Abstract
Background
Endoscopic ampullectomy is increasingly performed in patients with FAP (familial adenomatous polyposis)-associated ampullary adenomas. We sought to define the procedure-associated morbidities and long-term outcomes.
Methods
We performed a retrospective chart review of patients with FAP who underwent endoscopic ampullectomy at two tertiary institutions between 1999 and 2010. The severity of duodenal polyposis was classified according to Spigelman's classification.
Results
Of 26 FAP patients who underwent endoscopic ampullectomy, 21 arose in the setting of Spigelman's stage II duodenal polyposis. Adverse events associated with endoscopic ampullectomy included acute pancreatitis (19.2%), abdominal pain (7.6%), and bleeding (3.8%). The mean resected adenoma size was 0.99 ± 0.34 cm. Three adenomas (12.0%) contained foci of high-grade dysplasia. Follow-up data were available for 24 patients. The mean follow-up duration was 84.5 ± 36.2 months. Adenoma recurrence was observed in 14 patients (58.3%, 14/24) at a mean of 38.3 months after initial ampullectomy. Adenomas ≥ 10 mm recurred more frequently than smaller adenomas (76.9% vs. 36.4%, p=0.002). Positive margins were not associated with higher recurrence rates. No cancers were observed during long-term follow-up. Three patients underwent a Whipple procedure, but none was performed due to a recurrent ampullary adenoma.
Conclusions
Endoscopic ampullectomy in FAP can be performed safely. Because ampullary adenomas frequently recur after endoscopic ampullectomy, close surveillance is essential. Smaller tumors are less likely to recur, suggesting a benefit for early recognition of these lesions.
Introduction
Familial adenomatous polyposis (FAP) is an autosomal dominantly inherited disorder caused by germline mutations in the APC gene. In the classic form of FAP, colonic polyposis develops in over 90% of affected individuals by age 35. The lifetime risk of colorectal cancer is nearly 100% in the absence of colectomy1. The duodenum is the second most common site of polyp formation. Observed in up to 90% of individuals with FAP, duodenal polyps are usually recognized 10-20 years after the diagnosis of colonic polyposis. Duodenal/periampullary adenocarcinoma is the second most common cause of cancer death in FAP, and the cumulative risk of duodenal cancer is estimated to be as high as 10% by age 60 years, which is 100-300-fold higher than the general population.1, 2
Ampullary tumors develop via an adenoma-to-carcinoma sequence, as observed in the colon.3 These lesions are increasingly identified in asymptomatic patients by surveillance endoscopy. Management options include local or extended surgical resection (including pancreaticoduodenectomy (PD), pancreas-sparing duodenectomy (PSD), or transduodenal excision (TDE)) and endoscopic resection.4-6 Although outcomes from surgery may be excellent, morbidity and mortality rates are not insignificant (range; 4 to 15%) and depend upon the experience of the hospital and surgeon.7 Furthermore, FAP patients may be poor operative candidates due to abdominal desmoid disease.
Although surgery is indicated in cases of duodenal and ampullary cancer, less invasive endoscopic approaches are now commonly performed in FAP patients with benign ampullary adenomas. However, there are limited data on long-term outcome associated with this procedure. It is unknown what long-term recurrence rates are and whether removal of ampullary adenomas prevents adenocarcinoma. The goal of this study was to define procedure-associated morbidities and long-term recurrence rates after endoscopic ampullectomy for ampullary adenomas in FAP patients.
Patients and Methods
We performed a retrospective chart review and identified patients who underwent endoscopic ampullectomy for a histologically defined ampullary adenoma and also carried a diagnosis of FAP at Massachusetts General Hospital and Brigham and Women's Hospital between the years 2000 to 2010. Patients who had a diagnosis of ampullary adenocarcinoma were excluded, and those who developed ampullary adenomas in the absence of a diagnosis of FAP were excluded. The diagnosis of FAP was established by the presence of a germline APC mutation and/or clinical criteria including diffuse colonic adenomatous polyposis, extracolonic manifestations including fundic gland polyposis, duodenal or ampullary adenomas, osteomas, desmoid tumors, and a family history of FAP. APC gene testing was performed commercially in Clinical Laboratory Improvement Act (CLIA) -approved laboratories.
Data extracted from medical records included age, gender, extracolonic manifestations, results of upper and lower gastrointestinal endoscopic exams, colectomy, endoscopic ampullectomy, and genetic testing. The severity of duodenal polyposis was assessed by the Spigelman classification.8 The severity of acute pancreatitis was assessed by Ranson's severity index. All slides from resected ampullary adenomas were reviewed by one pathologist for the presence of dysplasia and the involvement of lateral and deep resection margins. This study was approved by the Institutional Review Boards of Massachusetts General Hospital and Brigham and Women's Hospital.
