Abstract
Aims
The aim of this study was to investigate the role of gap junctions in atrial fibrillation (AF) by analysing the effects of a gap junction enhancer and blocker on AF vulnerability and electrophysiological properties of isolated hearts.
Methods and results
The acute atrial stretch model of AF in the isolated rabbit heart was used. Sustained AF (SAF) was induced by a burst of high-frequency stimulation of the Bachmann's bundle. The effective refractory period (ERP) was measured, and the total conduction time (TCT) and the pattern of conduction of the anterior surface of the left atrium were monitored by using an optical mapping system. The effect of enhancing gap junction function by 100–1000 nM rotigaptide (ZP123) and block by 30 μM carbenoxolone on these parameters was measured. SAF inducibility was increased with an elevation of intra-atrial pressure. Enhanced gap junction conductance induced by treatment with 100–1000 nM rotigaptide reduced SAF inducibility, and the gap junction blocker carbenoxolone increased SAF inducibility. In the absence of gap junction enhancer or blocker, normal conduction was observed at 0 cmH2O. When intra-atrial pressure was raised to 12 cmH2O, the conduction pattern was changed to a heterogeneous zig-zag pattern and TCT was prolonged. Conduction pattern was not affected by either agent. Rotigaptide shortened TCT, whereas carbenoxolone prolonged TCT. ERP was significantly shortened with an increase in intra-atrial pressure, but ERP was unaffected by either agent.
Conclusion
Gap junction modulators changed AF inducibility through their effects on atrial conduction, not by altering ERP.
Keywords: Atrial fibrillation, Gap junction, Rotigaptide, Carbenoxolone
1. Introduction
Atrial fibrillation (AF) frequently occurs in patients with heart failure or acute myocardial infarction.1,2 Although the underlying electrophysiological mechanisms of AF are not fully understood, atrial stretch is reported3 to be one of the key factors to cause AF via slowing in conduction velocity4 and/or shortening of effective refractory period (ERP).5–7
Gap junctions form low resistance pathways for the spread of electrical activity between cardiomyocytes.8 Reduced conduction velocity during AF can be induced by reduced gap junction conductance; however, the role of gap junctions on the occurrence of AF is controversial. In models of chronic heart failure, enhanced gap junction conductance by rotigaptide was reported to inhibit the occurrence of AF in the rabbit9 and either inhibit10 or have no effect11 in the dog. The modulation of gap junctions on the occurrence of AF has not previously been examined in the atrial stretch model.5–7
To investigate the role of gap junction in the occurrence of AF, the effects of a gap junction enhancer (rotigaptide) and a blocker (carbenoxolone) on AF vulnerability, ERP and conduction velocity were analysed in the isolated rabbit heart.
2. Methods
The protocol of this study was approved by the Animal Care and Use Committee at the Research Institute of Environmental Medicine, Nagoya University. This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Hearts were isolated from rabbits (male, 1.7–2.0 kg) under anaesthesia with intravenous thiamylal sodium (30–40 mg/kg). When removing the heart, the pulmonary artery (PA), superior vena cava (SVC), pulmonary veins, and left SVC (LSVC) were recognized for preparing the experimental model.
Isolated hearts were perfused with a modified Krebs–Ringer solution that contained in mM: NaCl 120, KCl 4.0, NaHCO3 25.2, CaCl2 1.8, NaH2PO4 1.2, MgSO4 1.3, and glucose 5.8, gassed with 95% O2 and 5% CO2 (37°C, pH 7.40).
After stabilization of Langendorff preparation, acute stretch of the atria was performed using the method originally described by Ravelli and Allessie5 (Figure 1). PA, SVC, right superior pulmonary vein (RSPV), and LSVC were cannulated, and the other vessels such as inferior vena cava etc. were ligated. Cannulas for the PA and SVC were connected to a manometer for monitoring atrial pressure. Intra-atrial pressure was adjusted by moving up/down the drainage orifice from the RSPV and LSVC.
