Abstract
Objectives
To investigate the number of leiomyoma patients-exposed to bisphenol A (BPA) and to observe whether the serum concentration of BPA is related to leiomyoma growth.
Methods
A total of 158 patients were recruited for this study. Leiomyoma patients were divided into three groups, mild (n = 48), moderate (n = 32) and severe (n = 28), according to the size of leiomyomas. The control (n = 30) group was defined as having no leiomyomas. Transvaginal ultrasonography was used to identify and measure the leiomyomas. Serum BPA concentrations were measured by enzyme-linked immunosorbent assay.
Results
BPA was detected in 87.0% out of a total of 158 samples, and in 86.0% out of 108 leiomyoma patients. In detail, the detection rates of serum BPA were 88.0% in the control group, 77.2% in the mild group, 90.0% in the moderate group and 96.0% in the severe group. The mean BPA concentration in the control group was 0.558 ± 0.097 ng/mL, the leiomyoma groups, the mean BPA concentrations were 0.274 ± 0.063 ng/mL (mild), 0.346 ± 0.064 ng/mL (moderate) and 0.647 ± 0.039 ng/mL (severe) (P = 0.0003). Values represent the mean ± standard error.
Conclusion
The detection rates of serum BPA in the control and leiomyoma groups were 88.0% and 86.0%, respectively. However, there was no significant difference in the serum BPA concentrations between the control and leiomyoma groups. To verify the effect of BPA on leiomyoma growth, a close and sequential monitoring is recommended for people who are at risk for uterine leiomyoma.
Keywords: Endocrine disruptors, Leiomyoma, Uterus
Uterine leiomyoma is the most common type of benign neoplasm in the female reproductive system, and is the most common indication for hysterectomy in premenopausal women.1 However, despite its frequent occurrence, the causes of uterine leiomyoma remain unclear. It is known that the size of uterine leiomyoma is increased by exposure female hormones, such as estrogen secreted by the ovaries, because uterine leiomyoma usually occurs in young women. Such leiomyomas decrease in size after menopause, and can be effectively treated by gonadotropin-releasing hormone (GnRH) agonist.1 The levels of estrogen receptor in leiomyomas are higher than in normal myometrium, indicating that leiomyoma is more sensitive to stimulation by estrogen.2
Recently, concerns about environmental pollution have increased. It has been reported that synthetic chemicals disturb the reproductive system by acting as endocrine disruptors. In 2008, bisphenol A (BPA), an endocrine disrupting chemical, was regulated as a hazardous substance for the first time worldwide in Canada, when sales of baby bottles with BPA were prohibited there.3 BPA has two unsaturated phenol rings and is mostly found in plastic and canned products, and it is taken into the body through ingestion. BPA binds estrogen receptors, acts as an estrogen and is detectable in the serum.4 However, there were no difference in BPA levels between a group of women with simple endometrial hyperplasia and a control group,4 while Newbold et al.5 found that exposure to BPA for 18 months increased the occurrence of myoma in mice. Elucidating the relationship between BPA and the development of uterine leiomyoma will be invaluable for reducing the incidence and size of uterine leiomyomas in women. We investigated human BPA exposure levels by measuring serum BPA levels in women with uterine leiomyomas and the relationship between BPA level and leiomyoma growth by stratifying women according to leiomyoma size.
Material and Methods
The subjects of this study were 200 women who visited 2 University Hospital and were diagnosed with uterine leiomyoma by transvaginal ultrasound from March 10, 2010 to July 31, 2013. Thirty women who visited the hospital for gynecological check-ups without uterine leiomyoma and other diseases were placed into the control group. The uterine leiomyoma patients were stratified into 3 groups by leiomyoma size. The mild group consisted of patients with 1-2 uterine leiomyomas under 3 cm, the moderate group consisted of patients with more than 1 uterine leiomyoma around 3-5 cm, and the severe group consisted of patients with more than 1 uterine leiomyoma over 5 cm. Blood was collected from patients who provided informed consent after an explanation of the study purpose and of the blood collection method. Blood was drawn from a brachial vessel and stored in a tube coated with ethylenediaminetetraacetic acid. The serum was separated and placed in a -70℃ freezer until analysis.