Statistical Analysis
Statistical analysis included the chi-square test, Fisher's exact test, Cox proportional hazard regression analysis, Kaplan-Meier method and Log-Rank test. Data were presented as mean ± SD. The chi-square test was used to determine whether there was an association between positive margins and the resection method (en bloc vs. piecemeal resection), or association between positive margins and ampullectomy technique (submucosal injection of saline). Cox regression analysis was used to evaluate the impact and correlation of multiple variables to recurrence. The recurrence free function was estimated by Kaplan-Meier method, and the significance was analyzed by the Log-Rank test. A P value of < 0.05 was considered statistically significant.
Results
Patient Characteristics
The study group consisted of twenty-six patients with FAP that underwent endoscopic ampullectomy between 2000 and 2010. 17 patients were female (65%) and 9 were male (35%). 15 patients had deleterious germline mutations in the APC gene. Genetic testing was not performed in the remaining 11, and their diagnoses were established by clinical criteria including: a personal history of colonic polyposis, FAP-associated extracolonic manifestations, and a positive family history of FAP. The mean age at diagnosis of FAP was 27.8 ± 9.9 years (range, 16-47 years). Among men, the mean age was 36.1 ± 7.1 years (range, 27-47 years) and the mean age of the women was 23.4 ± 8.2 years (range, 16-46 years) (Table 1) (p=0.001).
Table 1. Baseline features of FAP patients who underwent endoscopic ampullectomy (N=26).
| General Characteristics | |
|---|---|
| Sex (M: F), n (%) | 9 (34.6): 17(65.4) |
| Mean age at diagnosis of FAP, years (range) | 27.8 ± 9.9 (16-47) |
| FAP diagnoses, n (%) | 26 |
| FAP family | 18 (69.2) |
| FAP de novo | 5 (19.2) |
| AFAP* family | 1 (3.9) |
| AFAP de novo | 2 (7.7) |
| APC gene testing, n (%) | |
| Positive | 15 (57.7) |
| Not done | 11 (42.3) |
| Colectomy, n (%) | 23 (88.5) |
| Mean age at colectomy, years (range) | 30.7 ± 8.1 (17-47) |
| Spigelman's stage at diagnosis, n (%) | |
| Stage I | 1 (3.8) |
| Stage II | 21 (80.9) |
| Stage III | 3 (11.5) |
| Stage IV | 1 (3.8) |
| Diagnosis of ampullary adenoma, n (%) | |
| Made at initial screening endoscopy | 15 (57.7) |
| Made at surveillance endoscopy | 11 (42.3) |
AFAP: attenuated FAP
Ampullary adenomas were diagnosed at a mean age of 40.4 ± 13.8 years (range, 19-76 years). The mean age among men was 42.0±14.6 years (range, 26-76 years) and 39.5 ± 13.8 years among women (range, 19-63 years) (p=0.43). In 7 patients (26.9%), the diagnosis was made by biopsy of an endoscopically normal appearing ampulla.
Fifteen of the 26 cases (57.7%) were diagnosed at the first upper endoscopic exam performed for screening. The remaining 11 cases were diagnosed at subsequent surveillance examinations. 3 patients who were diagnosed at their second surveillance endoscopy did not have a targeted biopsy of a normal appearing ampulla at their first endoscopy. The distributions of Spigelman stages of duodenal polyposis at the time of initial diagnosis were: stage I (1 case, 3.8%), stage II (21 cases, 80.9%), stage III (3 cases, 11.5%), and stage IV (1 case, 3.8%). This patient with Spigelman stage IV disease had significant concurrent illnesses including morbid obesity, prior abdominal surgery, chronic obstructive lung disease, and insulin dependent diabetes. For these reasons, a nonsurgical approach was initially pursued.
Colectomies had been previously performed in 23 cases, and colorectal cancer was diagnosed in 3 cases. The mean age of colectomy was 30.7 ± 8.1 years (range, 17-47 years). Three patients did not undergo colectomy. In one patient, the number of colonic polyps was relatively stable and low (∼20). The second patient refused surgery. The third patient had attenuated FAP, and the relatively small number of colonic adenomas (∼ 3-4 polyps at each annual colonoscopic examination) was managed endoscopically. Six patients (23.1%) were diagnosed with an extra-colonic malignancy prior to ampullectomy, and these are described in Table 2.
