Abstract
Plasmodium falciparum merozoite antigens are under development as potential malaria vaccines. One aspect of immunity against malaria is the removal of free merozoites from the blood by phagocytic cells. However assessing the functional efficacy of merozoite specific opsonizing antibodies is challenging due to the short half-life of merozoites and the variability of primary phagocytic cells. Described in detail herein is a method for generating viable merozoites using the E64 protease inhibitor, and an assay of merozoite opsonin-dependent phagocytosis using the pro-monocytic cell line THP-1. E64 prevents schizont rupture while allowing the development of merozoites which are released by filtration of treated schizonts. Ethidium bromide labelled merozoites are opsonized with human plasma samples and added to THP-1 cells. Phagocytosis is assessed by a standardized high throughput protocol. Viable merozoites are a valuable resource for assessing numerous aspects of P. falciparum biology, including assessment of immune function. Antibody levels measured by this assay are associated with clinical immunity to malaria in naturally exposed individuals. The assay may also be of use for assessing vaccine induced antibodies.
Keywords: Immunology, Issue 89, Parasitic Diseases, malaria, Plasmodium falciparum, hemozoin, antibody, Fc Receptor, opsonization, merozoite, phagocytosis, THP-1
Introduction
The importance of antibodies for immunity to Plasmodium falciparum malaria was shown 40 years ago, when immunoglobulin from hyperimmune adults was passively transferred to children suffering from severe malaria resulting in alleviated disease1. Consequently, considerable effort has sought to identify targets of protective malarial immunity, mostly through measuring antibody titers to peptides or bacterially expressed proteins by ELISA. ELISA based serology has also proven highly variable between studies, and does not address antibody functionality2. Many malaria antigens induce a cytophilic IgG1 and IgG3 antibody profile, particularly merozoite surface antigens3. This subclass bias suggests that antibody-Fc-receptor (FcR) interactions with phagocytes are important for the effector functions of opsonizing antimerozoite antibodies4. Several merozoite antigen vaccines under development are designed to elicit phagocyte effector functions5,6 and although significant evidence for the importance of antibody-FcR interactions in models of rodent malaria exists7-9, and a few recent studies support the importance of functional antibodies and phagocyte effector functions for immunity to malaria in humans10,11, this area remains poorly studied. Study into merozoite specific opsonizing antibodies has been limited by two factors; the difficulty in isolating good quality merozoites; and variable phagocytosis responses from primary cells.
Until recently, high speed centrifugation or Percoll density gradients were utilized to isolate merozoites from culture supernatants of rupturing schizont cultures. These merozoites were rarely viable, and often further manipulated by density centrifugation and multiple wash steps12, or cryopreservation11 before use in assays. These processes potentially detach many peripherally associated proteins from the merozoite surface, proteins known to be antigenic targets of malarial immunity13. Recently the cysteine protease inhibitor trans-Epoxysuccinyl-L-leucylamido(4-guanidino)butane (E64) has been used to generate viable merozoites. E64 prevents schizont rupture, generating membrane enclosed merozoites14, which can be disrupted by filtration to liberate viable merozoites15,16. This technique has lead to the spatial resolution of numerous proteins during erythrocyte invasion15,17-19 and has clarified the stage specific effect of several antimalarial drugs16,20. However, the generation of viable merozoites remains technically challenging. To aid in the dissemination of this technique and application to functional assays of immunity, a detailed protocol for viable merozoite purification and their use in a standardized functional assay of antibody:cellular cooperation in opsonization and phagocytosis is described here.
This technique demonstrates a significant advance over previous in vitro assays of merozoite opsonization, such as neutrophil respiratory burst, Antibody Dependent Cellular Inhibition (ADCI), and alternative merozoite phagocytosis assays. These assays are poorly reproducible due to variation in parasite inputs and the activity of primary phagocytic cells11,21. Contaminating hemozoin may also profoundly affect phagocyte function22. A recently reported robust and reproducible merozoite phagocytosis assay23 utilizes the promonocytic cell line THP-124. This is an ideal cell type for high throughput flow cytometry assays as it is non-adherent and specifically performs Fc-Receptor mediated phagocytosis25,26. An additional complexity while investigating phagocytosis is that the degree of phagocytosis is dependent on the number of merozoites relative to THP-1 cells and plasma concentration. To ensure reproducibility between experiments, merozoites should be enumerated and a defined concentration used. Due to their small size, flow cytometric quantification is required.
The procedure described here removes hemozoin and generates viable merozoites, and describes an application of these merozoites for flow cytometric enumeration of merozoites followed by opsonization and phagocytosis. Although technically demanding, the techniques described may prove useful in elucidating the contribution of merozoite surface specific antibody responses to naturally acquired and vaccine induced immunity.
Protocol
NOTE: All steps, besides centrifugation and flow cytometry, should be performed within a laminar flow hood to maintain sterility. Ensure proper precautions are taken regarding the handling of human samples. The assay is very sensitive for small amounts of plasma. The dilutions provided are optimal for resolving responses ranging from 5 - 78% with plasma from semi-immune children from Papua New Guinea (PNG). The optimal dilution may vary with plasma sets being tested, and therefore it is recommended that plasma be titrated to determine experimental conditions for each application. Ensure the inclusion of 2 negative controls THP-1 cells with merozoites in absence of plasma; and THP-1 cells with merozoites opsonized with a pool of plasma from malaria naïve individuals. This will allow control for merozoite adherence to THP-1 cells and to enable rigorous flow-cytometry gating of phagocytosis events.
The use of PNG plasma samples was approved by the Medical Research Advisory Committee, Papua New Guinea Ministry of Health, The Walter and Eliza Hall Institute Human Research Ethics Committee (project number 04/04). Written consent was obtained from parents/guardians of all participants.
