | 3.5 | 3.8 | 10.4 | 20.9 | 61.0 | 0.4 | 27.0/13.5 | 246 |
| # patients | 9.02 | 5.84 | 7.97 | 11.51 | 13.52 | ||||
| One Down | % selected | 17.0 | 7.5 | 73.1 | 1.2 | 0.0 | 1.2 | 9.6/9.9 | 245 |
| # patients | 38.64 | 9.09 | 0.05 | 0.00 | 0.00 | ||||
| Look Ahead | % selected | 6.4 | 6.1 | 14.8 | 23.1 | 48.5 | 1.1 | 27.1/13.4 | 445 |
| # patients | 7.58 | 6.17 | 9.38 | 10.55 | 13.93 | (62.0) | |||
| Complete Case | % selected | 11.4 | 6.1 | 7.8 | 20.1 | 54.4 | 0.2 | 15.3/11.0 | 246 |
| # patients | 31.64 | 4.12 | 3.79 | 3.82 | 4.60 | ||||
| TiTE-CRM | % selected | 1.1 | 5.6 | 19.1 | 28.0 | 40.2 | 6.0 | 25.9/12.8 | 231 |
| # patients | 6.37 | 6.89 | 9.16 | 10.50 | 12.62 | ||||
| Scenario 6 | (0.20, 0.10) | (0.50, 0.19) | (0.52, 0.34) | (0.54, 0.44) | (0.56, 0.54) | ||||
| δtrue | 0.55 | 0.69 | 0.53 | 0.45 | 0.38 | ||||
| LO-EffTox | % selected | 20.3 | 63.4 | 13.9 | 1.8 | 0.4 | 0.2 | 20.8/10.9 | 246 |
| # patients | 11.86 | 20.78 | 10.87 | 3.45 | 0.96 | ||||
| One Down | % selected | 8.0 | 12.4 | 79.2 | 0.4 | 0.0 | 0.0 | 12.4/5.6 | 247 |
| # patients | 38.73 | 9.24 | 0.03 | 0.00 | 0.00 | ||||
| Look Ahead | % selected | 16.7 | 52.2 | 26.3 | 3.8 | 0.9 | 0.1 | 21.6/11.5 | 402 |
| # patients | 9.10 | 20.03 | 13.71 | 4.26 | 0.86 | (48.6) | |||
| Complete Case | % selected | 27.8 | 40.2 | 22.8 | 7.1 | 2.0 | 0.1 | 14.4/7.6 | 246 |
| # patients | 32.84 | 7.63 | 5.38 | 1.82 | 0.33 | ||||
| TiTE-CRM | % selected | 0.0 | 11.8 | 51.6 | 30.2 | 4.6 | 1.8 | 23.5/16.7 | 237 |
| # patients | 4.47 | 7.42 | 14.68 | 13.57 | 7.10 | ||||
| Scenario 8 | (0.02, 0.10) | (0.05, 0.25) | (0.35, 0.55) | (0.40, 0.60) | (0.50, 0.70) | ||||
| δtrue | 0.43 | 0.34 | 0.29 | 0.28 | 0.27 | ||||
| LO-EffTox | % selected | 0.0 | 1.9 | 8.1 | 0.4 | 0.1 | 89.5 | 7.9/13.6 | 117 |
| # patients | 3.36 | 7.21 | 13.99 | 4.18 | 1.79 | ||||
| One Down | % selected | 0.0 | 4.2 | 90.1 | 1.4 | 0.0 | 4.3 | 1.3/6.1 | 247 |
| # patients | 38.62 | 9.31 | 0.05 | 0.00 | 0.00 | ||||
| Look Ahead | % selected | 0.0 | 1.6 | 18.8 | 1.1 | 0.1 | 78.4 | 8.2/13.4 | 367 |
| # patients | 3.22 | 8.10 | 16.11 | 3.79 | 1.17 | (53.5) | |||
| Complete Case | % selected | 0.0 | 8.2 | 33.5 | 8.7 | 3.9 | 45.7 | 5.0/10.6 | 204 |
| # patients | 28.81 | 4.85 | 7.18 | 2.84 | 1.15 | ||||
| TiTE-CRM | % selected | 5.8 | 58.7 | 31.7 | 0.9 | 0.0 | 2.9 | 9.1/17.5 | 236 |
| # patients | 8.00 | 17.23 | 14.29 | 5.65 | 1.69 | ||||
5.5 Sensitivity Analyses
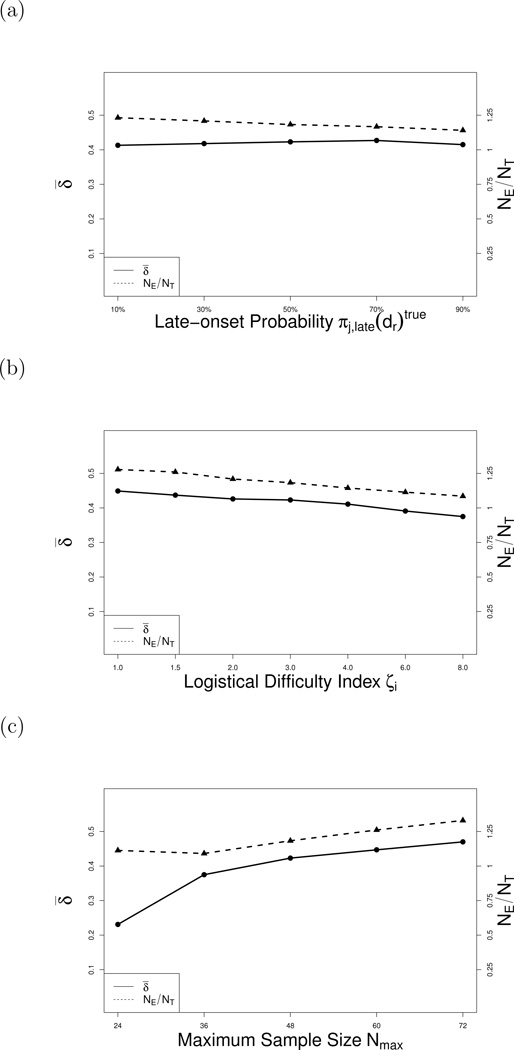
