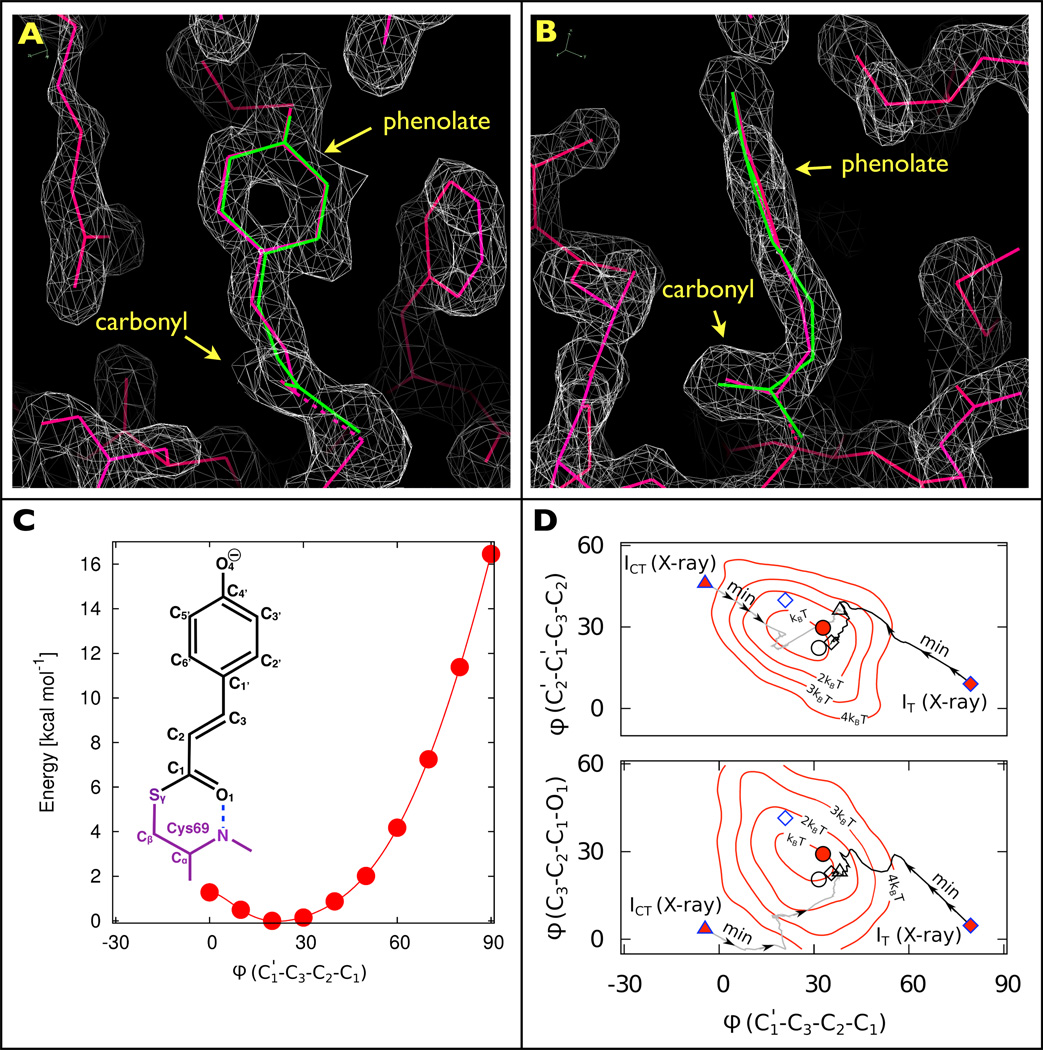
To the Editor — In the March 2013 issue of Nature Chemistry, Jung et al.1 reported time-resolved X-ray structures of early intermediates in the photocycle of photoactive yellow protein (PYP), a model system that has long served as a paradigm for understanding photoisomerization in proteins. In agreement with Schotte and colleagues2, the experimental electron density map recovered by Jung et al. for their first intermediate, which they denote IT, unveils a highly contorted p-coumaric acid (pCA) chromophore with its carbonyl group oriented nearly perpendicular to the phenolate ring (Fig. 1a,b). However, the X-ray structures assigned to IT and a subsequent intermediate denoted ICT are inconsistent with density functional theory (DFT) calculations and previously published structures. Here, we report new DFT calculations that lend additional support for the structures reported in Schotte et al. and help reconcile contradictions arising from the X-ray structures of IT and ICT.
