Abstract
Epidemiologic studies have consistently shown an association of long sleep (≥8 hr) with mortality and multiple morbidities. However, there has been little experimental investigation of the effects of sleep extension. The aim of this study was to examine the effects of time in bed (TIB) extension, on depression, anxiety, sleepiness, and systemic inflammation.
Following baseline, 14 healthy sleepers (31.79±10.94 years) were randomized to one of two one-week treatments: (1) a TIB extension treatment involving a fixed sleep schedule in which TIB was increased by 3 hours/night compared with the participants’ median baseline TIB; (2) a control treatment involving a fixed schedule in which TIB was the same as the participants’ median baseline TIB. Actigraphic recording of sleep was assessed throughout both weeks. Self-reported depression, state anxiety, sleepiness, and sleep quality, as well as blood pressure and inflammation, were assessed at baseline and following the treatment week.
Compared with baseline, TIB increased by 127.12±3.92 min and total sleep time increased by 119.88±18.52 min during TIB extension, but decreased slightly in the control treatment.
Depression was elevated more following TIB extension (effect size (ES)=−0.86) vs. control (ES=−0.50). Interleukin-6 levels increased by 2-fold following TIB extension (ES=−0.65), but did not change following the control treatment. Sleepiness increased after TIB extension, but decreased after the control treatment.
The results revealed negative effects of TIB extension on mood and inflammation. Larger-scale studies involving more prolonged, but less profound sleep extension, are warranted.
Keywords: Sleep extension, Inflammation, Depression, Healthy adults
INTRODUCTION
Epidemiologic studies have consistently shown a “U-shaped” association of sleep duration with mortality, with progressively increased mortality associated with short (< 6 h) and long sleep (≥ 8 h) [1–6]. Associations have also been found between short and long sleep duration and multiple morbidities, including depression [7], cognitive impairment [8–11], cardiovascular disease [12], diabetes [13], stroke [14], obesity [15,16], and inflammation [17].
However, such evidence does not establish causality, for which experimental studies are needed. Whereas there have been dozens of experimental studies of the potential risks of short sleep or sleep restriction [18–22], the potential risks of sleep extension or time-in-bed (TIB) extension have been largely ignored in experimental studies, and risks of long sleep suggested in epidemiologic studies have often been discounted as artifacts.
Nonetheless, studies of even 2–5 days of bed rest have revealed extremely hazardous effects [23,24]. Moreover, classic sleep extension studies have noted impairments in mood, performance and sleepiness [25,26]. However, many of these studies have failed to include measures of health or objective measures of sleep. Further experimental studies of more moderate TIB extension are needed to address potential risks of sleeping ≥ 8 hours.
There are many plausible mechanisms by which long sleep or long TIB could be harmful. Extending TIB would involve 1–2 hours of extra sedentary behavior, which has shown to be hazardous even at this “dose” when quantified in other ways (e.g., sitting) [27]. Moreover, long sleepers have been shown to have reduced daytime levels of physical activity [28], which could be attributed to the common phenomenon of feeling lethargic after long sleep duration, as well as to having less time available in the day. Another potential risk of prolonged TIB/TST is that it typically leads to sleep fragmentation, which has been shown to have negative health effects in epidemiologic studies [29] and in studies involving experimental induction of sleep fragmentation [30]. The aims of this pilot study were to examine the effects of TIB extension on depression, anxiety, sleepiness, and inflammation in young, healthy, normal sleeping adults.
MATERIALS AND METHODS
Participants
Fourteen healthy adults (10 females, 31.79±10.94 years) were assessed. Seven additional participants were excluded: three were excluded due to noncompliance (e.g, failure to arrive at appointments); two dropped out (one due to scheduling conflicts; one due to unwillingness to complete the TIB extension); one participant was enrolled in another research study that conflicted with the present study; and one participant was severely distraught after a traumatic life event. Participants were recruited via word-of-mouth, flyers, and advertisements. Following a brief phone screen, prospective participants met with staff in the laboratory for further study orientation, signing of informed consent approved by the University of South Carolina Institutional Review Board, and further screening.
A concerted effort was made to avoid expectancy or demand biases. Participants were informed on the consent form and verbally that there could be positive effects (e.g. feeling more rested), negative effects (e.g. feeling lethargic), or no effects of sleep extension. Moreover, the control treatment was presented as an active treatment to examine the effects of maintaining a stable sleep-wake schedule, for which participants were informed that there could be positive effects (e.g. better quality of sleep), negative effects (e.g., difficulty following a fixed schedule), or no effects.
