Abstract
Objective
Five core domains have been endorsed by Outcomes Measures in Rheumatology (OMERACT) for acute gout: pain, joint swelling, joint tenderness, patient global assessment, and activity limitation. The aim of this work was to evaluate instruments for these domains according to the OMERACT filter: truth, feasibility, and discrimination.
Methods
A systematic search strategy for instruments used to measure the acute gout core domains was formulated. For each method, articles were assessed by two reviewers to summarise information according to the specific components of the OMERACT filter.
Results
Seventy-seven articles and abstracts met the inclusion criteria. Pain was most frequently reported (76 studies, 20 instruments). The pain instruments used most often were 100mm visual analog scale (VAS) and 5-point Likert scale. Both methods have high feasibility, face and content validity, within- and between-group discrimination. Four-point Likert scales assessing index joint swelling and tenderness have been used in numerous acute gout studies; these instruments are feasible, with high face and content validity, and show within- and between-group discrimination. Five-point patient global assessment of response to treatment (PGART) scales are feasible and valid, and show within- and between-group discrimination. Measures of activity limitations were infrequently reported, and insufficient data were available to make definite assessments of the instruments for this domain.
Conclusion
Many different instruments have been used to assess the acute gout core domains. Pain VAS and 5-point Likert scales, 4-point Likert scales of index joint swelling and tenderness and 5-point PGART instruments meet the criteria for the OMERACT filter.
Keywords: gout, pain, measurement, outcome
Introduction
Acute gout is characterised by the sudden onset of intense pain and swelling of one or more joints, reaching a maximal level of severity within hours and usually resolving over 10-14 days. The aim of therapy for acute gout is rapid resolution of the attack. Typically, acute gout is treated with non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids or colchicine. There has been renewed interest in the treatment of acute gout since the identification of the central role of the NRP3 inflammasome and interleukin (IL)-1β in initiation of the inflammatory response to monosodium urate crystals (1). This has led to recent clinical trials of IL-1β inhibitors for management of acute gout.
Since 2002, the Outcome Measures in Rheumatology (OMERACT) Gout Special Interest Group has worked towards defining outcome measures for studies in gout (2-10). Five core domains have been endorsed by OMERACT for studies of acute gout: pain, joint tenderness, joint swelling, patient global assessment, and activity limitation (5). Although these domains have been endorsed for acute gout trials, the instruments for each of these domains have not been fully developed nor endorsed by the OMERACT process for this context. The aim of this systematic literature review was to evaluate instruments for the acute gout core domains according to the OMERACT filter: truth, feasibility, and discrimination (11).
Methods
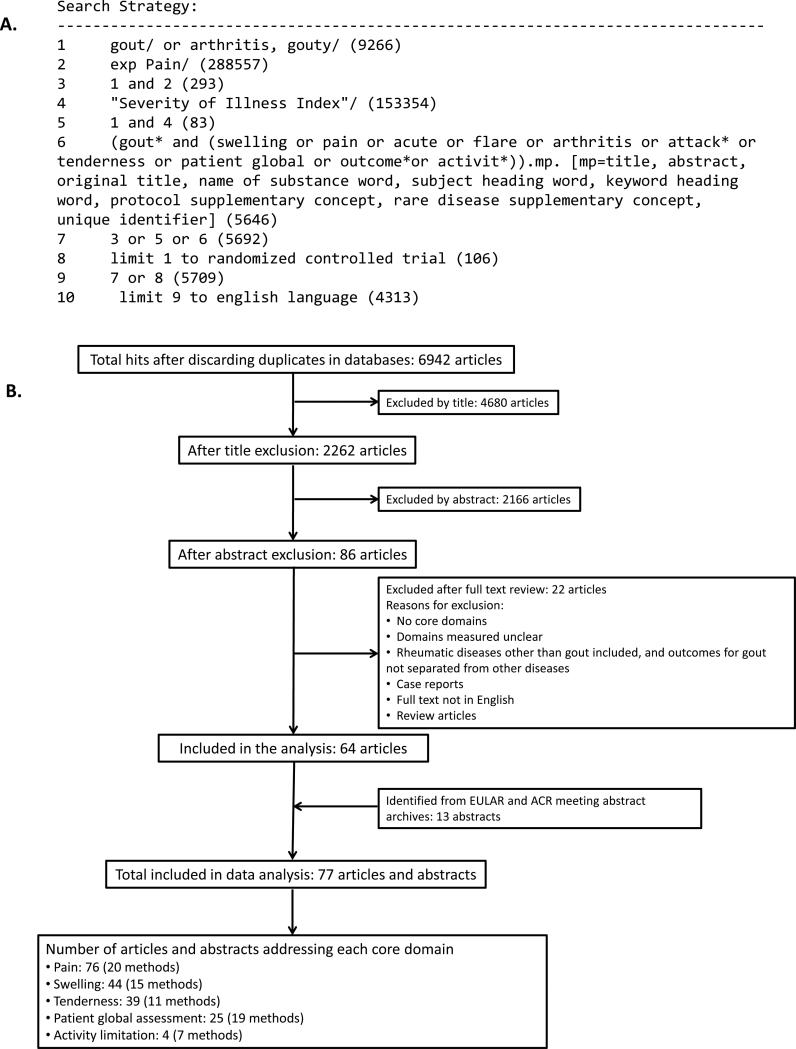
A systematic search strategy was formulated to provide a written summary of the evidence for instruments in the acute gout core domains endorsed by OMERACT. The research question was which instruments assessing the core domains in acute gout met the OMERACT filter. The following search keywords were used: “acute gout”, “gout flare”, ”gouty arthritis”, “gout pain”, “gout randomized control trial”, “gout attack”, “gout tenderness”, “gout swelling”, “gout patient global”, “gout outcome”, and “gout activity”. Searches were performed in the following electronic databases: PubMed, Medline, Cochrane Central Register of Controlled Trials (The Cochrane Library), Excerpta Medica Database (EMBASE), European League Against Rheumatism (EULAR) meeting abstract archive and American College of Rheumatology (ACR) Annual Scientific Meeting abstract archive. Bibliographical references of individual publications were also checked. Data sources were English publications from these databases and hand searches. No date restrictions were used (earliest database search date was 1946). The search was completed in December 2011. An example of the search strategy is shown in Figure 1A. Articles and abstracts were included if the participants had acute gout and at least one core domain was assessed in the study. The search results were further cross-checked with the results of an independent systemic literature review of randomised controlled trials (RCT) for treatments of acute gout to ensure that all relevant RCT studies were identified (12).
Figure 1. Search strategy and results.
A. Example of the search strategy. B. Summary of literature search results.
A total of 6,942 articles were generated by the search, with 4680 excluded by reviewing title as they did not relate to acute gout. Case reports, prevalence studies, studies of conditions other than acute gout, or those that did not address any aspect of the OMERACT filter were further excluded based on abstract or full text review. A total of 77 abstracts and full text articles met the inclusion criteria and were included in the analysis (Figure 1).
For each outcome domain, articles were assessed by two independent reviewers (CZ and RG) to summarize detailed information about each instrument according to the components of the OMERACT filter: feasibility, truth, and discrimination (11). Aspects of feasibility considered were: cost, training required, equipment required, and patient acceptability. Aspects of truth considered were: face validity (whether the method looks right), construct validity (whether the method relates to other methods of acute gout assessment in predicted ways, using correlation coefficients of patient level data), content validity (whether the method cover the relative issues adequately, including any patient assessments), and internal consistency (whether Cronbach alpha was reported). Aspects of discrimination that were considered were within-group change sensitivity (in prospective studies, reported as effect size where available), and between-group sensitivity (differences documented between different allocated treatment groups in prospective studies with relevant statistics reported).