1. THP-1 Culture
Maintain the human monocytic cell line THP-1 in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) and 55 μM 2-mercapthoethanol (THP-1 medium) and at 37 °C in a 5% CO2 humidified incubator.
Maintain cells at a density below 5 x 105 cells/ml. NOTE: Overgrowth will result in differentiation into adherent cells and down-regulation of Fc Receptors. Once cells have been passaged approximately 10 times, thaw a new vial to maintain cell phenotype.
2. Antibody Sample Preparation and Dilution
Heat inactivate plasma to be tested and malaria-naïve control plasma by incubating at 56 °C for 30 min
Serially dilute plasma samples to 1/2,000 in THP-1 medium.
Store diluted plasma at -20 °C.
3. Culture of Highly Synchronized P. falciparum
NOTE: The GFP-expressing parasite line D10-PfPHG27 was utilized due to its 48 hr life-cycle which assists synchronization of parasites and the controlled timing of E64 addition. Furthermore, because this line expresses GFP in the cytosol, this line allows detection of parasitaemia and free merozoites by flow cytometric detection of GFP. However, the GFP fluorescence intensity is not sufficient to provide visualization within THP-1 cells, and therefore, merozoites are counterstained with Ethidium Bromide (EtBr). Other parasite strains may be utilized, provided that tight synchrony is achievable. Synchronize parasites using a combination of sorbitol treatment to lyse mature forms28 and heparin to inhibit invasion15.
Maintain P. falciparum parasites in O+ human erythrocytes (RBC) at 3% hematocrit in RPMI-1640 medium (pH 7.4) supplemented with 25 mg/ml HEPES, 50 μg/ml hypoxanthine, 10% pooled human serum, 2 mg/ml sodium bicarbonate, and 20 μg/ml gentamycin (Parasite medium). Add 5 mg/ml Blasticidin S-hydrochloride to the culture medium to select for GFP+ parasites.
Incubate cultures in air tight boxes or alternatively double sealed culture flasks at 37 °C in an atmosphere of 1% O2, 4% CO2 and 95% N2.
Prepare thin smear slides, fix in 100% methanol for 10 sec, and stain with 10% Giemsa solution in 6.7 mM (pH 7.1) phosphate buffer (Giemsa solution) for 10 min to monitor parasitemia. After staining, rinse slide in water and air-dry. Assess parasitemia using a 100X oil immersion lens.
Maintain parasite cultures at a parasitemia below 5% infected RBC by splitting cultures and adding uninfected RBC as required.
To synchronize with heparin, add 20 IU/ml of medical-grade heparin to ring-stage cultures.
When the majority of parasites are at the schizont stage, pellet cells at 300 x g for 5 min, remove heparin-containing medium and resuspend in parasite medium to allow schizont rupture and merozoite invasion.
After 4 hr, add 20 IU/ml heparin back to cultures, blocking any further merozoite invasion. NOTE: Several cycles of sorbitol and heparin treatment may be required before parasites are sufficiently synchronized. To generate suitable numbers of merozoites, prepare 150 ml of parasite culture at 3 - 5% parasitaemia.
4. Isolation of Late Stage P. falciparum Trophozoites
Thirty-six hr after returning heparin to cultures, pellet cultured cells at 300 x g for 5 min, and resuspend pellet in parasite medium at 25% hematocrit.
Attach a large magnetic column (matrix volume of 6.3 ml) to a magnet, and equilibrate the column with parasite medium, making sure all air bubbles are removed.
Add the resuspended P. falciparum culture to the column, and adjust flow rate to one drop per sec.
Once culture has passed through the column, wash the column with parasite medium until the flow-through runs clear.
Elute parasites from the column in 30 ml of 37 °C parasite medium.
Prepare a thin smear of parasites, fix in 100% methanol for 10 sec, and stain with Giemsa solution for 3 min. Examine the slide under the microscope, and ensure that parasites almost fill the red blood cell, and appear to be in early schizont stages. If the parasites have not reached the appropriate maturation stage, return purified parasites to a 37 °C incubator in an atmosphere of 1% O2, 4% CO2 and 95% N2 until suitably developed. NOTE: Purity of greater than 90% infected red blood cells is required to ensure RBCs do not contaminate merozoite preparations.
Treat isolated schizonts with 10 μM E64 (Epoxysuccinyl-L-leucylamido(4-guanidino)butane (E64) for 6 - 10 hr. NOTE: Longer incubation times with E64 are possible, however the merozoites produced will no longer be invasive.
5. Isolation of P. falciparum Merozoites
Following incubation with E64, smear parasites, fix cells in 100% methanol, and stain with Giemsa solution to assess the formation of membrane-enclosed merozoites. NOTE: A good yield of merozoites will be obtained if greater than 50% of parasites have formed membrane enclosed merozoites.
Pellet E64-treated schizonts at 1,900 x g for 8 min. Wash with 50 ml of RT RPMI-1640 medium (pH 7.4) supplemented with 25 mg/ml HEPES, 50 μg/ml hypoxanthine, 2 mg/ml sodium bicarbonate, and 20 μg/ml gentamycin (wash medium) to remove remaining human serum.
Resuspend the pellet in 2 ml of wash medium. NOTE: Using FCS-containing THP-1 media will produce frothing during filtration.
Filter the 2 ml of resuspended E64-schizonts through a 1.2 μm/32 mm syringe filter. Collect the filtrate in a 10 ml tube. NOTE: The filtrate contains merozoites and hemozoin crystals, with un-ruptured schizonts and debris collected in the filter.
Attach a small magnetic column to a magnet, and equilibrate with 500 μl THP-1 medium.
Pass the filtrate over the magnetic column. Collect the flow-through. NOTE: Hemozoin will bind to the column, while merozoites will pass through.
Pass the flow-through over the column two more times to remove hemozoin, collecting the flow through each time.
Rinse the column with 2 ml of RT THP-1 medium to collect any remaining merozoites.