The pretreatment and serum extraction processes were performed in two steps. First, enzymatic hydrolysis was induced by β-glucuronidase treatment to convert BPA from the glucuronidated form to the free form in serum. Through this process, the total BPA level in the serum was measured. Second, high molecular weight proteins, carbohydrates, and lipid components in the serum were excluded, and samples were concentrated using an solid phase extraction (SPE) column (Isolute M-M cartridge) as the solvent. A 100 µL serum sample was taken and mixed with 200 µL acetic acid buffer solution (pH 5.0 [1.2 g sodium acetate, 1 mL acetic acid, 0.15 g 1-ascorbic acid, 0.01 g EDTA-2Na, 100 mL distilled water]) and 10 µL β-glucuronidase (from Helix pomatia, 22,000 U/mL; Sigma-Aldrich, St. Louis, MO, USA). The samples were incubated for 18 h at 37℃.
After incubation, the mixtures were processed with an Isolute M-M column (sorbent mass: 500 mg, reservoir volume: 3 mL; Biotage), which was pretreated with 10 mL methanol and 6 mL distilled water. The column was cleaned with 6 mL 35% methanol, and eluted with 2.5 mL 100% methanol. The nitrogen gas was uniformly evaporated by eluted methanol, and the sample extract was then adjusted to contain 10% methanol by mixing it with 10 µL of 100% methanol. BPA from the sample, which was dissolved in methanol, was analyzed.
Enzyme linked immunosorbent assay (ELISA) with an Ecologiena® Supersensitive BPA ELISA kit (Japan EnviroChemicals, Ltd., Tokyo, Japan) was used to measure BPA. This method was developed based on the principle that BPA monoclonal antibody bonds with BPA specifically. It is known that lot-to-lot variation rarely occurs because of the qualitative superiority of monoclonal antibodies. It is also known that results in this measurement range are rare. The measuring range was 0.05-10 µg/L (ppb), and this range is highly sensitive to measuring BPA in serum. According to the manufacturer's information, the results were reproducible and repeatable, and the coefficient of variation (CV) was below 10%. In addition, the cross-reactivities (CR) with other endocrine disruptors and estrogen were rare with the BPA kit that was used in this study.
Two independent sample t-tests were used to compare the average level of BPA between the control group and the uterine leiomyoma group. Comparisons of the average level among the control group and the three stratified uterine leiomyoma groups were performed by one-way analysis of variance (ANOVA), and a post-hoc multiple comparison was performed by two independent sample t-tests. P values < 0.05 were considered statistically significant.
Results
The total number of samples was 158, including 48 in the mild group, 32 in the moderate group, 28 in the severe group, and 50 in the control group. There were no statistically significant differences in the mean age or body mass index (BMI) among each group (each, P = 0.836, P = 0.854) (Table 1).
Table 1.
Statistical comparisons of the four patient groups
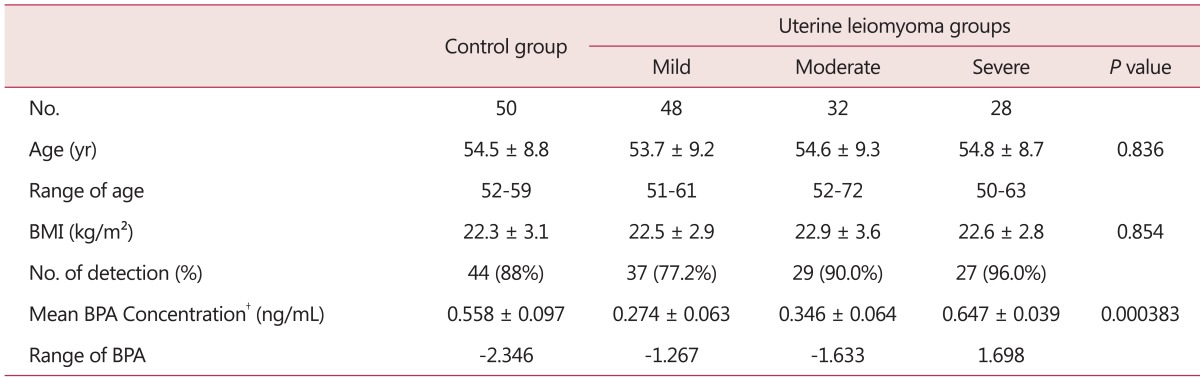
Mean ± standard deviation, *mean ± standard error, †non-detected.