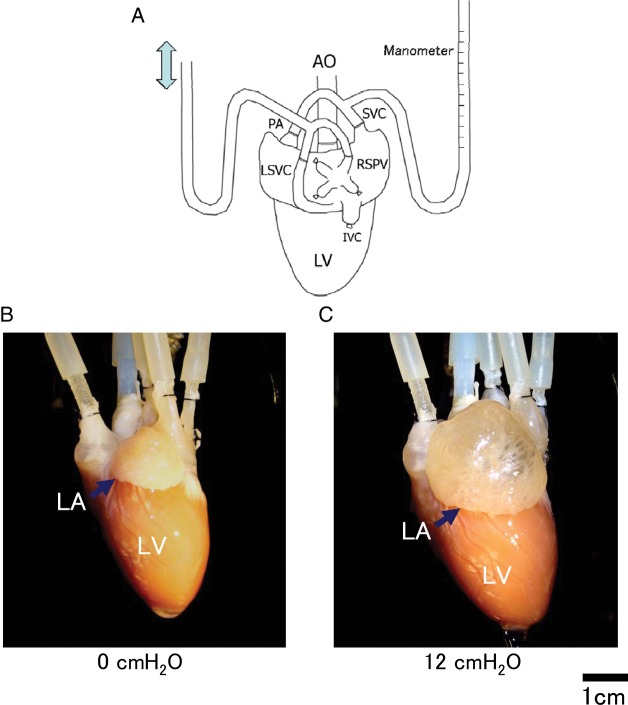
Figure 1.
Atrial stretch model of rabbit heart. (A) Schematic model of isolated heart preparation. Atrial septum was perforated for securing the same pressure of both atria. Atrial pressure was measured by manometer connected to the PA and SVC. RSPV and LSVC were cannulated for drainage. (B and C) Dilatation of LA is recognized in response to an elevation of intra-atrial pressure from 0 cmH2O (B) to 12 cmH2O (C).
Artificial perforation through the atrial septum for securing the same pressure between the right atrium and left atrium (LA) was created by using an 18-gauge needle with gentle pressing. Bilateral intra-atrial pressure was increased in 2 cmH2O steps from 0 to 20 cmH2O.
We attempted to create atrio-ventricular block by mechanical destruction, alcohol injection, or radiofrequency ablation. However, recovery of atrio-ventricular conduction was recognized in some cases and a certain amount of atrial muscle damage was unavoidably associated with atrio-ventricular block. For these reasons, we decided not to create an artificial atrio-ventricular block for this study. To reduce the influence of ventricular contraction, direct current (DC, 9.0 V) was applied to the ventricle for induction of ventricular fibrillation to reduce the effects of ventricular contractions.
AF was induced by a burst of high-frequency electrical stimulation (bipolar, 40 Hz, 3–10 s, pulse width: 2 ms, electrode interval: 0.5 mm) applied to Bachmann's bundle. The burst stimulation was applied 10 times in each pressure, applied in 2 cmH2O step-ups. Atrial electrogram was recorded from the epicardial surface (electrode interval: 2 mm) of the left atrial appendage (LAA) and right atrial appendage (RAA). AF was recognized as irregular reflections over 30 Hz in both LAA and RAA. When the induced AF did not terminate spontaneously, AF was stopped promptly by decreasing the intra-atrial pressure to 0 cmH2O without DC shock or drug administration. After termination of AF by decreased pressure, intra-atrial pressure was raised to the original pressure and the experiment was continued.
ERP of atrial muscle was measured at Bachmann's bundle by 5 Hz basic cycle length (BCL, S1) and extra stimulus (S2) applied at a voltage twice the pacing threshold. ERP was measured at 0, 4, 8, 12, 16, and 20 cm H2O. When intra-atrial pressure was elevated or depressed, ERP alteration was stabilized within 2 min. Therefore, ERP measurement was performed 2 min after a change in intra-atrial pressure. Based on the ERP alteration by elevated/depressed intra-atrial pressure, AF induction by burst stimulation was carried out over 2 min after intra-atrial pressure was changed.
The conduction of gap junctions was enhanced by rotigaptide (100–1000 nM) dissolved in the modified Krebs–Ringer solution. Measurements were made after 15–20 min exposure to the compound. Gap junctions were blocked by carbenoxolone (30 μM) in a similar manner. Higher concentrated carbenoxolone (100–1000 μM) were also tried; however, because S1 pacing was not captured in some cases, these data were excluded. The stock solutions of rotigaptide and carbenoxolone were prepared with distilled water.
Optical mapping was performed essentially as described previously.12–16 The heart was stained by the voltage-sensitive dye, di-4-ANEPPS and an excitation–contraction uncoupler (2,3-butanedione monoxime, 15 mM) was applied to reduce motion artefacts. The LA anterior surface was illuminated with bluish green light emitting diodes (NSPE-590S, Nichiha, Japan), and fluorescence was recorded by a high-speed digital camera (Fast-Max, Phontron, Japan) with 10-bit grey scale images (256 × 256 pixels, sampling: 1000 frames/s). LA anterior surface signals were recorded with optically shielded left ventricle to reduce noise artefact. Conduction time and conduction patterns of the LA anterior surface (unipolar pacing from the top of LAA) were evaluated by this system.