Table 2. Individual features of 26 FAP patients at the time of endoscopic ampullectomy.
| Patient No. | Age at diagnosis of ampullary adenoma | Sex | Spigelman stage at diagnosis | Ampulla pathology | Malignancies prior to ampullectomy |
|---|---|---|---|---|---|
| A#1 | 19 | F | Stage II | TA* with LGD | Thyroid carcinoma |
| A#2 | 53 | F | Stage IV | Adenoma with HGD, LM(+)**/DM(+)*** |
|
| A#3 | 45 | M | Stage II | TA with LGD, LM(+)/DM(+) |
|
| A#4 | 26 | M | Stage II | Adenoma with LGD | |
| A#5 | 20 | F | Stage II | TA with LGD | |
| A#6 | 49 | F | Stage II | TA with LGD | Endometrial carcinoma |
| A#7 | 63 | F | Stage II | TA with LGD LM(+)/DM(+) |
Skin cancer |
| A#8 | 32 | F | Stage II | TA with LGD | |
| A#9 | 38 | F | Stage II | TA with LGD | |
| A#10 | 28 | F | Stage II | TA with LGD, DM(+) | |
| A#11 | 23 | F | Stage I | TA with LGD, DM(+) | |
| A#12 | 45 | M | Stage III | TA with LGD | Rectal cancer |
| A#13 | 48 | M | Stage II | Adenoma with LGD LM(+)/DM(+) |
Sigmoid cancer |
| A#14 | 55 | F | Stage II | TA with LGD LM(+) |
|
| A#15 | 36 | M | Stage II | TA with LGD DM(+) |
|
| B#1 | 42 | F | Stage III | Adenoma with HGD, LM(+)/DM(+) |
|
| B#2 | 39 | F | Stage II | TA with LGD, LM(+) | Papillary thyroid cancer |
| B#3 | 28 | F | Stage III | TA with HGD, LM(+) | |
| B#4 | 34 | F | Stage II | TA with LGD, LM(+) | Endometrial adenocarcinoma |
| B#5 | 33 | M | Stage II | TA with LGD | Rectal cancer |
| B#6 | 59 | F | Stage II | failure to retrieve ampullectomy tissue | |
| B#7 | 52 | F | Stage II | TA with LGD, LM(+)/DM(+) |
|
| B#8 | 32 | M | Stage II | TA with LGD | |
| B#9 | 38 | F | Stage II | Adenoma with LGD LM(+)/DM(+) |
|
| B#10 | 76 | M | Stage II | TA with LGD LM(+)/DM(+) |
Duodenal cancer |
| B#11 | 37 | M | Stage II | TA with LGD LM(+) |
TA: tubular adenoma
LM(+): lateral resection margin positive
DM(+): deep resection margin positive
Ampullectomy technique
All ampullectomies were performed on an outpatient basis. Submucosal lifting with saline injection was performed in 5 patients. There were 2 methods used for ampullectomy, en bloc resection in 17 patients (65.4%) and piecemeal resection in the remaining 9 (34.6%). Post-ampullectomy stent placement was attempted in all patients and was successful in 19 (73.1%). In 9 patients, both a pancreatic and a biliary stent were placed. A single pancreatic stent was placed in 9 patients, and a single biliary stent was placed in 1 patient. Stents were not successfully placed in the remaining 7 cases (26.9%).
Pathology of ampullectomy specimens
25 of the 26 resected ampullary polyps were evaluated pathologically (Table 3). In 1 case, the sample was not successfully retrieved. All the ampullary tumors were benign adenomas. The mean adenoma size was 0.99 ± 0.34 cm (range, 0.4-2.0cm). In 3 patients (12.0%), high-grade dysplasia (HGD) was identified within the adenoma, and the mean size of these 3 adenomas was 1.10 ± 0.20 cm (range, 0.9-1.3 cm). The mean size of adenomas resected en bloc was 0.90 ± 0.28 cm (range, 0.4-1.2 cm) and 1.16 ± 0.40 cm (range, 0.7-2.0 cm) for those resected piecemeal.
Table 3. Pathology of ampullectomy samples.
| Number (%) | |
|---|---|
| Mean size of ampullary adenoma | 0.99 ± 0.34 cm (range 0.4-2.0 cm) |
| Histopathology * (n=25) | |
| Resection margins | |
| Positive | 16 (64.0) |
| Deep margin positive | 11 (44.0) |
| Lateral margin positive | 13 (52.0) |
| Both margins positive | 8 (32.0) |
| Negative | 9 (36.0) |
| High-grade dysplasia | 3 (12.0) |
Failure to retrieve ampullectomy specimen in 1 patient
Positive resection margins were identified in 16 cases (64.0%). Of these 16, 13 patients (52.0%) had lateral resection margin involvement by adenomatous tissue, and 11 patients (44.0%) showed deep margin involvement. In 8 patients (32.0%), both resection margins were involved by adenomatous tissue. There was no difference in the rate of positive resection margins with respect to the method of resection (en bloc resection vs. piecemeal resection, p=0.495) or submucosal injection of saline (p=0.22).