BMI: body mass index, BPA: bisphenol A
The median of the control group was 0.558 ng/mL, that of the mild group was 0.274 ng/mL, that of the moderate group was 0.346 ng/mL, and that of the severe group was 0.647 ng/mL. BPA was detected in 137 cases out of 158 (87.0%). BPA was detected in 26 (86.7%) cases out of 30 in the control group, and 84 (83.1%) cases out of 101 in the uterine leiomyoma groups taken together. BPA was detected in 37 cases (77.2%) in the mild group, 29 cases (90.0%) in the moderate group, and 27 (96.0%) in the severe group (Table 1).
The mean ± standard deviation of the BPA level for all subjects was 0.448 ± 0.047 ng/mL. There was no significant difference in the mean ± standard deviation of the BPA level between the uterine leiomyoma group (0.403 ± 0.049 ng/mL) and the control group (0.558 ± 0.097 ng/mL) (P = 0.08). However, there were significant differences in the mean ± standard deviation of the BPA level among the control group and three subgroups, the latter of which had 0.274 ± 0.063 ng/mL in the mild group, 0.346 ± 0.064 ng/mL in the moderate group, and 0.647 ± 0.039 ng/mL in the severe group (P = 0.0003) (Table 1).
The average level of BPA in the control group was significantly higher than the level in the mild and the moderate groups by post-hoc multiple comparisons (each, P = 0.007, P = 0.03). The average level of BPA in the severe group was also significantly higher than the mild and the moderate groups by post-hoc multiple comparisons (each, P = 0.0001, P = 0.003). However, there were no significant differences in the average level of BPA between the control group and the severe group or between the mild group and the moderate group (each, P = 0.48, P = 0.43).
Discussion
Estrogens exhibit biologic effects via estrogen receptors within target cells.6 Environmental estrogens have chemical structures similar to human estrogen; therefore, they also can bind with estrogen receptors, and are classified as phyto-estrogens or xeno-estrogens. Typical xeno-estrogens are BPA, benzo(a)pyrene (B[a]P), diethylstilbestrol (DES), and selective estrogen receptor modulators (SERMs). Thus, xeno-estrogens may disturb natural estrogen function and act as antagonists of estrogen function in vivo in cases of long term exposure.7 Most are classified as endocrine disruptors, which are associated with feminization such as early puberty and breast buds, and are related to breast cancers.8,9 Although little is known about the relationship between xeno-estrogens and uterine leiomyoma, the causal relationships between uterine leiomyoma and natural human estrogen have been well known.10
BPA, which is a xeno-estrogen, is mostly found in plastic products, and is used in nursing bottles, dishes, food containers, polycarbonate plastics (used in vinyl wrap), cans, and epoxy resin (which is in the amalgam used for dental treatment).11 It enters the body through food in most cases, although exposure can also occur through water, air, and dust. BPA was detected in the serum of pregnant women and fetuses, the human placenta, and breast milk.12~14 The level of BPA in containers depends on temperature rather than the amount of time they contain food.15 It has been reported in animal studies that BPA may result in reproductive disorders when long exposure occurs during the developmental process even at minimal levels.16 Few studies have investigated the effects of BPA exposure on human development and disorders, and studies measuring serum BPA, in particular, are rare. Takeuchi and Tsutsumi et al.17 reported that BPA was measured at 1.49 ± 0.11 ng/mL (mean ± standard error) in males and 0.64 ± 0.1 ng/mL in females. This is similar to the results of the present study.
The levels of estrogen receptors are increased in uterine leiomyomas, which also have lower levels of apoptosis than normal myometrium.18 GnRH agonist therapy produces estrogen ablation, which decreases the size of uterine leiomyomas. This shows that leiomyoma cells are hypersensitive to estrogen stimulation. Therefore, leiomyomas are a potential target of endocrine disruption by xeno-estrogens. It is important to understand that xeno-estrogens act as agonists on the myometrium via the estrogen receptors. In the present study, we assessed the relationship between uterine leiomyoma and BPA levels in the blood. Although there were no differences in the blood BPA level among groups, some statistical differences were present among subgroups classified by size of uterine leiomyoma; the more severe the uterine leiomyoma was, the higher the average level of BPA.
This is the first multi-center trial to investigate BPA levels in uterine leiomyoma patients. However, we are limited in our ability to conclude there is a causal relationship between existing uterine leiomyoma and BPA level in the blood. To further examine the pathogenesis of uterine leiomyoma, a closer look at high risk subjects and sequential studies are needed, and many factors related to uterine leiomyoma incidence should be considered. Additional studies are also needed to clarify the identification of the molecular mechanisms that determine agonist activity on the myometrium of the uterus. Furthermore, studies of the relationships between BPA and other gynecological disorders, such as adenomyosis are needed.
