Abstract
Objective To examine the relative benefits and disadvantages of non-steroidal anti-inflammatory drugs (NSAIDs) and opioids for the management of acute renal colic.
Data sources Cochrane Renal Group's specialised register, Cochrane central register of controlled trials, Medline, Embase, and reference lists of retrieved articles.
Review methods Randomised controlled trials comparing any opioid with any NSAID in acute renal colic if they reported any of the following outcomes: patient rated pain, time to pain relief, need for rescue analgesia, rate of recurrence of pain, and adverse events.
Results 20 trials totalling 1613 participants were identified. Both NSAIDs and opioids led to clinically important reductions in patient reported pain scores. Pooled analysis of six trials showed a greater reduction in pain scores for patients treated with NSAIDs than with opioids. Patients treated with NSAIDs were significantly less likely to require rescue analgesia (relative risk 0.75, 95% confidence interval 0.61 to 0.93). Most trials showed a higher incidence of adverse events in patients treated with opioids. Compared with patients treated with opioids, those treated with NSAIDs had significantly less vomiting (0.35, 0.23 to 0.53). Pethidine was associated with a higher rate of vomiting.
Conclusions Patients receiving NSAIDs achieve greater reductions in pain scores and are less likely to require further analgesia in the short term than those receiving opioids. Opioids, particularly pethidine, are associated with a higher rate of vomiting.
Introduction
Renal colic, typically characterised by the sudden onset of severe pain radiating from the flank to the groin, is most commonly caused by the passage of calculi through the urinary tract. Renal colic has an annual incidence of around 16 per 10 000 people and a life time incidence of 2-5%.1,2
The pain of renal colic is due to obstruction of urinary flow, with subsequent increasing wall tension in the urinary tract. Rising pressure in the renal pelvis stimulates the local synthesis and release of prostaglandins, and subsequent vasodilation induces a diuresis which further increases intrarenal pressure. Prostaglandins also act directly on the ureter to induce spasm of the smooth muscle.
As most renal calculi pass spontaneously, acute management should focus on rapid pain relief, confirmation of the diagnosis, and recognition of complications requiring immediate intervention.3 Both non-steroidal anti-inflammatory drugs (NSAIDs) and opioids provide pain relief in acute renal colic.4,5 Opioids have the advantages of cheapness, titratability, potency, and familiarity, but there are concerns over dependency and drug seeking behaviour presenting as renal colic. Opioids do not act directly on the cause of pain and need to be given parenterally, which may limit their usefulness.6 NSAIDs act directly on prostaglandin release (the main cause of pain) and have been shown to be effective, particularly when given intravenously.7 Compared with opioids, however, they are generally not titratable, have well recognised side effects (including renal failure and gastrointestinal bleeding), and may be less immediate and potent in their action. A meta-analysis in 1994 suggested that NSAIDs were at least as effective as opioids in treating the pain of acute renal colic but this study did not specifically examine the difference in efficacy between NSAIDs and opioids.8
Opioids and NSAIDs are currently recommended for acute renal colic, both alone and in combination.9,10 The choice of agent is based on clinician's preference, personal experience, and institutional culture. Two studies examining the combined effect of opioids and NSAIDs have given conflicting results, and there is currently no evidence that NSAIDs reduce the amount of opioid required for control of pain.11,12
We examined the relative benefits and disadvantages of NSAIDs and opioids, and aimed to determine which type of drug is most appropriate for the management of pain in acute renal colic.
Methods
We obtained relevant trials from the Cochrane Renal Group's specialised register of randomised controlled trials; the Cochrane central register of controlled trials 2003; Medline and PreMedline (1966 to 31 January 2003); Embase (1980 to 31 January 2003); reference lists of nephrology textbooks, review articles and relevant trials; and the abstracts of conference proceedings from nephrology meetings. Our search strategy was not limited by language, date, or publication status.13
Trials were included for review if they were randomised controlled trials, compared any NSAID with any opioid by any route, studied adults with a clinical diagnosis of acute renal colic, and had at least one of the predetermined outcomes of interest. We included combination therapies which contained an opioid or NSAID. NSAIDs included aspirin and cyclo-oxygenase-2 inhibitors but not paracetamol or dipyrone. The efficacy of dipyrone in renal colic has been reviewed previously.14
Outcomes of interest were patient rated pain on a validated pain scale, time to pain relief, need for rescue analgesia, rate of pain recurrence, and number of patients with one or more adverse events. Major adverse events were defined as gastrointestinal bleeding, renal failure, hypotension, and respiratory depression. Minor adverse events were defined as gastrointestinal disturbance without bleeding (vomiting, diarrhoea, pain), dizziness, and sleepiness.
