Abstract
The antidepressant and sleep-promoting effects of light exposure might be useful for treating age-related mood and sleep disorders. In view of recent evidence suggesting beneficial effects of morning light, this study examined the associations of mood and sleep with morning light exposure, 24 h environmental illumination, and the degree to which the volunteers’ bedroom windows were covered in the morning. We examined 459 postmenopausal women participating an ancillary study of the Women’s Health Initiative conducted at the University of California, San Diego Clinical Center, San Diego, CA, USA. At baseline, volunteers completed a 4-week sleep-recall questionnaire. Volunteers were then assessed for 5–7 days in their home environments with actigraphic wrist monitors. During home recording, self-reported mood was assessed. Morning illumination during the first 4 h after arising, 24-h illumination mesor (cosine-fitted mean), and illumination acrophase (cosine-fitted peak time) were calculated. Sleep was scored each night using validated wrist actigraphic methods. A sleep diary was completed each morning. During two 24-h periods, urine was collected approximately every 2 h during wakefulness and following any voidings during the sleep period. Cosine-fitting established the acrophase of urinary 6-sulfatoxymelatonin (aMT6s) excretion. Morning illumination and 24-h illumination were modestly associated with better mood and sleep. Associations of light with mood and sleep were consistently greater for subjects whose body clocks were delayed relative to the group median. Less morning window covering in the subjects’ bedrooms was associated with more morning light and less depressed mood. The results suggest that both morning and 24-h light exposure may be beneficial for older adults.
Keywords: Actigraphy, bright light, Circadian phase, older adults
INTRODUCTION
Higher levels of environmental illumination have been associated with better sleep and mood.1–3 Moreover, experimental increases in light exposure can elicit profound sleep-promoting4 and antidepressant effects.5,6
Several studies have suggested that antidepressant effects of bright light are greatest in the morning, especially for seasonal affective disorder.7–11 Also, there is intriguing evidence of antidepressant effects following simulated dawn light exposure.12–14 Indeed, a recent study found significantly greater antidepressant effects following relatively low-intensity dawn simulation during sleep (maximum intensity 250 lux) compared with morning bright light treatment (10 000 lux).14 In older individuals, morning bright light can also promote night-time sleep,15,16 though equally robust effects have been observed following afternoon or evening light.4
The mechanistic explanation for a therapeutic advantage of morning light has not been clearly established. Some evidence indicates that antidepressant effects of morning light are correlated with its circadian phase-advancing effects.17 A phase-shifting mechanism might be especially important for older adults (particularly older women), who might have a higher prevalence of circadian malsynchronization and sleep and mood morbidities than young adults.18 If morning light promotes sleep and mood via a phase-advancing mechanism, then it can be predicted that these effects would be greater in people who have relatively delayed body clock timing.
A phase-advancing mechanism might also explain the therapeutic effects of dawn light exposure. In animals, the suprachiasmatic nucleus (SCN) is apparently more responsive to light stimuli during sleep,19 which might make dawn light relatively important compared to light later in the day. One potential practical implication of these data is that having unobscured bedroom windows in the morning might be preferable to having darkly covered windows.20
Another potential advantage of morning light might be a greater biological sensitivity to light following night-time darkness. Retinal sensitivity to light is greater in the morning than at other times.21 Moreover, alerting or ‘energizing’ effects of bright light22,23 might be greatest in the morning. Since studies in urban environments have consistently shown that adults are generally exposed to lower levels of illumination over 24 h than might be expected,1,24 exposure to the dawn signal and morning illumination may play an important role in normal regulation of mood, sleep, and circadian timing.
Apart from our preliminary work,25 we are unaware of any studies that have specifically examined exposure to morning illumination in the home environment. Therefore, the aims of this study were to compare the associations of morning illumination versus 24-h illumination with mood and sleep in postmenopausal women. The degree of covering of the subjects’ bedroom windows during the morning was considered a proxy for dawn exposure, and was examined in relation to illumination, sleep, and mood. The assessments were made during a 3.5-year period with an equivalent number of volunteers assessed during the Spring, Summer, Fall, and Winter.
METHODS
Subjects
We examined 459 postmenopausal women ages 50–81 years (67.7 ± 0.4 SE years). The volunteers were recruited by writing to and calling women who had already entered the observational study of Women’s Health Initiative (WHI), a large multicenter study that includes clinical trials and observational studies of an ethnically representative sample. The exclusion criteria for the study have been reported elsewhere.26 All volunteers were women living independently in San Diego, CA, USA. No exclusions based on medication or sleep disorders were made. All volunteers signed informed consent approved by the University of California Institutional Review Board. Other data from these subjects have been reported elsewhere.27–29
Retrospective sleep assessment
Prior to home recording, volunteers completed a 4-week recall questionnaire of sleep habits, which included Likert-scale assessments (Table 1) of the frequency of (i) trouble falling asleep; (ii) waking up often during the night; (iii) awakening earlier than planned; (iv) daytime napping; (v) the sleep quality; and (vi) total sleep time on a ‘typical’ night.30
Table 1.