Add EtBr at 10 μg/ml (final concentration) and stain for 30 min at RT. Protect from light to prevent photobleaching. Note: Ensure that all EtBr contaminated liquid and plastic waste is disposed of in a manner suitable for cytotoxic chemicals. Merozoites are no longer invasive following this incubation.
Spin down merozoites at 4,000 x g for 10 min.
Discard supernatant into appropriate waste container.
Resuspend merozoite pellet in 4 ml THP-1 medium, and spin down at 4,000 x g for 10 min.
Add THP-1 medium up to a volume of 15 ml, and protect from light.
6. Quantifying Merozoite Concentration by Flow Cytometry
Bring counting beads to RT.
Prepare PBS with 0.5% BSA for diluting merozoites.
Count merozoites at 3 dilutions; 1 in 100; 1 in 50 and 1 in 25. Prepare 3 FACS tubes with 940, 930 and 910 μl of PBS+0.5% BSA, respectively. Add 10, 20 and 40 μl of purified merozoites per tube, respectively.
Vortex counting beads for 30 sec, and add 50 μl of beads per FACS tube containing diluted merozoites.
Run the three diluted merozoite samples on a flow cytometer equipped with a 488 nm laser. Set a stringent gate for merozoites based on EtBr and GFP fluorescence dual fluorescence, and gate counting beads by fluorescence in a separate channel. Acquire events until 2,000 beads are collected. Note: These instructions refer to counting GFP+ merozoites that have been counter-stained with EtBr. If not utilizing GFP expressing parasites then set merozoite gates based on side scatter and EtBr fluorescence.
Determine the ratio of merozoites:beads by dividing the number of merozoite events by the 2,000 counting bead events, calculate a ratio for each dilution. Use the counting bead batch specifications to determine the number of beads in the 50 μl of beads added per tube.
Multiply the number of beads in 50 μl by the dilution factor and by the merozoite:bead ratio for that dilution factor. This number equates to the concentration of merozoites per ml. Perform these steps for each dilution.
Average the merozoite concentrations measured across the three dilutions. NOTE: Standard deviations in excess of 10% for concentrations determined for 1 in 100, 1 in 50, and 1 in 25 dilutions indicate technical inaccuracies in preparing counting samples.
Resuspend merozoites at 8 x 106 merozoites/ml in THP-1 medium to ensure a final ratio of four merozoites per THP-1 cell. NOTE: Other merozoite to THP-1 ratios may be used, and concentrations should be modified accordingly.
7. Phagocytosis Assay
Add 200 μl of heat inactive FCS per well of 96 well U-bottom plates, and leave at RT O/N to block plates. Following incubation, remove FCS and wash wells twice with 200 μl sterile PBS. Remove PBS and store plates at 4 °C until required.
Calculate the number of THP-1 cells required to test plasma samples and controls, allowing triplicate wells per sample at 1 x 105 THP-1 cells per well.
Determine THP-1 culture concentration by loading 10 µl of culture on to a haemocytometer slide. Count the number of cells present in 4 sets of 16 corner squares of the haemocytometer under a microscope, then average the counts. Multiply the average count by 10,000 to calculate the number of cells per ml.
Spin down the required number of THP-1 cells at 500 x g for 5 min, and resuspend at 6.7 x 105 cells/ml in THP-1 medium.
Add 150 μl of THP-1 cells in THP-1 medium per well to a FCS-blocked 96 well U-bottom plate, resulting in 105 cells/well. Prepare 3 wells per sample that will be tested. Return plate to incubator until ready to add merozoites.
Add 150 μl of merozoite preparation at 8 x 106/ml per well to a separate FCS-blocked 96 well U-bottom plate. Prepare one well per sample tested
Add 10 μl of prepared diluted plasma samples per well, to the plate that contains the merozoites, and mix well to ensure homogenous solution.
Remove the THP-1 plate from the incubator, and add 50 μl of the merozoite/plasma solution per well containing THP-1 cells, with triplicate wells per plasma sample.
Mix well, and incubate at 37 °C in 5% CO2, humidified incubator for 40 min. Cover with foil to protect from light.
After 40 min, centrifuge samples for 5 min at 500 x g in a 4 °C prechilled centrifuge to arrest phagocytosis.
Remove supernatant, and wash cells twice in ice-cold PBS+0.5% BSA+2 mM EDTA (FACS buffer). NOTE: EDTA will facilitate in maintaining a single cell suspension for flow cytometry analysis.
Fix cells in 90 μl of 2% paraformaldehyde (PFA) in PBS+0.5% BSA+2 mM EDTA (FACS fixative). Leave on ice until acquisition, protected from light.
8. Flow Cytometry
Acquire samples using a flow cytometer equipped with a 488 nm laser and high throughput plate reader attachment. NOTE: This will enable up to 400 plasma samples to be tested from one merozoite preparation. Cells can also be transferred to tubes for manual sample loading.
Gate viable THP-1 cells by forward and side scatter.
Set the EtBr positive gate based on the ‘THP-1 cells with merozoites and no plasma’ control.
Acquire a minimum of 10,000 events per sample.
9. Data Analysis
Analyze data using flow cytometry analysis software, and set stringent gates for EtBr positive events.
Calculate the percentage phagocytosis for each sample: the % of EtBr+ cells for the sample minus the % positive cells with non-immune plasma.
Average results across triplicates. NOTE: Standard deviations for a triplicate in excess of 10% indicate experimental inaccuracies. Some background EtBr positive events may be observed as a result of merozoite adhesion to the surface of THP-1 cells, but should be consistent across all samples containing merozoites. Setting the gate based on the ‘THP-1 cells with merozoites and no plasma’ control, and then subtracting any background of the ‘THP-1 cells with non-immune plasma’ control will result in % phagocytosis that reflects the fluorescence due to internalized/phagocytosed merozoites only.