To investigate robustness, we conducted additional simulations in which we varied each of πj,late(d)true, logistical difficulty index ζ, Nmax, the event time distributions, K = number of sets in the piecewise exponential event time distribution partition, and the association parameter, ϕ. Each sensitivity analysis was conducted for all scenarios, and the results are summarized in Supplementary Tables S2 – S8. Table S2 shows that the design is not sensitive to changes in values of πj,late(d)true over the range 0.10 to 0.90, illustrated in Figure 4a. Tables S3 and S4 summarize sensitivity to the logistical difficulty index for the values ζ = 3.0 to 24.0 in Case 1 and ζ = 9.0 to 54.0 in Case 2. As ζ increases, the ratio NE/NT and δ̄ both decrease, illustrated in Figure 4b. Table S5 shows, as expected, that the design’s performance improves with larger Nmax, illustrated in Figure 4c. Simulations with XE and XT generated from several combinations of the Weibull and Log-logistic(Table S6) showed that LO-EffTox is robust to the true event time distributions. Table S7 shows that the number of sets in the partition of the piecewise exponential has little effect on the method’s performance for K = 6 to 12. Varying the association parameter from ϕ = 0.1 to 2.5 had almost no effect on performance (see Table S8).
Figure 4.
Sensitivity analyses under Scenario 2. Solid lines represent the desirability weighted selection percentages δ̄. Dashed lines represent the means of NE/NT.
6 Discussion
We have proposed a general methodology to address the problem of late-onset outcomes in phase I–II clinical trials. The method treats unobserved binary outcomes are as nonignorable missing data, uses data augmentation to impute the missing outcomes, and applies the design’s decision rules using the completed data. Simulations show that, in most cases, the proposed design performs better than alternative approaches to the late onset problem in phase I–II trials. Our results suggest that the general approach of imputing binary vectors Y by utilizing time-to-event variables used to define Y may improve the logistics of any outcome-adaptive procedure based on the distribution of Y.
Supplementary Material
Acknowledgments
This research was supported by NIH/NCI Cancer Center Support Grant CA016672 36. Yuan and Jin acknowledge support from NIH grant R01 CA154591. Peter Thall’s research was supported by NIH/NCI grant R01 CA 83932.
Appendix
The probability of YE = 1 with the known YT value is
where Sab and πa,b are defined by equation (2) and (3), respectively.
The probability of YT = 1 with the known YE value is
where Sab and πa,b are defined by equation (2) and (3), respectively.
When YT and YE are unknown, the probability of (YE, YT) is
where where Sab and πa,b are defined by equation (2) and (3), respectively, and S00 = 1.