4-week Recall Questionnaire Likert Scales
| Variables | Likert Scale |
|---|---|
| Frequency of: | |
| Trouble falling asleep; | 1 = Not in past 4 weeks |
| Waking up often during the night; | 2 = Less than once per week |
| Awakening earlier than planned; | 3 = 1–2 times per week |
| Daytime napping | 4 = 3–4 times per week |
| 5 = 5 or more times per week | |
| Overall sleep quality on typical night | 1 = Very restless |
| 2 = Restless | |
| 3 = Average quality | |
| 4 = Sound or restful | |
| 5 = Very sound or restful | |
| Hours of sleep on typical night | 5 h or Less |
| 6 h | |
| 7 h | |
| 8 h | |
| 9 h | |
| 10+ h | |
Home assessment
Following the volunteers’ consent to participate and completion of the 4-week recall sleep questionnaire, laboratory staff went to the volunteers’ homes to instruct the volunteers regarding the remainder of the study.
Window covering
During home visits, research staff categorized the degree to which the subjects’ bedroom windows were covered at morning wake times. Virtually all of the subjects slept in bedrooms with windows. The categorization comprised three levels: (i) uncovered (n = 64); (ii) light shades or drapes (n = 193); and (iii) completely blacked out or heavy shades or drapes (n = 173).
Actillume recording
Volunteers were then monitored for 5–7 consecutive days in their usual environments. Volunteers were asked to maintain their usual sleep–wake schedules. Throughout recording, volunteers wore Actillume wrist monitors (Ambulatory Monitoring, Ardsley, NY, USA). Each minute, the Actillume recorded illumination exposure via a photometer and wrist activity via a linear accelerometer. The photometer was sensitive to light levels ranging from 0.1 lux (almost complete darkness) to over 100 000 lux (bright outdoor illumination). Actillume photometers were calibrated at regular intervals against external photometers (traceable to the US National Institute of Standards and Technology). Brief intervals in which the Actillume was removed for bathing etc. were deleted from the analyses.
Actillume illumination metrics
Three illumination metrics were established: (i) the log of the mesor (mean of the 24-h cosine-fitted lux measures); (ii) the log of the mean illumination during the first 4 h after awakening (AM-LIGHT); and (iii) illumination acrophase (peak time of 24-h fitted cosine). These values were averaged across all days of recording. Mean illumination during the last 2 h before awakening was also assessed, but it appeared that the low values that were observed (1.5 ± 0.2 lux) were largely confounded by bed-clothes covering the Actillumes, so these data were not further considered.
Actillume and diary sleep metrics
For each night during the recording week, sleep and wakefulness were estimated via an established algorithm associating wrist movement with electroencephalographically assessed sleep.31 Bedtimes and wake-times were estimated each day using Actillume activity and illumination data combined with sleep diary data. The Actillume-assessed in-bed sleep variables selected for this study were: (i) sleep latency; (ii) total sleep time; (iii) wakefulness after sleep onset; and (iv) sleep efficiency (i.e. hours of sleep/time-in-bed). These were averaged across all available nights of recording. A daily sleep diary also recorded subjective estimates of: (i) sleep latency; (ii) total sleep time; and (iii) total duration of napping, which were averaged for the week.
Circadian phase assessment
During two 24-h periods (usually days 3 and 6 of the sleep recordings), urine was collected approximately every 2 h during wakefulness and following any nocturnal voidings. Volunteers recorded the timing and volume of each voiding, and froze samples (2 cc) in duplicate. Following completion of recording, the samples were transferred to a laboratory −70°C freezer. Urinary concentrations of 6-sulfatoxymelatonin (aMT6s), the primary metabolite of melatonin, was established using an ELISA assay. Cosine-fitting of excretion rate (ng/ h) estimated the acrophase (peak time) of excretion.
Mood assessment
During the week of recording, subjects completed the CESD-6,32 a 6-item subset of the 20-item Center for Epidemiologic Studies depression inventory containing the following items:
You felt depressed.
Your sleep was restless.
You enjoyed life (scored with reverse scale).
You had crying spells.
You felt sad.
You felt that people disliked you.
The items were answered on a 4-point Likert scale indicating the frequency of symptoms during the previous week (rarely or none, 1–2 days, 3–4 days, or 5–7 days), and each item was scored from 0 (rarely or none) to 3 (5–7 days). A CESD-6 composite score was calculated by averaging the scores on the individual items.
Data analysis
Variables that were not normally distributed were log-transformed prior to correlational and regression analyses.
Association of illumination with depressed mood, sleep, and aMT6s acrophase
The associations of AM-LIGHT, illumination mesor, and illumination acrophase with CESD-6, sleep, and aMT6s acrophase were assessed with Pearson product-moment correlations. The associations of illumination with depressed mood and sleep were further compared between volunteers whose aMT6s acrophases were delayed versus advanced relative to the median aMT6s acrophase. To make these comparisons, the magnitude of the correlations were compared.33 The phase-angle between the aMT6s acrophase and wake-time was also compared between advanced versus delayed groups because this phase-angle influences sensitivity to morning light.