Results
Summary of search results
The literature search identified 77 articles and abstracts that met the criteria for inclusion in the review. The search summary is outlined in Figure 1B. No studies explicitly addressed internal consistency using the specified definitions. Reproducibility data were not available for any instrument in the assessment of acute gout.
Pain
Pain was the most frequently reported domain (in 76 of the 77 studies assessed, Figure 1). Twenty different instruments were used in these studies to assess the pain of acute gout. The three most frequently used instruments are shown in Table 1. All three methods were considered feasible with high face and content validity. The 100mm (10cm) pain VAS has been used in 16 studies of acute gout. Sensitivity to change for the pain VAS has been demonstrated with an effect size of 9.3 after 72 hours following canakinumab 150mg treatment (13). This instrument has also documented between group discrimination in two separate clinical trials (14, 15).
Table 1.
Summary of pain instruments used in studies of acute gout.
| Method | Description | Number and type of studies with references | Feasibility | Truth | Within-group discrimination (ES) | Between-group discrimination (estimate or statistic with p-value) |
|---|---|---|---|---|---|---|
| Visual analogue pain scale (VAS) (10cm/100mm) | A 10 cm/100mm, horizontal VAS with the far left end (0) represents no pain and the far right end (10cm/100mm) represents the most severe pain the patient has ever experienced. | Total: 16 Controlled: 11 (13-15, 18, 30-36) Observational: 5 (26, 37-40) |
Inexpensive, no training required, no specialist equipment required, acceptable to patients | High face validity. Reduction of pain scores was accompanied by reduction of joint swelling and tenderness, C-reactive protein value, and patient global assessment (18, 26). Similar reductions reported in pain, tenderness, swelling, erythema (15). Unable to calculate correlation coefficients with available information. Measure has been endorsed by OMERACT for use in chronic gout studies (7). |
All articles reported significant reduction in pain scores over time. In an RCT of prednisolone (PRED) vs. naproxen (NAP), the decrease of the pain from baseline to Day 4 was 44·7 mm for PRED and 46·0 mm for NAP, ES on Day 4 =2.00 for PRED, and 2.21 for NAP (32). In an RCT of canakinumab (CAN) vs. triamcinolone acetonide (TA), the % change from baseline in pain score after 72 hours was −84.6%, ES =9.3 for CAN 150mg; and −57.8%, ES =4.5 for TA (13). |
In an RCT of CAN vs. TA, significantly lower pain scores were reported for CAN 150mg vs. TA 72 hours post dose (least square mean difference −9.7 mm, p=0.0005) (14). In an RCT of high dose colchicine,73% patients in the colchicine group and 36% patients in the placebo group improved pain score by 50% after 48 hours (p<0.05) (15). |
| 5-point Likert scale (range 0-4) | 0= no pain, 1= mild pain, 2= moderate pain, 3= severe/strong pain and 4= excruciating pain/ very severe/ extreme/very strong | Total: 16 Controlled*: 12 (17-21, 27, 34, 41-45) Observational: 4 (16, 46-48) *Navarra and Schlesinger references were post hoc analysis of Rubin and Schumacher studies. |
Inexpensive, no training required, no specialist equipment required, acceptable to patients | High face validity. Reduction in pain score was accompanied by reduction in other secondary end points (joint tenderness, joint swelling and joint erythema and global assessments of response to treatment, C-reactive protein) (17, 18). Unable to calculate correlation coefficients with available information. Patients with both monoarticular and oligoarticular disease had a clinical response, but the response was greater in those with monoarticular disease, p<0.001 (42). Patients with both moderate pain and severe/extreme pain at baseline had a clinical response, but the response was greater in those with severe/extreme pain, p<0.001 (42). Good construct validity: significant differences in pain scores between patients categorized into None/Fair vs. Good/Excellent based on responses to patient and investigator global assessment of response to therapy (p<0.0001) (27). |
All articles reported significant reduction in pain scores over time. In untreated acute gout, pain decreased from 3.7 at baseline (Day 1) to 2.5 on Day 7. ES Day 2=0.05 and Day 7 = 0.87 (16). In an RCT comparing etoricoxib (ETO) and indomethacin (IND), score decreased by nearly 1.0 point from baseline to four hours after the first dose in both groups ES at Day 2 =2.17 for ETO and 2.47 for IND; at Day 8, ES= 3.48 for ETO and 3.77 for IND (17). |
In an RCT of CAN vs. TA, 92% patients in CAN 150mg group and 56% in TA group had no or mild pain after 48 hours (p < 0.05). The reduction in pain intensity from baseline was also significantly greater for CAN 150 mg, compared with TA from 48 hours to 7 days post-dose (p < 0.05) (18). High-dose celecoxib led to a greater reduction in pain intensity on Day 2 compared with low-dose celecoxib (least squares mean difference −0.46, p=0.0014) (19). |
| 4-point Likert scale (range 0-3) | 0= no pain, 1= mild/slight pain, 2= moderate pain and 3= severe pain | Total: 9 Controlled: 5 (49-53) Observational: 4 (54-57) |
Inexpensive, no training required, no specialist equipment required, acceptable to patients | High face validity. Scores for pain, redness, tenderness, restriction of movement and swelling showed similar reductions at timepoints tested (49, 52-55). Unable to calculate correlation coefficients with available information. |
All articles reported significant reduction in pain scores over time. Following ketoprofen treatment, pain decreased from 2.7 at baseline (Day 1) to 1.08 on Day 2, to 0.52 on Day 5, and 0.37 on Day 8. Following IND, the pain score on respective days were 2.76, 0.91, 0.50 and 0.30 (p<0.05 for each timepoint compared with baseline in both treatment groups) (49). ES could not be calculated from available data. |
No significant difference in change in pain scores between ketoprofen and IND groups (49), in percentage improvement in pain scores between meclofenamate sodium and IND treatment groups (51), or % with no/mild pain between tiaprofenic acid and ketoprofen groups (52) |
The properties of the three methods used most frequently have been shown. All pain scores were patient reported. No articles reported internal validity, feasibility, test-retest reproducibility. Effect size (ES) is provided wherever possible. If the ES could not be calculated, the statistic and associated p-value is provided.
Similarly, the 5-point Likert pain scale has been used in 16 studies of acute gout, including a study of untreated acute gout (16). Sensitivity to change for the 5-point Likert scale has been demonstrated with effect sizes of 2.17-2.47 following two days of NSAID treatment (17). Between-group discrimination has been demonstrated in two separate clinical trials (18, 19).
The 4-point Likert pain scale has been reported in nine studies of acute gout. Sensitivity to change over time has been reported in many studies, although data were not available to allow calculation of effect sizes. Between group discrimination has not been demonstrated.
Joint swelling
Joint swelling has been reported in 44 studies, using 15 different instruments (Figure 1). The three instruments most frequently used are shown in Table 2. All three instruments were considered to be feasible, although some observer training is required. Physician assessment of joint swelling in the index joint using a 4-point Likert scale (range 0-3) has been used in eight studies of acute gout. This method has high face validity as it captures the degree of swelling in the affected joint, which is particular relevant to acute gout, which frequently presents as a monoarthritis (17). Sensitivity to change over time has been reported in many studies, although data were not available to allow calculation of effect sizes. Between group discrimination has been reported in a clinical trial of canakinumab vs. triamcinolone using this instrument (18). Several randomised controlled trials comparing two NSAIDs have not shown difference in change in joint swelling using this instrument (17, 20).