Statistical comparisons were performed by two-way ANOVA with a post hoc Bonferroni multiple comparison test to compare all groups. Differences were considered significant when P < 0.05.
3. Results
3.1. Elevated intra-atrial pressure increased the inducibility of AF
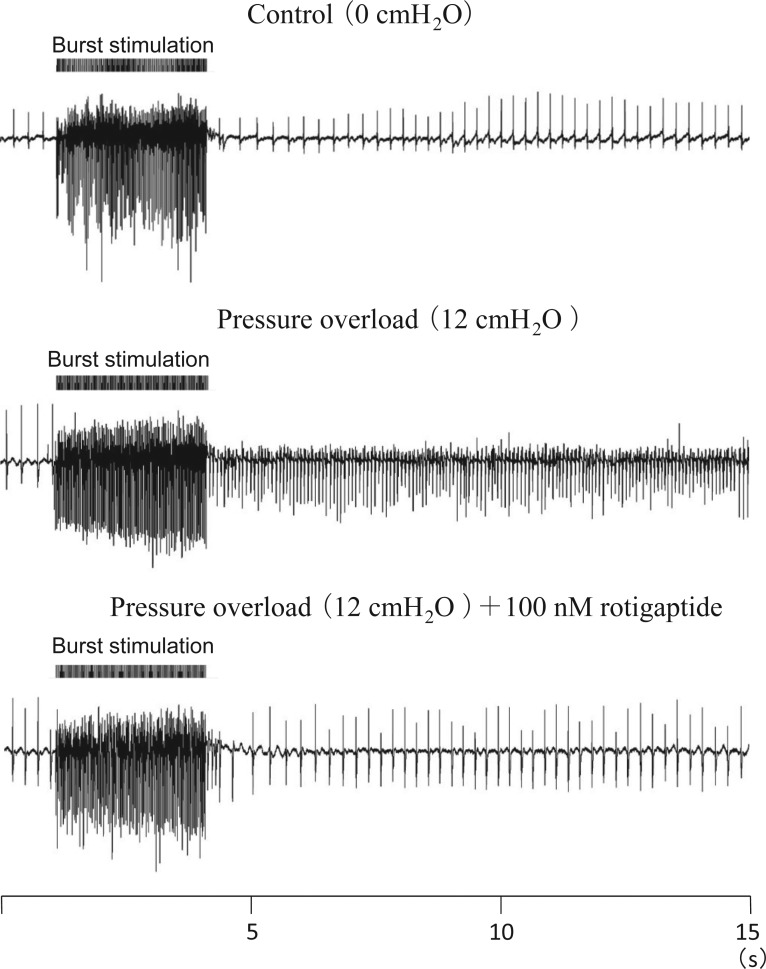
AF was induced by a burst of high-frequency electrical stimulation applied to Bachmann's bundle. Sustained AF (SAF) was defined as AF which continued over 30 s. When the atria were not dilated, SAF was not inducible (Figure 2, top panel). SAF was initiated by burst stimulation when intra-atrial pressure was elevated to 12 cmH2O (Figure 2, middle panel). At pressures of 14–20 cmH2O, SAF was achieved in all experiments. SAF inducibility was a sigmoid function of intra-atrial pressure (Figure 3). The pressure level of 50% SAF inducibility was achieved at 10.3 cmH2O by estimation with pressure-response curve (Figure 3A).
Figure 2.
AF induced by raised intra-atrial pressure. SAF was not induced at 0 cmH2O of intra-atrial pressure (without dilated atria) by high-frequency burst stimulation. SAF was initiated at 12 cmH2O. After 15 min administration of gap junction enhancer (100 nM rotigaptide), SAF was not induced at the same intra-atrial pressure (12 cmH2O).