Association of window covering with depressed mood, sleep, and aMT6 acrophase
The association of window covering (3 levels) with AM-LIGHT, illumination mesor, illumination acrophase, CESD-6, sleep, and aMT6s acrophase, was assessed via Kruskal–Wallis non-parametric tests. As with the analyses of illumination, the analyses of window covering further compared volunteers who were phase-delayed versus phase-advanced relative to the median aMT6s acrophase.
RESULTS
Morning illumination, 24-h illumination, and illumination acrophase
The mean (± SE) AM-LIGHT was 999 ± 66 lux (median = 653 lux). Twenty-four hour illumination mesor was 614 ± 52 lux (median = 411 lux). The mean illumination acrophase was 13 : 47 ± 0 : 03 (median = 13 : 45). AM-LIGHT and the illumination mesor were significantly correlated (r = 0.48, P < 0.001), however, neither was significantly correlated with the illumination acrophase.
Self-reported mood
Depressed mood assessed with the CESD-6 was 1.88 ± 0.11 (median = 1.00).
Sleep
Sleep data are displayed in Tables 2 and 3. In the 4-week recall questionnaire (Table 2), volunteers indicated average duration of sleep, average quality of sleep and only moderate complaints regarding trouble falling asleep, awakening often during the night, awakening earlier than planned, or daytime napping. Actigraphic and sleep diary-assessments of sleep also suggest roughly ‘average’ sleep.
Table 2.
Four week recall questionnaire (mean ± SE). See Table 1 for Likert Scale
| Variable | Likert Score |
|---|---|
| Frequency of: | |
| Trouble falling asleep | 2.0 ± 0.06 (<1 time per week) |
| Waking up often during night | 3.3 ± 0.07 (~1–2 times per week) |
| Awakening earlier than planned | 2.2 ± 0.07 (~<1 time per week) |
| Daytime napping | 2.5 ± 0.06 (~1–2 times per week) |
| Overall sleep quality | 3.4 ± 0.05 (Average quality) |
| Hours of sleep | 6.7 ± 0.05 hr |
Table 3.
Objective and sleep diary (mean ± SE)
| Variable | Actigraphic sleep | Sleep diary |
|---|---|---|
| Sleep latency (min) | 28.90 ± 1.00 | 28.30 ± 1.30 |
| Total sleep time (min) | 359.80 ± 2.60 | 409.00 ± 3.00 |
| Wake after sleep onset (min) | 87.50 ± 1.70 | – |
| Sleep efficiency (%) | 81.80 ± 0.30 | – |
| Final wake time | 6.92 + 0.050 | – |
| Daytime napping (min) | – | 17.80 ± 1.10 |
–, not measured.
aMT6s acrophase, phase-delayed and phase advanced volunteers
The mean aMT6s acrophase was 03:47 h ± 6 min. Data comparing subjects whose aMT6s acrophases were earlier than the median aMT6s acrophase (i.e. earlier than 3:38, the advanced group) or later than the median acrophase (the delayed group) are displayed in Table 4.
Table 4.
Comparisons between phase-delayed versus phase-advanced subjects
| Variable | aMT6s Acrophase < Median (Advanced) | aMT6s Acrophase > Median (Delayed) |
|---|---|---|
| aMT6s Acrophase (h) | 2.45 ± 0.08 | 5.11 ± 0.12** |
| Age (years) | 65.93 ± 0.58 | 69.07 ± 0.56** |
| CESD-6 | 1.67 ± 0.16 | 1.99 ± 0.18 |
| Final Wake Time (h) | 6:30 ± 0.07 | 7:18 ± 0.08** |
| Phase Angle: aMT6s Acrophase-to-Wake Time (h) | 4.04 ± 0.093 | 2.23 ± 0.12** |
| AM-LIGHT (lux) | 1071.34 ± 120.96 | 1014.12 ± 92.06 |
| Illumination Mesor (lux) | 683.46 ± 76.44 | 622.59 ± 95.79 |
| Light Acrophase (h) | 13.36 ± 0.07 | 14.04 ± 0.08* |
| Phase Angle: aMT6s Acrophase-to-Light Acrophase (h) | 11.08 ± 0.12 | 8.98 ± 0.14** |
P < 0.001 comparing advanced with delayed groups.
Association of aMT6s acrophase with aMT6s acrophase-to-wake time phase-angle
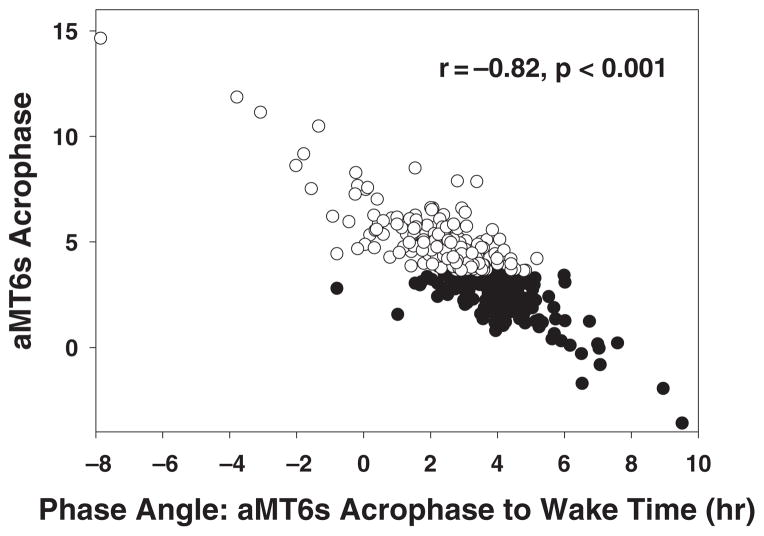
The correlation of aMT6s acrophase with phase-angle between the aMT6 acrophase and wake time was 0.82 (P < 0.001) (Fig. 1). This phase-angle was significantly longer in the phase-advanced group (4.04 ± 0.09 h) versus the phase-delayed group (2.23 ± 0.12 h) (P < 0.001).