Follow-up after ampullectomy and adverse events
Two patients did not return for follow-up upper endoscopic examinations after the initial ampullectomy. The remaining 24 patients who presented for follow-up were analyzed. The mean duration of follow-up was 84.5 ± 36.2 months (range, 19-153 months). 208 follow-up procedures were performed among these 24 patients, and those included 180 EGDs, 21 ERCPs and 7 enteroscopies (Table 4). The mean interval between follow-up procedures was 12.2 ± 5.6 months (range 1-56 months). The mean number of follow-up endoscopies was 8.6 ± 6.0 per patient (range 2-26 exams).
Table 4. Post-ampullectomy follow-up examinations.
| Ampullectomy follow-up (n=24) | |
|---|---|
| Mean total follow-up duration, months (range) | 84.5 ± 36.2 (19-153) |
| Total examinations, n (%) | 208 |
| EGD | 180 (86.5) |
| ERCP | 21 (10.1) |
| Enteroscopy | 7 (3.4) |
| Mean interval between follow-up procedures, months (range) | 12.2 ± 5.6 (1-56) |
| Mean number of follow-up exams per patient, n (range) | 8.6± 6.0 (2-26) |
| Adverse events with follow-up procedures, n (%) | 7 patients (29.2) |
| Post-ERCP acute pancreatitis | 4 (16.7) |
| Post-ampullectomy stenosis | 4 (16.7) |
| Post-ERCP abdominal pain | 1 (4.2) |
| Biopsy-induced pancreatitis | 1 (4.2) |
| Atrial fibrillation | 1 (4.2) |
| Ampullary adenoma recurrence, n (%) | 14 (58.3) |
| Mean time interval to recurrence, months (range) | 38.3 ± 37.9 (7-110) |
8 out of the 26 patients (30.8%) experienced an acute adverse event after the initial ampullectomy. The most frequent adverse event was acute pancreatitis that required admission (n=5, 19.2%). Three had one Ranson's severity criteria of acute pancreatitis. The remaining two patients met no severity criteria. Pancreatitis resolved in all cases with conservative treatment. The median length of hospital stay was 2.8 ± 1.3 days (range, 2-5 days). Acute pancreatitis occurred in 2 patients who received a pancreatic stent and also in 3 who did not.
One patient had severe abdominal pain attributed to spontaneous biliary stent migration into the small bowel. This resolved with conservative management. Another patient developed epigastric pain and was hospitalized for 2 days. The third patient had hematemesis and hematochezia and was admitted for 3 days with transfusion of 2 units of red blood cells. Bleeding was controlled with epinephrine injection to the ampullectomy site.
Seven patients experienced adverse events related to procedures performed during the follow-up surveillance interval (29.2%, 7/24). The most frequent adverse events in this post-ampullectomy surveillance interval were acute pancreatitis (16.7%, 4/24) and ampullary stenosis (16.7%, 4/24) (Table 4). One case of pancreatitis was severe and was complicated by ARDS. This procedure involved biopsy of two small nodules at the ampullectomy site, and the pathologic analysis revealed adenoma. No stent insertion was performed. The patient required hospitalization for 14 days (including an ICU stay for 10 days). The second patient developed post-ERCP pancreatitis without placement of a pancreatic stent and had a second episode at an ERCP 9 years later that did include pancreatic stent placement. The third patient developed pancreatitis 2 days after ERCP. The fourth patient developed pancreatitis after an ampullary biopsy. These latter three patients recovered within 2-3 days of admission with conservative management.
Post-ampullectomy stenosis occurred in 4 patients at a mean interval from the initial ampullectomy of 75.0 ± 39.3 months (range 19-111 months). Sphincterotomy with placement of biliary and pancreatic stents was performed in 3 patients, and balloon papilloplasty was performed for mild stenosis in the 4th patient.