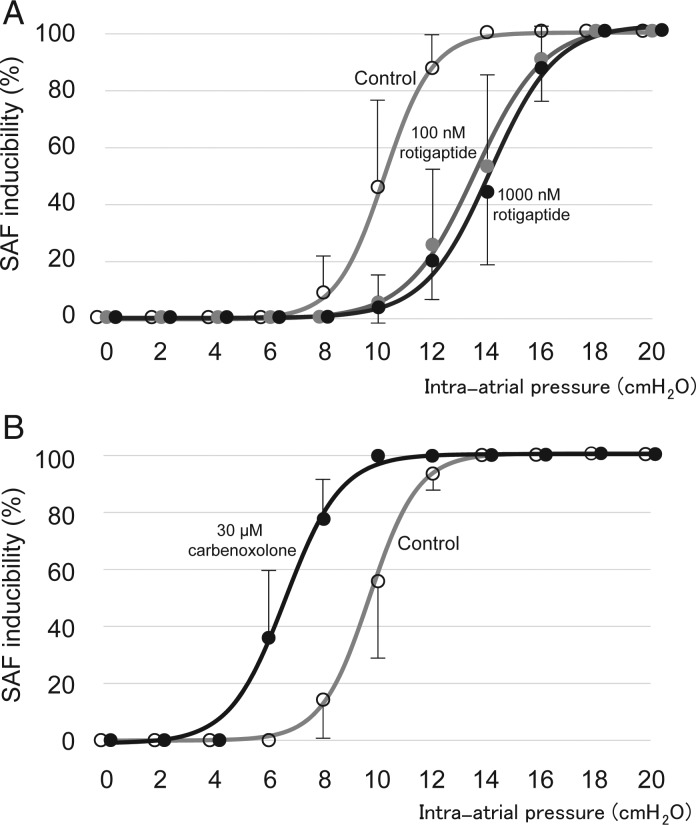
Figure 3.
Intra-atrial pressure and inducibility of SAF. (A) Gap junction enhancer (rotigaptide) inhibited AF caused by an elevation of intra-atrial pressure. Open circles: Control; grey shaded circles: 100 nM rotigaptide; black circles: 1000 nM rotigaptide. (B) Gap junction blocker (carbenoxolone) facilitated AF caused by an elevation of intra-atrial pressure. Open circles: Control; black circles: 30 μM carbenoxolone.
3.2. Gap junction enhancer inhibited AF
Rotigaptide was applied at 100–1000 nM to evaluate the effect of enhanced gap junction conduction on SAF inducibility. The pressure-response curve was shifted to the right by this compound. At 12 cmH2O, rotigaptide reduced SAF inducibility from 88.8 ± 11.3 to 26.3 ± 25.0 and 20.0 ± 13.1% (P < 0.05, n = 8, 100 and 1000 nM, respectively, Figure 3A). The pressure level of 50% SAF inducibility was increased from 10.3 to 13.5 and 13.9 cmH2O (100 and 1000 nM, respectively). No significant difference was recognized between 100 and 1000 nM rotigaptide in SAF inducibility at each pressure.
3.3. Block of gap junctions facilitated AF
The gap junction blocker carbenoxolone (30 μM) increased the inducibility of SAF caused by an elevation of intra-atrial pressure. At 8 cmH2O, carbenoxolone increased SAF inducibility from 15.0 ± 14.1 to 77.5 ± 15.8% (P < 0.05, n = 8), and the pressure-response curve was shifted to the lower pressure direction. The pressure level of 50% SAF inducibility was decreased from 9.6 to 6.6 cmH2O (Figure 3B).
3.4. Effects on conduction time and conduction pattern by an elevation of intra-atrial pressure
Total conduction time (TCT) and conduction patterns of the LA anterior surface were evaluated by optical mapping. In the absence of gap junction enhancer or blocker, normal conduction was observed at 0 cmH2O (Figure 4A and D). When intra-atrial pressure was raised to 12 cmH2O, the conduction pattern was changed to a heterogeneous zig-zag pattern (Figure 4B and E). TCT was prolonged from 31.9 ± 1.1 ms (0 cmH2O) to 38.0 ± 1.7 ms (P < 0.05, n = 7, Figure 4G).
