Abstract
Two existing and widely applied protocols of embryonic stem (ES) cell differentiation have been developed to enable in vitro generation of neurons resembling neocortical projection neurons in monolayer culture and from embryoid bodies. The monolayer approach offers advantages for detailed in vitro characterizations and potential mechanistic and therapeutic screening. We investigated whether mouse ES cells undergoing largely undirected neocortical differentiation in monolayer culture recapitulate progressive developmental programs of in vivo progenitor and postmitotic differentiation and whether they develop into specific neocortical subtypes. We find that ES-derived mitotic cells that have been dorsalized by the sonic hedgehog antagonist cyclopamine, and that express, as a total population, cardinal markers of telencephalic progenitors, are, in fact, molecularly heterogeneous. We next show that these progenitors subsequently generate small numbers of heterogeneous neocortical-like neurons that are “stalled” at an immature stage of differentiation, based on multiple developmental criteria. Although some aspects of neocortical development are recapitulated by existing protocols of ES cell differentiation, these data indicate that mouse ES-derived neocortical progenitors both are more heterogeneous than their in vivo counterparts and seemingly include many incorrectly specified progenitors. Furthermore, these ES-derived progenitors spontaneously differentiate into sparse, and incompletely and largely imprecisely differentiated, neocortical-like neurons that fail to adopt specific neuronal identities in vitro. These results provide both foundation and motivation for refining and enhancing directed differentiation of clinically important neocortical projection neuron subtypes.
INDEXING TERMS: directed differentiation, neocortex, projection neuron, pallial progenitors, corticogenesis
Neocortical projection neurons undergo distinct molecular refinements at progenitor (Molyneaux et al., 2005; Chen et al., 2005; Chen et al., 2008; Azim et al., 2009a) and postmitotic (Weimann et al., 1999; Arlotta et al., 2005; Alcamo et al., 2008; Britanova et al., 2008; Lai et al., 2008; Joshi et al., 2008; Azim et al., 2009b; Tomassy et al., 2010; Cederquist et al., 2013) stages of development. These molecular refinements individually represent distinct developmental programs that, in sequential combinations, control neocortical development. In the absence of these critical transcriptional regulators that control any of these stages, the precise molecular identity, laminar/area positioning, and projection patterns of neocortical projection neuron subtypes are disrupted in vivo. These transcriptional controls, therefore, are good candidates for rigorous characterization of in vitro neocortical-like neurons derived from embryonic stem (ES) cells.
Recent advances in mouse ES-cell-directed neocortical differentiation recapitulate some, but not all, aspects of corticogenesis (Gaspard et al., 2008; Eiraku et al., 2008; Hansen et al., 2011; Nasu et al., 2012). Importantly, populations of ES-derived neocortical-like neurons sequentially express single genes characteristic of neocortical neurons in vivo. However, many of these genes (e.g., Pax6, Ctip2, Satb2) are not specific only to the neocortex but are expressed in other regions of the developing neural tube. For example, Pax6 is differentially expressed throughout the rostrocaudal extent of the neural tube ventricular zone (Ericson et al., 1997; Osumi et al., 1997; Briscoe et al., 2000; Alaynick et al., 2011), and Ctip2 is also expressed in striatum, olfactory bulb, and hippocampus (Leid et al., 2004; Arlotta et al., 2005, 2008).
With deeper analysis and multiple markers, it is increasingly apparent that ES-derived neocortical-like neurons are incompletely specified in vitro. First, a substantial fraction of these neurons expresses combinations of molecular markers that are not described for the neocortex in vivo (e.g., Reelin/Ctip2; Gaspard et al., 2008). Second, ES-derived neocortical neurons often display mixed subtype-specific molecular characteristics, such as coexpression of deep- and superficial-layer markers in individual hES-derived neurons (Mariani et al., 2012; Shi et al., 2012). Finally, these neurons display skewed areal specification and projection patterns to visual and limbic targets (Gaspard et al., 2008; Espuny-Camacho et al., 2013). These subtle but distinct deficiencies in the differentiation of ES-derived neocortical neurons suggest incomplete differentiation, which might hinder neocortical subtype acquisition and limit the interpretability of these in vitro models of corticogenesis.
More refined characterizations of in vitro neocortical differentiation are now possible, given recent advances in the study of neocortical development (Molyneaux et al., 2007; Woodworth et al., 2012; Custo Greig et al., 2013). Pax6, often used to mark the pallium exclusively, is not a specific marker of the pallial tissue, given its expression throughout the neural tube (Alaynick et al., 2011). In the absence of positional information in vitro, characterization of Pax6-expressing “pallial” progenitors is incomplete without the presence of additional markers of pallial progenitors (e.g., Sox6; Azim et al., 2009a; Otx2, Acampora et al., 1999) or the absence of other markers coexpressed with Pax6 outside of the pallium.
Sox6 is a transcription factor that controls the development of pallial progenitors independently from Pax6; its absence results in misspecification of pallial progenitors, by ectopic expression of subpallial genes (Azim et al., 2009a). As with Pax6, Sox6 is not specific to the pallium; it is also expressed by postmitotic, subpallium-derived interneurons. However, when Sox6 is assessed in combination with Pax6, the presence of both markers greatly increases the specificity for pallial progenitors. To date, this combination has not been used for the identification of pallial progenitors in vitro.
Postmitotic neocortical neurons in vivo undergo a prolonged maturation process, during which gene expression becomes progressively restricted to particular subtypes (Lai et al., 2008; Britanova et al., 2008; Alcamo et al., 2008; Chen et al., 2008; Joshi et al., 2008; Azim et al., 2009b; Woodworth et al., 2012; Cederquist et al., 2013; Custo Greig et al., 2013). These neurons initially coexpress transcriptional controls characteristic of multiple neocortical projection subtypes (e.g., Tbr1, Ctip2, Satb2, Clim1, Lmo4) and multiple neocortical area identities (e.g., CoupTF1, Bhlhb5, Lmo4) before distinct subtype identities emerge. Together, this process of molecular refinement involves, at minimum, coordinated neuronal maturation, neocortical projection neuron class distinction, and neocortical area subtype distinction. These three stage-specific features of neocortical identity refinement form the basis for our approach to characterizing neocortical identity in vitro, presented here.
We assessed mouse ES cell-derived neocortical-like neurons at progenitor and postmitotic stages and identified multiple characteristics consistent with stalled maturation. First, ES-derived neocortical-like progenitors are more heterogeneous than has been previously reported from single-marker analyses. Second, neocortical-like neurons are stalled at a maturation stage resembling midcorticogenesis, as indicated by overlapping expression of multiple subtype-specific markers that do not resolve with time. Additionally, area-specific differentiation is abnormal, because ES-derived neocortical neurons are deficient in the sensorimotor cortex regulator of neocortical development Bhlhb5. Overall, this approach rigorously investigates the refinement of ES-derived neocortical differentiation and indicates directions for refining specific differentiation of clinically important neocortical projection neurons.
MATERIALS AND METHODS
Cell culture and differentiation
Nagy ES cell line G4 (MMRRC stock No. 011987-MU) or feeder-free E14Tg2a (Baygenomics) mouse embryonic stem cells were propagated by using standard procedures (Ying et al., 2003) on gelatin-coated (0.1% gelatin; Stem Cell Technologies) cell-culture-treated plastic dishes. Nagy ES cells were cultured on mouse embryonic fibroblast feeder cells (Millipore EmbryoMax PMEF-N). Mouse ES cell media is GMEM (Invitrogen, Carlsbad, CA) supplemented with 10% ESC-certified fetal bovine serum (vol/vol; Invitrogen), 0.1 mM nonessential amino acids (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma, St. Louis, MO), 50 U/ml penicillin/streptomycin, and 1,000 U/ml leukemia inhibitor factor (ESGRO).
For differentiation, Nagy G4 and E14Tg2a ES cells were plated at low density (5,000 cells/cm2) on gelatin-coated plastic dishes in ES cell medium, and cultured as described by Gaspard et al. (2009). Briefly, ESCs were trypsinized, dissociated, and plated on gelatin-coated cell culture plates. Medium was changed to DDM after 1 day. DDM consists of DMEM/F12 (Invitrogen- Gibco) supplemented with N2 supplement (N2 supplement consists of 8.61 µM insulin, 1 mM transferrin, 2 µM progesterone, 10 mM putrescine, and 3 µM selenite; Invitrogen-Gibco), 2 mM glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 0.5 mg/ml bovine serum albumin fraction V (all from Invitrogen-Gibco), and 0.1 mM β-mercaptoethanol (Sigma).
Cyclopamine (Calbiochem, La Jolla, CA) or Ag1.3 (a gift from Lee Rubin, Harvard University) was added from day 2 to day 10 in the differentiation medium at a final concentration of 1 µM. After 10–14 days of differentiation, cells were trypsinized, dissociated, plated on poly-lysine/laminin (Becton-Dickinson, San Jose, CA)-coated glass coverslips, and allowed to grow for 4–14 days in N2B27 medium. N2B27 medium consists of a 1:1 mixture of DDM and Neurobasal medium supplemented with B27 (without vitamin A; Invitrogen-Gibco) and 2 mM glutamine.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde (wt/vol) for 30 minutes, and washed three times in phosphate-buffered saline (PBS). Widefield image acquisition was performed with a Nikon 90i epifluorescence microscope with a Clara DR-328G cooled CCD digital camera (Andor Technology, Belfast, Northern Ireland). Confocal imaging was performed with a Bio-Rad Radiance 2100 Rainbow laser scanning confocal microscope based on a Nikon E800 microscope. Images were assembled in Adobe Photoshop and Illustrator (CS3, CS5), with adjustments for contrast, brightness, and color balance to obtain optimal visual reproduction of data.