Adenoma Recurrence
Among the 24 patients who had follow-up endoscopic exams, adenoma recurrence was detected in 14 patients (58.3%) over a mean follow-up period of 84.5 ± 36.2 months. The mean interval to recurrence was 38.3 ± 37.9 months (range, 7-110 months). Biopsy-proven recurrence was detected in 2 patients with endoscopically normal ampullae (14.3%, 2/14). Specific details regarding these 14 recurrences are described in Table 5. Among these 14, six patients were diagnosed at their first follow-up upper gastroduodenal endoscopy. Patients who had an adenoma less than 10 mm had a mean recurrence free interval of 95.8±9.7 months (range: 16-110 months, median: 104 months), and the overall recurrence rate in this group was 36.4% (4/11). However, those with an adenoma greater than or equal to 10 mm had a significantly shorter recurrence free interval of 34.7 ± 8.9 months (range: 7-56 months, median: 16 months), and the overall recurrence rate increased to 76.9% (10/13) (p=0.002) (Fig. 1). Cox multivariate regression analysis indicated that larger adenoma size was an independent predictor of recurrence (p=0.04), and there was no significant correlation between age, Spigelman stage, resection method, positive margin, history of duodenal adenoma and/or fundic gland polyps and recurrence of ampullary adenoma (p=0.93, 0.07, 0.19, 0.71, 0.64, respectively). No invasive ampullary cancers were diagnosed during follow-up.
Table 5. Features of 14 recurrent ampullary adenomas.
| Patient Number | Histology | Size of initial adenoma (cm) | Recurrence free interval (mo) | Number of surveillance exams when recurrence diagnosed | Treatment |
|---|---|---|---|---|---|
| A#2 | TA*, VA**, with HGD*** | 1.3 | 7 | 1 | Endoscopic biopsy |
| A#3 | TA | 1.0 | 11 | 1 | Endoscopic biopsy |
| A#5 | TA | 0.8 | 91 | 7 | Endoscopic biopsy |
| A#6 | Adenoma with HGD | 0.8 | 110 | 15 | 2nd ampullectomy |
| A#7 | TA | 1.2 | 10 | 2 | Endoscopic biopsy |
| A#9 | TA | 1.1 | 16 | 2 | APC fulguration |
| A#11 | TA | 0.7 | 104 | 5 | Endoscopic biopsy |
| A#12 | TA | 1.0 | 13 | 1 | 2nd ampullectomy |
| A#15 | TA with LGD**** | 1.2 | 16 | 1 | APC fulguration |
| B#1 | Adenoma | 0.9 | 16 | 2 | Endoscopic biopsy |
| B#3 | TA | 1.1 | 56 | 1 | 2nd ampullectomy |
| B#5 | Adenoma | 1.2 | 23 | 4 | Endoscopic biopsy |
| B#8 | Adenoma | 2.0 | 7 | 1 | Endoscopic biopsy |
| B#11 | Adenoma | 1.0 | 56 | 3 | 2nd ampullectomy |
TA: tubular adenoma
VA: villous adenoma
HGD: high grade dysplasia
LGD: low grade dysplasia
Figure 1. Risk of adenoma recurrence based upon adenoma size.
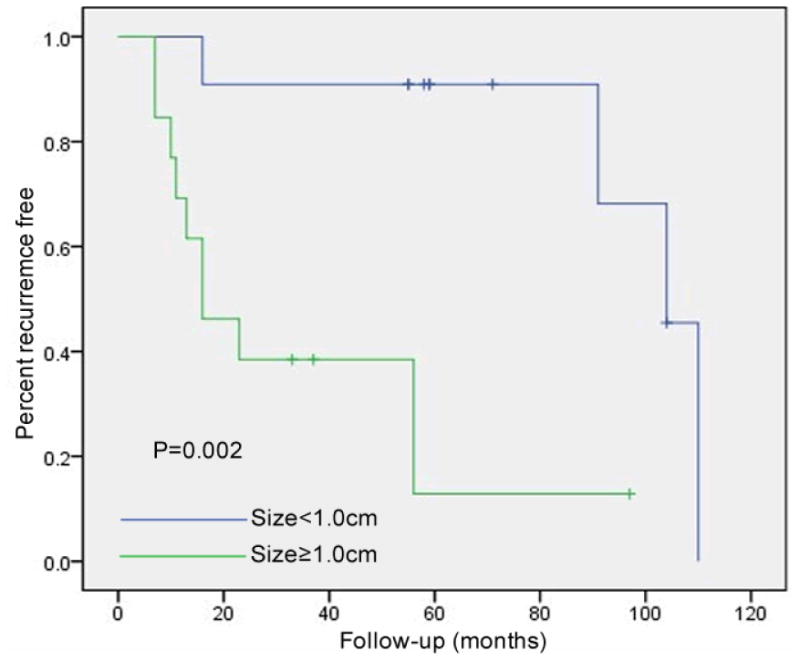
Adenoma size is correlated with risk of recurrence. Patients with an adenoma greater than or equal to 10 mm demonstrated an increased rate of recurrence (76.9% vs. 36.4%, p=0.002).