Table 2.
Summary of joint swelling instruments used in studies of acute gout.
| Method | Description | Number and type of studies with references | Feasibility | Truth | Within-group discrimination (ES) | Between-group discrimination (estimate or statistic with p-value) |
|---|---|---|---|---|---|---|
| Physician assessment of swelling in the index joint using a 4-point Likert scale (range 0-3) | 0= no swelling; 1= mild swelling; 2=moderate swelling; 3= severe swelling (or bulging beyond joint margins) | Total: 8 Controlled: 6 (17, 18, 20, 41, 44, 58) Observational: 2 (54, 57) |
Inexpensive, some training required, no specialist equipment required, acceptable to patients | High face validity. The reduction in the number of patients with severe or moderate swelling was accompanied by greater proportion of patients reporting no or mild pain on the 5- point Likert scale, the increasing proportion of patients reporting normalisation of C-reactive protein, and better responses from patient and investigator global assessment of response to treatment (18). The joint swelling scores showed similar reductions with those for pain and joint tenderness (17) and erythema (44). Unable to calculate correlation coefficients with available information. Measure captures degree of swelling in affected joint. |
All articles reported significant reduction in joint swelling scores over time, typically by 72 hours. In an RCT comparing etoricoxib (ETO) and indomethacin (IND), the least squares mean change (95% CI) from baseline to Days 2-8 was −1.45 (CI −1.61 to −1.29) for ETO and −1.45 (−1.62 to −1.28) for IND (17). In another RCT comparing ETO and IND, the least square mean change (95% CI) from baseline to the mean of Days 2-5 was −1.65 (−1.80 to −1.50) for ETO, and −1.56 (−1.72 to −1.40) for IND (20). ES could not be calculated from available data. |
In an RCT of canakinumab (CAN) vs. triamcinolone acetonide (TA), the CAN 150 mg group had a lower swelling score compared with the TA group from 72 hours to seven days post-dose. The odds ratio favouring CAN 150mg was 2.7 (95% CI, 1.09–6.5) (18). In two RCTs comparing ETO and IND, there was no difference between the least square mean difference in swelling scores between the ETO and IND groups (17, 20). |
| Physician measurement of index joint circumference/perimeter | The circumference/perimeter of the affected joint measured by tape measure, reported in cm. | Total: 7 Controlled: 3 (21, 22, 31) Observational: 4 (37, 46, 47, 59) |
Inexpensive, some training required, no specialist equipment required (tape measure only), acceptable to patients | Reduction in joint circumference was accompanied by reduction in pain, swelling, erythema and joint impairment (21, 31, 46, 59). Unable to calculate correlation coefficients with available information. Large variation in circumference measured depending on affected joint eg. 8cm for big toe and 36.5cm for knee (21). |
All articles reported significant reduction in joint swelling scores over time, typically at 72 hours. In an observational study of intravenous indoprofen, the average joint circumference of the affected joint decreased from 31.6cm at baseline to 27.3cm on Day 3, ES =0.46 (46). In a clinical trial of tenoxicam (TEN) dosing, the average joint circumference of the affected joint decreased from 23.6cm at baseline to 18.1cm on Day 6 following treatment with TEN 40mg, ES= 0.46 on Day 6 (22). |
In an RCT of ice therapy, the mean (SD) reduction for the ice group was 5.90 (3.84) cm compared with 3.83 (4.19) cm for controls after 1 week (p=0.14) (31). In a clinical trial of two dosage regimens of indoprofen, the mean (SD) reduction for the iv bolus/24 hour infusion arm was 2.4 (1.0) and for high dose single iv bolus was 2.5 (0.53) (p=0.82) after 48 hours of treatment (21). In a clinical trial of TEN dosing, there was no significant difference between 20mg and 40mg daily dosing in joint swelling (p>0.05) (22). |
| Physician assessment of the number of swollen joints (swollen joint count, SJC) | Total: 3 Controlled: 2 (23, 28) Observational: 1 (26) |
Inexpensive, some training required, no specialist equipment required, acceptable to patients | The reduction in the SJC was accompanied by improvement in tender joint count, pain score, C-reactive protein value, Leeds Foot Impact Scale and score of Lower Limb Task Questionnaire score (26). Unable to calculate correlation coefficients with available information. Monoarticular flares are common in patients; in an RCT comparing etoricoxib (ETO) and indomethacin (IND), 99/150 patients had a single joint affected (17). Risk of floor effect. Degree of swelling not captured within the measure. |
All treatments led to significant reduction in SJC over time, with the exception of the herbal formula Danggui-Nian-Tong-Tang (DNTT) (28). Following IND treatment, mean (SD) SJC reduced from 1.3 (0.7) at baseline to 0.6 (0.5) after 72 hours ES=0.22 (28). In an observational study, mean (SD) SJC reduced from 3 (3) at baseline to 0 (1) at the follow up visit (>1 month after treatment). ES =0.67 (26). |
In an RCT comparing DNTT with IND, there was a significant difference between the groups in SJC after 72 hours of treatment; mean (SD) SJC for DNTT 1.9 (1.2) and IND 0.6 (0.5), p<0.0001 (28). In an RCT of two dosage regimens of piroxicam, there was no significant difference in SJC between high dose and low dose piroxicam (23). |
The properties of the three methods used most frequently have been shown. No articles reported internal validity, feasibility, test-retest reproducibility. Effect size (ES) is provided wherever possible. If the ES could not be calculated, the statistic and associated p-value is provided.
Physical measurement of the circumference of the affected joint using a tape measure has been reported in seven acute gout studies. Although this method also allows assessment of the affected joint, there is a large variation in measurement depending on the size of the joint when large joints, such as the knee, and also small joints, such as those in the toes, are included (21). Sensitivity to change over time has been demonstrated with an effect size of 0.46 following three days of NSAID treatment (22). Between group discrimination has not been reported using this method.
Physician assessment of the swollen joint count (SJC) has been reported in three studies of acute gout. This instrument has the ability to measure the extent of disease in polyarticular gout, but does not capture of the degree of swelling in an affected joint. This may reduce the sensitivity of the measure in patients with monoarticular gout, and SJC is not appropriate for studies of monarticular gout. Within-group and between-group discrimination has been reported using this instrument (Table 2).
Joint tenderness
Joint tenderness has been reported in 39 studies, using 11 different instruments (Figure 1). The three instruments most frequently used are shown in Table 3. All three instruments were considered to be feasible, although some observer training is required. All instruments assessing joint tenderness may cause some patient distress, as joints affected by acute gout may be extremely tender. Physician assessment of joint tenderness in the index joint using a 4-point Likert scale (range 0-3) has been used in 17 studies of acute gout. This method has high face validity as it captures the degree of tenderness in the affected joint, which is particular relevant to acute gout, which frequently presents as a monoarthritis (17). Sensitivity to change over time has been reported in many studies, with effect size calculated as 2.5 following three days of high dose piroxicam (23). Between group discrimination has been reported in a clinical trial of canakinumab vs. triamcinolone using this instrument (18). Several randomised controlled trials comparing two NSAIDs have not shown differences in change in joint tenderness using this instrument (17, 20).
Table 3.