Antibody characterization
See Table 1 for a list of all antibodies used. The Pax6 antiserum has been widely characterized and used in the field and does not label Pax6 null cells. It is confirmed to be specific to the developmental pallial domain by immunocytochemistry. The Sox6 antiserum does not stain samples of postnatal brain from a Sox6 knockout mouse (manufacturer’s data sheet). Identical results in embryonic brain tissue were obtained from the Macklis laboratory (Azim et al., 2009a). The Mash1 antiserum recognizes a single band of 34 kDa molecular weight by SDS-PAGE (per manufacturer), with specific staining of the mouse subpallium (Yun et al., 2002). The Gsh2 antiserum recognizes a single band of 35 kDa molecular weight by SDS-PAGE (per manufacturer) and is confirmed to be specific to the developmental subpallial domain by immunocytochemistry. The Nkx2.1/TTF1 antiserum staining is abolished when the diluted primary antibody is preincubated with 0.1 µM of the immunizing peptide (Moreno and Gonzalez, 2007) and is confirmed to be specific to the developmental subpallial domain by immunocytochemistry. The Tbr1 antiserum recognizes a single band of 74 kDa molecular weight by SDS-PAGE and stained a pattern of cellular morphology and distribution in the mouse brain that is identical to that in previous reports (Hevner et al., 2001). The Ctip2 antiserum detects two bands representing Ctip2 at about 120 kDa by SDS-PAGE. No staining is seen on tissue from a Ctip2 knockout mouse (Arlotta et al., 2005, 2008). The Satb2 antiserum detects one 81-kDa band representing Satb2 by SDS-PAGE. No staining is seen on tissue from a Satb2 knockout mouse (Britanova et al., 2008). The Er81 antiserum produced a pattern of immunoreactivity that was identical to previous descriptions of E16.5 mouse brain sections (Stenman et al., 2003; Yoneshima et al., 2006). The GAD67 antiserum had no detectable cross-reactivity with GAD65 by Western blot on rat brain lysate (manufacturer’s data sheet). The TuJ1/β-tubulin III antiserum is well characterized and highly reactive to neuron-specific class III β-tubulin (βIII). TuJ1 does not identify β-tubulin found in glial cells. The MAP2 antiserum localizes the high-molecular-weight forms of MAP2 (MAP2a and MAP2b) but shows no reactivity with MAP2c (manufacturer’s data sheet). No cross-reactivity is observed with MAP1, MAP5, tubulin, or tau. The NeuN (neuronal nuclei) antiserum recognizes two major bands of 40 and 50 kDa by SDS-PAGE. The antiserum recognizes residues 1–106 at the N-terminal of Fox-3. It is specific to two Fox-3 isoforms, based on absent NeuN staining in Fox-3 null SK-N-SH cells and identical colocalization of Fox-3 and NeuN antisera (Kim et al., 2009). The Bhlhb5 antiserum does not stain tissue from a Bhlhb5 knockout mouse (data not shown; Joshi et al., 2008). The CoupTF1 antiserum did not stain tissue from a CoupTF1 knockout mouse (Tripodi et al., 2004; Tomassy et al., 2010).
TABLE 1.
Primary Antibodies Used
| Antigen | Immunogen | Manufacturer, species, mono- vs. polyclonal, catalog No. |
Dilution used |
|---|---|---|---|
| Pax6 | QVPGSEPDMSQYWPRLQ derived from the C-terminus of mouse Pax-6 protein | Covance, rabbit polyclonal, PRB-278P | 1:300 |
| Sox6 | Synthetic peptide derived from <800 residues to the C-terminus of mouse SOX, conjugated to KLH | Abcam, rabbit polyclonal, AB30455 | 1:200 |
| Mash1 | Full-length recombinant rat Mash1 protein | BD, mouse monoclonal, 556604 | 1:500 |
| Gsh2 | Synthetic peptide derived from a region between amino acids 1–46 of human GSH2 | Abcam, rabbit polyclonal, 26255 | 1:500 |
| Nkx2.1/TTF1 | Synthetic peptide containing residues 110–122 at the N-terminus of rat Nkx2.1 | BioPat, mouse monoclonal, PA0100 | 1:5,000 |
| Tbr1 | Synthetic peptide derived from within residues 50–150 of mouse TBR1, conjugated to KLH | Abcam, rabbit polyclonal, 31940-100 | 1:500 |
| Ctip2 | Synthetic protein derived from within residues 1–150 of human CTIP2 | Abcam, rat monoclonal [25B6], 18465-100 | 1:500 |
| Satb2 | Recombinant human Satb2 protein containing a fragment of the C-terminal | Abcam, mouse monoclonal [SATBA4B10], 51502 | 1:200 |
| Er81 | Synthetic mouse Er81 C-terminal peptide sequence, CNPHPYNEGYVY, conjugatd to KLH | Abcam, rabbit polyclonal, AB36788 | 1:100 |
| GAD67 | Recombinant GAD67 protein | Millipore, mouse monoclonal [clone 1G10.2], MAB5406 | 1:1,000 |
| TuJ1/β-tubulin III | Synthetic peptide containing residues 441–450 of human b-tubulin III (Ala446 to Ser446 substitu- tion) with N-terminal added cysteine, conjugated to KLH | Sigma, rabbit polyclonal, T2200-200uL | 1:1,000 |
| TuJ1/β-tubulin III | Microtubules derived from rat brain | Covance, mouse monoclonal, MMS-435P | 1:1,000 |
| Map2 | Full-ength recombinant bovine Map2 | Sigma, mouse monoclonal, M1406 | 1:500 |
| NeuN/Fox-3 | Purified cell nuclei from mouse brain; recognizes residues 1–106 of Fox-3 | Millipore, mouse monoclonal [A60], MAB377 | 1:250 |
| Bhlhb5 | Synthetic protein containing the N-terminus of hamster Bhlhb5 | Santa Cruz Biotechnology, goat polyclonal, 6045 | 1:300 |
| CoupTF1 | Synthetic protein containing the first 203 residues of mouse COUP-TFI | Rabbit polyclonal, gift of the Michele Studer labora- tory, Institute of Biology Valrose, Nice, France | 1:500 |
Secondary antibodies were from the Invitrogen Molecular Probes Alexa series. Specificity was tested with omission of primary antibodies (data not shown). Nuclei were stained with Hoechst 33342 (1:3,000; Sigma).
Mice
All mouse studies were approved by the Harvard University and/or Massachusetts General Hospital IACUCs and were performed in accordance with institutional and federal guidelines. The date of vaginal plug detection was designated E0.5, and the day of birth as P0. Wild-type CD1 mice were used in all experiments (Charles River Laboratories).
Brains were fixed by using standard methods (Fricker-Gates et al., 2002; Arlotta et al., 2005). Briefly, brains were fixed by transcardial perfusion with PBS-heparin (10 U/ml), followed by 4% paraformaldehyde, and postfixed overnight at 4°C in 4% paraformaldehyde. Brains were sectioned coronally at 50 µm on a vibrating microtome (Leica). Coverslips or floating sections were blocked in 1% BSA (Sigma) and 0.1% Triton X-100 (Sigma) for 20 minutes at room temperature, before incubation in primary antibody.
RESULTS
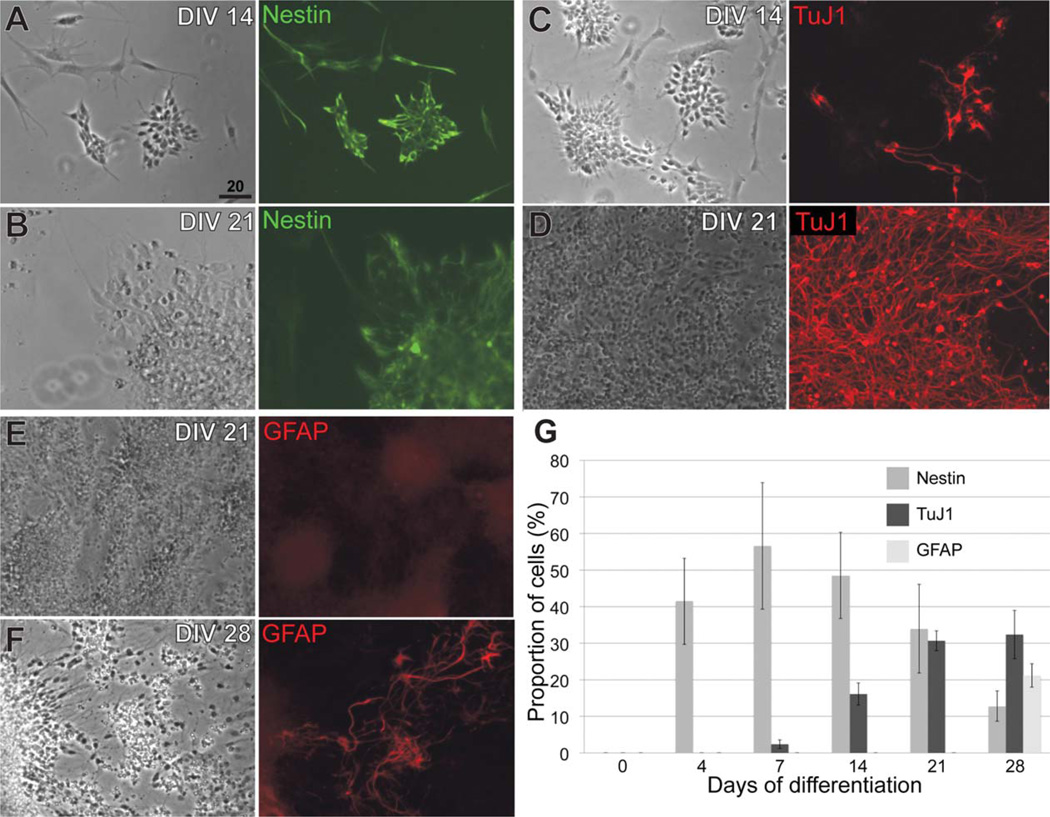
To begin characterizing ES-derived neocortical-like cells, we cultured mouse ES cells, and directed their differentiation to neocortical fates using an established monolayer cell culture protocol (Gaspard et al., 2008, 2009). This protocol allows rostral and dorsal differentiation by plating ES cells at low density, removing serum and retinoids, and antagonizing residual Shh morphogen signaling with cyclopamine. We replicated this protocol and generated sequential waves of broad neural populations (neural progenitors, immature neurons, and astroglia) from E14Tg2a mouse ES cells over the course of 28 days (Fig. 1), closely replicating the originally published results (Gaspard et al., 2008). After 2 weeks in culture, 55% ± 6.8% (mean ± s.e.m.) of ES-derived cells express nestin, an intermediate filament protein, broadly marking neural progenitors. Similar results were obtained with Nagy G4 mouse ES cells (data not shown). These results show that both the timing of neural induction and the sequential generation of neural progenitors, neurons, and astroglia are nearly identical to previously published results (Gaspard et al., 2008).