Representative Results
The maturation stage of parasites prior to E64 treatment is critical for generating membrane-enclosed merozoites. Figure 1Ai shows the appropriate maturation stage of schizonts for adding E64. The parasites should be large and almost fill the red blood cell. A dappled appearance of Giemsa stain indicates merogony has commenced, and E64 should be added to yield membrane-enclosed merozoites (Figure 1Aii). If E64 is added earlier to trophozoite stage parasites, membrane enclosed merozoites are not generated even after 12 hr of E64. Instead parasites take on abnormal morphology such as enlargement of the digestive vacuole (Figure 1Bi and 1Bii), and merozoites are not formed. If E64 is added later to schizonts, membrane-enclosed merozoites are not generated as schizont rupture is uninhibited (Figure 1Ci and 1Cii). A high level of parasite synchrony is required, otherwise a range of all three outcomes described will be seen after E64 treatment.
Removal of hemozoin is another critical step for purifying merozoites. If merozoites are not purified from hemozoin then merozoite-hemozoin clusters form which cannot be dislodged by pipetting. These aggregates run on the cytometer as single events, albeit with different forward-scatter and side-scatter characteristics than single merozoites, resulting in inaccurate merozoite counting by flow cytometry (Figure 2A). As THP-1 cells can also phagocytose hemozoin, these merozoite-hemozoin clusters can also be phagocytosed (Figure 2B). These aggregates are highly EtBr fluorescent as they contain multiple merozoites. Phagocytosis of aggregates results in a THP-1 EtBr fluorescence profile equivalent to that observed for the phagocytosis of multiple individual merozoites. Hence, if hemozoin is not removed, the EtBr fluorescence of THP-1 cells will be an overestimate of the opsonizing potential for the plasma being tested. Hemozoin is also reported to significantly alter phagocyte function. GFP positive merozoites are counter stained with EtBr to increase visualization of merozoites phagocytosed by THP-1 cells. Using the conditions described in this protocol, all GFP positive merozoites are counterstained with EtBr which has a brighter fluorescence intensity (Figure 3A). Phagocytosis assessment by EtBr fluorescence enables superior resolution of merozoite phagocytosis than GFP fluorescence (Figure 3B).
The number of merozoites added to the assay will modulate the amount of phagocytosis observed. For this reason, accurate counting by flow cytometry is critical. Increasing ratios of merozoites:THP-1 will result in increased adherence of merozoites to THP-1 cells in the absence of plasma (Figure 4A). Increasing merozoite:THP-1 ratios also results in increased phagocytosis responses when merozoites are opsonized (Figure 4B). A 4:1 merozoite:THP-1 ratio is recommended for robust phagocytic responses. Using this ratio, and the dilution of plasma indicated, this assay is able to resolve low, intermediate and high levels of opsonizing antibodies. Between 0 and 78 percent phagocytosis responses have been observed using PNG plasma samples and the other conditions described. Such responses are recently shown to associate with naturally acquired clinical immunity to malaria10. Figure 5 shows examples of the 4 quartiles of phagocytosis responses from PNG individuals.
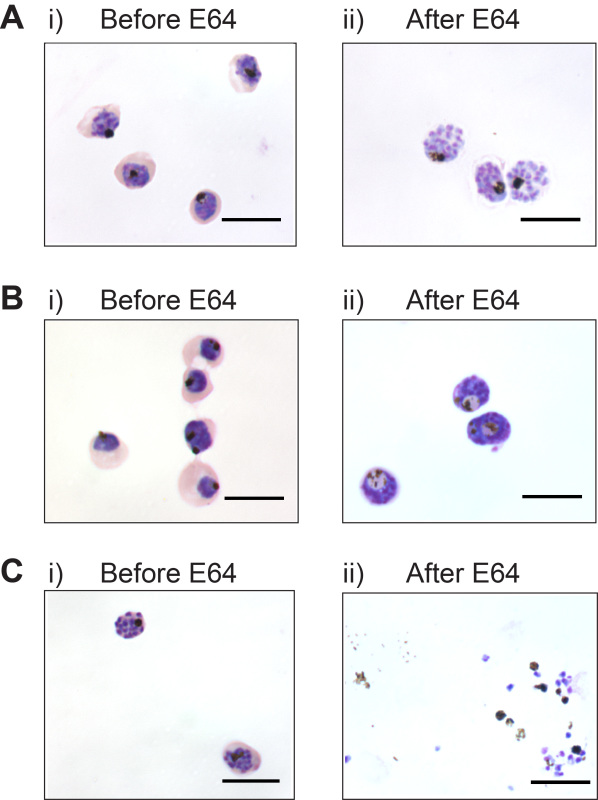 Figure 1. Timing of E64 addition is critical for generating membrane-enclosed merozoites. (A) Appropriate maturation stage of parasites i) prior to E64 treatment, and ii) membrane-enclosed merozoites produced after 6 hr of E64 treatment. (B) membrane enclosed merozoites are not generated if E64 is added to immature parasites; i) Late stage trophozoites, and ii) after 12 hr of E64. (C) Schizont rupture is not inhibited if E64 is added to late segmented schizonts; i) Late stage schizonts, and ii) after 6 hr of E64. Scale bar represents 10 μm.
Figure 1. Timing of E64 addition is critical for generating membrane-enclosed merozoites. (A) Appropriate maturation stage of parasites i) prior to E64 treatment, and ii) membrane-enclosed merozoites produced after 6 hr of E64 treatment. (B) membrane enclosed merozoites are not generated if E64 is added to immature parasites; i) Late stage trophozoites, and ii) after 12 hr of E64. (C) Schizont rupture is not inhibited if E64 is added to late segmented schizonts; i) Late stage schizonts, and ii) after 6 hr of E64. Scale bar represents 10 μm.