Inclusion criteria included being between 18–55 years of age and having average self-reported sleep duration between 6–9 hours with no sleep complaints. Exclusion criteria included engaging in shift-work schedules; significant health problems; and conditions and medication use which could influence measures of inflammation, including smoking, obesity (body mass index, BMI >30 kg/m2), rheumatoid arthritis, and nonsteroidal anti-inflammatory drugs.
Baseline
During a 1-week baseline, participants followed their usual sleep-wake schedules. The baseline was used to assess pretreatment measures and also served as a final screen to establish that the participants had normal sleep.
Experimental treatments
Following baseline, participants were randomly assigned to one of two 1-week experimental treatments: (1) TIB extension (n=8) or (2) a usual sleep control treatment (n=6). During both conditions, participants were instructed to refrain from altering their usual diet or physical activity habits.
TIB Extension
For the TIB extension treatment, participants followed a fixed sleep schedule, in which their TIB was three hours longer per night than their median baseline TIB. Participants were told to stay in bed for the additional three hours without any distractions (i.e., phone, television) and to attempt to sleep during this time. According to personal schedules and preferences, the TIB extension was accomplished by advancing bedtime, delaying arise time, or some combination of both.
Usual sleep control
For the usual sleep control treatment, participants followed a fixed sleep schedule, in which their TIB was the same as their median baseline TIB. As with the TIB extension treatment, participants were assigned fixed bed and arise times and were instructed to maintain this schedule throughout the week.
Measures
Sleep
Throughout baseline and the 1-week intervention, sleep was assessed continuously via a wrist actigraphic monitor (Philips Respironics Actiwatch Spectrum), which included light channels which facilitated determination of lights out and time of arising. The actigraphic monitor was supplemented with a daily sleep diary. The following variables were averaged for the baseline week and the treatment week: time-in-bed, total sleep time, sleep efficiency (time spent asleep within the TIB interval), and sleep fragmentation index, (the sum of the percentage of mobile bouts and percentage of immobile bouts less than 1-minute to the number of immobile bouts).
Subjective Sleep Measures
Following baseline and the treatment week, the following self-reported sleep measures were assessed: sleepiness (Epworth Sleepiness Scale, ESS) [31], sleep quality (Pittsburgh Sleep Quality Index, PSQI, global score) [32], and the short version of the Functional Outcomes of Sleepiness Questionnaire (FOSQ-10) [33]. Higher scores on the ESS and the PSQI represent impairment, whereas lower scores on the FOSQ-10 represent more impaired daytime functioning.
Physical Activity
A pedometer was worn continuously on the waist to estimate physical activity. Mean pedometer activity counts in the form of “steps” were assessed each week.
Self-Reported Depression and Anxiety
Following baseline and the treatment week, depression was assessed with the Beck Depression Inventory II (BDI) [34], and state anxiety was assessed with the Spielberger State-Trait Anxiety Inventory, Form Y (STAI) [35].
Blood Pressure, Heart Rate, and Inflammation
At the end of baseline and the end of the treatment week, subjects refrained from exercise for 24 hours and underwent a 12-hr fast, prior to reporting to the laboratory in the morning. Following a 10-minute seated resting period, resting heart rate and blood pressure were assessed [36]. Participants reported any medications taken during each week.
A 20-gauge polyethylene catheter was placed in an antecubital vein to withdraw 7 ml of blood from each research participant. Immediately, blood was centrifuged at 3000 RPM for 15 minutes and plasma was collected and stored at −20 C°. Plasma IL-6, C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and adiponectin levels were measured using Enzyme Linked Immunosorbent Assays (ELISA). Standard procedure was followed for protein measurement by ELISA according to manufacturer recommendations (BD OptEIA kit, BD BIOSCIENCES). The processing of the assays was completed in a blinded fashion.
Analyses
Data were compared between treatments with ANCOVA of end of study values with covariate control for baseline levels. However, since this was a pilot study with a small number of subjects, Cohen’s effect sizes (d) were also calculated (i.e., mean final value-mean baseline value, divided by the pooled standard deviation). According to convention, small, moderate, and large effect sizes were defined as d= .2, .5, and .8, respectively.