Figure 1.
Sequential generation of neural progenitors, neurons, and astroglia in an established monolayer ES cell protocol is reproducible. A,B: Nestin expression decreases, as a proportion of total cells, from day in vitro (DIV) 14 to DIV 21. C,D: TuJ1 expression increases, as a proportion of total cells, from day 14 to day 21. E,F: GFAP expression begins by day 28. G: Quantification of nestin, TuJ1, and GFAP expression over the course of 28 days. Data are represented as mean ± s.e.m. (N = 3). Scale bar = 20 µm.
Distinct subsets of pallial progenitors are generated from ES cells
We first assessed the proportion of rostral, dorsal, pallial-like differentiation by ES-derived nestin-expressing neural progenitors at in vitro day 14. Approximately half of nestin-expressing progenitors are pallial-like, based on coexpression of Pax6 (Fig. 2A). All Pax6- expressing cells coexpress nestin, and Pax6 is not expressed by any TuJ1 (β-tubulin III)-expressing immature neurons (data not shown). Together these data suggest that Pax6 expression is restricted to about half of ES-derived neural progenitors.
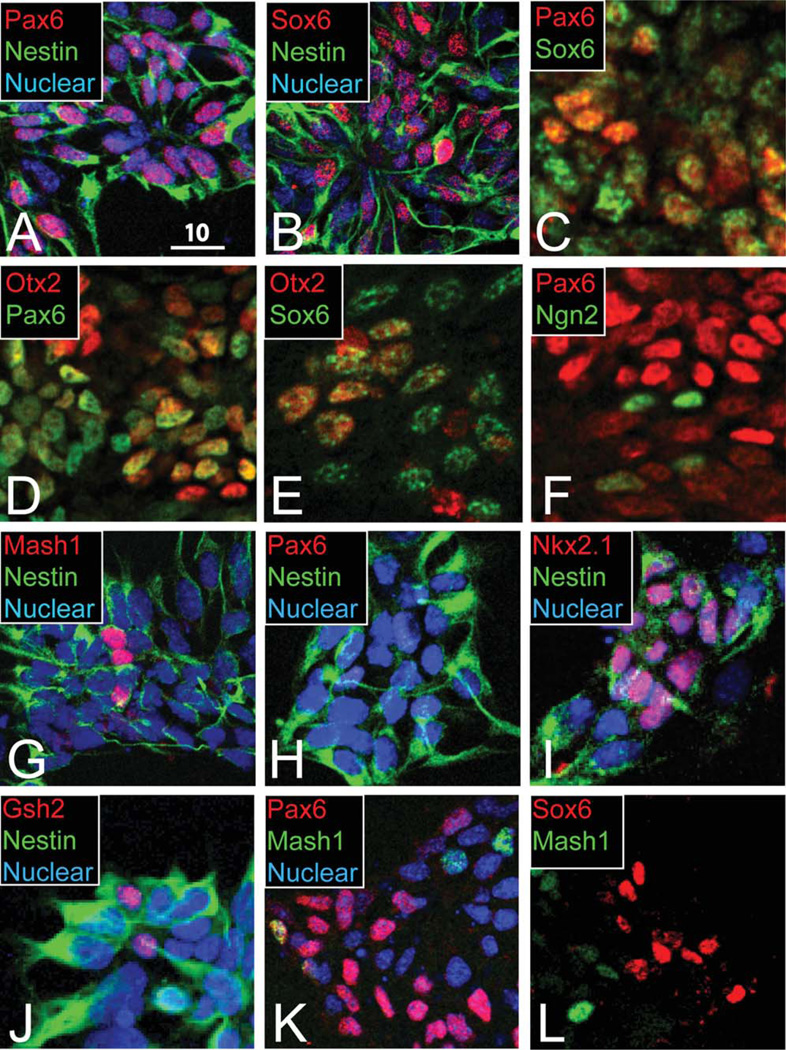
Figure 2.
Pallial-like progenitors generated by ES-derived progenitors are molecularly heterogeneous. A,B: Half of nestin-expressing progenitors coexpress Pax6 (A) or Sox6 (B). C: About 20% of progenitors express both Pax6 and Sox6. D,E: Most Pax6 (D) and Sox6 (E) pallial progenitors coexpress Otx2. F: Ngn2 is expressed by some Pax6-low or -negative progenitors. G: Mash1/nestin subpallial-like progenitors represent ~10% of cells. H–J: Ventralized ES cells lose Pax6 expression (H) and increase subpallial Nkx2.1 (I) and Gsh2 (J). K,L: Pax6/Mash1 (K) and Sox6/Mash1 (L) cellular subsets are mostly distinct. A magenta-green version of this figure is available online in the Supporting Information. Scale bar = 10 µm.
We next asked whether these Pax6-expressing neural progenitors at in vitro day 14 display other characteristics of pallial progenitors. We hypothesized that correctly specified pallial progenitors will coexpress Sox6 (Azim et al., 2009a). As with the proportion of Pax6-expressing progenitors, we find that approximately half of nestin-expressing progenitors also express Sox6 (Fig. 2B). However, Pax6 and Sox6 are coexpressed by only approximately 20% of progenitors (Fig. 2C), which is strikingly dissimilar to their highly overlapping expression in vivo (Azim et al., 2009a). Overall, the combined distribution of Pax6 and Sox6 expression accounts for the majority of nestin-expressing progenitors at day 14, but these pallial transcription factor controls are largely not expressed by the same cells.
Because most Pax6-expressing cells do not coexpress Sox6, we hypothesized that some Pax6-expressing cells might possess identities characteristic of a position in the neural tube caudal to the telencephalon. Otx2, expressed throughout the ventricular zone of the neural tube rostral to the hindbrain, demarcates the midbrain–hindbrain boundary and is required for early specification of forebrain and midbrain (Acampora et al., 1999). We find that the majority of Pax6-expressing pallial-like progenitors coexpress Otx2, consistent with a forebrain progenitor identity (Fig. 2D). Otx2 is also coexpressed by most Sox6-expressing progenitors (Fig. 2E). These data suggest that many Pax6-and Sox6-expressing progenitors resemble forebrain pallial progenitors, but the absence of Otx2 coexpression in many progenitors indicates further heterogeneity not observed in vivo.
To assess whether downstream pallial molecular programs are intact in cells differentiating under these conditions, we assessed expression of Ngn2 in these ES-derived pallial-like progenitors. In the developing pallium, Pax6 and Sox6 are both upstream of Ngn2, a proneurogenic transcription factor that has cell-cycle-dependent expression in progenitors undergoing neurogenesis (Schuurmans et al., 2004; Kageyama et al., 2008; Ma et al., 2008; Azim et al., 2009a). We find that Ngn2 is highly expressed by cells with low Pax6 expression, suggesting that ES-derived pallial-like progenitors are undergoing neurogenesis with dynamic regulation of Pax6 and Ngn2 (Fig. 2F).
To investigate whether pallial-like progenitors appropriately exclude markers of subpallial identity, we tested for molecular markers of these populations at in vitro day 14. Mash1, also called Ascl1, is a transcription factor expressed in the subpallium (both lateral and medial ganglionic eminences) and at the adjacent pallial–subpallial boundary; in concert with Dlx1/2, it is essential for the proper specification of subpallium-derived neurons (Long et al., 2009). We find that Mash1 is coexpressed by approximately 10% of nestin-expressing ES-derived progenitors (Fig. 2G).
To investigate whether Mash1-expressing progenitors display other characteristics of subpallial progenitors, we assessed their coexpression with Gsh2 and/or Nkx2.1. Gsh2 is a transcription factor expressed by early progenitors of the lateral ganglionic eminence and, to a lesser extent, the medial ganglionic eminence; Gsh2 functions upstream of Mash1 activation and represses Pax6 transcription (Corbin et al., 2003; Wang et al., 2009; Azim et al., 2009a; Batista-Brito et al., 2009; Pei et al., 2011). Nkx2.1 is another subpallial control expressed in the medial ganglionic eminence (Butt et al., 2008). Gsh2 and Nkx2.1 are individually coexpressed with Mash1 in the subpallium, in distinct compartments; we hypothesized that some Mash1-expressing progenitors might coexpress one or both these subpallial transcription factors. However, we find that Gsh2 and Nkx2.1 expression is absent in ES-derived progenitors (data not shown).