Figure 1.
Association of aMT6s acrophase with phase angle between aMT6s acrophase and wake time for volunteers whose aMT6s acrophases were advanced (closed circles) or delayed (open circles) relative to the sample median.
Associations of illumination with mood
AM-LIGHT (r = −0.12, P = 0.012) and illumination mesor (r = −0.17, P < 0.001) were significantly inversely associated with the CESD-6 depression rating. The illumination acrophase was not significantly associated with CESD-6.
The correlation of AM-LIGHT and CESD-6 was significantly greater in the phase-delayed volunteers (r = −0.209) versus the phase-advanced subjects (r = −0.063) (P = 0.005). The associations of CESD-6 with illumination mesor and illumination acrophase were not significantly different between the phase-delayed versus phase-advanced volunteers.
Associations of illumination with sleep
Correlations of illumination with sleep are displayed in Tables 5 and 6.
Table 5.
Correlations of AM-LIGHT with sleep
| Significant correlates | Entire sample Pearson r (P) | Advanced Subjects Pearson r (P) | Delayed Subjects Pearson r (P) |
|---|---|---|---|
| Actigraphic Sleep | |||
| Sleep Latency | −0.21 (<0.001) | −0.33 (<0.001) | −0.12 (0.108) |
| Total Sleep Time | 0.14 (0.003) | 0.17 (0.027) | 0.20 (0.007) |
| Wake After Sleep Onset | −0.06 (0.222) | −0.15 (0.046) | 0.01 (0.893) |
| Final Awakening Time | 0.19 (<0.001) | 0.28 (<0.001) | 0.18 (0.017) |
| Diary Sleep | |||
| Sleep Latency | −0.17 (<0.001) | −0.24 (0.001) | −0.12 (0.120) |
| Total Sleep Time | 0.21 (<0.001) | 0.11 (0.133) | 0.38 (<0.001)* |
| Naps | −0.05 (0.353) | −0.18 (0.052) | 0.01 (0.937) |
| 4-Week Recall | |||
| Trouble Falling Asleep | −0.10 (0.032) | 0.04 (0.597) | −0.20 (0.006)* |
| Wake Up Often in Night | −0.16 (0.001) | −0.09 (0.236) | −0.19 (0.011) |
| Wake Up Too Early | −0.16 (0.001) | −0.09 (0.260) | −0.24 (0.001) |
| Total Sleep Time | 0.17 (<0.001) | 0.04 (0.602) | 0.34 (<0.001)* |
| Sleep Quality | 0.15 (0.002) | 0.02 (0.800) | 0.23 (0.001)* |
Significantly different correlation for phase-advanced versus phase-delayed volunteers.
Table 6.
Correlations of illumination mesor with sleep
| Significant Correlates | Entire Sample Pearson r (P) | Advanced subjects aMT6s < 0338 hr Pearson r (P) | Delayed subjects aMT6s > 0338 hr Pearson r (P) |
|---|---|---|---|
| Actigraphic Sleep | |||
| Sleep Latency | −0.24 (<0.001) | −0.33 (<0.001) | −0.17 (0.023) |
| Total Sleep Time | −0.12 (0.011) | −0.12 (0.116) | −0.09 (0.223) |
| Wake After Sleep Onset | −0.14 (0.004) | −0.25 (0.001) | −0.06 (0.409)* |
| Final Awakening Time | −0.17 (<0.001) | −0.13 (0.088) | −0.12 (0.103) |
| Diary Sleep | |||
| Sleep Latency | −0.24 (<0.001) | −0.31 (<0.001) | −0.16 (0.025) |
| Total Sleep Time | 0.05 (0.309) | −0.06 (0.407) | 0.18 (0.016)* |
| Naps | 0.00 (0.939) | −0.11 (0.227) | 0.08 (0.354) |
| 4-Week Recall | |||
| Trouble Falling Asleep | −0.20 (<0.001) | −0.01 (0.920) | −0.26 (<0.001)* |
| Wake Up Often in Night | −0.18 (<0.001) | −0.09 (0.218) | −0.23 (0.002) |
| Wake Up Too Early | −0.11 (0.018) | −0.07 (0.376) | −0.21 (0.004) |
| Total Sleep Time | 0.07 (0.145) | −0.10 (0.166) | 0.20 (0.005)* |
| Sleep Quality | 0.18 (<0.001) | 0.02 (0.749) | 0.26 (<0.001)* |
Significantly different correlation for phase-advanced versus phase-delayed volunteers.