RESULTS AND DISCUSSION
Results
The treatment effects for TIB and TST were statistically significant, but none of the other ANCOVA results were statistically significant. Descriptive data and Cohen’s d effects sizes for all variables are displayed in Table 1.
Table 1.
Baseline and control week means, standard deviations (±), and Cohen’s d effect sizes (ES) for each variable in the control group.
| Control | Experimental | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Normal | ES | Baseline | Extension | ES | ||
| Sleep | |||||||
| TIB | 8:03±0:44 | 7:36±0:34 | 0.69 | 7:56±1:02 | 10:03±0:58 | −2.11 | |
| TST | 6:57±0:21 | 6:41±0:13 | 0.91 | 6:47±0:35 | 8:47±0:54 | −2.65 | |
| Fragmentation | 38.54±11.11 | 37.88±9.79 | 0.06 | 29.59±11.74 | 34.76±5.73 | −0.56 | |
| Efficiency | 85.80±3.41 | 86.59±3.00 | 0.25 | 86.36±5.24 | 87.59±3.71 | −0.27 | |
| PSQI | 3.00±1.90 | 3.67±1.75 | −0.37 | 3.88±0.99 | 4.75±1.75 | −0.61 | |
| Sleepiness | |||||||
| ESS | 4.67±2.73 | 3.83±3.37 | 0.27 | 4.50±3.42 | 5.13±3.64 | −0.18 | |
| FOSQ | 17.89±2.61 | 18.15±1.63 | −0.12 | 17.25±2.21 | 17.00±2.12 | 0.12 | |
| Mood | |||||||
| BDI | 4.83±6.31 | 6.00±9.23 | −0.15 | 2.38±1.77 | 5.25±4.37 | −0.86 | |
| STAI | 35.17±14.03 | 33.00±12.93 | 0.16 | 31.25±7.42 | 33.50±9.68 | −0.26 | |
| Inflammation | |||||||
| IL-6 (pg/mL) | 2.12±1.79 | 2.21±1.63 | −0.05 | 0.63±1.08 | 1.47±1.47 | −0.65 | |
| TNF-a (pg/mL) | 0.69±1.67 | 0.96±1.33 | −0.18 | 0.33±0.75 | 0.41±0.95 | −0.09 | |
| CRP (ng/mL) | 78.33±9.00 | 78.09±13.55 | 0.02 | 63.01±23.48 | 64.03±22.47 | −0.04 | |
| Adiponectin (pg/mL) | 1580.95±689.79 | 1594.15±668.65 | −0.02 | 1640.95±913.46 | 1719.71±1046.84 | −0.08 | |
| BP/HR | |||||||
| Systolic | 115.56±11.52 | 112.90±7.05 | 0.28 | 112.19±9.28 | 112.88±10.84 | −0.07 | |
| Diastolic | 70.28±8.13 | 73.00±8.42 | −0.33 | 71.56±8.62 | 71.42±8.51 | 0.02 | |
| HR | 78.11±9.93 | 81.63±7.09 | −0.41 | 75.38±16.62 | 72.58±12.75 | 0.19 | |
| Activity | |||||||
| Steps | 5662.37±1625.11 | 6246.17±1583.66 | −0.36 | 6442.39±1772.44 | 7413.02±2281.76 | −0.48 | |
Abbreviations: BDI: Beck Depression Inventory II; STAI: State-Trait Anxiety Inventory; BP: Blood Pressure; CRP: C-Reactive Protein; ES: Effect Size; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcomes Of Sleep Questionnaire; HR: Heart Rate; IL-6: Interleukin 6; PSQI: Pittsburgh Sleep Quality Index; FOSQ: Functional Outcomes Of Sleepiness Questionnaire; TIB: Time In Bed; TNF-Α: Tumor Necrosis Factor-Alpha, TST: Total Sleep Time
Sleep
Actigraphic TIB increased from 7:56±1:02 to 10:03±0:58 in the sleep extension treatment (ES=−2.11), but decreased moderately in the control treatment (8:03±0:44 to 7:36±0:34, ES=0.69), F(2,11)=71.825, p<.0001. An increase in actigraphic total sleep duration (TST) was found in the TIB extension treatment [(from 6:47±0:35 to 8:47±0:54 (ES=−2.66)], but TST decreased from 6:57±0:21 to 6:41±0:13 in the control treatment (ES=0.91), F(2,11)=27.75, p<.0001.