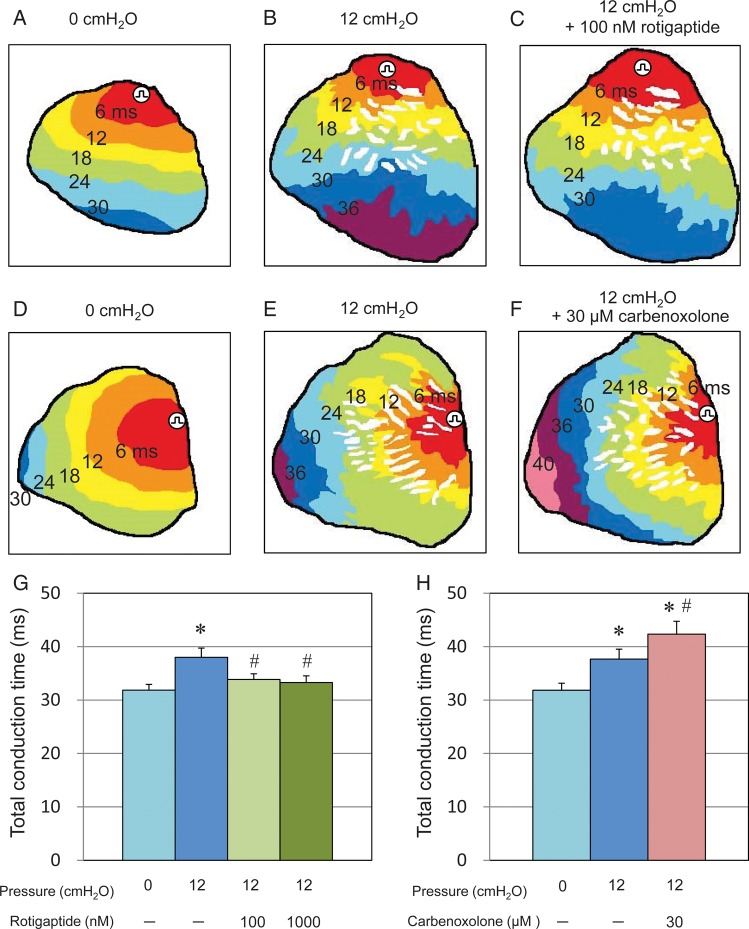
Figure 4.
Conduction pattern and conduction time evaluated by optical mapping. (A–E) Conduction pattern with constant pacing (4 Hz) from the top of left atrium anterior surface. (A and D) Conduction pattern when intra-atrial pressure was 0 cmH2O. Smooth conduction patterns were recognized. (B and E) Conduction pattern when intra-atrial pressure was 12 cmH2O. Heterogeneous zig-zag pattern was recognized and in some part of thin wall area conduction was blocked. (C and F) Conduction pattern (heterogeneous zig-zag pattern) was not affected by gap junction enhancer (C) or blocker (F). (G) Gap junction enhancer reduced TCT from 38.0 ± 1.7 to 33.9 ± 1.1 and 33.3 ± 1.3 ms at 12 cmH2O (n = 7, 100 and 1000 nM, respectively). *P < 0.05 vs. 0 cmH2O without rotigaptide. #P < 0.05 vs. 12 cmH2O without rotigaptide. (H) Gap junction blocker prolonged TCT from 37.7 ± 1.9 to 42.3 ± 2.4 ms at 12 cmH2O (n = 6). *P < 0.05 vs. 0 cmH2O without carbenoxolone. #P < 0.05 vs. 12 cmH2O without carbenoxolone.
3.5. Influences of gap junction enhancer and blocker on conduction time and conduction pattern
When the atria were not dilated, both gap junction enhancer (100–1000 nM rotigaptide) and blocker (30 μM carbenoxolone) did not change TCT or conduction pattern (data not shown). When intra-atrial pressure was raised to 12 cmH2O, the heterogeneous zig-zag pattern of conduction was not altered by either the gap junction enhancer or blocker (Figure 4C and F). However, rotigaptide slightly, but significantly shortened TCT from 38.0 ± 1.7 to 33.9 ± 1.1 and 33.3 ± 1.3 ms (P< 0.05, n = 7, 100 and 1000 nM, respectively, Figure 4G) and carbenoxolone prolonged TCT from 37.7 ± 1.9 to 42.3 ± 2.4 ms (P < 0.05, n = 6, Figure 4H). There was no significant difference between 100 and 1000 nM rotigaptide in TCT.
Thus, conduction velocity was accelerated by gap junction enhancer and reduced by gap junction blocker without influence on the pattern of conduction.