AM-LIGHT and sleep
AM-LIGHT was positively correlated with 4-week recall, actigraphic, and sleep diary estimates of total sleep time; actigraphically defined final time of awakening, and 4-week recall estimates of sleep quality. AM-LIGHT was inversely correlated with actigraphic and diary estimates of sleep latency, and 4-week recall estimates of trouble falling asleep, waking up often during the night, and waking up earlier than planned.
The correlations of AM-LIGHT with better sleep were more evident among volunteers with delayed versus advanced aMT6s acrophases. For four variables, significantly larger correlations of AM-LIGHT with sleep were found for the phase delayed versus phase-advanced individuals: diary-assessed total sleep time (r = 0.38 vs r = 0.11, respectively, P = 0.004); 4-week recall estimates of trouble falling asleep (r = −0.20 vs r = 0.04, P = 0.010), sleep quality (r = 0.23 vs r = 0.02, P = 0.020), and total sleep time (r = 0.34 vs r = 0.04, P = 0.002).
Illumination mesor and sleep
Illumination mesor was significantly positively correlated with 4-week recall estimates of sleep quality. Illumination mesor was: (i) inversely correlated with 4-week recall estimates of trouble falling asleep, and actigraphic and diary estimates of sleep latency; (ii) inversely correlated with 4-week recall complaints of wakefulness during the night and actigraphically recorded wakefulness during the night; and (iii) inversely correlated with 4-week recall estimates of waking up earlier than planned.
The correlations of illumination mesor with better sleep tended to be more evident in phase-delayed versus phase advanced volunteers. For four variables, the correlation of illumination mesor with better sleep was significantly larger among phase-delayed versus phase-advanced subjects: diary-assessed total sleep time (r = 0.18 vs r = −0.06, P = 0.011); and 4-week recall estimates of trouble falling asleep (r = −0.26 vs r =−0.01, P = 0.007), sleep quality (r = 0.26 vs r = 0.02, P = 0.011), and total sleep time (r = 0.20 vs r = −0.10, P < 0.001). For one variable, the correlation of illumination mesor with better sleep was significantly less in the phase-delayed vs phase-advanced subjects: actigraphically assessed wake after sleep onset (r = −0.06 vs r = −0.25, P = 0.034).
Illumination acrophase and sleep
Illumination acrophase was significantly correlated with only one sleep variable: actigraphically defined final time of awakening (r = 0.64, P < 0.001). The association of illumination acrophase with sleep efficiency was significantly different (P < 0.001) among the phase-delayed (r = 0.17, P = 0.047) compared with the phase-advanced volunteers (r = − 0.04, P = 0.647).
Associations of illumination with aMT6s acrophase
The aMT6s acrophase was significantly associated with the light acrophase (r = 0.36, P < 0.001), but not with the illumination mesor, nor with AM-LIGHT, even after controlling for wake time.
The correlation of AM-LIGHT with aMT6s acrophase was negative for the phase-delayed subjects (i.e. the greater the AM-LIGHT, the earlier the acrophase) (r = −0.12), and positive for the phase-advanced subjects (r = 0.24); the difference in correlations was significant (P < 0.001).
The correlation of illumination mesor with aMT6s acrophase was also marginally different (P = 0.08) and opposite in direction for the phase-delayed subjects (r = −0.14; the greater the illumination the earlier the aMT6s acrophase) versus the phase-advanced subjects (r = 0.012; the greater the illumination the later the aMT6s acrophase). No difference in correlations of illumination acrophase and aMT6s acrophase was found between the phase-delayed and phase-advanced volunteers.
Window covering
Associations with illumination
Kruskal–Wallis non-parametric tests revealed significant associations of window covering with AM-LIGHT (Chi-Square = 7.89, P = 0.019) and illumination mesor (Chi-Square = 10.56, P = 0.019). Mean AM-LIGHT was 856 ± 60 lux for volunteers who had black out covering or heavy shades on their bedroom windows (n = 168); 954 ± 95 lux for volunteers with light shades or drapes (n = 184); and 1629 ± 321 lux for volunteers with uncovered windows. Illumination mesor was 546 ± 86, 586 ± 56, and 627 ± 55, for volunteers with black out covering/heavy shades, light shades/drapes, and uncovered windows, respectively. Window covering was not significantly associated with illumination acrophase. The associations of window covering with illumination did not differ significantly between the phase-delayed versus phase-advanced volunteers.
Associations with mood
Kruskall–Wallis analysis indicated a marginal association between window covering and the CESD-6 measure of depressed mood (Chi-Square = 4.6, P = 0.102). CESD-6 was 2.21 ± 0.21, 1.63 ± 0.14, and 1.63 ± 0.27 among volunteers with blacked-out or heavy shades, light shades or drapes, and uncovered windows, respectively. The association of window covering with CESD-6 was not significantly different between phase-delayed versus phase-advanced subjects.