Patients who had their adenomas removed en bloc had a 46.7% recurrence rate (7/15), and patients who had a piecemeal resection exhibited a 77.8% recurrence rate (7/9) (p=0.29). The mean recurrence-free intervals were 74.3 ± 10.7 months (range: 13-104 months, median: 91 months) and 46.6±16.3 months (range: 7-110 months, median: 16 months), respectively. Although there was a trend towards a greater recurrence-free survival with en bloc resection at 60 months (70.7% vs 28.6%), this was not statistically significant (p=0.15) (Fig. 2).
Figure 2. Risk of adenoma recurrence based upon resection method.
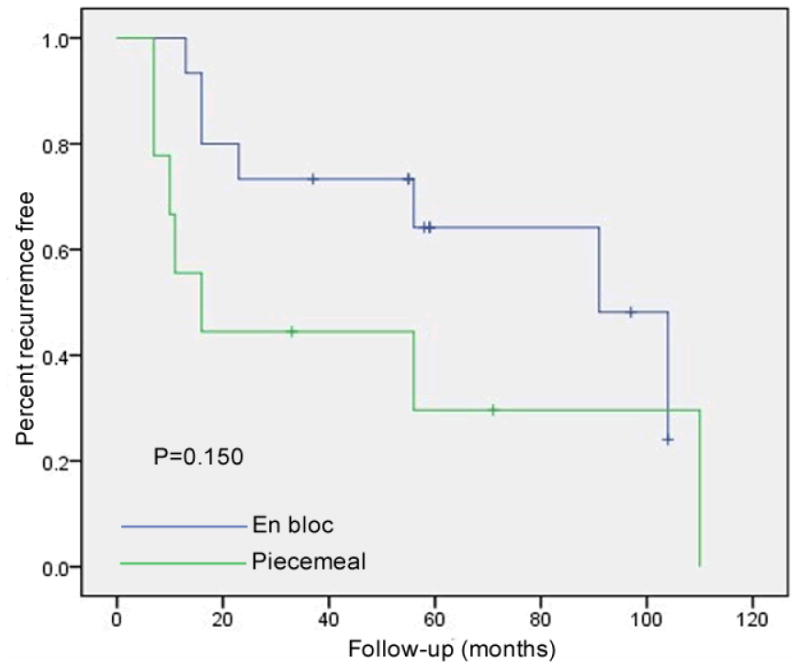
The recurrence-free rate of patients with en bloc resection was much higher than those with piecemeal resection (57.1% vs. 22.2%). However, it was not statistically significant (p=0.150).
There was no correlation between recurrence and resection margins. Recurrences were observed in 6 patients with negative margins (66.7%, 6/9) with a mean recurrence-free interval of 64.1 ± 16.5 months (7-110 months) and in 8 patients with positive margins (57.1%, 8/14) with a mean recurrence-free interval of 60.9 ± 12.2 months (7-104 months). The 5-year recurrence free survival rate was 56.1% for those with negative resection margins and 47.2% for those with positive resection margins (p=0.72) (Fig. 3).
Figure 3. Risk of adenoma recurrence based upon histologic margins.
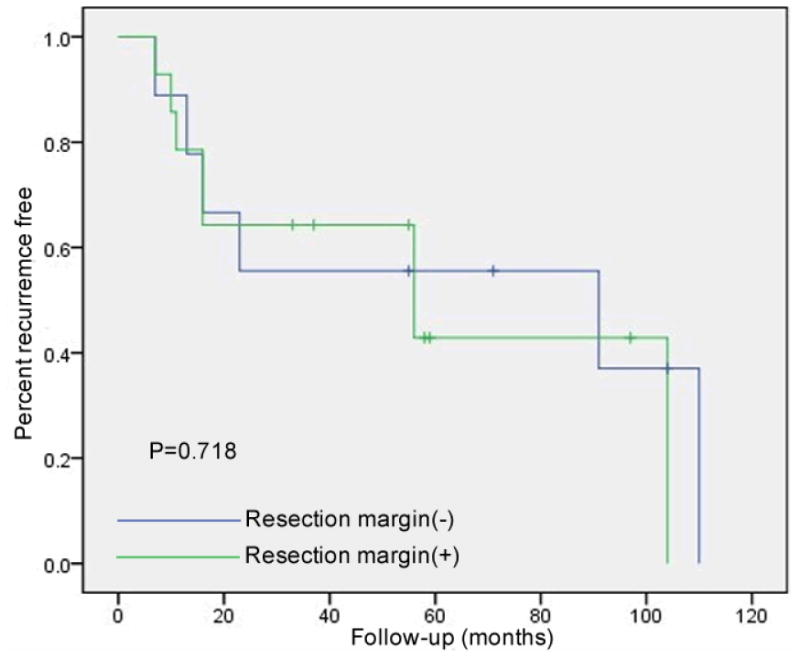
There was a trend toward a lower recurrence rate in cases with an initial complete resection based upon negative histologic margins. The recurrence free rate was 33.3% in the patients who had negative margins on their initial ampullectomy specimens, and 46.2% in patients with positive margins (p=0.718).