Summary of joint tenderness instruments used in studies of acute gout.
| Method | Description | Number and type of studies with references | Feasibility | Truth | Within-group discrimination (ES) | Between-group discrimination (estimate or statistic with p-value) |
|---|---|---|---|---|---|---|
| Physician assessment of tenderness in the index joint using a 4-point Likert scale (range 0-3). | 0=no pain; 1=mild/ patient states there is pain when touched, 2=moderate/patient states there is pain and winces, 3= severe/patient states there is pain, winces and withdraws | Total: 17 Controlled*: 12 (15, 17, 18, 20, 23, 41, 42, 44, 49-52) Observational: 5 (54-57, 60) *Navarra reference was post hoc analysis of Rubin and Schumacher studies. |
Inexpensive, some training required, no specialist equipment required, may cause patient distress | High face validity. The reduction in the number of patients with severe or moderate tenderness was accompanied by greater proportion of patients reporting no or mild pain on the 5-point Likert scale, the increasing proportion of patients reporting normalisation of C-reactive protein, and better responses from PGART and IGART (18). Unable to calculate correlation coefficients with available information. Patients with both monoarticular and oligoarticular disease had a clinical response, but the response was greater in those with monoarticular disease, p<0.001 (42). Patients with both moderate pain and severe/extreme pain at baseline had a clinical response, but the response was greater in those with severe/extreme pain, p<0.05 (42). Measure captures degree of tenderness in affected joint. |
All articles reported significant reduction in joint tenderness scores over time, typically by 72 hours. In a clinical trial of two doses of piroxicam, mean tenderness score reduced from 2.10 at baseline to 0.54 on Day 3 and 0.15 on Day 7 in the high dose piroxicam group. ES =2.5 on Day 3 and 2.9 on Day 7 (23). |
In an RCT of canakinumab (CAN) vs. triamcinolone acetonide (TA), the CAN 150 mg group had a lower tenderness score compared with the TA group seven days post-dose. The odds ratio favouring CAN 150mg was 3.2 (95% CI, 1.27–7.9) (18). In two RCTs comparing etoricoxib (ETO) and indomethacin (IND), there was no difference between the least square mean difference in tenderness scores between the ETO and IND groups (17, 20). |
| Physician assessment of tenderness in the index joint using Likert 0-4 (5-point) scale | 0= no tenderness, 1=mild tenderness, 2= moderate tenderness, 3=severe tenderness and 4=very severe tenderness | Total: 5 Controlled: 2 (21, 43) Observational: 3 (16, 46, 47) |
Inexpensive, some training required, no specialist equipment required, may cause patient distress | High face validity. Reduction in tenderness was accompanied by similar reduction in pain, swelling and restriction of joint movement (21, 46). Unable to calculate correlation coefficients with available information. Measure captures degree of tenderness in affected joint. |
All articles reported significant reduction in joint tenderness scores over time, typically by 72 hours following treatment. In untreated acute gout, tenderness scores were 3.9 at baseline, (Day 1), 3.9 on Day 2, and 3.1 on Day 7. ES=0.0 on Day 2; and 0.9 on Day 7 (16). In clinical study of different dosing regimens of intravenous indoprofen, high dose bolus indoprofen lead to reduction of tenderness scores from 3.54 at baseline to 2.54 after 2 hours, 1.46 after 4 hours, 1.08 after 24 hours, and 0.09 after 48 hours. The ES from 2, 4, 24, and 48 hours after the start of treatment were 2.1, 4.3, 5.1, and 7.2 respectively (21). |
In a clinical trial of two dosage regimens of indoprofen, there was no difference in the reduction in tenderness between two regimens (21). In a clinical trial comparing two anti-inflammatory agents (IND and proquazone), there was no difference in the reduction in tenderness between two agents (43). |
| Physician assessment of the number of tender joints (tender joint count, TJC) | Total: 3 Controlled: 2 (23, 28) Observational: 1 (26) |
Inexpensive, some training required, no specialist equipment required, may cause patient distress | The reduction in the TJC was accompanied by reduction in tender joint count, pain VAS score, and C-reactive protein value, Leeds Foot Impact Scale and increase in the mean score of Lower Limb Task Questionnaire (26). Unable to calculate correlation coefficients with available information. Monoarticular flares are common in patients; in an RCT comparing etoricoxib (ETO) and indomethacin (IND), 99/150 patients had a single joint affected (17). Risk of floor effect. Degree of tenderness not captured within the measure. |
All treatments lead to significant reduction in TJC over time, with the exception of the herbal formula Danggui-Nian-Tong-Tang (DNTT) (28). Following IND treatment, mean (SD) TJC reduced from 1.4 (0.8) at baseline to 0.6 (0.7) after 72 hours ES=0.22 (28). In an observational study, mean (SD) TJC reduced from 8 (9) at baseline to 1 (1) at the follow up visit (>1 month after treatment). ES =0.78 (26). |
In an RCT comparing DNTT with IND, there was a significant difference between the groups in TJC after 72 hours of treatment; mean (SD) TJC for DNTT 2.6 (2.4) and IND 0.6 (0.7), p=0.001 (28). In an RCT of two dosage regimens of piroxicam, there was no significant difference in TJC between high dose and low dose piroxicam (23). |
The properties of the three methods used most frequently have been shown. No articles reported internal validity, feasibility, test-retest reproducibility. Effect size (ES) is provided wherever possible. If the ES could not be calculated, the statistic and associated p-value is provided.
Physician assessment of joint tenderness in the index joint using a 5-point Likert scale (range 0-4) has been used in five studies of acute gout. As outlined above for the 4-point Likert scale, this method has high face validity as it captures the degree of tenderness in the affected joint. Sensitivity to change over time has been reported in a study of untreated acute gout, with effect size calculated as 0.9 on Day 7 (16). A clinical study of intravenous indoprofen showed effects sizes of 2.1 after 2 hours of treatment and 7.2 after 48 hours (21). Between group discrimination has not been demonstrated.
Physician assessment of the tender joint count (TJC) has been reported in three studies of acute gout. As with the SJC, this instrument has the ability to measure the extent of disease in polyarticular gout, but does not capture of the degree of tenderness in an affected joint. This may reduce the sensitivity of the measure in patients with monoarticular gout, and TJC is not appropriate for studies of monarticular gout. Within-group and between-group discrimination has been reported using this instrument (Table 3).
Patient global assessment
Patient global assessment has been reported in 25 studies of acute gout, using 19 different methods (Figure 1). Both patient global assessment of response to therapy (PGART) and patient global assessment of disease activity (PGA) have been reported. Of the 19 instruments, 10 were variations of the 5-point PGART instrument, using different descriptors, ranges and methods of data collection. The three instruments used most frequently are shown in Table 4. All three methods were considered feasible with high face and content validity.
Table 4.