The sleep fragmentation index moderately increased from 29.59±11.74 to 34.76±5.73 following the TIB extension (ES=−0.56), and showed no change following the control treatment (38.54±11.11 to 37.88±9.79, ES=0.06). Sleep efficiency slightly increased following TIB extension treatment (from 86.36±5.24 to 87.59±3.71) (ES=−0.27), as well following the control treatment (85.80±3.41 to 86.59±3.00, ES=0.25).
Subjective sleep measures
ESS scores increased from 4.50±3.42 to 5.13±3.64 following sleep extension (ES=−0.18), but decreased from 4.67±2.73 to 3.83±3.37 following the control treatment (ES=0.27). The FOSQ-10 scores did not change following TIB extension (17.25±2.21 to 17.00±2.12, ES=0.12) or the control treatment (17.89±2.61 to 18.15±1.63, ES=−0.12).
The PSQI global scale increased (indicating worse sleep) from 3.88±.99 to 4.75±1.75 (ES=−0.61) following TIB extension, and also increased from 3.00±1.90 to 3.67±1.75 (ES=−0.37) following the control treatment. These effect sizes differed by over 1.5-fold.
Activity
Increases in the number of average weekly steps were observed following sleep extension (6442.39±1772.44 to 7413.02±2281.76, ES=−0.48), and following the control treatment (5662.37±1625.11 to 6246.17±1583.66, ES=−.36).
Self-reported depression and anxiety
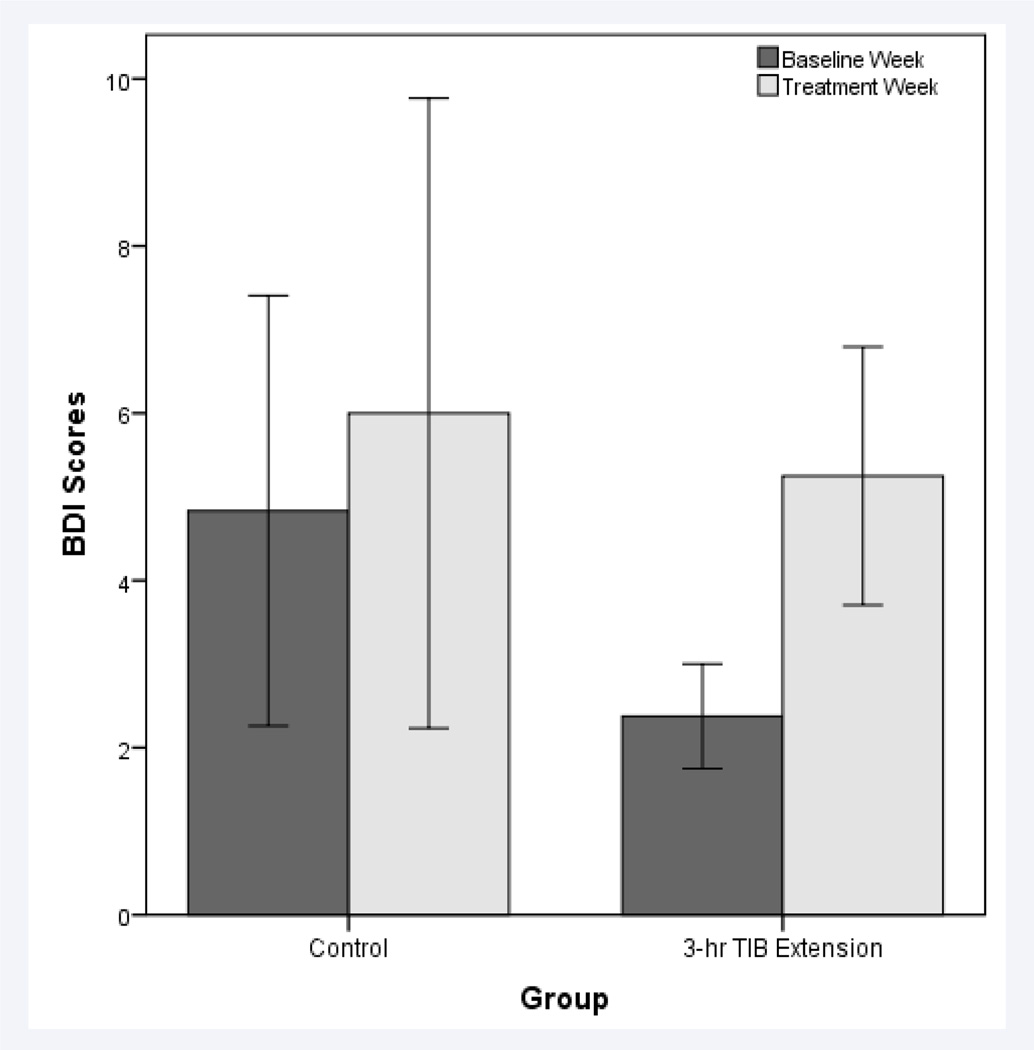
Mean BDI scores increased from 2.38±1.77 to 5.25±4.37 (ES=−0.86) following TIB extension, but less so following the control (from 4.83±6.31 to 6.00±9.23, ES=−0.15). These effect sizes differed by over 5-fold (Figure 1).