3.6. Effects on ERP by gap junction enhancer and blocker
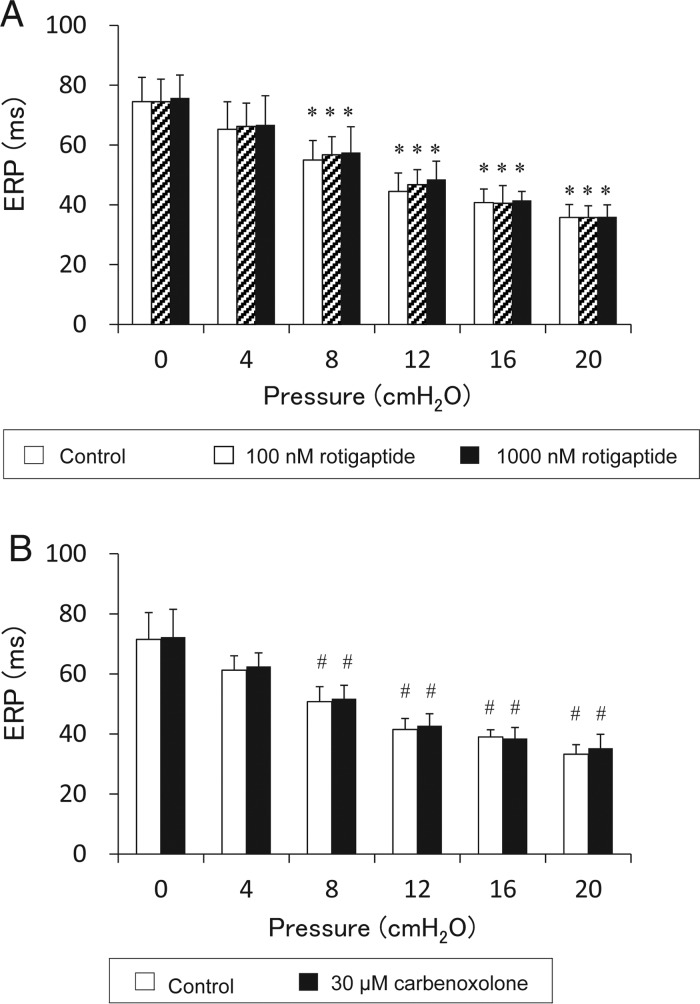
Under control conditions, the ERP was significantly shortened by an increase in intra-atrial pressure. When intra-atrial pressure was raised from 0 to 20 cmH2O, ERP was significantly shortened from 74.5 ± 8.1 to 35.8 ± 4.3 ms (n = 8, Figure 5).
Figure 5.
Changes of ERP in response to acute atrial dilatation. (A) ERP changes caused by raised intra-atrial pressure and effects of gap junction enhancer (100 and 1000 nM rotigaptide). BCL: 200 ms, n = 8. *P < 0.05 vs. 0 cmH2O. (B) ERP changes caused by raised intra-atrial pressure and effects of gap junction blocker (30 μM carbenoxolone). BCL: 200 ms, n = 8. #P < 0.05 vs. 0 cmH2O. Either gap junction enhancer (rotigaptide) or blocker (carbenoxolone) did not affect the ERP shortening caused by raised intra-atrial pressure.
When the atria were not dilated, rotigaptide did not change ERP. ERP was 74.5 ± 8.1, 74.5 ± 7.5, and 75.8 ± 7.7 ms (Control, 100 nM rotigaptide, and 1000 nM rotigaptide, respectively, no significant difference, Figure 5A). Carbenoxolone also did not affect ERP. ERP was 71.5 ± 8.9 ms in control and 72.3 ± 9.3 ms in 30 μM carbenoxolone (no significant difference, n = 8, Figure 5B).
Rotigaptide did not affect ERP shortening caused by an elevation of intra-atrial pressure. When intra-atrial pressure was raised to 20 cmH2O, the ERP was 35.8 ± 4.3, 35.8 ± 3.9, and 36.0 ± 4.0 ms (Control, 100 nM rotigaptide, and 1000 nM rotigaptide, respectively, no significant difference, Figure 5A).
Carbenoxolone also did not affect ERP shortening caused by an elevation of intra-atrial pressure. When intra-atrial pressure was raised to 20 cmH2O, the ERP was 33.3 ± 3.2 ms in control and 35.3 ± 4.7 ms in the presence of 30 μM carbenoxolone (no significant difference, n = 8, Figure 5B).
4. Discussion
In this study, the role of gap junctions in the initiation of AF was analysed by enhancing or blocking of gap junction conduction in the rabbit acute stretch model. Major findings of the study were as follows:
Enhanced gap junction conductance induced by rotigaptide decreased, while block by carbenoxolone facilitated the incidence of stretch-induced AF.
Rotigaptide decreased, whereas carbenoxolone increased the conduction time (TCT) of the LA anterior surface.
ERP shortening caused by atrial stretch was not affected by either gap junction modulator.
The regulation of gap junction conductance plays a substantial role in initiation and maintenance of AF.