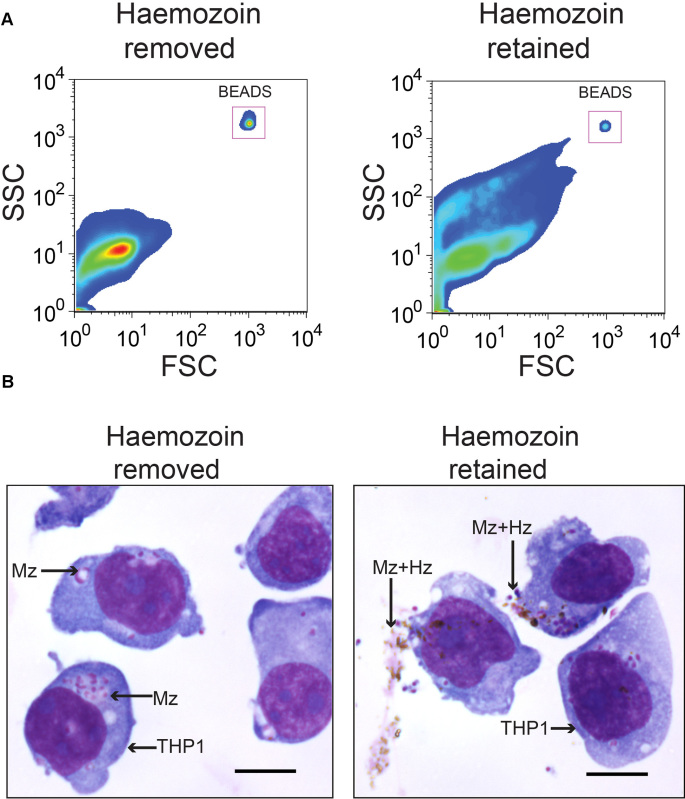 Figure 2. Hemozoin removal is critical to generate a single-cell merozoite suspension. Merozoite-hemozoin aggregates form following filtration of membrane enclosed merozoites, unless hemozoin is magnetically separated from merozoites. (A) Forward-scatter and side-scatter plots of merozoites with hemozoin removed and hemozoin retained. (B) Hemozoin-merozoite aggregates can be phagocytosed by THP-1 cells. Diff-Quick stained cyto-spin slides of THP-1 cells incubated with PNG plasma opsonized merozoite preparations with or without hemozoin. Scale bar represents 10 μm.
Figure 2. Hemozoin removal is critical to generate a single-cell merozoite suspension. Merozoite-hemozoin aggregates form following filtration of membrane enclosed merozoites, unless hemozoin is magnetically separated from merozoites. (A) Forward-scatter and side-scatter plots of merozoites with hemozoin removed and hemozoin retained. (B) Hemozoin-merozoite aggregates can be phagocytosed by THP-1 cells. Diff-Quick stained cyto-spin slides of THP-1 cells incubated with PNG plasma opsonized merozoite preparations with or without hemozoin. Scale bar represents 10 μm.
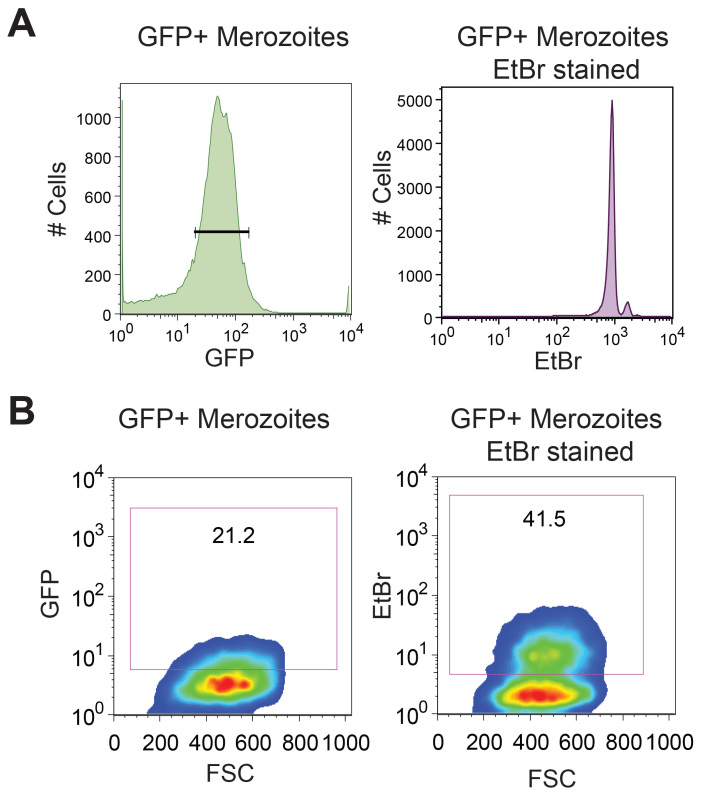 Figure 3. Ethidium Bromide staining allows for superior resolution of merozoite phagocytosis. D10-GFP purified merozoites are counterstained with EtBr to improve fluorescence intensity. (A) Flow cytometry histrogram of purified merozoites with gating on GFP positive merozoites, and a dot blot showing EtBr fluorescence within this gated GFP positive population. (B) Forward scatter versus GFP and forward scatter versus EtBr of THP-1 cells following phagocytosis of GFP and EtBr dual fluorescent merozoites. Gates were drawn based on the THP-1 cells with merozoites and no plasma control.
Figure 3. Ethidium Bromide staining allows for superior resolution of merozoite phagocytosis. D10-GFP purified merozoites are counterstained with EtBr to improve fluorescence intensity. (A) Flow cytometry histrogram of purified merozoites with gating on GFP positive merozoites, and a dot blot showing EtBr fluorescence within this gated GFP positive population. (B) Forward scatter versus GFP and forward scatter versus EtBr of THP-1 cells following phagocytosis of GFP and EtBr dual fluorescent merozoites. Gates were drawn based on the THP-1 cells with merozoites and no plasma control.