Acknowlegement
This paper was supported by Wonkwang University in 2013.
References
- 1.Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104:393–406. doi: 10.1097/01.AOG.0000136079.62513.39. [DOI] [PubMed] [Google Scholar]
- 2.Hunter DS, Hodges LC, Eagon PK, Vonier PM, Fuchs-Young R, Bergerson JS, et al. Influence of exogenous estrogen receptor ligands on uterine leiomyoma: evidence from an in vitro/in vivo animal model for uterine fibroids. Environ Health Perspect. 2000;108(Suppl 5):829–834. doi: 10.1289/ehp.00108s5829. [DOI] [PubMed] [Google Scholar]
- 3.Cao XL, Dufresne G, Belisle S, Clement G, Falicki M, Beraldin F, et al. Levels of bisphenol A in canned liquid infant formula products in Canada and dietary intake estimates. J Agric Food Chem. 2008;56:7919–7924. doi: 10.1021/jf8008712. [DOI] [PubMed] [Google Scholar]
- 4.Hiroi H, Tsutsumi O, Takeuchi T, Momoeda M, Ikezuki Y, Okamura A, et al. Differences in serum bisphenol a concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocr J. 2004;51:595–600. doi: 10.1507/endocrj.51.595. [DOI] [PubMed] [Google Scholar]
- 5.Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 2007;24:253–258. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosselli M, Reinhart K, Imthurn B, Keller PJ, Dubey RK. Cellular and biochemical mechanisms by which environmental oestrogens influence reproductive function. Hum Reprod Update. 2000;6:332–350. doi: 10.1093/humupd/6.4.332. [DOI] [PubMed] [Google Scholar]
- 7.Hiroi H, Tsutsumi O, Momoeda M, Takai Y, Osuga Y, Taketani Y. Differential interactions of bisphenol A and 17beta-estradiol with estrogen receptor alpha (ERalpha) and ERbeta. Endocr J. 1999;46:773–778. doi: 10.1507/endocrj.46.773. [DOI] [PubMed] [Google Scholar]
- 8.Stratakis CA, Vottero A, Brodie A, Kirschner LS, DeAtkine D, Lu Q, et al. The aromatase excess syndrome is associated with feminization of both sexes and autosomal dominant transmission of aberrant P450 aromatase gene transcription. J Clin Endocrinol Metab. 1998;83:1348–1357. doi: 10.1210/jcem.83.4.4697. [DOI] [PubMed] [Google Scholar]
- 9.Hess-Wilson JK, Boldison J, Weaver KE, Knudsen KE. Xenoestrogen action in breast cancer: impact on ER-dependent transcription and mitogenesis. Breast Cancer Res Treat. 2006;96:279–292. doi: 10.1007/s10549-005-9082-y. [DOI] [PubMed] [Google Scholar]
- 10.Baird DD, Newbold R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod Toxicol. 2005;20:81–84. doi: 10.1016/j.reprotox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Haishima Y, Hayashi Y, Yagami T, Nakamura A. Elution of bisphenol-A from hemodialyzers consisting of polycarbonate and polysulfone resins. J Biomed Mater Res. 2001;58:209–215. doi: 10.1002/1097-4636(2001)58:2<209::aid-jbm1009>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 13.Kuruto-Niwa R, Tateoka Y, Usuki Y, Nozawa R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere. 2007;66:1160–1164. doi: 10.1016/j.chemosphere.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 14.Schonfelder G, Flick B, Mayr E, Talsness C, Paul M, Chahoud I. In utero exposure to low doses of bisphenol A lead to long-term deleterious effects in the vagina. Neoplasia. 2002;4:98–102. doi: 10.1038/sj.neo.7900212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi T, Tsutsumi O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun. 2002;291:76–78. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- 18.Hodges LC, Hunter DS, Bergerson JS, Fuchs-Young R, Walker CL. An in vivo/in vitro model to assess endocrine disrupting activity of xenoestrogens in uterine leiomyoma. Ann N Y Acad Sci. 2001;948:100–111. doi: 10.1111/j.1749-6632.2001.tb03991.x. [DOI] [PubMed] [Google Scholar]