Simultaneous analysis of AF inducibilities with a gap junction enhancer and blocker was not performed previously. In previous studies, Haugan et al.,9 Guerra et al.,10 and Shiroshita-Takeshita et al.11 (acute ischaemia model) reported that the gap junction enhancer rotigaptide inhibited AF. Our data support the conclusions of these studies. Acute atrial stretch is quite similar to acute atrial ischaemia in the relevance of shortening ERP and reduction in conduction velocity. On the other hand, Shiroshita-Takeshita et al.11 reported that, in a model of chronic heart failure, gap junction enhancer had no effect on AF inducibility. This apparent discrepancy may be explained by different species, AF models, or variable arrhythmia substrate.
In the absence of gap junction enhancer or blocker, when intra-atrial pressure was elevated, the TCT of the LA anterior surface was significantly prolonged. The mechanisms of reduced atrial conduction velocity by acute stretch can be explained by inactivation of gap junction channel associated with Ca overload induced by Na and Ca influx by activation of stretch-activated channel.17 Moreover, it has been assumed that modulation of reduced atrial conduction regulates AF occurrence caused by atrial stretch.
In our study, gap junction enhancement improved the conduction of atrial muscle and reduced AF occurrence. In contrast, gap junction block exaggerated conduction and promoted AF occurrence. Thus, these data indicate that the modulation of gap junction may regulate stretch-induced AF occurrence by influencing the conduction velocity of atrium. On the other hand, the heterogeneous zig-zag conduction pattern caused by acute stretch was not changed by gap junction modulators, although conduction velocity was affected.
When intra-atrial pressure was elevated, ERP was significantly shortened. The mechanisms of ERP shortening by atrial stretch are considered not due to gap junction modulation, and usually explained by the following factors: (i) the increase of outward current such as IKAch, ICl, and IK1 activated by acute stretch and (ii) the inactivation of Ca channel associated with Ca overload through Na and Ca influx by activation of stretch-activated channel.17
Thus, together with previous studies, our findings indicate that altered conduction is the main mechanism regulating the occurrence of AF in the atrial stretch model.
Ten times higher dose (1000 nM) of rotigaptide does not provide any additional benefit than the 100 nM rotigaptide in AF inducibilities (Figure 3A) and atrial conduction velocity (Figure 4G). Our data are compatible to the previous studies, indicating that 100 nM rotigaptide should be the best effective dose for enhancing gap junction conductance and preserving/ improving atrial conduction time.18,19 The 100 nM rotigaptide may be the adequate dose to reduce stretch-induced AF caused by increased atrial pressure.
4.1. Study limitation
In this study, rotigaptide was administrated prior to the induction of atrial stretch, which does not translate to the clinical setting.
As far as we have searched on previous studies, rotigaptide and carbenoxolone have no effects other than gap junction. But, we could not deny unknown effects of these drugs on ion channels.
The role of gap junctions in persistent or permanent AF was not investigated. It is unknown if the results obtained here are unique to the rabbit or atrial stretch model. Thus, it will be necessary to consider other animal species and models in the future.
Funding
This work was supported by Grants-in-Aid for Scientific Research (C) 26461066 from the Japanese Society for Promotion of Sciences. Funding to pay the Open Access publication charges for this article was provided by the Japanese Society for Promotion of Sciences.
Acknowledgements
We thank Michael C. Sanguinetti (Department of Physiology, The University of Utah) for his generous advice and comments on this manuscript. Rotigaptide was kindly supplied from James K. Hennan (Wyeth Research).
Conflict of interest: none declared.