Associations with sleep
Kruskal–Wallis tests revealed significant associations of the degree of window covering with 4-week recall reports of sleep quality (Chi-Square = 8.92, P = 0.01) and more awakening during the night (Chi-Square = 6.01, P = 0.05). However, the least complaints were associated with light shades/drapes. Overall sleep quality was rated 3.38 ± 0.12, 3.55 ± 0.07, 3.24 ± 0.08 for subjects with uncovered windows, light shades or drapes, and blacked out/heavy shades, respectively. Complaints of awakening often during the night were 3.50 ± 0.18, 3.09 ± 0.11, 3.45 ± 0.11 for subjects with uncovered windows, light shades or drapes, and blacked out/heavy shades, respectively. Post-hoc analyses revealed significant quadratic (U–Shaped) associations of window covering with awakening often (F= 3.20; P = 0.042) and overall sleep quality (F = 4.25; P = 0.015). No significant association of window covering was found in any of the other 4-week recall, actigraphic, or diary estimates of sleep. No significant association of window covering with aMT6s acrophase was found. Associations of window covering with sleep did not differ significantly between phase-delayed and phase-advanced subjects.
DISCUSSION
The analyses confirm that both morning light and 24 h light are modestly associated with better mood and sleep. Less window covering was associated with more morning light and more 24-h illumination. The beneficial effects of light were greater for phase-delayed than for phase-advanced subjects.
Morning light and 24 h illumination were inversely correlated with CESD-6 to an equivalent extent. In contrast, our group did not find that evening light was associated with better mood.29 Assessment of the associations of light with depression were limited because the shortened 6-item version of this scale (CESD-6) that was available has only modest validity.32
No relative advantage of AM-LIGHT or illumination mesor was found for sleep. Both were associated with some measures of better sleep. The negative association of illumination mesor with actigraphically assessed total sleep time, which has been reported previously,34 is possibly confounded by people spending less time in bed when their sleep durations are short. In post-hoc analyses, controlling for time-in-bed, there were no longer significant associations of illumination mesor with total sleep time.
The marginal association of less morning window covering with better mood is potentially important. If the effects could be confirmed experimentally, they might offer a simple, but valuable strategy for improving health. Whether an association of less window covering with better mood can be explained by exposure to the dawn light signal is unclear. Numerous factors influence dawn light exposure, e.g. whether one’s eyes are open, whether one’s face is buried in a pillow, weather patterns, etc. The significant correlations of window covering with AM-LIGHT and mesor illumination might reflect simply that individuals with brighter bedroom environments at dawn tend to also have brighter environments at other times-of-day. The ‘U–shaped’ association of window covering with better sleep was contrary to prediction. Perhaps light shades/ drapes provide an optimal compromise of avoiding light during the night, but allowing sufficient exposure to morning light.
Several lines of evidence indicate that AM-LIGHT and illumination mesor were more closely associated with better mood and sleep in the phase-delayed than the phase-advanced subjects. The mood data provide some support for the hypothesis that antidepressant effects of light are mediated by a circadian phase-advancing effect. Indeed, higher levels of AM-LIGHT and illumination mesor were associated with advanced aMT6s acrophases in the phase-delayed subjects, but delayed acrophases in the phase-advanced subjects. This difference is consistent with phase-group difference in aMT6s acrophase-to wake time phase angles (Fig. 1) and the phase-response curve for light depicting the direction and magnitude of phase shifts depending upon the circadian phase of light exposure. AM-LIGHT occurred during a more sensitive advance region of the light PRC in the phase-delayed subjects (approximately 2–6 h after the aMT6 acrophase) compared with the phase-advanced subjects (approximately 4–8 h after the aMT6s acrophase).
Therapeutic effects of phase-advances might be limited to those with delayed circadian rhythms. Most data suggestive of particular beneficial effects of morning light have been observed in patients with seasonal affective disorder, a disorder that is associated with a delayed circadian system. It is plausible, conversely, that phase-delaying effects of evening light might promote optimal mood in people with advanced body clocks. Of course, it is known that morning and evening light have better sleep-promoting efficacy in people with delayed and advanced sleep–wake syndromes, respectively.
Notwithstanding the phase-group results in the present study, it is noteworthy that the association of illumination acrophase with aMT6s acrophase was quite weak (albeit significant) for both phase-groups. Likewise, numerous studies have indicated that while light has antidepressant, sleep-promoting, and phase-shifting effects, these effects are not necessarily correlated.35–37 Other hypotheses to explain therapeutic effects of light are that light promotes circadian phase stability or feelings of alertness/energy.22,23
A noteworthy finding was that the phase-delayed group was significantly older than the phase-advanced group. Whereas several studies have shown an age-associated advance in circadian timing,38 others have shown no significant age-group difference.39 Other work has indicated that in the restricted range over age 55 years, aging is associated with a delay in circadian phase.40
The age difference of phase-delayed versus phase-advanced subjects led us to hypothesize that other differences between the phase groupings might be confounded by age. However, in post-hoc analyses comparing subjects who were above or below the median age of the sample, none of the significant effects of group-phase were confirmed.
In summary, the results indicate significant, but modest, associations of AM-LIGHT and illumination mesor with better mood and sleep. These effects were consistently greater for subjects who were relatively phase-delayed versus phase-advanced. Having a lightly covered or uncovered bedroom window can increase morning light exposure, perhaps resulting in better sleep and mood.