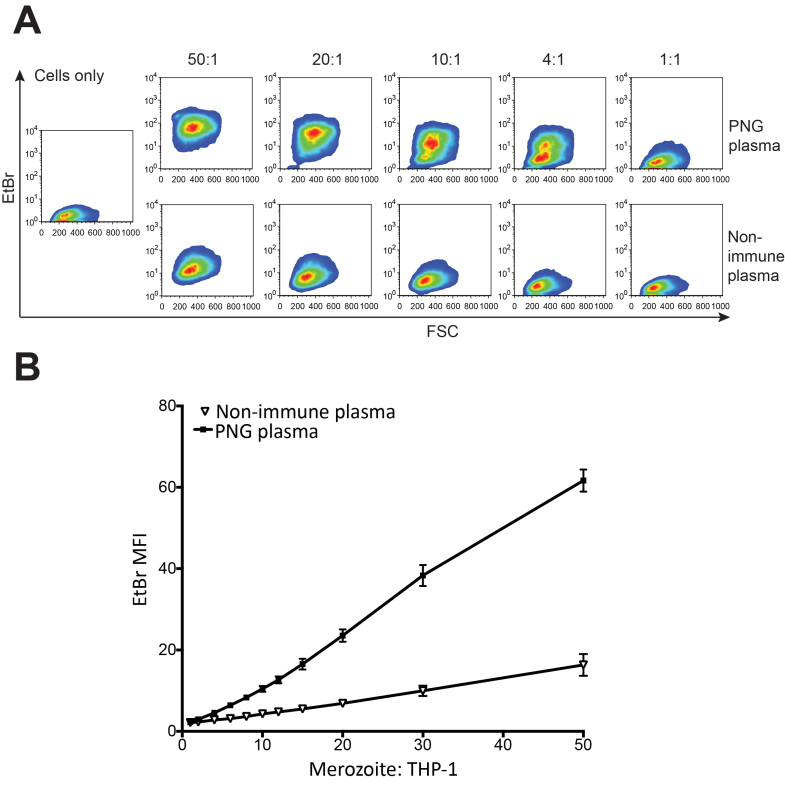 Figure 4. The merozoite:THP-1 ratio influences the degree of phagocytosis observed. (A) Five merozoite:THP-1 ratios are depicted; 50:1, 20:1, 10:1, 4:1, 1:1. Cells only control indicates background fluorescence due to THP-1 cells. THP-1 EtBr fluorescence for each ratio is indicated for merozoites opsonized with a pool of PNG plasma or with nonimmune Australian plasma. (B) Mean fluorescence intensity of THP-1 cells for different merozoite:THP-1 ratios, and opsonization with a pool of PNG plasma or nonimmune Australian plasma (mean+SEM). Please click here to view a larger version of this figure.
Figure 4. The merozoite:THP-1 ratio influences the degree of phagocytosis observed. (A) Five merozoite:THP-1 ratios are depicted; 50:1, 20:1, 10:1, 4:1, 1:1. Cells only control indicates background fluorescence due to THP-1 cells. THP-1 EtBr fluorescence for each ratio is indicated for merozoites opsonized with a pool of PNG plasma or with nonimmune Australian plasma. (B) Mean fluorescence intensity of THP-1 cells for different merozoite:THP-1 ratios, and opsonization with a pool of PNG plasma or nonimmune Australian plasma (mean+SEM). Please click here to view a larger version of this figure.
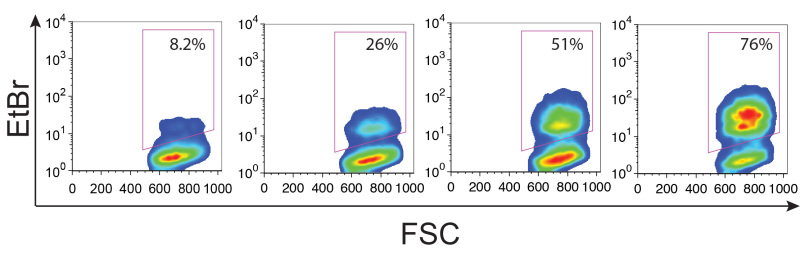 Figure 5. A large range of opsonizing antibodies can be measured. Merozoites were opsonized with plasma from PNG individuals and incubated with THP-1 cells. Four representative phagocytosis responses are shown. Gates were drawn based on the THP-1 cells with merozoites and no plasma control, and numbers indicate the %phagocytosis after subtraction of the THP-1 cells with non-immune plasma control. Please click here to view a larger version of this figure.
Figure 5. A large range of opsonizing antibodies can be measured. Merozoites were opsonized with plasma from PNG individuals and incubated with THP-1 cells. Four representative phagocytosis responses are shown. Gates were drawn based on the THP-1 cells with merozoites and no plasma control, and numbers indicate the %phagocytosis after subtraction of the THP-1 cells with non-immune plasma control. Please click here to view a larger version of this figure.
Discussion
To measure merozoite phagocytosis, proficiency in two techniques is required: purification of merozoites and THP-1 phagocytosis assay. The most critical steps for combining these two techniques are: 1) Highly synchronized parasites; 2) Adding E64 at the correct time to yield membrane enclosed merozoites; 3) Removing hemozoin to avoid aggregates; 4) Accurate merozoite counting by flow cytometry; 5) the dilution of plasma used; and 6) maintaining low cell density and passage number of THP-1 cells. Careful consideration of these key aspects will ensure robust phagocytosis responses are observed.