Among the 14 recurrences, a second ampullectomy was performed in 4 patients (15.4%, 4/26) at a mean interval of 58.8±39.7 months after the initial ampullectomy. In the remaining 10 recurrences, APC fulguration was performed in 2 patients, and polypectomy with biopsy forceps was performed in the remaining 8 patients.
Three patients ultimately had a Whipple procedure performed. None was performed for ampullary adenoma recurrence. In 2 cases, the indications were progressive duodenal polyposis with high grade dysplasia. The Spigelman stages at the time of surgery were III and IV. The 3rd patient elected to have a Whipple procedure because of concerns over future complications with endoscopic surveillance in the setting of prior severe pancreatitis. In this case, the Spigelman stage was II at the time of surgery.
Discussion
As prophylactic colectomy has become the standard of care in FAP patients, concerns over the development of associated extracolonic malignancies have become more prevalent.9 The management of ampullary adenomas in FAP is not standardized, and we sought to define long-term outcome with an endoscopic approach. We identified a high recurrence rate (58.3%) of ampullary adenomas following endoscopic ampullectomy over an 85 month period. Adenoma size ≥ 10 mm was associated with a higher recurrence rate (76.1% vs 36.4%), but there was no correlation with resection method, positive margins, or Spigelman stage of duodenal polyposis. Pancreatitis was the most common complication (19.2%) of endoscopic ampullectomy, and there were no deaths. No ampullary cancers were identified during follow-up.
Our observed recurrence rate is significantly higher than prior reports.10-12 Patel reported a recurrence rate of 16% after endoscopic ampullectomy over a mean follow-up period of only 17.2 months.13 Only 9 of the 38 cases in this series were associated with FAP. In another series of 193 patients who underwent endoscopic ampullectomy, most had sporadic disease. The adenoma recurrence rate in the sporadic group was 6% over a follow-up period of 32 months but rose to 17% in the sub-group of 17 FAP patients over a follow-up period of 48 months.14 Catalano reported on 103 patients undergoing endoscopic ampullectomy, 31 of whom had FAP.15 A 4% recurrence rate was observed in the sporadic group vs. 23% in the FAP group over a 36 month follow-up period. Our study was not designed to determine whether recurrent lesions represented re-growth of previously resected adenomas or emergence of new adenomas, and this distinction is difficult to make. However, because our study population was composed entirely of FAP patients, it is reasonable to speculate that the higher rate we observed was attributable to the underlying lifelong genetic risk for new adenoma formation. Our series has the longest follow-up interval (>7 years), and these data indicate that with longer follow-up, ampullary adenomas will recur in most FAP patients.
Microscopic adenomatous change is relatively common in FAP patients. Burke et al.16 noted that 54% of FAP patients harbored ampullary adenomas, even when the ampullae had a normal appearance on direct examination. In our series, 26.9% of adenomas were diagnosed histologically when biopsies were obtained of macroscopically normal appearing ampullae at the original examination, and in 14.3% of cases at follow-up.
Although the current study is one of the largest series of FAP-associated ampullary adenomas with long-term follow-up, the relatively small number of patients does not permit broad generalizations. However, we suggest endoscopic ampullectomy can be used as a first line therapeutic option. Recurrences after ampullectomy are much more common than previously appreciated but can be managed safely with additional endoscopic therapy. Follow-up exams should be accompanied by a biopsy at the ampullectomy site, even if it appears normal endoscopically. Because 9/13 recurrences were diagnosed within 2 years after the initial ampullectomy, we agree with endoscopic surveillance at 6-month intervals in the first two years after ampullectomy.17 Subsequently, annual surveillance is desirable to detect late recurrences. No invasive ampullary cancers were identified during surveillance in our series, suggesting a possible protective benefit of endoscopic ampullectomy. Despite the high recurrence rate, only a minority of patients ultimately required Whipple surgery, and the primary indication for surgery was progressive duodenal polyposis. For those with ampullary adenomas in the setting of advanced duodenal polyposis (Spigelman stage IV), surgery may be a more appropriate first-line approach. Because of the correlation between smaller adenoma size and lower risk of recurrence, early recognition and treatment of ampullary adenomas may be warranted in FAP patients.