To determine whether this protocol is competent to generate cells with appropriate subpallial characteristics, we directed the ventralization of ES-derived neural progenitors with Shh agonism. In the presence of the Shh agonist Ag1.3, Pax6 expression is appropriately lost (Fig. 2H), whereas expression of Nkx2.1 and Gsh2, individually is increased (Fig. 2I,J). Mash1 expression was not affected (data not shown). These data provide a positive control for the absence of Gsh2 and Nkx2.1 expression with cyclopamine-mediated dorsal differentiation, confirming that subpallial gene expression by ES-derived progenitors is Shh dependent, as expected in vivo. In contrast, Mash1 expression by a subpopulation of these cells appears to be independent of subpallial specification.
We next asked whether Mash1-expressing progenitors are instead pallial-like, given previous reports of cells with Mash1 expression in the dorsal pallium and at the pallial–subpallial boundary in vivo (Britz et al., 2006; Ge et al., 2006). Although pallial progenitors expressing Pax6 (Fig. 2K) or Sox6 (Fig. 2L) are mostly distinct from Mash1-expressing progenitors, we find that approximately 15% of Pax6-expressing progenitors coexpress Mash1 (Fig. 2K). These findings suggest that many Mash1-expressing ES-derived progenitors are potentially pallial. This interpretation is consistent with the broad dorsalization induced by cyclopamine in ES cell differentiation but again highlights a high degree of heterogeneity within ES-derived pallial-like progenitors by day 14.
A small subset of ES-derived neurons is neocortical, based on multiple markers
At 21 days of differentiation, 31% ± 3.3% (mean ± s.e.m.) of cells express TuJ1 and can be considered immature neurons, although this proportion is highly variable (Figs. 1G, 3A,B). Previous reports with this protocol have indicated that a higher proportion of ES-derived neurons is generated (Gaspard et al., 2008, 2009), which raises specific methodological points that might explain the quantitative differences that we observe. First, the ES-derived cells produced by this monolayer protocol do not remain a monolayer after greater than 7 days of differentiation; at later times, we observe cell overgrowth and “clumping” of cells with heterogeneous morphologies. We use confocal imaging to localize TuJ1 staining more precisely near areas of dense cell overgrowth at day 21. Counting total nuclei within aggregates of cells has not proved reliable, so we excluded neurons found within these dense aggregates. Second, we maintained strict criteria for counting TuJ1-expressing neurons: TuJ1 staining must minimally encompass a hemicircle around the nucleus and display a polarized, neuron-like morphology. Third, because TuJ1 expression is not entirely specific to neurons (e.g., TuJ1 is expressed by fibroblasts; Vierbuchen et al., 2010), we excluded non-neuronal TuJ1-expressing cells based on multiple exclusion criteria: comparatively lower intensity of TuJ1 expression, fibroblast-like morphology, or any nuclei surrounded by an exceedingly high density of neurites from adjacent neurons, which can sometimes incorrectly resemble distinct neurons. Finally, TuJ1 expression is not distributed uniformly in vitro across a coverslip, and all characterizations were performed on selected imaging fields containing substantial numbers of neurons.
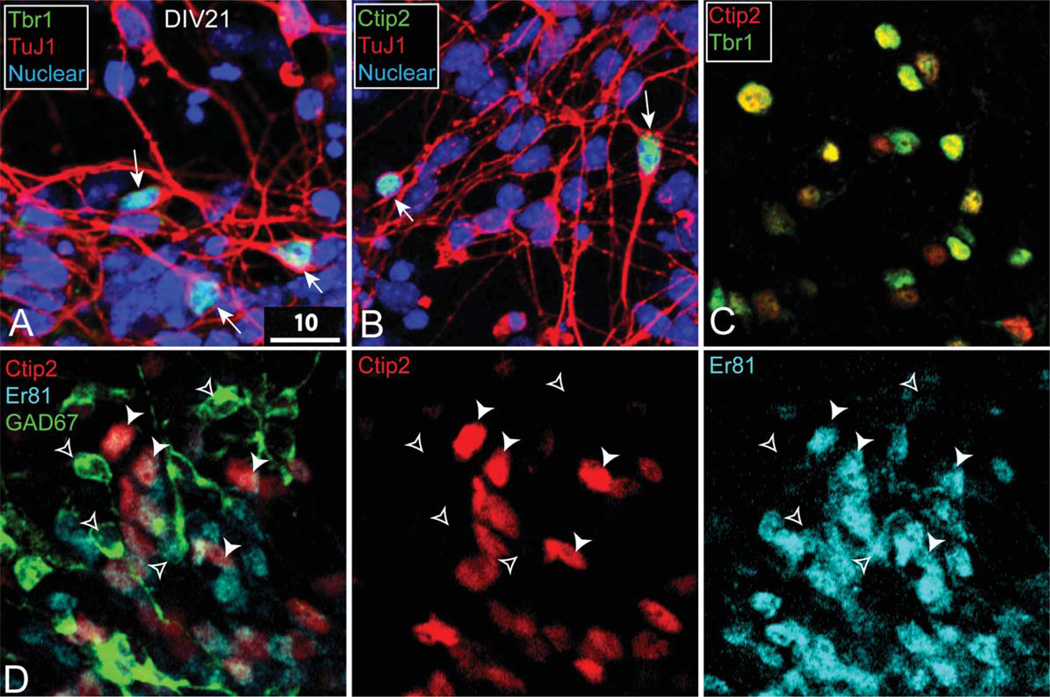
Figure 3.
ES-derived, Ctip2-expressing neurons are neocortical-like. A: Tbr1-expressing neurons coexpress TuJ1. B: Ctip2-expressing neurons coexpress TuJ1. C: Ctip2-expressing neurons coexpress Tbr1. D: Ctip2-expressing cells are distinct from GAD67-expressing cells; Er81 is coexpressed by Ctip2 neurons (solid arrowheads, Ctip2/Er81; open arrowheads, GAD67). A partial magenta-green version of this figure is available online in the Supporting Information. Scale bar = 10 µm.
To investigate the potentially neocortical identity of these ES-derived neurons at day 21, we performed immunostaining for multiple neuronal markers. We first assessed the expression of Tbr1, which is expressed briefly by all postmitotic pyramidal neurons generated in the developing pallium, before its expression becomes restricted to corticothalamic projection neurons (CThPN) and callosal projection neurons (CPN) in layer VI (Englund et al., 2005; Hevner et al., 2001). Tbr1 is expressed in few brain areas other than neocortex, and Tbr1-expressing neurons are glutamatergic (Hevner et al., 2001; Bedogni et al., 2010; McKenna et al., 2011). Approximately 10–20% of TuJ1-expressing neurons in vitro also express Tbr1 (Fig. 3A). Given the low percentage of ES-derived neurons expressing Tbr1, we imaged selected fields containing relatively high concentrations of Tbr1-expressing neurons for further subtype characterization.
To identify cells with properties of early neocortical neurons, and potentially of specific deep-layer subtypes, we focused on expression of Ctip2. As with Tbr1, Ctip2 is a critical transcription factor expressed at distinct levels (off, low, high) by multiple newly postmitotic neocortical subtypes; later in development, Ctip2 controls corticofugal projection neuron (CFuPN) axon outgrowth and fasciculation, with refined laminar expression specific to deep layers, low level by CThPN in layer VI and high level by SCPN in layer V (Arlotta et al., 2005). Expression of Ctip2 by immature CPN, and therefore coexpression with CPN marker Satb2, is lost by late embryogenesis (Alcamo et al., 2008; Britanova et al., 2008; Chen et al., 2008). Notably, Ctip2 is highly expressed in brain regions other than the neocortex, most highly by medium-sized spiny neurons in the striatum (Leid et al., 2004; Arlotta et al., 2005, 2008).
We find that Ctip2, similarly to Tbr1, is expressed by a modest fraction of TuJ1-expressing neurons (approximately 10–20% of neurons in selected fields containing positive Ctip2 staining; Fig. 3B). We hypothesized that, if these Ctip2-expressing neurons are neocortical-like, most should also express Tbr1. Consistent with this prediction, Ctip2 and Tbr1 display nearly complete coexpression after 21 days in culture (Fig. 3C). These data suggest that this sparse population of ES-derived Ctip2- expressing neurons is glutamatergic and most closely resembles immature deep-layer projection neurons.
To investigate rigorously whether these Ctip2- and Tbr1-coexpressing neurons represent non-neocortical neurons, we performed coexpression analysis of Ctip2 with Er81 and GAD67. Er81 is expressed in neocortical deep layers, olfactory bulb (interneurons), amygdala, and thalamus but not in striatum (Stenman et al., 2003; Yoneshima et al., 2006); the intersection of Er81 and Ctip2 expression is fairly exclusive to neocortex. We find that Ctip2-expressing neurons coexpress Er81 in the cytoplasm (Fig. 3D), which indicates that they are not striatal. Many important striatal genes, such as Darpp32, Foxp1, and Foxp2, are expressed both in cortex and in striatum, so we examined expression of GAD67, which is expressed only by GABAergic inhibitory populations, such as medium-sized spiny neurons and subpallium-derived cortical interneurons (for review see Gord and Bernardo, 2011). We find that the ES-derived neurons expressing Ctip2 do not coexpress GAD67 and, therefore, are not GABAergic (Fig. 3D). Together, the coexpression of Ctip2, Er81, and Tbr1 and the absence of GAD67 strongly support the interpretation that a small proportion of ES-derived neurons under these relatively undirected conditions adopts properties of immature neocortical neurons in vitro.