Figure 1.
Mean (±SE) Beck Depression Inventory (BDI-II) scores following the baseline week and treatment week for the control and TIB extension treatments.
State anxiety increased slightly after TIB extension (31.25±7.42 to 33.50±9.68, ES=−0.26), but decreased slightly in the control treatment (35.17±14.03 to 33.00±12.93, ES=0.16). These effect sizes differed by over 1.5 fold.
Blood pressure, heart rate, and inflammation
Systolic blood pressure did not change following TIB extension (112.19±9.28 to 112.88±10.84, ES=−0.07), although a small decrease was observed following the control treatment (115.56±11.52 to 112.90±7.05, ES=0.28). Diastolic blood pressure did not change following TIB extension (71.56±8.62 to 71.42±8.51, ES=0.02), but a small increase was observed following the control treatment (70.28±8.13 to 73.00±8.42, ES=−0.33). Heart rate showed no changes following TIB extension (75.38±16.62 to 72.58±12.75, ES=0.19), while a small increase was observed following the control treatment (78.11±9.93 to 81.63±7.09, ES=−0.41).
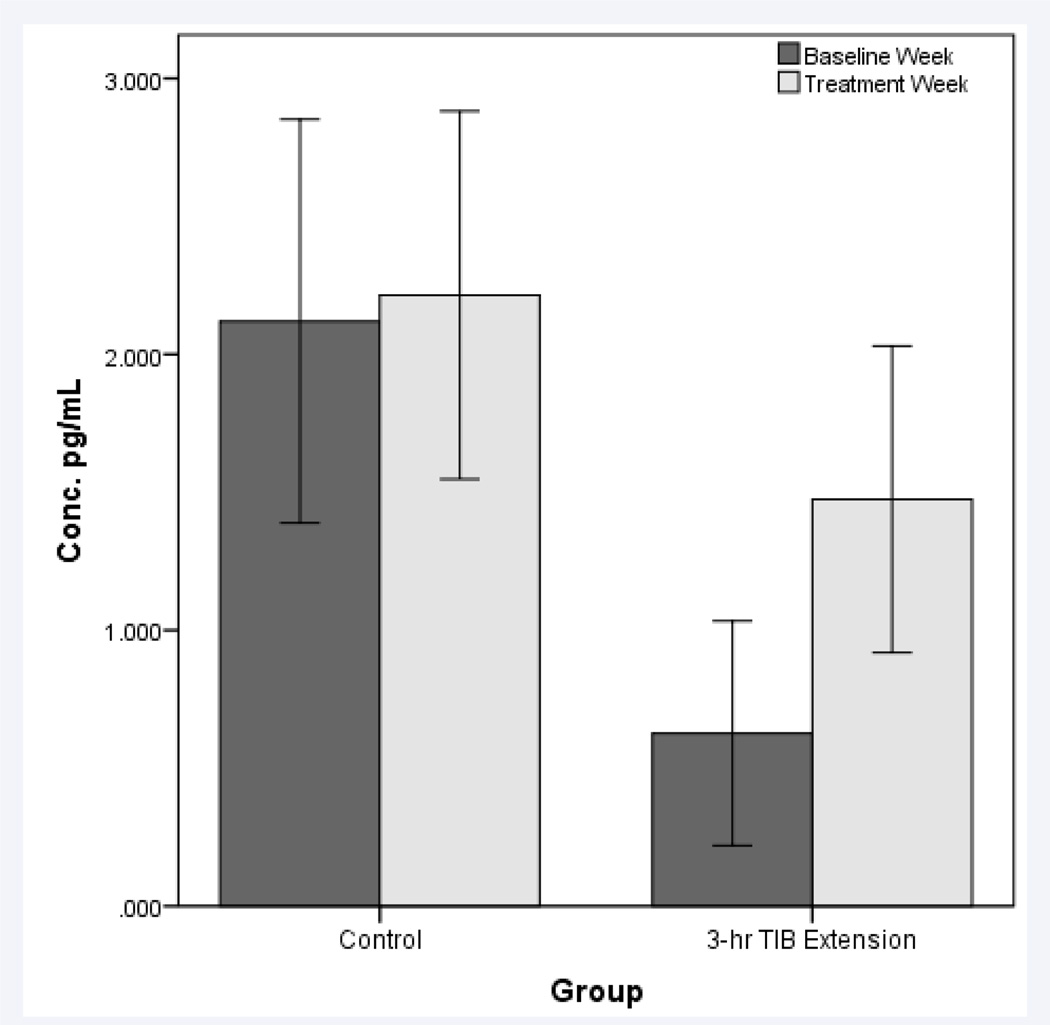
Two participants randomized to the TIB extension condition were not included in the inflammation analyses due to inability to obtain blood samples from these participants. IL-6 levels demonstrated a 2-fold increase in the TIB extension group (N=6, 0.626±1.08 pg/mL to 1.47±1.47, ES=−0.65), but did not change following the control treatment. (N=6, 2.12±1.79 to 2.21±1.63, ES=−0.05). These effect sizes differed by approximately 5-fold (Figure 2). No changes in TNF-α, adiponectin, or CRP were observed following the TIB extension treatment or the control treatment.
Figure 2.
Mean (±SE) interleukin-6 (IL-6) values following the baseline week and treatment week for the control and TIB extension treatments.
DISCUSSION
Except for TIB and TST, none of the treatment effects were statistically significant for this small pilot study. Nonetheless, effect sizes revealed apparent detrimental effects of sleep extension compared with the control condition for depression, anxiety, and systemic inflammation.
Adherence to the protocol was marginal. Although participants in the TIB extension treatment were asked to increase their TIB by 3 hours, mean TIB extension was only 2h 7 min, and only one participant increased the TIB by ≥3h. Nonetheless, it is noteworthy that the 2h 7min TIB extension resulted in a TST increase of 1h 59min, which suggests feasibility of increasing TST by extending TIB.