Acknowledgments
Research supported by HL55983, HL61280, and AG15763. Joseph D. Assmus, Mary Anne Mowen, and Katharine M. Rex assisted with this study.
References
- 1.Youngstedt SD, Kripke DF, Elliott JA, Baehr EK, Sepulveda RS. Light exposure, sleep quality, and depression in older adults. In: Holick MF, Jung EG, editors. Biologic Effects of Light 1998. Boston: Kluwer Academic Publishers; 1999. pp. 427–35. [Google Scholar]
- 2.Beauchemin KM, Hays P. Sunny hospital rooms expedite recovery from severe and refractory depressions. J Affect Disord. 1996;40:49–51. doi: 10.1016/0165-0327(96)00040-7. [DOI] [PubMed] [Google Scholar]
- 3.Espiritu RC, Kripke DF, Ancoli-Israel S, et al. Low illumination by San Diego adults: association with atypical depressive symptoms. Biol Psychiat. 1994;35:403–7. doi: 10.1016/0006-3223(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 4.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41:829–36. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 5.Kripke DF. Light treatment for nonseasonal depression: speed, efficacy, and combined treatment. J Affect Dis. 1998;49:109–17. doi: 10.1016/s0165-0327(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 6.Lam RW. Seasonal affective disorder and beyond: A commentary. In: Lam RW, editor. Seasonal Affective Disorder and Beyond. Washington: American Psychiatric Press; 1998. pp. 305–22. [Google Scholar]
- 7.Terman M, Terman JS, Quitkin FM, McGrath PJ, Stewart JW, Rafferty B. Light therapy for seasonal affective disorder: a review of efficacy. Neuropsychopharmacol. 1989;2:1–22. doi: 10.1016/0893-133x(89)90002-x. [DOI] [PubMed] [Google Scholar]
- 8.Lewy AJ, Bauer VK, Cutler NL, et al. Morning vs evening light treatment of patients with winter depression. Arch General Psychiat. 1998;55:890–6. doi: 10.1001/archpsyc.55.10.890. [DOI] [PubMed] [Google Scholar]
- 9.Eastman CI, Young MA, Fogg LF, Liu L, Meaden PM. Bright light treatment of winter depression. Arch General Psychiat. 1998;55:883–9. doi: 10.1001/archpsyc.55.10.883. [DOI] [PubMed] [Google Scholar]
- 10.Benkelfat C, Seletti B, Palmour RM, Hillel J, Ellenbogen M, Young SN. Tryptophan depletion in stable lithium-treated patients with bipolar disorder in remission. Arch General Psychiat. 1995;52:154–6. doi: 10.1001/archpsyc.1995.03950140072010. [DOI] [PubMed] [Google Scholar]
- 11.Terman M, Terman JS, Ross DC. A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch General Psychiat. 1998;55:875–82. doi: 10.1001/archpsyc.55.10.875. [DOI] [PubMed] [Google Scholar]
- 12.Terman M, Schlager DS, Fairhurst S, Perlman B. Dawn and dusk simulation as a therapeutic intervention. Biol Psychiat. 1989;25:966–70. doi: 10.1016/0006-3223(89)90276-x. [DOI] [PubMed] [Google Scholar]
- 13.Avery DH, Bolte MAP, Wolfson JK, Kazaras AL. Dawn simulation compared with a dim red signal in the treatment of winter depression. Biol Psychiat. 1994;36:181–8. doi: 10.1016/0006-3223(94)91223-8. [DOI] [PubMed] [Google Scholar]
- 14.Avery DH, Eder DN, Bolte MA, et al. Dawn simulation and bright light in the treatment of SAD. A controlled study. Biol Psychiat. 2001;50:205–16. doi: 10.1016/s0006-3223(01)01200-8. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi R, Kohsaka M, Fukuda N, Sakakibara S, Honma H, Koyama T. Effects of morning bright light on sleep in healthy elderly women. Psychiat Clin Neurosci. 1900;53:237–8. doi: 10.1046/j.1440-1819.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 16.Kohsaka M, Fukuda N, Kobayashi R, et al. Effect of short duration morning bright light in elderly men: sleep structure. Psychiat Clin Neurosci. 2000;54:367–8. doi: 10.1046/j.1440-1819.2000.00718.x. [DOI] [PubMed] [Google Scholar]
- 17.Terman JS, Terman M, Lo ES, Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Arch General Psychiat. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 18.Youngstedt SD, Kripke DF, Elliott JA, Klauber MR. Circadian abnormalities in older adults. J Pineal Res. 2001;31:264–72. doi: 10.1034/j.1600-079x.2001.310311.x. [DOI] [PubMed] [Google Scholar]
- 19.Antle MC, Ogilvie MD, Pickard GE, Mistlberger RE. Response of the mouse circadian system to serotonin 1A/ 2/7 agonists in vivo: surprisingly little. J Biol Rhythms. 2003;18:145–58. doi: 10.1177/0748730403251805. [DOI] [PubMed] [Google Scholar]