Neocortical neurons are relatively immature
To investigate whether neocortical-like Ctip2-expressing neurons display appropriate features of stage-specific differentiation, we first assessed basic markers of neuronal maturation. Nearly all CNS neurons activate common programs of neuronal maturation, as marked by TuJ1, Map2, and NeuN/Fox-3 (Kim et al., 2009). Very few mature neurons in the CNS lack NeuN expression, most notably Purkinje neurons and gamma spinal motor neurons (Friese et al., 2009).
We find that these ES-derived neurons are relatively immature, based on the low abundance of NeuN expression after 21 or 28 days (approximately 5–10% of TuJ1-positive neurons coexpress NeuN, assessed in selected fields in vitro). Given the importance of neuronal maturation for the timing of postmitotic neocortical subtype refinement, we asked whether the small population of neocortical-like neurons that coexpress Ctip2, Tbr1, and Er81 is mature or immature. We find that all Ctip2-expressing neurons coexpress TuJ1 (Fig. 3B). Approximately one-third of these neurons express both Map2 and NeuN (Fig. 4A). These neurons are not uniformly or completely mature, but some display crucial hallmarks of at least early maturation.
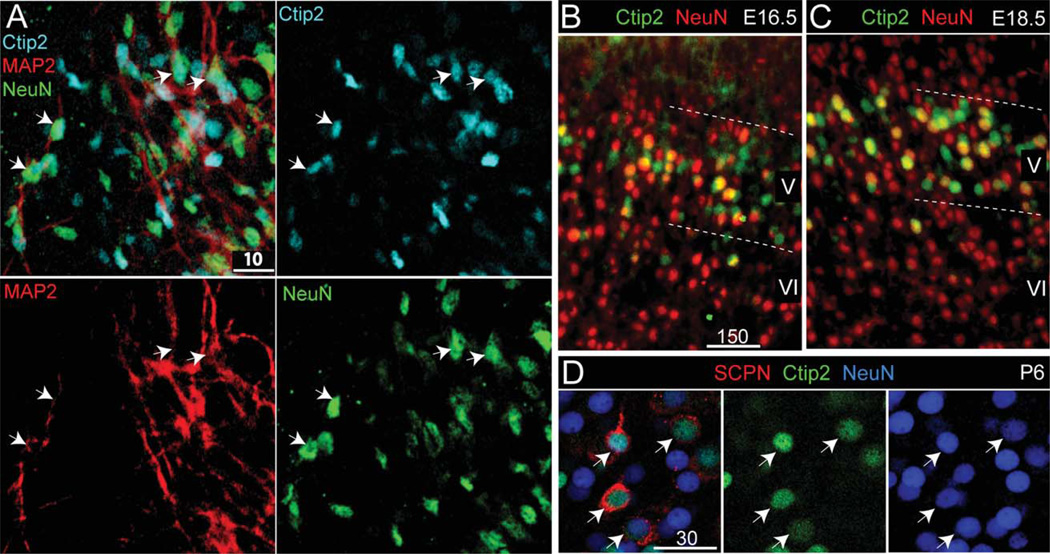
Figure 4.
ES-derived, Ctip2-expressing neurons are immature, consistent with NeuN expression at midcorticogenesis in vivo. A: Approximately one-third of ES-derived, Ctip2-expressing neurons coexpress Map2 and NeuN in vitro (arrows, Ctip2/Map2/NeuN). B,C: In vivo, NeuN is normally expressed by one-third of E16.5 (B) and E18.5 (C) Ctip2-high neurons. D: By P6, in vivo, all retrogradely labeled SCPN coexpress Ctip2 and NeuN, indicating completion of a next stage of progressive maturation. A partial magenta-green version of this figure is available online in the Supporting Information. Scale bars = 10 µm in A; 150 µm in B (applies to B,C); 30 µm in D.
We next investigated whether the extent of NeuN expression might indicate an equivalent stage in development. In vivo at E16.5–E18.5, NeuN is expressed by approximately one-third of of Ctip2-expressing neocortical neurons (Fig. 4B,C). Later, at P6, all Ctip2-expressing neocortical neurons also express NeuN (Fig. 4D). Between E16.5 and P6, Ctip2-expressing cortical neurons in vivo extend axons to their targets in the midbrain, brainstem, and spinal cord and begin the process of pruning collateral connections (Stanfield, 1992; Arlotta et al., 2005). In contrast, ES-derived neocortical neurons in culture develop to a relatively immature state most highly resembling midcorticogenesis.
Impaired subtype distinction of immature ES-derived CFuPN
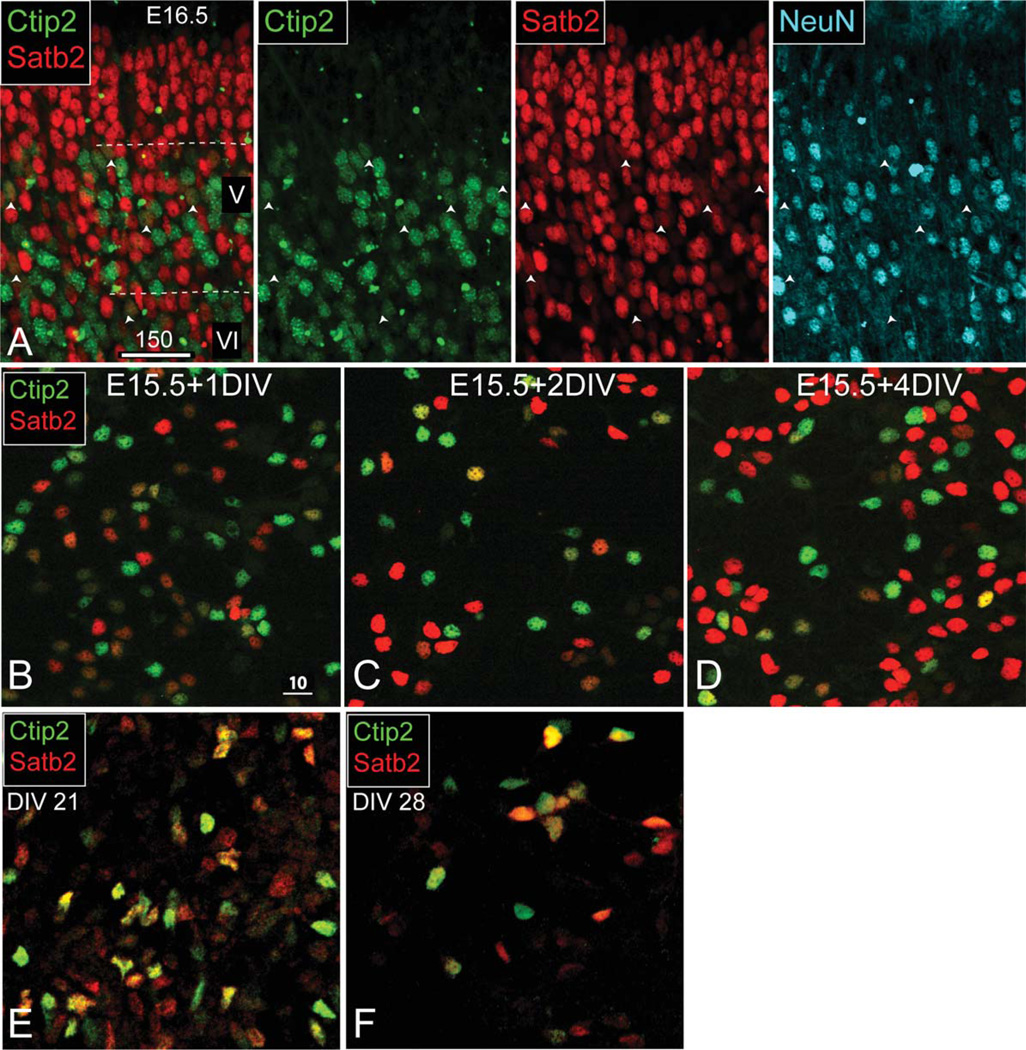
We next assessed whether ES-derived, Ctip2-expressing, immature neurons are appropriately molecularly distinct from other subtypes. During midcorticogenesis, in vivo, when only a small percentage of neurons expresses NeuN, neocortical projection neurons coexpress markers characteristic of multiple subtypes. By the first week of postnatal neocortical development, this molecular coexpression resolves into a refined, subtype-specific molecular identity, termed “subtype refinement” (Lai et al., 2008; Joshi et al., 2008; Azim et al., 2009b; Lickiss et al., 2012; Cederquist et al., 2013). One example transcription factor, Satb2, is transiently expressed by early-stage CFuPN but is later restricted to specific expression by CPN and other associative neocortical neurons (Alcamo et al., 2008; Britanova et al., 2008; Lickiss et al., 2012). To investigate specifically this subtype refinement of Ctip2 and Satb2 in vivo, for comparison with the events in culture, we assessed E16.5 neocortex and found significant Ctip2/Satb2 coexpression in layer V; these immature Ctip2/Satb2 coexpressing postmitotic neurons consistently do not express NeuN (Fig. 5A).
Figure 5.
ES-derived, Ctip2-expressing neurons do not resolve immature projection neuron marker expression over 1 week in vitro. A: At E16.5 in vivo, Ctip2- and Satb2-coexpressing neurons are relatively immature, indicated by the absence of NeuN colabeling. B–D: Dissociated primary E15.5 neocortical cells initially coexpress Ctip2 and Satb2, but this immature expression resolves during the course of 4 days in vitro (DIV). E,F: Under the same culture conditions, ES-derived neocortical-like neurons coexpress Ctip2 and Satb2 at 21 DIV (E), and this coexpression persists at 28 DIV (F). A magenta-green version of this figure is available online in the Supporting Information. Scale bars = 150 µm in A; 10 µm in B (applies to B–F).