References
- 1.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology ; The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–463. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 3.Ravelli F. Mechano-electric feedback and atrial fibrillation. Prog Biophys Mol Biol. 2003;82:137–149. doi: 10.1016/s0079-6107(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 4.Eijsbouts SC, Majidi M, van Zandvoort M, Allessie MA. Effects of acute atrial dilation on heterogeneity in conduction in the isolated rabbit heart. J Cardiovasc Electrophysiol. 2003;14:269–278. doi: 10.1046/j.1540-8167.2003.02280.x. [DOI] [PubMed] [Google Scholar]
- 5.Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96:1686–1695. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 6.Bode F, Katchman A, Woosley RL, Franz MR. Gadolinium decreases stretch-induced vulnerability to atrial fibrillation. Circulation. 2000;101:2200–2205. doi: 10.1161/01.cir.101.18.2200. [DOI] [PubMed] [Google Scholar]
- 7.Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature. 2001;409:35–36. doi: 10.1038/35051165. [DOI] [PubMed] [Google Scholar]
- 8.Desplantez T, Dupont E, Severs NJ, Weingart R. Gap junction channels and cardiac impulse propagation. J Membr Biol. 2007;218:13–28. doi: 10.1007/s00232-007-9046-8. [DOI] [PubMed] [Google Scholar]
- 9.Haugan K, Miyamoto T, Takeishi Y, Kubota I, Nakayama J, Shimojo H, Hirose M. Rotigaptide (ZP123) improves atrial conduction slowing in chronic volume overload-induced dilated atria. Basic Clin Pharmacol Toxicol. 2006;99:71–79. doi: 10.1111/j.1742-7843.2006.pto_432.x. [DOI] [PubMed] [Google Scholar]
- 10.Guerra JM, Everett TH, IV, Lee KW, Wilson E, Olgin JE. Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. 2006;114:110–118. doi: 10.1161/CIRCULATIONAHA.105.606251. [DOI] [PubMed] [Google Scholar]
- 11.Shiroshita-Takeshita A, Sakabe M, Haugan K, Hennan JK, Nattel S. Model-dependent effects of the gap junction conduction-enhancing antiarrhythmic peptide rotigaptide (ZP123) on experimental atrial fibrillation in dogs. Circulation. 2007;115:310–318. doi: 10.1161/CIRCULATIONAHA.106.665547. [DOI] [PubMed] [Google Scholar]
- 12.Takemoto Y, Takanari H, Honjo H, Ueda N, Harada M, Kato S, Yamazaki M, Sakuma I, Opthof T, Kodama I, Kamiya K. Inhibition of intercellular coupling stabilizes spiral-wave reentry, whereas enhancement of the coupling destabilizes the reentry in favor of early termination. Am J Physiol Heart Circ Physiol. 2012;303:H578–H586. doi: 10.1152/ajpheart.00355.2012. [DOI] [PubMed] [Google Scholar]
- 13.Harada M, Tsuji Y, Ishiguro YS, Takanari H, Okuno Y, Inden Y, Honjo H, Lee JK, Murohara T, Sakuma I, Kamiya K, Kodama I. Rate-dependent shortening of action potential duration increases ventricular vulnerability in failing rabbit heart. Am J Physiol Heart Circ Physiol. 2011;300:H565–H573. doi: 10.1152/ajpheart.00209.2010. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguro YS, Honjo H, Opthof T, Okuno Y, Nakagawa H, Yamazaki M, Harada M, Takanari H, Suzuki T, Morishima M, Sakuma I, Kamiya K, Kodama I. Early termination of spiral wave reentry by combined blockade of Na+ and L-type Ca2+ currents in a perfused two-dimensional epicardial layer of rabbit ventricular myocardium. Heart Rhythm. 2009;6:684–692. doi: 10.1016/j.hrthm.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki M, Honjo H, Nakagawa H, Ishiguro YS, Okuno Y, Amino M, Sakuma I, Kamiya K, Kodama I. Mechanisms of destabilization and early termination of spiral wave reentry in the ventricle by a class III antiarrhythmic agent, nifekalant. Am J Physiol Heart Circ Physiol. 2007;292:H539–H548. doi: 10.1152/ajpheart.00640.2006. [DOI] [PubMed] [Google Scholar]
- 16.Amino M, Yamazaki M, Nakagawa H, Honjo H, Okuno Y, Yoshioka K, Tanabe T, Yasui K, Lee JK, Horiba M, Kamiya K, Kodama I. Combined effects of nifekalant and lidocaine on the spiral-type re-entry in a perfused 2-dimensional layer of rabbit ventricular myocardium. Circ J. 2005;69:576–584. doi: 10.1253/circj.69.576. [DOI] [PubMed] [Google Scholar]
- 17.Dobrev D, Carlsson L, Nattel S. Novel molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov. 2012;11:275–291. doi: 10.1038/nrd3682. [DOI] [PubMed] [Google Scholar]
- 18.Haugan K, Olsen KB, Hartvig L, Petersen JS, Holstein-Rathlou NH, Hennan JK, Nielsen MS. The antiarrhythmic peptide analog ZP123 prevents atrial conduction slowing during metabolic stress. J Cardiovasc Electrophysiol. 2005;16:537–545. doi: 10.1111/j.1540-8167.2005.40687.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Zemlin C, Hennan JK, Petersen JS, Veenstra RD. Enhancement of ventricular gap-junction coupling by rotigaptide. Cardiovasc Res. 2008;79:416–426. doi: 10.1093/cvr/cvn100. [DOI] [PMC free article] [PubMed] [Google Scholar]