Acknowledgments
Grant Support: Kate J. and Dorothy L. Clapp Fund (DCC), NCI/NIH K24CA11343 (SS)
Footnotes
Disclosure: The authors indicated no potential conflicts of interest. No writing assistance was used.
References
- 1.Vasen HF, Bulow S, Myrhoj T, Mathus-Vliegen L, Griffioen G, Buskens E, Taal BG, Nagengast F, Slors JF, de Ruiter P. Decision analysis in the management of duodenal adenomatosis in familial adenomatous polyposis. Gut. 1997;40:716–719. doi: 10.1136/gut.40.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjork J, Akerbrant H, Iselius L, Bergman A, Engwall Y, Wahlström J, Martinsson T, Nordling M, Hultcrantz R. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology. 2001;121:1127–1135. doi: 10.1053/gast.2001.28707. [DOI] [PubMed] [Google Scholar]
- 3.Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:301–309. doi: 10.1007/s00534-004-0898-3. [DOI] [PubMed] [Google Scholar]
- 4.Brosens LA, Keller JJ, Offerhaus GJ, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54:1034–1043. doi: 10.1136/gut.2004.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarmiento JM, Thompson GB, Nagorney DM, Donohue JH, Farnell MB. Pancreas-sparing duodenectomy for duodenal polyposis. Arch Surg. 2002;137:557–562. doi: 10.1001/archsurg.137.5.557. discussion 562-563. [DOI] [PubMed] [Google Scholar]
- 6.van Heumen BW, Nieuwenhuis MH, van Goor H, Mathus-Vliegen LE, Dekker E, Gouma DJ, Dees J, van Eijck CH, Vasen HF, Nagengast FM. Surgical management for advanced duodenal adenomatosis and duodenal cancer in Dutch patients with familial adenomatous polyposis: a nationwide retrospective cohort study. Surgery. 2012;151:681–690. doi: 10.1016/j.surg.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 8.Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783–785. doi: 10.1016/s0140-6736(89)90840-4. [DOI] [PubMed] [Google Scholar]
- 9.Attard TM, Cuffari C, Tajouri T, Stoner JA, Eisenberg MT, Yardley JH, Abraham SC, Perry D, Vanderhoof J, Lynch H. Multicenter experience with upper gastrointestinal polyps in pediatric patients with familial adenomatous polyposis. Am J Gastroenterol. 2004;99:681–686. doi: 10.1111/j.1572-0241.2004.04115.x. [DOI] [PubMed] [Google Scholar]
- 10.Wong RF, DiSario JA. Approaches to endoscopic ampullectomy. Curr Opin Gastroenterol. 2004;20:460–467. doi: 10.1097/00001574-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Bertoni G, Sassatelli R, Nigrisoli E, Bedogni G. Endoscopic snare papillectomy in patients with familial adenomatous polyposis and ampullary adenoma. Endoscopy. 1997;29:685–688. doi: 10.1055/s-2007-1004281. [DOI] [PubMed] [Google Scholar]
- 12.Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45–49. doi: 10.1007/s00464-008-9866-3. [DOI] [PubMed] [Google Scholar]
- 13.Patel R, Davitte J, Varadarajulu S, Wilcox CM. Endoscopic resection of ampullary adenomas: complications and outcomes. Dig Dis Sci. 2011;56:3235–3240. doi: 10.1007/s10620-011-1826-4. [DOI] [PubMed] [Google Scholar]
- 14.Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, Gluck M, Jiranek G, Patterson D, Schembre D, Traverso LW, Kozarek RA. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923–932. doi: 10.1016/j.gie.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Catalano MF, Linder JD, Chak A, Sivak MV, Jr, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225–232. doi: 10.1016/s0016-5107(03)02366-6. [DOI] [PubMed] [Google Scholar]
- 16.Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49:358–364. doi: 10.1016/s0016-5107(99)70013-1. [DOI] [PubMed] [Google Scholar]
- 17.Standards of Practice Committee. Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, Rajan E, Shen B, Zuckerman MJ, Fanelli RD, Van Guilder T, Baron TH. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849–854. doi: 10.1016/j.gie.2006.08.044. [DOI] [PubMed] [Google Scholar]