As a further, direct comparison, we next assessed postmitotic subtype refinement by primary developing neocortical neurons in vitro using dissociated E12.5 neocortical cells cultured under the same conditions as for day-14–21 ES-derived neocortical neurons. We find that these primary neurons reduce their initially high levels of Ctip2 and Satb2 coexpression and increase the intensity of either Ctip2 or Satb2 over the course of 4 days in vitro (Fig. 5B–D), confirming that primary neurons are capable of subtype-specific transcription factor refinement during maturation in vitro.
We then investigated whether the small population of ES-derived, Ctip2-expressing, immature neocortical neurons similarly displays molecular profiles consistent with midcorticogenesis and whether this molecular identity is refined to subtype specificity over time. We find that most Ctip2-expressing neurons continue to coexpress Satb2 at 21 days (1 week after the onset of in vitro neurogenesis; Fig. 5E). Strikingly, Ctip2/Satb2 coexpression is still maintained after 28 days of postmitotic differentiation (Fig. 5F), in contrast to primary dissociated E15.5 neocortical neurons cultured for only 4 days under the same conditions in vitro (Fig. 4B–D). Moreover, these ES-derived neocortical-like neurons express a continuum of low, medium, and high expression levels of Ctip2 and Satb2, in contrast to primary dissociated E15.5 neocortical neurons, which have distinctly high, low, or absent expression levels of Ctip2 or Satb2 when cultured under the same conditions in vitro.
Incomplete molecular area refinement of ES-derived CFuPN
It has been previously reported that some ES cell-derived neurons, when grafted in the white matter tracts ventral to the neocortex of P0/P1 mice, project axons to intracortical and subcerebral (mostly visual) targets after 1 month (Gaspard et al., 2008). The expression of a single caudal neocortex marker, CoupTF1, was used to explain these biased projection patterns. Since that publication, multiple transcription factors (e.g., Bhlhb5, CoupTF1, and Lmo4) have been characterized as important postmitotic controls over neocortical area specification in vivo (Armentano et al., 2007; Joshi et al., 2008; Huang et al., 2009; Tomassy et al., 2010; Cederquist et al., 2013). In striking parallel to initially broad expression of genes that are refined over time to define precise subtype identity, these postmitotic area controls are initially coexpressed broadly in all neocortical areas, then become refined in expression during the first postnatal week (Woodworth et al., 2012; Custo Greig et al., 2013).
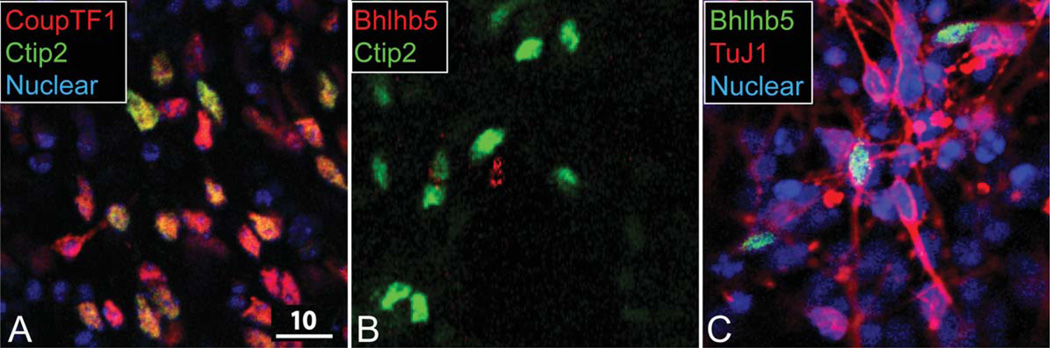
To investigate whether immature ES-derived CFuPN might have area-specific molecular identity, we assessed putative CFuPN marked by high Ctip2 expression. We find that nearly all ES cell-derived Ctip2-expressing neurons coexpress CoupTF1 (Fig. 6A), which is consistent with in vivo broad expression (caudal-high to rostral-low gradient) in the neocortex at midcorticogenesis. In striking contrast, Ctip2-expressing neurons do not coexpress Bhlhb5 (Fig. 6B), although Bhlhb5 is expressed by other ES-derived neurons (Fig. 6C). These data indicate that the absence of Bhlhb5 coexpression is inappropriate for the same stage of development in vivo and might represent deficits in area-specific differentiation by ES-derived neocortical neurons.
Figure 6.
ES-derived, Ctip2-expressing neurons do not complete postmitotic area refinements. A: All ES-derived Ctip2-expressing neurons coexpress CoupTF1. B: All ES-derived Ctip2-expressing neurons cells exclude Bhlhb5. C: Bhlhb5 is expressed by other ES-derived neurons (N = 4; approximately 1,000 neurons were screened). A magenta-green version of this figure is available online in the Supporting Information. Scale bar = 10 µm.
DISCUSSION
The experiments presented here are the first to investigate deeply the differentiation of neocortical-like neurons derived from ES cells, using the current and rapidly advancing knowledge in the field, and the results identify maturation deficits of these neurons. We demonstrate the utility of coordinating markers of neuronal maturation with markers of neocortical subtypes to assess the stage and extent of neocortical differentiation. Previous reports of ES-derived neocortical neuronal subtypes have assessed the presence of individual markers or, less commonly, combinations of very limited and relatively broad markers to identify neocortical subtypes (Gaspard et al., 2008; Eiraku et al., 2008; Ideguchi et al., 2010; Nasu et al., 2012; Mariani et al., 2012; Shi et al., 2012; Espuny-Camacho et al., 2013). However, most neocortical subtype-specific markers are only truly specific during transient developmental stages, in defined anatomical locations, and are not individually specific to the neocortex (Molyneaux et al., 2007; Woodworth et al., 2012; Custo Greig et al., 2013).
Developmental stage-specific characterizations of ES-derived neocortical-like neurons in vitro suggest that these neurons most resemble in vivo immature, unrefined neocortical neurons at midcorticogenesis. This conclusion is based on three distinct developmental criteria. First, fewer than one-third of TuJ1-expressing neocortical- like neurons express mature neuronal markers (MAP2, NeuN), consistent with the proportion of neocortical neurons that express NeuN in vivo at E16.5–E18.5 (Fig. 4). These data provide a metric for comparison with a similar developmental stage in vivo; we use this information to interpret the stage-specific expression of subtype markers. Second, neocortical-like neurons coexpress multiple subtype-specific transcription factors (e.g., Tbr1, Ctip2, Satb2) in a continuum of low, medium, and high expression levels consistent with in vivo coexpression of these genes during early to midcorticogenesis but that is in striking contrast to the more mature expression of these transcription factors by primary, dissociated E15.5 neocortical neurons cultured under the same conditions in vitro (Figs. 3 and 5). Third, neocortical-like neurons appropriately coexpress some, but not all, postmitotic controls over area-specific differentiation (e.g., CoupTF1, Bhlhb5; Fig. 6); although this expression profile is most consistent with caudal fates, it does not reflect the broad patterns of area-specific markers during midcorticogenesis.
Neocortical projection neurons are not the only population that displays increasingly restricted expression of subtype-specific transcription factors during maturation; indeed, spinal motor neurons (SMN) follow a similar process of refinement and diversity generation in vivo (Jessell, 2000; Dasen and Jessell, 2009; Alaynick et al., 2011). Initially, early postmitotic SMN express the transcription factors Hb9, Islet1, and Lhx3 (Sharma et al., 1998), and, with continued maturation and position-dependent differentiation (Sürmeli et al., 2011), expression of each transcription factor becomes progressively restricted to distinct SMN subtype identities, including medial, lateral, and hypaxial motor column subtypes. However, in vitro subtype-specific molecular refinements by heterogeneous ES-derived SMN are not distinct at early, immature stages of differentiation (Wichterle et al., 2002; Soundararajan et al., 2006; Peljto and Wichterle, 2011). Our findings, though directed toward characterizing neocortical neuronal identities, also reveal unresolved, immature subtype refinement in vitro.
Although ES-derived neocortical-like neurons recapitulate some aspects of immature neocortical development specific to a stage approximating midcorticogenesis, these data also indicate that these neurons are “stalled” in maturation in vitro. This conclusion is based on the comparison of subtype refinement by primary dissociated neocortical cells and ES-derived neocortical neurons under the same culture conditions (Fig. 5). The immature subtype marker profiles in ES-derived neurons do not resolve over the course of 2 weeks in vitro, in contrast to the timing observed in vivo or to primary neurons cultured with the same conditions in vitro. The conclusion that ES-derived neocortical-like neurons are stalled in differentiation, rather than permanently mis-specified, is supported by evidence of continued neuronal maturation, based on the extension of long-range axons to forebrain and midbrain targets but not by resolution of subtype-specific molecular markers, following transplantation into early postnatal mice (Gaspard et al., 2008).
Increasingly, more refined analyses of ES-derived neuron physiology and subtype identity indicate stalled or incomplete neuronal differentiation following directed differentiation in vitro. For example, in one protocol of SMN generation from mouse ES cells, in vitro maturation is limited; only after 5 days of myotube coculture do ES-derived SMN express more mature physiologic properties of postnatal spinal motor neurons (Miles et al., 2004). Recently, more detailed analyses of ES-derived photoreceptor neurons (Eiraku and Sasai, 2012), midbrain-like dopaminergic neurons (Kriks et al., 2011), and spinal nociceptor neurons (Chambers et al., 2012) similarly suggest variability and limitations in the extent of neuronal subtype maturation in vitro and after grafting in vivo.