Summary of patient global assessments used in studies of acute gout.
| Method | Description | Number and type of studies with references | Feasibility | Truth | Within-group discrimination (ES) | Between-group discrimination (estimate or statistic with p-value) |
|---|---|---|---|---|---|---|
| Patient global assessment of response to treatment (PGART) (5-point numerical scale) | 0=excellent, 1=very good, 2=good, 3=fair and 4=poor response to treatment. | Total: 4 Controlled*: 4 (17, 20, 27, 42) Observational: 0 *Navarra and Schlesinger references were post hoc analysis of Rubin and Schumacher studies. |
Inexpensive, no training required, no specialist equipment required, acceptable to patients | High face validity. Low PGART scores were associated with reductions in tenderness and pain scores over Days 2-5 (17, 20). Unable to calculate correlation coefficients with available information. Patients with both monoarticular and oligoarticular disease had a clinical response, but the response was greater in those with monoarticular disease, p<0.001 (42). Good construct validity: significant differences in pain scores between patients categorized into None/Fair vs. Good/Excellent based on responses to PGART (p<0.0001) (27). Baseline assessment of disease severity not captured using this measure. |
All articles reported significant reduction in PGART scores over time. In an RCT comparing etoricoxib (ETO) and indomethacin (IND), the least squares mean change (95% CI) from baseline to Days 2-8 was 1.42 (1.20 to 1.65) for ETO and 1.33 (1.10 to 1.56) for IND (17). In another RCT comparing ETO and IND, the least square mean change (95% CI) from baseline to the mean of Days 2-5 was 1.58 (1.37-1.79) for ETO, and 1.70 ( 1.48 -1.92) for IND (20). ES could not be calculated from available data. |
In two RCTs comparing ETO and IND, there was no difference between the least square mean difference in PGART scores between the ETO and IND groups (17, 20). |
| Patient global assessment of response to treatment (PGART) (5-point descriptive scale) | Excellent, good, acceptable, slight, poor-response to treatment | Total: 2 Controlled: 2 (13, 18) Observational: 0 |
Inexpensive, no training required, no specialist equipment required, acceptable to patients | High face validity. Excellent and good PGART responses were accompanied by reductions in pain, tenderness, swelling and erythema (18) and C-reactive protein (13). Unable to calculate correlation coefficients with available information. Baseline assessment of disease severity not captured using this measure. |
In an RCT of canakinumab (CAN) vs. triamcinolone acetonide (TA), good or excellent response to treatment reported in 88.8% patients receiving CAN 150mg after 72 hours and in 92.6% after 7 days, and in 53.5% patients receiving TA after 72 hours and in 55.3% after 7 days (13). ES could not be calculated from available data. |
In an RCT of CAN vs. TA, good or excellent response to treatment was observed more often in patients receiving any CAN dose compared with TA; at 72 hours odds ratio (OR) 2.0 (p=0.02) and at 7 days OR 2.3 (p=0.01) (13). In another RCT of CAN vs. TA, CAN 150mg was associated with significant better responses compared with T, OR favouring CAN 150mg versus TA ≈4.0, p=0.002 (18). |
| Patient global assessment (PGA) of overall condition | 1=very good; 2= good; 3= fair 4=poor and 5=very poor. | Total: 3 Controlled: 2 (24, 25) Observational: 1 (61) |
Inexpensive, no training required, no specialist equipment required, acceptable to patients | High face validity. Improvements in PGA were accompanied by similar reductions in pain, tenderness and swelling (24, 25, 61). Unable to calculate correlation coefficients with available information. |
All articles reported reduction in PGA scores over time. In a clinical trial of etodolac (ETD) and naproxen (NAP),, the mean scores at baseline and on Days 2, 4, and 7 were 4.3, 3.2, 2.3, and 1.8 respectively for ETD, and 4.0, 3.5, 2.7 and 2.1 for NAP, p<0.05 for both groups at each timepoint compared with baseline (24). In another clinical trial of ETD and NAP, no patients described their condition as good or very good at baseline. At the last study visit (Days 3-7), good or very good condition was reported by 76% in the ETD group and 81% NAP group (25). p-value not reported. ES could not be calculated from available data. |
In the two RCTs of ETD and NAP, there was no significant difference between the two treatment groups in the PGA scores over time (24, 25). |
The properties of the three methods used most frequently have been shown. No articles reported internal validity, feasibility, test-retest reproducibility. Effect size (ES) is provided wherever possible. If the ES could not be calculated, the statistic and associated p-value is provided.
In contrast to the PGA, the PGART is a measure of change and does not allow measurement of patient assessment at baseline. A 5-point numerical PGART scale has been reported in three articles (see Table 4 for details of this scale). Sensitivity to change over time has been reported, although data were not available to allow calculation of effect sizes. Several randomised controlled trials comparing two NSAIDs have not shown between-group differences in PGART response using this instrument (17, 20).
A 5-point descriptive PGART scale has been reported in two clinical trials (see Table 4 for details of scale). Sensitivity to change over time has been reported, although data were not available to allow calculation of effect sizes. Two separate randomised controlled trials comparing canakinumab with triamcinolone acetonide have shown between group discrimination using this PGART instrument (13, 18).
A 5-point PGA scale has been reported in three acute gout studies. Sensitivity to change over time has been reported in these studies, although data were not available to allow calculation of effect sizes. Two randomised controlled trials comparing two NSAIDs have not shown differences in change in PGA using this instrument (24, 25).
Activity limitation
Activity limitation has been measured infrequently in studies of acute gout, with only four studies reporting this domain, using seven different instruments (Figure 1). Only two instruments, the Health Assessment Questionnaire (HAQ) and the Short form (36) Health Survey (SF-36) physical function (PF) domain have been reported in more than one study. Properties for these two instruments are shown in Table 5. Both instruments were considered to be feasible with high content and face validity. Both instruments have been endorsed by OMERACT for studies of chronic gout (3, 7).
Table 5.
Summary of activity limitation instruments used in studies of acute gout.
| Method | Description | Number and type of studies with references | Feasibility | Truth | Within-group discrimination (ES) | Between-group discrimination (estimate or statistic with p-value) |
|---|---|---|---|---|---|---|
| Health Assessment Questionnaire (HAQ) | 0-3 composite scale (0=no disability, 3=complete disability) | Total: 2 Controlled: 1 (18) using 20 item HAQ-DI Observational: 1 (26) using 10 item HAQ-II |
Inexpensive, no training required, no specialist equipment required, acceptable to patients | High face validity. Improvement in the HAQ-DI score was accompanied by similar reductions in joint tenderness and swelling, pain score, C-reactive protein and PGART (18). Improvement in HAQ scores was accompanied by similar improvements in other measures of disability including the SF-36 PF score (18), Leeds Foot Impact Scale and the Lower Limb Task Questionnaire (26). Unable to calculate correlation coefficients with available information. Measure has been endorsed by OMERACT for use in chronic gout studies (7). |
Both articles reported significant reduction in HAQ scores over time. In an observational study, mean (SD) HAQ-II score reduced from 1.9 (0.6) at baseline to 0.9 (0.6) at the follow up visit (>1 month after treatment). ES =1.43 (26). In an RCT of canakinumab (CAN) vs. triamcinolone acetonide (TA), reductions in HAQ-DI scores ranged from 0.46 - 0.67 at Day 7, and 0.52 - 0.85 at Week 8 across the groups (18). |
In an RCT of CAN vs. TA, there was no significant difference between the treatment groups in HAQ-DI scores over time (18). |
| Short form (36) Health Survey (SF-36) physical function (PF) domain | Scores range from 0 to 100, where 0 represents the worst possible physical function and 100 is perfect physical function | Total: 2 Controlled: 2 (18): SF36 PF reported, (44) SF-36 PF not reported separately Observational: 0 |
Licensed, no training required, no specialist equipment required, acceptable to patients | High face validity. Improvement in SG-36 PF score (compared to baseline) was accompanied by reductions in pain, PGART, joint tenderness, swelling and erythema (18). Unable to calculate correlation coefficients with available information. SF-36 questionnaire has been endorsed by OMERACT for measurement of health related quality of life in chronic gout studies (3). |
In an RCT of CAN vs. TA, improvements in SF-36 PF scores were observed in both groups. Mean SF-36 PF scores rapidly improved in the CAN 150 mg group from 41.5 at baseline to 80.0 at seven days post-dose (a mean increase of 39.0 points), and exceeded the value for the US general population by eight weeks post-dose (86.1 vs. 84.2 for the US general population) (18). ES could not be calculated from available data. |
In patients with acute gout, mean SF-36 PF scores were much lower than those for the general US population: 31.1 to 41.5 (US general population, 84.2) (18). In an RCT of CAN vs. TA, differences between SF-36 PF scores were not reported between groups over time (18). |
The properties of the two methods used most frequently have been shown as no other methods have been used in >1 study. No articles reported internal validity, feasibility, test-retest reproducibility. Effect size (ES) is provided wherever possible. If the ES could not be calculated, the statistic and associated p-value is provided.