That the increase in TST was nearly the same as the increase in TIB and sleep efficiency actually increased in the TIB extension treatment were surprising findings, as we expected that the extra TIB would lead to considerable sleep interruption. These data provide some insight into competing narratives about sleep extension. Some scientists have argued that it is impossible to acquire too much sleep; that an increase in duration and efficiency of sleep when given the opportunity must reflect a chronic sleep debt; and that obtaining extra sleep will have positive consequences. On the other hand, epidemiologic and experimental sleep extension trials have suggested that it is indeed possible to obtain too much sleep, analogous to harmful effects of excessive amounts of other healthy behaviors, such as exercise, sunlight exposure, and water intake. The increased level of physical activity following sleep extension was contrary to the hypothesis that prolonged sleep might elicit reductions in physical activity.
The similar increases in TIB and TST in this study make it difficult to draw conclusions regarding the extent to which negative effects could be attributed to TIB or TST. Future larger scale studies, perhaps including a treatment involving extending TIB while disallowing increases in TST, could better address this issue.
The increases in depression and anxiety following sleep extension compared with the control sleep treatment are consistent with other research [25], as well as anecdotal accounts of negative affect associated with sleep extension on weekends and holidays [37]. Anecdotally, participants had far more physical complaints (e.g., aches and pains) following TIB extension, though physical complaints were not objectively recorded.
The increase in IL-6 following sleep extension is consistent with evidence of greater inflammation in long sleepers [17], and experimental evidence of increased inflammation following bed rest [38]. However, neither CRP, TNF-α, nor adiponectin were altered appreciably by sleep extension compared with the control treatment.