We speculate that the maturation deficits in ES-derived neocortical neurons are the result of both intrinsic and extrinsic deficits. First, recent mouse studies demonstrate that the absence of specific intrinsic factors might accelerate, delay, or interrupt mature laminar or area positioning (e.g., Sox5 in Lai et al., 2008; FoxG1 in Miyoshi and Fishell, 2012; Bhlhb5 in Joshi et al., 2008; CoupTF1 in Tomassy et al., 2010, and Alfano et al., 2011). The finding that Bhlhb5 is absent in ES-derived neocortical-like neurons at midcorticogenesis is consistent with at least one intrinsic deficit in area-specific transcriptional refinement. Second, simplified growth and media conditions in vitro might exclude extrinsic factors necessary for neocortical subtype distinction (for review see Tiberi et al., 2012b). Coculturing with astrocytes might be beneficial, particularly for synaptic maturation and other refinements that occur later in postnatal development (Johnson et al., 2007; Foo et al., 2011), although the deficits of subtype-specific molecular refinement by ES-derived neocortical neurons occur prior to the stage that coincides with postnatal gliogenesis. Third, the absence of cell–cell interactions in adherent cell culture might impede subtype-specific refinements; strikingly, subtype marker overlap does not appear to be as severe in aggregate-based protocols of ES-derived neocortical differentiation, possibly indicating the utility of cell–cell interactions within self-organized ES-derived aggregates (Eiraku et al., 2008; Nasu et al., 2012). Similarly, subtype-specific maturation of ES-derived neocortical-like neurons might occur when transplanted as individually isolated neurons in vivo into embryonic or postnatal neocortex, although such subtype characterizations have not been performed in situ (Gaspard et al., 2008). Finally, intrinsic deficits in the chromatin landscape might contribute to the stalled maturation of ES-derived neocortical-like neurons; recent studies suggest that chromatin remodeling is important at multiple stages of corticogenesis (MacDonald and Roskams, 2009; Tiberi et al., 2012b; Baranek et al., 2012). We speculate that some of these deficits might contribute to the insufficiency of ES-derived progenitors, by multiple protocols, to generate distinct superficial-layer neuron subtypes (Hansen et al., 2011).
Early deficits in pallial progenitor specification might explain the sparse enrichment and stalled maturation of postmitotic neocortical neurons. The data presented here describe heterogeneity of pallial and forebrain markers (e.g., Pax6, Sox6, Otx2, and Mash1) and absence of subpallial markers in ES-derived progenitors (Fig. 2). Although these data suggest that dorsalization of ES-derived progenitors is highly efficient, the heterogeneity and minimally overlapping expression of multiple pallial markers (e.g., Pax6 and Sox6) strongly indicate an incomplete extent of pallial differentiation by most ES-derived progenitors. In particular, the strikingly low efficiency of neocortical-like neuron generation (at most 20% of ES-derived neurons express Tbr1, Ctip2, or Satb2) supports the interpretation that most ES-derived pallial-like progenitors are incompletely specified. We speculate that the small population of Pax6- and Sox6-coexpressing progenitors (~20% of total progenitors; Fig. 2C) most closely resembles true pallial progenitors and likely accounts for the small population of neocortical-like neurons; the prospective isolation of these ES-derived pallial-like progenitors might allow further study of neocortical subtype specification in future studies. Together, these data suggest that deficits in neocortical-like neuron subtype specification might originate with incomplete pallial progenitor specification.
Judging from the typically exceptional specificity of neocortical neuronal subtype involvement with specific neurodegenerative diseases (e.g., CSMN and spinal motor neurons in ALS; corticostriatal projection neurons in Huntington’s disease), the utility of directed differentiation for studying neocortical biology, pathologic mechanisms, and potential therapies likely hinges on its close approximation to in vivo development. Although the results presented in this report suggest caution in utilizing ES-derived neocortical cells as a model for cortical development, with further refinements these protocols might be substantially improved. For example, the same protocol for ES-derived neocortical directed differentiation was recently used as a model system to identify Bcl6 as a regulator of neocortical progenitors, and this pathway was verified in vivo (Tiberi et al., 2012a). Absent a mechanistic understanding of the deficits of ES-derived neocortical neuron differentiation, these data indicate specific directions for the continued refinement of directed differentiation to approximate neocortical development more closely. For example, deficits in the transcriptional state or chromatin landscape of ES-derived neurons might be targeted for manipulation to enhance neocortical differentiation (Juliandi et al., 2012).
Taken together, the data from these experiments and from prior work by other groups indicate that ES-derived neocortical differentiation is limited in vitro, with multiple maturation deficits not consistent with in vivo development. The stage-specific, multiple-marker methodology presented here promises to be increasingly useful for the characterization of neocortical subtypes and for potentially directing the differentiation of refined subtypes. These results provide both foundation and motivation for refining, enhancing, and enriching for directed differentiation of clinically important CFuPN as a class and of distinct cortical projection neuron subtypes.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Kim and M. Abrams for excellent technical assistance, Dr. T. Wuttke for help with primary neuron isolation, Dr. H. Padmanabhan for expert advice, and other members of the Macklis laboratory for discussion and critical reading of the manuscript. We also thank Dr. Lee Rubin, A.W. Gee, Dr. K. Haston, C. Goldstein, Dr. M. Hayhurst, Dr. L. Katsimpardi, and other members of the Rubin laboratory for generous advice, training, sharing of equipment and reagents, and expert discussions.
Grant sponsor: National Institutes of Health; Grant numbers: NS41590; NS49553; NS45523; NS75672; T32 GM007753-30 (to C.S.); T32 AG000222-18 (to C.S.); Grant sponsor: Harvard Stem Cell Institute Nervous System Diseases Program; Grant sponsor: Jane and Lee Seidman Fund for Central Nervous System Research; Grant sponsor: Emily and Robert Pearlstein Fund for Nervous System Repair (to J.D.M.).
Footnotes
Additional Supporting Information may be found in the online version of this article at Wiley Online Library.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ROLE OF AUTHORS
Designed the research: CS, JDM. Performed the research: CS. Analyzed data: CS, JDM. Wrote the manuscript: CS, JDM.
LITERATURE CITED
- Acampora D, Barone P, Simeone A. Otx genes in corticogenesis and brain development. Cereb Cortex. 1999;9:533–542. doi: 10.1093/cercor/9.6.533. [DOI] [PubMed] [Google Scholar]
- Alaynick W, Jessell T, Pfaff S. SnapShot: spinal cord development. Cell. 2011;146:178–1780. doi: 10.1016/j.cell.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcamo E, Chirivella L, Dautzenberg M, Dobreva G, Fariñas I, Grosschedl R, McConnell S. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Alfano C, Viola L, Heng J, Pirozzi M, Clarkson M, Flore G, De Maio A, Schedl A, Guillemot F, Studer M. COUPTFI promotes radial migration and proper morphology of callosal projection neurons by repressing Rnd2 expression. Development. 2011;138:4685–4697. doi: 10.1242/dev.068031. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux B, Chen J, Inoue J, Kominami R, Macklis J. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci. 2008;28:622–632. doi: 10.1523/JNEUROSCI.2986-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano M, Chou SJ, Tomassy GS, Leingartner A, O’Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Azim E, Jabaudon D, Fame R, Macklis J. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009a;12:1238–1247. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Shnider S, Cederquist G, Sohur U, Macklis J. Lmo4 and Clim1 progressively delineate cortical projection neuron subtypes during development. Cereb Cortex. 2009b;19(Suppl 1):62–69. doi: 10.1093/cercor/bhp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek C, Dittrich M, Parthasarathy S, Bonnon C, Britanova O, Lanshakov D, Boukhtouche F, Sommer J, Colmenares C, Tarabykin V, Atanasoski S. Protooncogene Ski cooperates with the chromatin-remodeling factor Satb2 in specifying callosal neurons. Proc Natl Acad Sci U S A. 2012;109:3546–3551. doi: 10.1073/pnas.1108718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, Pachnis V, Fishell G. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron. 2009;63:466–481. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni F, Hodge R, Elsen G, Nelson B, Daza R, Beyer R, Bammler T, Rubenstein J, Hevner R. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci U S A. 2010;107:13129–13134. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell T, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Britanova O, De Juan Romero C, Cheung A, Kwan K, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, Šestan N, Molnár Z, Tarabykin V. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Britz O, Mattar P, Nguyen L, Langevin L, Zimmer C, Alam S, Guillemot F, Schuurmans C. A role for proneural genes in the maturation of cortical progenitor cells. Cereb Cortex. 2006;16(Suppl 1):i138–i151. doi: 10.1093/cercor/bhj168. [DOI] [PubMed] [Google Scholar]
- Butt S, Sousa V, Fuccillo M, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederquist G, Azim E, Shnider S, Padmanabhan H, Macklis J. Lmo4 establishes rostral motor cortex projection neuron subtype diversity. J Neurosci. 2013;33:6321–6332. doi: 10.1523/JNEUROSCI.5140-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S, Qi Y, Mica Y, Lee G, Zhang X-J, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi S-H, Studer L. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30:715–720. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci U S A. 2008;105:11382–11387. doi: 10.1073/pnas.0804918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J, Rutlin M, Gaiano N, Fishell G. Combinatorial function of the homeodomain proteins Nkx2.1 and Gsh2 in ventral telencephalic patterning. Development. 2003;130:4895–4906. doi: 10.1242/dev.00717. [DOI] [PubMed] [Google Scholar]