Figure 1.
Structures and stereochemistry of early intermediates following photoactivation of PYP. a, Front and b, side views of the pCA chromophore and its immediate surroundings. The two electron density maps for IT (grey), contoured at 1.64σ, clearly show the carbonyl oriented perpendicular to the phenolate plane. Although quite different stereochemically, both X-ray (red) and DFT (green) pCA structures thread through the electron density maps with high fidelity. c, Labelling scheme for the pCA chromophore and DFT energies (calculated at the D-B3LYP/def2-TZVP level) for the C3=C2 dihedral angle (ϕ) of pR0. d, Dihedral angles of early intermediates reported by Jung et al. (blue outline: IT as diamonds; ICT as triangles), and Schotte et al. (black outline: pR0 as circles), for both X-ray refined structures (red-filled symbols) and DFT-optimized structures (open symbols). The X-ray structures for IT and ICT were found to be unstable: during DFT structure optimization, both converged to structures similar to pR0 (the black and grey lines, labelled ‘Min’, indicate the dihedral projections along the energy minimization pathways obtained during the respective structure optimizations). The DFT calculations reported in Schotte et al. included 176 atoms (D-BP86/def2-SVP); Jung et al. included 157 atoms (B97-1/6-31G(d)/3-21G). Underlaid are dihedral/dihedral free-energy contours computed from a 5 ps hybrid quantum/classical mechanics (QM/MM) simulation2,8 of the pR0 intermediate with 143 QM atoms/2,171 MM atoms (simulations were performed using CHARMM/Q-Chem: D-BP86/def2-SVP/CHARMM27). Note that in this projection, the Jung et al. DFT structure for IT differs from the IT, ICT and pR0 DFT-optimized cluster by only ~1 kT in free energy.
The unusual pCA conformation in Fig. 1a,b begs a question regarding how this twist is accommodated stereochemically among the three dihedral angles between the phenolate and the carbonyl (Fig. 1c). Of key mechanistic importance is the central dihedral angle involving the C3=C2 double bond, whose trans-to-cis photoisomerization drives the structural transitions that ultimately lead to the signalling state of PYP. The 79°/81° central dihedral angle found in the Jung et al. X-ray structures for IT (PDB ID: 3VE3/4I38) is in stark contrast with the 21° angle extracted from their DFT-optimized structure, and contradicts the assertion that the DFT results support their interpretation of IT. Whereas a ~90° angle “halfway between the trans- and cisisomers” has been predicted for the electronic excited state of pCA (ref. 3), the persistence of IT far beyond the ~2 ps excited state lifetime of PYP (ref. 4) suggests that it corresponds to a ground-state species. More plausible is their DFT structure for IT, a strained cis conformation that is consistent with the Schotte et al. X-ray and DFT structures for the corresponding pR0 intermediate2 (PDB ID: 4B9O; Fig. 1d).
Although quite different stereochemically, the X-ray and DFT structures for IT thread through the experimental IT electron density maps with high fidelity (Fig. 1a,b). Indeed, the pCA atomic coordinates differ by only 0.18 Å r.m.s., and when refined against 1.6 Å diffraction data, the difference in their crystallographic R factor was found to be negligible (ΔRcryst = 0.0001). For this comparison, PHENIX was used to refine atomic models against the structure-factor amplitudes reported for IT (PDB ID: 4I38), for which Rcryst = 0.2266 when the three dihedral angles between the pCA phenolate and carbonyl were fixed at the values found in the X-ray structure of Jung et al., versus Rcryst = 0.2267 when fixed at the corresponding values in their DFT structure. The electron density maps in Fig. 1a,b were extrapolated from low- occupancy (<10%) difference maps, and lack the spatial resolution required to refine the coordinates of individual pCA chromophore atoms without chemical restraints. During their X-ray structure refinement, Jung et al. gradually released the dihedral angle restraint across the C3=C2 bond and recovered a structure whose central dihedral angle deviated significantly from that found in their DFT structure. Using the DFT methods reported by Schotte and colleagues2, we found the IT X-ray structure of Jung et al. to be unstable: it converged to a conformation similar to their IT DFT structure and the pR0 structures of Schotte et al. (Fig. 1D).