The HAQ has been reported in two acute gout studies. Sensitivity to change over time has been reported, with effect size in an observational study of acute gout calculated as 1.43 after >1 month following treatment (26). A randomised controlled trial comparing canakinumab with triamcinolone acetonide has not shown between-group discrimination .
The SF-36 has been reported in two studies of acute gout. However, data specifically related to the PF score has only been reported in one acute gout study, a clinical trial of canakimumab vs. triamcinolone (18). Sensitivity to change over time was observed in this study, although data were not available to allow calculation of effect sizes. Differences between SF-36 PF scores were not reported between groups. However, this study did report that mean SF-36 PF scores in patients with acute gout were much lower than those for the general US population.
Conclusions
A key finding of this systematic literature review is that many different instruments have been used to assess the acute gout core domains. The wide variation observed in this review supports the need to standardise measurement of key domains in gout.
All of the instruments identified within this review were considered feasible; these are low cost tools that can be easily and rapidly administered without the need for specialist equipment. Any method that assesses joint tenderness may cause patient discomfort, particularly in the context of acute gout, which can cause exquisite joint tenderness. As in other articular diseases, careful training of observers is required to ensure that assessment of joint swelling and tenderness in patients with acute gout is undertaken in a manner that does not cause undue patient distress.
Most of the instruments commonly used to measure acute gout core domains have high face validity. Gout frequently presents as a monoarthritis (17). Thus, assessment of swelling and tenderness in an index joint may have higher face validity than enumeration of the number of affected joints. In particular, TJC and SJC are not appropriate instruments for studies of monoarticular gout. Calculation of correlation coefficients to analyse the relationships between various aspects of acute gout was not possible using published data, although one study has reported a highly significant relationship between changes in the 5-point Likert pain score and the 5-point descriptive PGART (27). Ideally, the relationship between a patient global assessment and all other instruments should be reported. Based on previous qualitative work (5), we would expect patient global assessment to correlate highly with pain and activity limitation, moderately with tender joint assessment and less with swollen joint assessment. A further validity issue was raised when considering assessment of joint swelling by tape measurement of the index joint, noting the wide variation in sizes of joints frequently affected by gout.
Aspects of discrimination within the OMERACT filter include reproducibility and change sensitivity. No published data were available for reproducibility for any of the acute gout instruments assessed in this review. Although test-retest reproducibility may be difficult to measure and unreliable in the context of acute gout where treatment leads to rapid improvement in the clinical features of inflammation, interobserver reproducibility could be assessed for investigator assessment of swollen and tender joints.
With respect to change sensitivity, acute gout is typically self-limiting over 10-14 days. Thus, even in the absence of treatment, measures of acute gout severity improve over time. This was clearly demonstrated in a study of untreated acute gout, which showed significant reduction in measures of pain, tenderness and swelling over seven days (16). Furthermore, because of the severe nature of pain caused by acute gout, it is now considered unethical to undertake placebo controlled trials of acute gout. The majority of clinical trials identified in the literature search were equivalence and safety NSAID studies, typically with indomethacin as the active comparator. Thus, assessment of between-group discrimination for the purposes of the OMERACT filter is somewhat limited. However, several studies did allow analysis of between-group discrimination, particularly a placebo-controlled study of colchicine published in 1987 (15), a randomised controlled trial comparing high-dose and low-dose celecoxib (19), several RCTs comparing canakinumab with triamcinolone (13, 18), and a study comparing a Chinese herbal medication with indomethacin (28). Although the minimal important difference has not been reported for instruments assessing acute gout, statistical differences could be detected both within- and between-groups, for the following measures: pain VAS, 5-point pain Likert score, 4-point physician assessments of index joint swelling and tenderness, TJC, SJC and PGART.
With regards to the OMERACT Filter Cube taxonomy of discrimination (29), all studies report statistical differences since the minimal relevant difference or important differences have not been determined for acute gout, so all change indices are located in the first column of the Cube. All studies look at group settings so all change indices are located in the front face of the Cube. For the studies that report a within-group change, those data are clearly in the second floor of the Cube but for between-group differences, some comparisons concerned change scores (top floor of the Cube) and others concerned final scores (bottom floor of the Cube).
In summary, many different instruments have been used to assess the acute gout core domains. Pain VAS and 5-point Likert scales, 4-point Likert scales of index joint swelling and tenderness and 5-point PGART instruments meet the criteria for the OMERACT filter. Further research is required to validate measures of activity limitation for studies of acute gout.
Acknowledgements
The authors wish to thank Susan Foggin, Medical & Health Information Services Manager, University of Auckland for assistance with literature searches.
Source of support: This work was supported by a University of Auckland summer studentship (CSZ).
Footnotes
Disclosures: ND has received consultant fees from Ardea Biosciences, Metabolex, Novartis and Takeda. Her institution has received funding from Fonterra and she is a named inventor on a patent related to milk products and gout. DK has received consultant fees from Ardea, Takeda, Novartis, and Savient, as well as have been a Speaker Bureau for Savient. PPK serves on the speakers bureau for Takeda. JAS has received research grants from Takeda and Savient and consultant fees from Savient, Takeda, Ardea, Regeneron, Allergan, URL pharmaceuticals and Novartis. JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 36 companies; a member of the American College of Rheumatology's Guidelines Subcommittee of the Quality of Care Committee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee. CSZ, RG, FMM, and WJT have no conflicts of interest to declare.