References
- Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: Efficient dose escalation with overdose control. Statistics in Medicine. 1998;17:1103–1120. doi: 10.1002/(sici)1097-0258(19980530)17:10<1103::aid-sim793>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Bekele BN, Ji Y, Shen Y, Thall PF. Monitoring late-onset toxicities in phase I trials using predicted risks. Biostatistics. 2008;9:442–457. doi: 10.1093/biostatistics/kxm044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekele BN, Shen Y. A Bayesian approach to jointly modeling toxicity and biomarker expression in a phase I/II dose-finding trial. Biometrics. 2005;61:343–354. doi: 10.1111/j.1541-0420.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- Braun TM. The bivariate continual reassessment method: Extending the CRM to phase I trials of two competing outcomes. Controlled Clinical Trials. 2002;23:240–256. doi: 10.1016/s0197-2456(01)00205-7. [DOI] [PubMed] [Google Scholar]
- Braun TM. Generalizing the TiTE-CRM to adapt for early- and late-onset toxicities. Statistics in Medicine. 2006;25:2071–2083. doi: 10.1002/sim.2337. [DOI] [PubMed] [Google Scholar]
- Bryant J, Day R. Incorporating toxicity considerations into the design of two-stage phase II clinical trials. Statistics in Medicine. 1995;51:1372–1383. [PubMed] [Google Scholar]
- Cheung Y, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56:1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- Clayton D. A model for association in bivariate life tables and its application in epidemiological studies of familial tendency in chronic disease incidence. Biometrika. 1978;65:141–151. [Google Scholar]
- Conaway M, Dunbar S, Peddada S. Design for single- or multiple-agent phase I trials. Biometrics. 2004;60:661–669. doi: 10.1111/j.0006-341X.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- Gehan E. Estimating survival functions from the life table. Journal of Chronic Diseases. 1969;21:629–644. doi: 10.1016/0021-9681(69)90035-6. [DOI] [PubMed] [Google Scholar]
- Gelman A, Jakulin A, Pittau MG, Su Y. A weakly informative default prior distribution for logistic and other regression models. The Annals of Applied Statistics. 2008;2:1360–1383. [Google Scholar]
- Gilks WR, Best NG, Tan KKC. Adaptive rejection Metropolis sampling within Gibbs sampling. Applied Statistics. 1995;44:455–472. [Google Scholar]
- Gooley TA, Martin PJ, Fisher LD, Pettinger M. Simulation as a design tool for phase I/II clinical trials: An example from bone marrow transplantation. Controlled Clinical Trials. 1994;15:450–462. doi: 10.1016/0197-2456(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Murtaugh P, Fisher I. Bivariate binary models of efficacy and toxicity in dose-ranging trials. Communications in Statistics, Part A - Theory and Methods. 1990;19:2003–2020. [Google Scholar]
- O’Quigley J, Hughes MD, Fenton T. Dose-finding designs for HIV studies. Biometrics. 2001;57:1018–1029. doi: 10.1111/j.0006-341x.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- O’Quigley J, Pepe M, Fisher L. Continual reassessment method: A practical design for phase I clinical trials in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- Simon RM. Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. [PubMed] [Google Scholar]
- Tanner MA, Wong WH. The calculation of posterior distributions by data augmentation (with discussion) Journal of the American Statistical Association. 1987;82:528–550. [Google Scholar]
- Thall P, Cook J. Dose-finding based on efficacy-toxicity trade-offs. Biometrics. 2004;60:684–693. doi: 10.1111/j.0006-341X.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Thall P, Lee J, Tseng C-H, EH E. Accrual strategies for phase I trials with delayed patient outcome. Statistics in Medicine. 1999;18:1155–1169. doi: 10.1002/(sici)1097-0258(19990530)18:10<1155::aid-sim114>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Thall PF, Russell KE. A strategy for dose-finding and safety monitoring based on efficacy and adverse outcomes in phase I/II clinical trials. Biometrics. 1998;54:251–264. [PubMed] [Google Scholar]
- Thall PF, Simon RM. Practical bayesian guidelines for phase IIB clinical trials. Biometrics. 1994;50:337–349. [PubMed] [Google Scholar]
- Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Statistics in Medicine. 1995;14:357–379. doi: 10.1002/sim.4780140404. [DOI] [PubMed] [Google Scholar]
- Yin G, Li Y, Ji Y. Bayesian dose-finding in phase I/II clinical trials using toxicity and efficacy odds ratios. Biometrics. 2006;62:777–787. doi: 10.1111/j.1541-0420.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Yin G. Bayesian phase I/II adaptively randomized oncology trials with combined drugs. The Annals of Applied Statistics. 2011a;5:924–942. doi: 10.1214/10-AOAS433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Yin G. Robust EM continual reassessment method in oncology dose finding. Journal of the American Statistical Association. 2011b;106:818–831. doi: 10.1198/jasa.2011.ap09476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sargent DJ, Mandrekar S. An adaptive dose-finding design incorporating both toxicity and efficacy. Statistics in Medicine. 2006;25:2365–2383. doi: 10.1002/sim.2325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.