Although, described here is the preparation of merozoites for assessing phagocytosis, the technique can be utilized for a wide range of applications. Irrespective of the down stream methodology, optimal merozoite preparations depend on correctly timing E64 addition in order to generate merozoites. The E64 method described here has been shown to yield invasive merozoites for use in high resolution microscopy of invasion events and drug sensitivity assays15-20. While merozoite viability is not essential for phagocytosis, integrity of the merozoite surface coat is required. Therefore the E64 method described here allows high quality merozoites to be produced for assessing antibody responses to the surface coat. As outlined in Figure 1, if E64 is added too early or too late membrane enclosed merozoites are not formed. For this reason, highly synchronous parasite cultures are required. Here, the use of sorbitol and heparin treatments to tightly synchronize D10-GFP parasite cultures to a window of 2 hr is described. Heparin can promote gametocytogenesis in other lab isolates, and hence should be used carefully. Alternative synchronization methods such as alanine may also be used, provided that tightly synchronized parasites are produced29. If E64 is added to asynchronous parasites, a lower proportion of membrane-enclosed merozoites will be produced and remaining parasite-infected RBC will either rupture normally or develop abnormal morphology. The presence of parasites that have not developed into membrane enclosed merozoites will result in clogging of the filter and significantly reduce the merozoite yield obtained.
During filtration of membrane-enclosed merozoites, hemozoin is liberated from the digestive vacuoles and is present as free crystals in solution. Hemozoin is highly proinflammatory and has been reported to modulate monocyte and macrophage phagocytosis responses30,31 . In addition to modulating phagocytosis, as shown in Figure 2, hemozoin can form aggregates with merozoites in solution. As THP-1 cells can phagocytose these aggregates, this can be a major confounder for the resolution of antibody mediated phagocytosis. Therefore, removal of hemozoin is necessary in this assay to avoid additional complexity of hemozoin on phagocyte biology. In addition, failure to remove hemozoin may also be deleterious when using this technique to generate free merozoites for other applications, especially where merozoite quantification is required.
As outlined in Figure 4, the number of merozoites added to the assay can influence the degree of phagocytosis by THP-1 cells. Although flow cytometry allows for enumeration of merozoite concentration, careful pipetting and replicate counts of merozoites are necessary to improve accuracy. This is especially critical if multiple parasite lines are to be tested side-by-side. It has previously been demonstrated that plasma from semi-immune children from PNG can be significantly diluted before responses decline23. Described here is the optimal dilution of plasma (1/120,000 final dilution of plasma) for a cohort of 5 - 12 year old PNG children that produced the large range of phagocytosis responses described in Figure 5. This range allowed for stratification of responses into four groups (0 - 19%, 20 - 39%, 40 - 59% and 60 - 79% phagocytosis), and regression modeling revealed that opsonizing responses were associated with protection from clinical disease and high-density parasitaemia10 For different cohort studies it may be necessary to adjust plasma dilution to ensure the phagocytosis by THP-1 cells is not saturated or below the level of detection.
Flow cytometry allows fast and quantifiable phagocytosis with improved accuracy over microscopy. This protocol is a high throughput, plate based and automated acquisition of 96 well plates to study naturally acquired humoral immunity. The method requires staining of merozoites with ethidium bromide, however alternative DNA stains such as SYBRgreen, DAPI and propidium iodine, membrane stains or protein stains could be utilized. This assay would be amenable to primary monocytes or neutrophils, and could be adapted if phagocyte or FcR biology was of interest. Primary cells or THP-1 cells differentiated in vitro with PMA can be used to study phagocytosis in malaria and other pathogens12,32-34. However using primary cells may be challenging as these cells are adherent and also display non-antibody mediated phagocytosis35. In addition, variability in purity, viability and functionality are some key limitations to the use of primary phagocytic cells. The Fc receptors involved in merozoite phagocytosis remain uncharacterized, and therefore using blocking antibodies to specific Fc Receptors could elucidate the contribution of each Fc Receptor to merozoite phagocytosis. As THP-1 phagocytosis is FcR dependent, this enables straightforward interpretation of the phagocytosis observed. This assay also lends itself to addressing the antigen specificity of opsonizing antibodies which could be achieved by utilizing knock-out parasites for merozoite surface antigens, or using affinity purified human antibodies to merozoite surface proteins. Furthermore, in depth studies of cytokine responses following merozoite phagocytosis are lacking, and could be achieved using this assay.
These two techniques constitute considerable advances to the study of functional antimerozoite antibodies. The purification of high quality merozoites is advantageous to using cryopreserved merozoites, or fluorescent microspheres coated with merozoite antigens. Although this assay has been used as a tool for evaluating naturally acquired immunity, it may prove an important tool for addressing the acquisition of immunity in response to vaccination. While it has been recently shown that the phagocytosis or opsonised merozoites is associated with protection from clinical malaria, it is not possible to draw direct conclusions from in vitro THP-1 phagocytosis to in vivo phagocytosis of merozoites as phagocytosis in this assay occurs under static conditions and in the absence of competing red blood cells. The potential adaptations of this assay may thus provide a versatile tool for further understanding of malaria and merozoite phagocytosis.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors wish to acknowledge the children and adult plasma donors, and the staff at the Papua New Guinea Institute of Medical Research. The authors would like to thank Amandine B Carmagnac, Catherine Q Nie, Danny W Wilson, Ivo Mueller and Diana S Hansen for their contribution to development of this technique, and thank the Australian Red cross for blood and serum packs. This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS. This work was supported by National Health and Medical Research Council grants # 1031212 and # 637406, and National Institutes of Health grant # AI089686.