- 20.Harada T, Matsumura A, Takeuchi H. Effects of the usage of a blacked-out curtain on the sleep-wake rhythm of Japanese University students. Sleep Biol Rhythms. 2003;1:179–81. [Google Scholar]
- 21.Tuunainen A, Kripke DF, Cress AC, Youngstedt SD. Retinal circadian rhythms in humans. Chronobiol Int. 2001;18:957–71. doi: 10.1081/cbi-100107971. [DOI] [PubMed] [Google Scholar]
- 22.Campbell SS, Dawson D. Enhancement of nighttime alertness and performance with bright ambient light. Physiol Behav. 1990;48:317–20. doi: 10.1016/0031-9384(90)90320-4. [DOI] [PubMed] [Google Scholar]
- 23.Clodore M, Foret J, Benoit O, et al. Psychophysiological effects of early morning bright light exposure in young adults. Psychoneuroendocrinol. 1990;15:193–205. doi: 10.1016/0306-4530(90)90030-d. [DOI] [PubMed] [Google Scholar]
- 24.Kripke DF, Juarez S, Cole RJ, et al. Adult illumination exposures and some correlations with symptoms. In: Hiroshige T, Honma K, editors. Evolution of Circadian Clock. Sapporo: Hokkaido University Press; 1994. pp. 349–60. [Google Scholar]
- 25.Kripke DF, Youngstedt SD. Illumination Levels in Wake and Sleep. In: Holick MF, Jung EG, editors. Biologic Effects of Light 1996. Berlin: Walter de Gruyter; 1995. pp. 332–9. [Google Scholar]
- 26.Matthews KA, Shumaker SA, Bowen DJ, et al. Women’s Health Initiative: Why now? What is it? What’s new? Am Psych. 1997;52:101–16. doi: 10.1037//0003-066x.52.2.101. [DOI] [PubMed] [Google Scholar]
- 27.Kripke DF, Elliott JA, Youngstedt SD, Smith JS. Melatonin: Marvel or marker? Ann Med. 1998;30:81–7. doi: 10.3109/07853899808999388. [DOI] [PubMed] [Google Scholar]
- 28.Tuunainen A, Kripke DF, Elliott JA, et al. Depression and endogenous melatonin in postmenopausal women. J Affect Dis. 2002;69:149–58. doi: 10.1016/s0165-0327(01)00303-2. [DOI] [PubMed] [Google Scholar]
- 29.Wallace-Guy GM, Kripke DF, Jean-Louis G, Langer RD, Elliott JA, Tuunainen A. Evening light exposure: Implications for sleep and depression. J Am Geriatr Soc. 2002;50:738–9. doi: 10.1046/j.1532-5415.2002.50171.x. [DOI] [PubMed] [Google Scholar]
- 30.Kripke DF, Brunner R, Freeman R, et al. Sleep complaints of postmenopausal women. Clin J Women’s Health. 2001;1:244–52. doi: 10.1053/cjwh.2001.30491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jean-Louis G, Kripke DF, Cole RJ, Assmus JD, Langer RD. Sleep detection with an accelerometer actigraph: comparisons with polysomnography. Physiol Behav. 2001;72:21–8. doi: 10.1016/s0031-9384(00)00355-3. [DOI] [PubMed] [Google Scholar]
- 32.Tuunainen A, Langer RD, Klauber MR, Kripke DF. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiat Res. 2001;103:261–70. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 34.Jean-Louis G, Kripke DF, Mason WJ, Ancoli-Israel S. Relationships among illumination, activity, and sleep patterns. In: Holick MF, Jung EG, editors. Biological Effects of Light 1998. Boston: Kluwer Academic Publishers; 1999. pp. 37–9. [Google Scholar]
- 35.Eastman CI, Gallo LC, Lahmeyer HW, Fogg LF. The circadian rhythm of temperature during light treatment for winter depression. Biol Psychiat. 1993;34:210–20. doi: 10.1016/0006-3223(93)90074-n. [DOI] [PubMed] [Google Scholar]
- 36.Thalen BE, Kjellman BF, Morkrid L, Wibom R, Wetter-berg L. Light treatment in seasonal and nonseasonal depression. Acta Psychiat Scand. 1995;91:352. doi: 10.1111/j.1600-0447.1995.tb09794.x. [DOI] [PubMed] [Google Scholar]
- 37.Yamada N, Martin-Iverson MT, Daimon K, Tsujimoto T, Takahashi S. Clinical and chronobiological effects of light therapy on nonseasonal affective disorders. Biol Psychiat. 1995;37:866–73. doi: 10.1016/0006-3223(94)00221-N. [DOI] [PubMed] [Google Scholar]
- 38.Duffy JF, Dijk D-J, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–87. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 39.Carrier J, Monk TH, Reynolds CF, Buysse DJ, Kupfer DJ. Are age differences in sleep due to phase differences in the output of the circadian timing system? Chronobiol Int. 1999;16:79–91. doi: 10.3109/07420529908998714. [DOI] [PubMed] [Google Scholar]
- 40.Lushington K, Dawson D, Kennaway DJ, Lack L. The relationship between 6-sulphatoxymelatonin rhythm phase and age in self-reported good sleeping controls and sleep maintenance insomniacs aged 55–80 years. Psychopharmacol. 1999;147:111–2. doi: 10.1007/s002130051150. [DOI] [PubMed] [Google Scholar]