References
- 1.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 2.Dalbeth N, McQueen FM, Singh JA, MacDonald PA, Edwards NL, Schumacher HR, Jr., et al. Tophus measurement as an outcome measure for clinical trials of chronic gout: progress and research priorities. J Rheumatol. 2011;38:1458–61. doi: 10.3899/jrheum.110272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grainger R, Taylor WJ, Dalbeth N, Perez-Ruiz F, Singh JA, Waltrip RW, et al. Progress in measurement instruments for acute and chronic gout studies. J Rheumatol. 2009;36:2346–55. doi: 10.3899/jrheum.090371. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher HR, Jr., Edwards LN, Perez-Ruiz F, Becker M, Chen LX, Furst DE, et al. Outcome measures for acute and chronic gout. J Rheumatol. 2005;32:2452–5. [PubMed] [Google Scholar]
- 5.Schumacher HR, Taylor W, Edwards L, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome domains for studies of acute and chronic gout. J Rheumatol. 2009;36:2342–5. doi: 10.3899/jrheum.090370. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher HR, Taylor W, Joseph-Ridge N, Perez-Ruiz F, Chen LX, Schlesinger N, et al. Outcome evaluations in gout. J Rheumatol. 2007;34:1381–5. [PubMed] [Google Scholar]
- 7.Singh JA, Taylor WJ, Simon LS, Khanna PP, Stamp LK, McQueen FM, et al. Patient-reported outcomes in chronic gout: a report from OMERACT 10. J Rheumatol. 2011;38:1452–7. doi: 10.3899/jrheum.110271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamp LK, Khanna PP, Dalbeth N, Boers M, Maksymowych WP, Schumacher HR, Jr., et al. Serum urate in chronic gout--will it be the first validated soluble biomarker in rheumatology? J Rheumatol. 2011;38:1462–6. doi: 10.3899/jrheum.110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor WJ, Schumacher HR, Jr., Baraf HS, Chapman P, Stamp L, Doherty M, et al. A modified Delphi exercise to determine the extent of consensus with OMERACT outcome domains for studies of acute and chronic gout. Ann Rheum Dis. 2008;67:888–91. doi: 10.1136/ard.2007.079970. [DOI] [PubMed] [Google Scholar]
- 10.Taylor WJ, Singh JA, Saag KG, Dalbeth N, MacDonald PA, Edwards NL, et al. Bringing it all together: a novel approach to the development of response criteria for chronic gout clinical trials. J Rheumatol. 2011;38:1467–70. doi: 10.3899/jrheum.110274. [DOI] [PubMed] [Google Scholar]
- 11.Boers M, Brooks P, Strand CV, Tugwell P. The OMERACT filter for Outcome Measures in Rheumatology. J Rheumatol. 1998;25:198–9. [PubMed] [Google Scholar]
- 12.Khanna P, Singh MK, FitzGerald JD, Bae S, Prakash S, Kaldas M, et al. Pharmacological Treatment of Acute Gout: A Systematic Review American College of Rheumatology Annual Scientific Meeting; Chicago. 2011.2011. [Google Scholar]
- 13.So A, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010;62:3064–76. doi: 10.1002/art.27600. [DOI] [PubMed] [Google Scholar]
- 14.Schlesinger N, Brown JP, Bardin T, Kiechle T, Shpilsky A, Alten R, et al. Comparison of Pain Intensity, Incidence of New Flares, Safety and Tolerability of Canakinumab Vs Triamcinolone Acetonide in Gouty Arthritis Patients with Cardiovascular Diseases or with Cardiovascular Risk Factors.. American College of Rheumatology Annual Scientific Meeting; 2011; Chicago: 2011. p. S401. [Google Scholar]
- 15.Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M. Does colchicine work? The results of the first controlled study in acute gout. Aust N Z J Med. 1987;17:301–4. doi: 10.1111/j.1445-5994.1987.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 16.Bellamy N, Downie WW, Buchanan WW. Observations on spontaneous improvement in patients with podagra: implications for therapeutic trials of non-steroidal anti-inflammatory drugs. Br J Clin Pharmacol. 1987;24:33–6. doi: 10.1111/j.1365-2125.1987.tb03132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schumacher HR, Jr., Boice JA, Daikh DI, Mukhopadhyay S, Malmstrom K, Ng J, et al. Randomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritis. BMJ. 2002;324:1488–92. doi: 10.1136/bmj.324.7352.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlesinger N, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab relieves symptoms of acute flares and improves health-related quality of life in patients with difficult-to-treat Gouty Arthritis by suppressing inflammation: results of a randomized, dose-ranging study. Arthritis Res Ther. 2011;13:R53. doi: 10.1186/ar3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumacher RH, Berger M, Li-Yu J, Perez-Ruiz F, Burgos Vargas R, Li C. Efficacy and Tolerability of Celecoxib in the Treatment of Moderate to Extreme Pain Associated with Acute Gouty Arthritis: A Randomized Controlled Trial.. American College of Rheumatology Annual Scientific Meeting; Atlanta. 2010; 2010. [DOI] [PubMed] [Google Scholar]
- 20.Rubin BR, Burton R, Navarra S, Antigua J, Londono J, Pryhuber KG, et al. Efficacy and safety profile of treatment with etoricoxib 120 mg once daily compared with indomethacin 50 mg three times daily in acute gout: a randomized controlled trial. Arthritis Rheum. 2004;50:598–606. doi: 10.1002/art.20007. [DOI] [PubMed] [Google Scholar]
- 21.Marcolongo R, Lucchese M, Caruso I, Fumagalli M, Bruni G, Sacchetti G. Intravenous indoprofen for prompt relief of acute gout: a regimen-finding study. J Int Med Res. 1980;8:326–32. doi: 10.1177/030006058000800504. [DOI] [PubMed] [Google Scholar]
- 22.Barclay CA, Traballi CA. Evaluation of tenoxicam in rheumatology--clinical trial results in Argentina and Brazil. Eur J Rheumatol Inflamm. 1987;9:26–50. [PubMed] [Google Scholar]
- 23.Tumrasvin T, Deesomchok U. Piroxicam in treatment of acute gout high dose versus low dose. J Med Assoc Thai. 1985;68:111–6. [PubMed] [Google Scholar]
- 24.Maccagno A, Di Giorgio E, Romanowicz A. Effectiveness of etodolac ('Lodine') compared with naproxen in patients with acute gout. Curr Med Res Opin. 1991;12:423–9. doi: 10.1185/03007999109111513. [DOI] [PubMed] [Google Scholar]
- 25.Lederman R. A double-blind comparison of Etodolac (Lodine®) and high doses of naproxen in the treatment of acute gout. Advances in Therapy. 1990;7:344–54. [Google Scholar]
- 26.Rome K, Frecklington M, McNair P, Gow P, Dalbeth N. Foot pain, impairment, and disability in patients with acute gout flares: A prospective observational study. Arthritis Care Res (Hoboken) 2012;64:384–8. doi: 10.1002/acr.20670. [DOI] [PubMed] [Google Scholar]
- 27.Schlesinger N, Norquist JM, Holmes R, Boice J, Watson DJ. Validation of a patient-reported assessment of pain in acute gouty arthritis. Ann Rheum Dis. 2007;66:S234. [Google Scholar]
- 28.Chou CT, Kuo SC. The anti-inflammatory and anti-hyperuricemic effects of Chinese herbal formula danggui-nian-tong-tang on acute gouty arthritis: a comparative study with indomethacin and allopurinol. Am J Chin Med. 1995;23:261–71. doi: 10.1142/S0192415X95000316. [DOI] [PubMed] [Google Scholar]
- 29.Beaton DE, Bombardier C, Katz JN, Wright JG, Wells G, Boers M, et al. Looking for important change/differences in studies of responsiveness. OMERACT MCID Working Group. Outcome Measures in Rheumatology. Minimal Clinically Important Difference. J Rheumatol. 2001;28:400–5. [PubMed] [Google Scholar]