The present study provided a unique experimental examination of multiple variables which have been associated with long sleep duration in epidemiologic studies. Effect sizes revealed several negative responses to TIB extension, including increased levels of systemic inflammation and self-reported increased depressive symptoms, increased sleepiness, and worsened sleep quality.
The results did not seem to be confounded by demand or expectancy biases. Anecdotally, most participants seemed enthusiastic about the prospect of sleeping longer for the study. Moreover, participants were given instructions designed to minimize these potential biases.
However, the study had several limitations. First, there were a small number of participants.
Second, the results from this study might not generalize to chronic and/or modest sleep extension. Three hours of TIB extension was apparently too difficult for the participants, as the average TIB extension was 127 min. Most of the participants were employed full-time and many had young children, which contributed to adherence difficulties. As in other areas of scientific inquiry (e.g., sleep deprivation), relatively extreme interventions are a first step in exploring potential effects.
Third, the study was restricted to young, healthy, and normal sleepers. It is unclear whether the results could generalize to children or individuals with disturbed sleep or other morbidities.
Fourth, the extreme degree of TIB extension was anecdotally more difficult for participants with inflexible habitual wake times, and these participants were able to extend their TIB only by advancing their bedtimes. Future studies of sleep extension in older adults may be more feasible, when many individuals have more flexibility in their sleep schedules.
Fifth, the findings of greater increases in depression and IL-6 following TIB extension were likely influenced by lower baseline levels in these variables for the TIB treatment (compared with the control). Increasing the sample size and/or stratified randomization based on baseline levels could help prevent this problem in future studies.
CONCLUSION
This was a comprehensive controlled trial of the effects of experimental TIB extension. Effect sizes revealed apparent detrimental effects of sleep extension compared with the control treatment for depression, anxiety, sleep factors, and systemic inflammation. Future studies should include larger sample sizes, and more chronic and less severe sleep extension. Conversely, further studies are need to explore the effects of chronic moderate sleep restriction in long sleepers [39–41].
ACKNOWLEDGEMENTS
This work is funded by NIH HL095799 (Shawn D. Youngstedt) and the Laura Griffin Award (University of South Carolina Psychology Department) awarded to Alexandria M. Reynolds.
ABBREVIATIONS
- BDI
Beck Depression Inventory II
- BMI
Body Mass Index
- STAI
State-Trait Anxiety Inventory
- BP
Blood Pressure
- CRP
C-Reactive Protein
- ELISA
Enzyme Linked Immunosorbent Assay
- ES
Effect Size
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- HR
Heart Rate
- IL-6
Interleukin 6
- PSQI
Pittsburgh Sleep Quality Index
- FOSQ
Functional Outcomes of Sleepiness Questionnaire
- TIB
Time in Bed
- TNF-Α
Tumor Necrosis Factor-Alpha
- TST
Total Sleep Time
REFERENCES
- 1.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 2.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 3.Amagai Y, Ishikawa S, Gotoh T, Doi Y, Kayaba K, Nakamura Y, et al. Sleep duration and mortality in Japan: the Jichi Medical School Cohort Study. J Epidemiol. 2004;14:124–128. doi: 10.2188/jea.14.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lan TY, Lan TH, Wen CP, Lin YH, Chuang YL. Nighttime sleep, Chinese afternoon nap, and mortality in the elderly. 2007;30:1105–1110. doi: 10.1093/sleep/30.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamakoshi A, Ohno Y JACC Study Group. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27:51–54. [PubMed] [Google Scholar]
- 6.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–1253. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimäki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–573. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–446. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav Sleep Med. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Jiang CQ, Lam TH, Liu B, Jin YL, Zhu T, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34:575–580. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 13.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi A, Giles WH, Croft JB, Bliwise DL. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurology. 1997;48:904–911. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- 15.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jean-Louis G, Williams NJ, Sarpong D, Pandey A, Youngstedt S, Zizi F, et al. Associations between inadequate sleep and obesity in the US adult population: analysis of the national health interview survey (1977–2009) BMC Public Health. 2014;14:290. doi: 10.1186/1471-2458-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–204. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riedel BW, Lichstein KL, Dwyer WO. Sleep compression and sleep education for older insomniacs: self-help versus therapist guidance. Psychol Aging. 1995;10:54–63. doi: 10.1037//0882-7974.10.1.54. [DOI] [PubMed] [Google Scholar]