According to the kinetic model of Jung et al., IT bifurcates into ICT (PDB ID: 3VE4/4I39) and pR1, with ICT supplanting the planar cis intermediate reported in prior time-resolved X-ray (PDB ID: 4BBT, 1TS8)2,5 and cryo-crystallography studies (PDB ID: 1OT9, 1UWP)6,7. This model implies that the well-characterized planar cis intermediate, denoted ICP by Ihee and colleagues5, does not exist. The opposite seems more plausible: we performed DFT calculations on ICT and found that its structure was unstable, and converged to a conformation similar to the DFT structures reported for IT and pR0 (Fig. 1d). Jung et al. invoked ICT to explain persistence of a twisted intermediate following the decay of IT, whereas Schotte et al. accounted for this persistence with a reversible transition between pR0 and pR1 (in the notation of Schotte et al., pR1 is similar to the intermediate ICP), a view that is supported by their similar DFT energies2. Had Jung et al. allowed for this reversibility, they could have accounted for their time-resolved electron density maps with ICP, whose structure is supported by both DFT and prior crystallography studies2,5.
How might differences in the buffer conditions influence the results? In ~2.8 M ammonium sulfate, two long-lived pR intermediates were required to account for the time-resolved diffraction data1,5. In 1.1 M NaCl and 2.5 M ammonium sulfate, which is arguably more physiologically relevant for halophilic bacteria, only one long-lived pR intermediate was required2. Whereas 1.1 M NaCl seems to simplify the PYP photocycle, it is difficult to rationalize how its absence could stabilize IT in a high-energy twisted state, or morph planar ICP into an unstable, twisted ICT conformation.
In conclusion, the contradiction between Jung and colleagues’ X-ray and DFT structures for IT arises from an empirical choice to loosen rather than tighten their dihedral angle restraint across the C3=C2 bond. This choice seems not to be compelled by their diffraction data, and results in an X-ray structure that is more consistent with the electronic excited state. If we make the more plausible assumption that IT is a ground-state intermediate, the unveiling of “… a long-hypothesized highly twisted intermediate along the trans-to-cis isomerization pathway” has not yet occurred, but may prove possible with a free-electron X-ray laser such as the Linac Coherent Light Source, where sub-ps time resolution can be achieved.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH. The Biowulf cluster at NIH (http://biowulf.nih.gov/) is acknowledged for computer time.
References
- 1.Jung YO, et al. Nature Chem. 2013;5:212–220. doi: 10.1038/nchem.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schotte F, et al. Proc. Natl Acad. Sci. USA. 2012;109:19256–19261. doi: 10.1073/pnas.1210938109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groenhof G, et al. J. Am. Chem. Soc. 2004;126:4228–4233. doi: 10.1021/ja039557f. [DOI] [PubMed] [Google Scholar]
- 4.Larsen DS, et al. Biophys. J. 2004;87:1858–1872. doi: 10.1529/biophysj.104.043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ihee H, et al. Proc. Natl Acad. Sci. USA. 2005;102:7145–7150. doi: 10.1073/pnas.0409035102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson S, Crosson S, Moffat K. Acta Crystallogr. D. 2004;60:1008–1016. doi: 10.1107/S090744490400616X. [DOI] [PubMed] [Google Scholar]
- 7.Kort R, Hellingwerf KJ, Ravelli RB. J. Biol. Chem. 2004;279:26417–26424. doi: 10.1074/jbc.M311961200. [DOI] [PubMed] [Google Scholar]
- 8.Woodcock HL, III, et al. J. Comput. Chem. 2007;28:1485–1502. doi: 10.1002/jcc.20587. [DOI] [PubMed] [Google Scholar]