- 30.Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49:670–7. doi: 10.1016/j.annemergmed.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlesinger N, Detry MA, Holland BK, Baker DG, Beutler AM, Rull M, et al. Local ice therapy during bouts of acute gouty arthritis. J Rheumatol. 2002;29:331–4. [PubMed] [Google Scholar]
- 32.Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854–60. doi: 10.1016/S0140-6736(08)60799-0. [DOI] [PubMed] [Google Scholar]
- 33.Schlesinger N, Lin HY, Mysler EF, Puig J, De Meulemeester M, Balfour A, et al. Long-term safety and efficacy of Canakinumab (ACZ885) in the prevention of flares in gouty arthritis patients. Ann Rheum Dis. 2011;70:181. doi: 10.1136/ard.2010.144063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlesinger N, Schumacher HR, Bardin T, Alten R, Bloch M, Brown JP, et al. Efficacy of Canakinumab versus Triamcinolone Acetonide in acute gouty arthritis patients: Results of the b-relieved ii study (response in acute flare and in prevention of episodes of re-flare in gout). Ann Rheum Dis. 2011;70:186. [Google Scholar]
- 35.So A, Alten R, Bardin T, Schumacher H, Bloch M, Rolfe A, et al. A controlled trial of Canakinumab vs Triamcinolone Acetonide in acute gouty arthritis patients: Results of the b-relieved study (response in acute flare and in prevention of episodes of re-flare in gout). Ann Rheum Dis. 2011;70:104. [Google Scholar]
- 36.Sunkureddi P, Bardin T, Alten R, Schlesinger N, Bloch M, Kiechle T, et al. Effect of IL-1 Inhibition with Canakinumab Compared to Triamcinolone Acetonide on Pain Intensity and New Flares in Gouty Arthritis Patients with Chronic Kidney Disease Stage 2–5.. American College of Rheumatology Annual Scientific Meeting; Chicago. 2011.2011. p. S398. [Google Scholar]
- 37.Bochev B, Toncheva A. Diclofenac Duo 0.075 for treatment of patients with coxarthrosis, gonarthrosis and gout. Ortopediya i Travmatologiya. 2003;39:65–70. [Google Scholar]
- 38.Shrestha M, Chiu MJ, Martin RL, Cush JJ, Wainscott MS. Treatment of acute gouty arthritis with intramuscular ketorolac tromethamine. Am J Emerg Med. 1994;12:454–5. doi: 10.1016/0735-6757(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 39.Zou R, Zhang HX, Zhang TF. Comparative study on treatment of acute gouty arthritis by electroacupuncture with different frequency. Chin J Integr Med. 2006;12:212–4. doi: 10.1007/BF02836525. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez C, Noguera R, Gonzalez JA, Pascual E. Treatment of acute attacks of gout with a small dose of intraarticular triamcinolone acetonide. J Rheumatol. 1999;26:2285–6. [PubMed] [Google Scholar]
- 41.Cheng TT, Lai HM, Chiu CK, Chem YC. A single-blind, randomized, controlled trial to assess the efficacy and tolerability of rofecoxib, diclofenac sodium, and meloxicam in patients with acute gouty arthritis. Clin Ther. 2004;26:399–406. doi: 10.1016/s0149-2918(04)90035-5. [DOI] [PubMed] [Google Scholar]
- 42.Navarra S, Rubin BR, Yu Q, Smugar SS, Tershakovec AM. Association of baseline disease and patient characteristics with response to etoricoxib and indomethacin for acute gout. Curr Med Res Opin. 2007;23:1685–91. doi: 10.1185/030079907x210750. [DOI] [PubMed] [Google Scholar]
- 43.Ruotsi A, Vainio U. Treatment of acute gouty arthritis with proquazone and indomethacin. A comparative, double-blind trial. Scand J Rheumatol Suppl. 1978:15–7. doi: 10.3109/03009747809095668. [DOI] [PubMed] [Google Scholar]
- 44.Willburger RE, Mysler E, Derbot J, Jung T, Thurston H, Kreiss A, et al. Lumiracoxib 400 mg once daily is comparable to indomethacin 50 mg three times daily for the treatment of acute flares of gout. Rheumatology (Oxford) 2007;46:1126–32. doi: 10.1093/rheumatology/kem090. [DOI] [PubMed] [Google Scholar]
- 45.Terkeltaub R, Schumacher R, Curtis C, Patterson N, Evans R, Wang J, et al. Evaluation of Rilonacept in Patients with Gouty Arthritis Experiencing an Acute Gout Attack.. American College of Rheumatology Annual Scientific Meeting; Atlanta. 2010 November 7-11.2010. p. 157. [Google Scholar]
- 46.Ambanelli U, Bassi G, Bianchi V, Carcassi U, Caruso I, Colombo B, et al. Indoprofen in rheumatic patients with acute episodes: a multicentre trial. J Int Med Res. 1982;10:399–407. doi: 10.1177/030006058201000603. [DOI] [PubMed] [Google Scholar]
- 47.Caruso I, Fumagalli M, Marcolongo R, Sacchetti G. Indoprofen for acute gouty arthritis. Arthritis Rheum. 1977;20:1438–9. doi: 10.1002/art.1780200729. [DOI] [PubMed] [Google Scholar]
- 48.Mease PJ, Willkens RF. Treatment of acute gout with oxaprozin. Seminars in Arthritis and Rheumatism. 1986;15:86–9. [Google Scholar]
- 49.Altman RD, Honig S, Levin JM, Lightfoot RW. Ketoprofen versus indomethacin in patients with acute gouty arthritis: a multicenter, double blind comparative study. J Rheumatol. 1988;15:1422–6. [PubMed] [Google Scholar]
- 50.Douglas G, Thompson M. A comparison of phenylbutazone and flufenamic acid in the treatment of acute gout. Ann Phys Med. 1970;10:275–80. doi: 10.1093/rheumatology/x.6.275. [DOI] [PubMed] [Google Scholar]
- 51.Eberl R, Dunky A. Meclofenamate sodium in the treatment of acute gout. Results of a double-blind study. Arzneimittelforschung. 1983;33:641–3. [PubMed] [Google Scholar]
- 52.Katona G, Burgos-Vargas R. Clinical experiences with the intramuscular injection of tiaprofenic acid in rheumatic diseases, with particular emphasis on time of onset and duration of the analgesic effect. Drugs. 1988;35(Suppl 1):72–80. doi: 10.2165/00003495-198800351-00016. [DOI] [PubMed] [Google Scholar]
- 53.Shi XD, Li GC, Qian ZX, Jin ZQ, Song Y. Randomized and controlled clinical study of modified prescriptions of Simiao Pill in the treatment of acute gouty arthritis. Chin J Integr Med. 2008;14:17–22. doi: 10.1007/s11655-007-9001-7. [DOI] [PubMed] [Google Scholar]
- 54.Cobra CJ, Cobra JF, Cobra Neto C. Use of piroxicam in the treatment of acute gout. Eur J Rheumatol Inflamm. 1983;6:126–33. [PubMed] [Google Scholar]
- 55.Murphy JE. Piroxicam in the treatment of acute gout: a multicentre open study in general practice. J Int Med Res. 1979;7:507–10. doi: 10.1177/030006057900700605. [DOI] [PubMed] [Google Scholar]
- 56.Tausch G, Eberl R. Efficacy, tolerance and safety of piroxicam in the treatment of acute gout. European Journal of Rheumatology and Inflammation. 1978;1:365–8. [Google Scholar]
- 57.Willkens RF, Case JB, Huix FJ. The treatment of acute gout with naproxen. J Clin Pharmacol. 1975;15:363–6. doi: 10.1002/j.1552-4604.1975.tb01465.x. [DOI] [PubMed] [Google Scholar]
- 58.Lomen PL, Turner LF, Lamborn KR, Winblad MA, Sack RL, Brinn EL. Flurbiprofen in the treatment of acute gout. A comparison with indomethacin. Am J Med. 1986;80:134–9. doi: 10.1016/0002-9343(86)90131-2. [DOI] [PubMed] [Google Scholar]
- 59.Valdes EF. Use of tenoxicam in patients with acute gouty arthritis. Eur J Rheumatol Inflamm. 1987;9:133–6. [PubMed] [Google Scholar]
- 60.Fletcher MA, Wade AG. Clinical experience with isoxicam in patients with acute gout. Br J Clin Pract. 1985;39:108–13. [PubMed] [Google Scholar]
- 61.Molina J. Fentiazac in acute gouty arthritis. Clin Ther. 1985;7:327–33. [PubMed] [Google Scholar]