- 19.Hoch CC, Reynolds CF, 3rd, Buysse DJ, Monk TH, Nowell P, Begley AE, et al. Protecting sleep quality in later life: a pilot study of bed restriction and sleep hygiene. J Gerontol B Psychol Sci Soc Sci. 2001;56:P52–P59. doi: 10.1093/geronb/56.1.p52. [DOI] [PubMed] [Google Scholar]
- 20.Youngstedt SD, Kline CE, Zielinski MR, Kripke DF, Devlin TM, Bogan RK, et al. Tolerance of chronic 90-minute time-in-bed restriction in older long sleepers. Sleep. 2009;32:1467–1479. doi: 10.1093/sleep/32.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds CF, 3rd, Serody L, Okun ML, Hall M, Houck PR, Patrick S, Maurer J. Protecting sleep, promoting health in later life: a randomized clinical trial. Psychosom Med. 2010;72:178–186. doi: 10.1097/PSY.0b013e3181c870a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider SM, Robergs RA, Amorim FT, de Serna DG, Duran-Valdez EE, Schade DS. Impaired orthostatic response in patients with type 2 diabetes mellitus after 48 hours of bed rest. Endocr Pract. 2009;15:104–110. doi: 10.4158/EP.15.2.104. [DOI] [PubMed] [Google Scholar]
- 24.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, et al. Physical Inactivity Rapidly Induces Insulin Resistance and Microvascular Dysfunction in Healthy Volunteers. Arterioscler Thromb Vasc Biol. 2007;27:2650–2656. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taub JM, Berger RJ. Performance and mood following variations in the length and timing of sleep. Psychophysiology. 1973;10:559–570. doi: 10.1111/j.1469-8986.1973.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 26.Taub JM. Effects of ad lib extended-delayed sleep on sensorimotor performance, memory and sleepiness in the young adult. Physiol Behav. 1980;25:77–87. doi: 10.1016/0031-9384(80)90185-7. [DOI] [PubMed] [Google Scholar]
- 27.Ford ES, Kohl HW, 3rd, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. Obes Res. 2005;13:608–614. doi: 10.1038/oby.2005.65. [DOI] [PubMed] [Google Scholar]
- 28.Patel SR, Blackwell T, Ancoli-Israel S, Stone KL Osteoporotic Fractures in Men-MrOS Research Group. Sleep characteristics of self-reported long sleepers. Sleep. 2012;35:641–648. doi: 10.5665/sleep.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett LS, Barbour C, Langford B, Stradling JR, Davies RJ. Health status in obstructive sleep apnea: relationship with sleep fragmentation and daytine sleepiness, and effects of continuous positive airway pressure treatment. Am J Respir Crit Care Med. 1999;159:1884–1890. doi: 10.1164/ajrccm.159.6.9808107. [DOI] [PubMed] [Google Scholar]
- 30.Stepanski EJ. The effect of sleep fragmentation on daytime function. Sleep. 2002;25:268–276. doi: 10.1093/sleep/25.3.268. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire. Sleep. 2009;32:915–919. doi: 10.1093/sleep/32.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 35.Spielberger CD. State-Trait Anxiety Inventory: Bibliography. 2nd ed. Palo Alto, CA: Consulting Psychologists Press; 1989. [Google Scholar]
- 36.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 37.Globus GG. A syndrome associated with sleeping late. Psychosom Med. 1969;31:528–535. doi: 10.1097/00006842-196911000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Højbjerre L, Sonne MP, Alibegovic AC, Nielsen NB, Dela F, Vaag A, et al. Impact of physical inactivity on adipose tissue low-grade inflammation in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2011;34:2265–2272. doi: 10.2337/dc11-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youngstedt SD, Kripke DF. Sleep duration and mortality: rationale for sleep restriction. Sleep Medicine Reviews. 2004;8:159–174. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Zielinski MR, Kline CE, Bogan RK, Kripke DF, Youngstedt SD. No effect of 8-week time-in-bed restriction on glucose tolerance in older long sleepers. Journal of Sleep Research. 2008;17:412–419. doi: 10.1111/j.1365-2869.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youngstedt SD, Jean-Louis G, Bootzin RR, et al. Chronic moderate sleep restriction in older long sleepers and average sleepers. Contemporary Clinical Trials. 2013;36:175–186. doi: 10.1016/j.cct.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]