- Custo Greig L, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development, and diversity. Nat Rev Neurosci. 2013;14:755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen J, Jessell T. Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol. 2009;88:169–200. doi: 10.1016/S0070-2153(09)88006-X. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Sasai Y. Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues. Nat Protoc. 2012;7:69–79. doi: 10.1038/nprot.2011.429. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza R, Bulfone A, Kowalczyk T, Hevner R. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell T, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Espuny-Camacho I, Michelsen K, Gall D, Linaro D, Hasche A, Bonnefont J, Bali C, Orduz D, Bilheu A, Herpoel A, Lambert N, Gaspard N, Péron S, Schiffmann S, Giugliano M, Gaillard A, Vanderhaeghen P. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Foo L, Allen N, Bushong E, Ventura P, Chung W-S, Zhou L, Cahoy J, Daneman R, Zong H, Ellisman M, Barres B. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71:799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker-Gates RA, Shin JJ, Tai CC, Catapano LA, Macklis JD. Late-stage immature neocortical neurons reconstruct interhemispheric connections and form synaptic contacts with increased efficiency in adult mouse cortex undergoing targeted neurodegeneration. J Neurosci. 2002;22:4045–4056. doi: 10.1523/JNEUROSCI.22-10-04045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese A, Kaltschmidt J, Ladle D, Sigrist M, Jessell T, Arber S. Gamma and alpha motor neurons distinguished by expression of transcription factor Err3. Proc Natl Acad Sci U S A. 2009;106:13588–13593. doi: 10.1073/pnas.0906809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, Van Den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann S, Gaillard A, Vanderhaeghen P. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Herpoel A, Naeije G, van den Ameele J, Vanderhaeghen P. Generation of cortical neurons from mouse embryonic stem cells. Nat Protoc. 2009;4:1454–1463. doi: 10.1038/nprot.2009.157. [DOI] [PubMed] [Google Scholar]
- Ge W, He F, Kim KJ, Blanchi B, Coskun V, Nguyen L, Wu X, Zhao J, Heng J, Martinowich K, Tao J, Wu H, Castro D, Sobeih MM, Corfas G, Gleeson JG, Greenberg ME, Guillemot F, Sun YE. Coupling of cell migration with neurogenesis by proneural bHLH factors. Proc Natl Acad Sci U S A. 2006;103:1319–1324. doi: 10.1073/pnas.0510419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gord F, Bernardo R. Mechanisms of inhibition within the telencephalon: where the wild things are. Annu Rev Neurosci. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D, Rubenstein J, Kriegstein A. Deriving excitatory neurons of the neocortex from pluripotent stem cells. Neuron. 2011;70:645–660. doi: 10.1016/j.neuron.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Huang Z, Kawase-Koga Y, Zhang S, Visvader J, Toth M, Walsh C, Sun T. Transcription factor Lmo4 defines the shape of functional areas in developing cortices and regulates sensorimotor control. Dev Biol. 2009;327:132–142. doi: 10.1016/j.ydbio.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideguchi M, Palmer TD, Recht LD, Weimann JM. Murine embryonic stem cell-derived pyramidal neurons integrate into the cerebral cortex and appropriately project axons to subcortical targets. J Neurosci. 2010;30:894–904. doi: 10.1523/JNEUROSCI.4318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Johnson M, Weick J, Pearce R, Zhang S-C. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PS, Molyneaux BJ, Feng L, Xie X, Macklis JD, Gan L. Bhlhb5 regulates the postmitotic acquisition of area identities in layers II-V of the developing neocortex. Neuron. 2008;60:258–272. doi: 10.1016/j.neuron.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliandi B, Abematsu M, Sanosaka T, Tsujimura K, Smith A, Nakashima K. Induction of superficial cortical layer neurons from mouse embryonic stem cells by valproic acid. Neurosci Res. 2012;72:23–31. doi: 10.1016/j.neures.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat Neurosci. 2008;11:1247–1251. doi: 10.1038/nn.2208. [DOI] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim J-W, Piao J, Ganat Y, Wakeman D, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal M, Surmeier D, Kordower J, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Leid M, Ishmael J, Avram D, Shepherd D, Fraulob V, Dollé P. CTIP1 and CTIP2 are differentially expressed during mouse embryogenesis. Gene Express Patterns. 2004;4:733–739. doi: 10.1016/j.modgep.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickiss T, Cheung A, Hutchinson C, Taylor J, Molnár Z. Examining the relationship between early axon growth and transcription factor expression in the developing cerebral cortex. J Anat. 2012;220:201–211. doi: 10.1111/j.1469-7580.2011.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Cobos I, Potter G, Rubenstein J. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb Cortex. 2009;19(Suppl 1):i96–i106. doi: 10.1093/cercor/bhp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y-C, Song M-R, Park J, Henry Ho H-Y, Hu L, Kurtev M, Zieg J, Ma Q, Pfaff S, Greenberg M. Regulation of motor neuron specification by phosphorylation of neurogenin 2. Neuron. 2008;58:65–77. doi: 10.1016/j.neuron.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J, Roskams A. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog Neurobiol. 2009;88:170–183. doi: 10.1016/j.pneurobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Mariani J, Simonini M, Palejev D, Tomasini L, Coppola G, Szekely A, Horvath T, Vaccarino F. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna W, Betancourt J, Larkin K, Abrams B, Guo C, Rubenstein J, Chen B. Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci. 2011;31:549–564. doi: 10.1523/JNEUROSCI.4131-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Yohn DC, Wichterle H, Jessell T, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24:7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. Dynamic FoxG1 expression coordinates the integration of multipolar pyramidal neuron precursors into the cortical plate. Neuron. 2012;74:1045–1058. doi: 10.1016/j.neuron.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Nasu M, Takata N, Danjo T, Sakaguchi H, Kadoshima T, Futaki S, Sekiguchi K, Eiraku M, Sasai Y. Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture. PLoS One. 2012;7:e53024. doi: 10.1371/journal.pone.0053024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi N, Hirota A, Ohuchi H, Nakafuku M, Iimura T, Kuratani S, Fujiwara M, Noji S, Eto K. Pax-6 is involved in the specification of hindbrain motor neuron subtype. Development. 1997;124:2961–2972. doi: 10.1242/dev.124.15.2961. [DOI] [PubMed] [Google Scholar]
- Pei Z, Wang B, Chen G, Nagao M, Nakafuku M, Campbell K. Homeobox genes Gsx1 and Gsx2 differentially regulate telencephalic progenitor maturation. Proc Natl Acad Sci U S A. 2011;108:1675–1680. doi: 10.1073/pnas.1008824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peljto M, Wichterle H. Programming embryonic stem cells to neuronal subtypes. Curr Opin Neurobiol. 2011;21:43–51. doi: 10.1016/j.conb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans C, Armant O, Nieto M, Stenman J, Britz O, Klenin N, Brown C, Langevin L-M, Seibt J, Tang H, Cunningham J, Dyck R, Walsh C, Campbell K, Polleux F, Guillemot F. Sequential phases of cortical specification involve neurogenin-dependent and -independent pathways. EMBO J. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, Potter S, Westphal H, Pfaff SL. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Smith J, Robinson H, Livesey F. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–486. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundararajan P, Miles GB, Rubin LL, Brownstone RM, Rafuse VF. Motoneurons derived from embryonic stem cells express transcription factors and develop phenotypes characteristic of medial motor column neurons. J Neurosci. 2006;26:3256–3268. doi: 10.1523/JNEUROSCI.5537-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield B. The development of the corticospinal projection. Prog Neurobiol. 1992;38:169–202. doi: 10.1016/0301-0082(92)90039-h. [DOI] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sürmeli G, Akay T, Ippolito G, Tucker P, Jessell T. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell. 2011;147:653–665. doi: 10.1016/j.cell.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiberi L, van den Ameele J, Dimidschstein J, Piccirilli J, Gall D, Herpoel Al, Bilheu Al, Bonnefont J, Iacovino M, Kyba M, Bouschet T, Vanderhaeghen P. BCL6 controls neurogenesis through Sirt1-dependent epigenetic repression of selective Notch targets. Nat Neurosci. 2012a;15:1627–1635. doi: 10.1038/nn.3264. [DOI] [PubMed] [Google Scholar]
- Tiberi L, Vanderhaeghen P, van den Ameele J. Cortical neurogenesis and morphogens: diversity of cues, sources and functions. Curr Opin Cell Biol. 2012b;24:269–276. doi: 10.1016/j.ceb.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, De Leonibus E, Jabaudon D, Lodato S, Alfano C, Mele A, Macklis J, Studer M. Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci U S A. 2010;107:3576–3581. doi: 10.1073/pnas.0911792107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi M, Filosa A, Armentano M, Studer M. The COUP-TF nuclear receptors regulate cell migration in the mammalian basal forebrain. Development. 2004;131:6119–6129. doi: 10.1242/dev.01530. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang Z, Kokubu Y, Südhof T, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Waclaw R, Allen Z, Guillemot F, Campbell K. Ascl1 is a required downstream effector of Gsx gene function in the embryonic mouse telencephalon. Neural Dev. 2009;4:5. doi: 10.1186/1749-8104-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann JM, Zhang YA, Levin ME, Devine WP, Brulet P, McConnell SK. Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron. 1999;24:819–831. doi: 10.1016/s0896-6273(00)81030-2. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Woodworth M, Custo Greig L, Kriegstein A, Macklis J. SnapShot: cortical development. Cell. 2012;151:918–9180. doi: 10.1016/j.cell.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Yoneshima H, Yamasaki S, Voelker C, Molnar Z, Christophe E, Audinat E, Takemoto M, Nishiwaki M, Tsuji S, Fujita I, Yamamoto N. Er81 is expressed in a subpopulation of layer 5 neurons in rodent and primate neocortices. Neuroscience. 2006;137:401–412. doi: 10.1016/j.neuroscience.2005.08.075. [DOI] [PubMed] [Google Scholar]
- Yun K, Fischman S, Johnson J, Hrabe de Angelis M, Weinmaster G, Rubenstein JL. Modulation of the notch signaling by Mash1 and Dlx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development. 2002;129:5029–5040. doi: 10.1242/dev.129.21.5029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.