References
- Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Fowkes FJI, Richards JS, Simpson JA, Beeson JG. Medicine PLoS., editor. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Medicine. 2010. [DOI] [PMC free article] [PubMed]
- Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. The Journal of experimental medicine. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarshad A, et al. Journal of immunology. Vol. 178. Baltimore, MD: 1950. A novel antibody-dependent cellular cytotoxicity mechanism involved in defense against malaria requires costimulation of monocytes FcgammaRII and FcgammaRIII; pp. 3099–3106. [DOI] [PubMed] [Google Scholar]
- Genton B, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. The Journal of Infectious Diseases. 2002;185:820–827. doi: 10.1086/339342. [DOI] [PubMed] [Google Scholar]
- Sirima SB, Cousens S, Druilhe P. Protection against malaria by MSP3 candidate vaccine. The New England journal of medicine. 2011;365:1062–1064. doi: 10.1056/NEJMc1100670. [DOI] [PubMed] [Google Scholar]
- Llewellyn D, et al. Assessment of antibody-dependent respiratory burst activity from mouse neutrophils on Plasmodium yoelii malaria challenge outcome. Journal of leukocyte biology. 2013. [DOI] [PMC free article] [PubMed]
- McIntosh RS, et al. The importance of human FcgammaRI in mediating protection to malaria. PLoS pathogens. 2007;3:72. doi: 10.1371/journal.ppat.0030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleass RJ, et al. Novel antimalarial antibodies highlight the importance of the antibody Fc region in mediating protection. Blood. 2003;102:4424–4430. doi: 10.1182/blood-2003-02-0583. [DOI] [PubMed] [Google Scholar]
- Hill DL, et al. Opsonizing Antibodies to P. falciparum Merozoites Associated with Immunity to Clinical Malaria. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos C, et al. Clinical Protection from Falciparum Malaria Correlates with Neutrophil Respiratory Bursts Induced by Merozoites Opsonized with Human Serum Antibodies. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0009871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaratilake LM, Ferrante A. Opsonization and phagocytosis of Plasmodium falciparum merozoites measured by flow cytometry. Clinical and diagnostic laboratory immunology. 2000;7:9–13. doi: 10.1128/cdli.7.1.9-13.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, et al. Identification and Prioritization of Merozoite Antigens as Targets of Protective Human Immunity to Plasmodium falciparum Malaria for Vaccine and Biomarker Development. The Journal of Immunology. 2013. [DOI] [PMC free article] [PubMed]
- Salmon BL, Oksman A, Goldberg DE. Malaria parasite exit from the host erythrocyte: a two-step process requiring extraerythrocytic proteolysis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:271–276. doi: 10.1073/pnas.011413198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MJ, et al. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proceedings of the National Academy of Sciences. 2010;107:14378–14383. doi: 10.1073/pnas.1009198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Langer C, Goodman CD, Mcfadden GI, Beeson JG. Defining the timing of action of anti-malarial drugs against Plasmodium falciparum. Antimicrobial Agents and Chemotherapy. 2013. pp. 1–46. [DOI] [PMC free article] [PubMed]
- Angrisano F, et al. Spatial Localisation of Actin Filaments across Developmental Stages of the Malaria Parasite. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0032188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riglar DT, et al. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host and Microbe. 2011;9:9–20. doi: 10.1016/j.chom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Zuccala ES, et al. Subcompartmentalisation of Proteins in the Rhoptries Correlates with Ordered Events of Erythrocyte Invasion by the Blood Stage Malaria Parasite. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0046160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marapana DS, et al. Malaria Parasite Signal Peptide Peptidase is an ER-Resident Protease Required for Growth but not for Invasion. Traffic. 2012;13:1457–1465. doi: 10.1111/j.1600-0854.2012.01402.x. [DOI] [PubMed] [Google Scholar]
- Rzepczyk CM, Lopez JA, Anderson KL, Alpers MP. Investigation of the effect of monocytes with Papua New Guinea sera on Plasmodium falciparum in culture. International Journal for Parasitology. 1988;18:401–406. doi: 10.1016/0020-7519(88)90151-8. [DOI] [PubMed] [Google Scholar]
- Schofield L, Mueller I. Clinical immunity to malaria. Curr Mol Med. 2006;6:205–221. doi: 10.2174/156652406776055221. [DOI] [PubMed] [Google Scholar]
- Hill DL, et al. Efficient measurement of opsonizing antibodies to Plasmodium falciparum merozoites. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0051692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S, et al. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) International journal of cancer Journal international du cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Ackerman ME, et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. Journal of Immunological Methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataíde R, et al. Using an Improved Phagocytosis Assay to Evaluate the Effect of HIV on Specific Antibodies to Pregnancy-Associated Malaria. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Crabb BS, Beeson JG. Development of fluorescent Plasmodium falciparum for in vitro growth inhibition assays. Malar J. 2010;9:152. doi: 10.1186/1475-2875-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Braun-Breton C, Rosenberry TL, da Silva LP. Induction of the proteolytic activity of a membrane protein in Plasmodium falciparum by phosphatidyl inositol-specific phospholipase. C. Nature. 1988;332:457–459. doi: 10.1038/332457a0. [DOI] [PubMed] [Google Scholar]
- Jaramillo M, Godbout M, Olivier M. Hemozoin induces macrophage chemokine expression through oxidative stress-dependent and -independent mechanisms. J Immunol. 2005;174:475–484. doi: 10.4049/jimmunol.174.1.475. [DOI] [PubMed] [Google Scholar]
- Schofield L, Tachado SD. Regulation of host cell function by glycosylphosphatidylinositols of the parasitic protozoa. Immunology and cell biology. 1996;74:555–563. doi: 10.1038/icb.1996.89. [DOI] [PubMed] [Google Scholar]
- Khusmith S, Druilhe P, Gentilini M. Enhanced Plasmodium falciparum merozoite phagocytosis by monocytes from immune individuals. Infection and immunity. 1982;35:874–879. doi: 10.1128/iai.35.3.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunov O, et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS. 2011;5:1657–1669. doi: 10.1021/nn2000756. [DOI] [PubMed] [Google Scholar]
- Tebo AE, Kremsner PG, Luty AJ. Fcgamma receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clinical and experimental immunology. 2002;130:300–306. doi: 10.1046/j.1365-2249.2002.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood. 2000;96:3231–3240. [PubMed] [Google Scholar]