Validity assessment and data abstraction
Study quality was assessed independently by the two reviewers without blinding to authorship or journal, using the checklist developed for the Cochrane Renal Group.15 Discrepancies were resolved by discussion. Criteria assessed were allocation concealment, intention to treat analysis, completeness to follow up, blinding of investigators, participants, and outcome assessors, and data analysis.
Identified titles and abstracts were screened independently by the two reviewers. Potentially relevant reviews were retained and the full text examined. Data extraction was carried out independently by the reviewers. When important data were not reported, we tried to contact the authors. Discrepancies between the reviewers were resolved by discussion.
Study characteristics and quantitative data synthesis
Whenever possible we classified the studies by age of participants, size and site of stones, and route and dose of drugs. Analysis was performed using meta-analytic software in Revman 4.1. The results for dichotomous outcomes (need for rescue analgesia, rate of pain recurrence, adverse event rate) are expressed as relative risks with 95% confidence intervals. We pooled data using the random effects model, but the fixed effects model was also analysed to ensure robustness. When continuous scales of measurement were used to assess the effects of treatment (patient rated pain scores, time to pain relief) we used weighted mean differences. Heterogeneity was analysed by a χ2 test (one degree of freedom), and a P value of 0.05 or less was considered statistically significant.
We explored possible sources of heterogeneity (for example, participants, treatments, study quality). When sufficient randomised controlled trials were identified, we attempted to assess publication bias with a funnel plot.16
Results
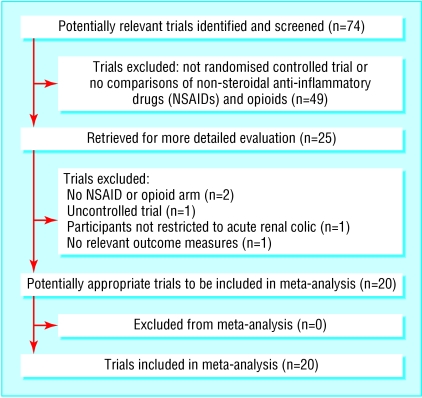
Of 74 potentially relevant studies, we excluded 49 on review of the abstract (fig 1). Twenty five articles were retrieved for more detailed evaluation, of which five were excluded for failure to meet our inclusion criteria, leaving 20 trials for review.4,11,12,17-33
Fig 1.
Flow of studies through trial
Study characteristics
The 20 trials were conducted in nine countries, included 1613 participants, and were published between 1982 and 1999 (table 1). Most studies included only those participants with renal calculi confirmed on subsequent testing using a variety of techniques, specifically excluding patients without such a confirmation. Overall, the trials used five different NSAIDs and seven different opioids, although each trial used only one type of each drug. All but two trials used fixed doses of drugs, regardless of the patient's weight.22,32 Drugs were given by the parenteral route (intravenous or intramuscular) in all but three trials. In these three trials, NSAIDs were given orally or rectally and opioids were given parenterally.18,20,31
Table 1.
Characteristics of included studies of efficacy of non-steroidal anti-inflammatory drugs compared with opioids for pain relief in acute renal colic
| Study | Country | Group (No of participants; No of men), interventions | Outcomes | Notes |
|---|---|---|---|---|
| al-Sahlawi and Tawfik 199612 | Kuwait | Group 1 (50; 34), indomethacin 100 mg intravenously; group 2 (50; 37), pethidine 100 mg, lysine-acetyl salicylate 1.8 g intravenously | Complete relief at 30 minutes, rescue analgesia, adverse events | Group 3 excluded from analysis because of drug type (acetyl salicylate) |
| Amau et al 199117 | Spain | Group 1 (116; 63), diclofenac 75 mg intramuscularly; group 2 (118; 61), pethidine 100 mg intramuscularly | Pain score*, rescue analgesia, adverse events | Two groups (n=217) not included because of drug type (dipyrone) |
| Cordell et al 199418 | United States | Group 1 (31; 18), indomethacin 100 mg rectally; group 2 (20; 18), morphine 5-10 mg intravenously | Adverse events | Crossover trial, post crossover data not included |
| Cordell et al 199611 | United States | Group 1 (35; 28), meperidine 50 mg intravenously; group 2 (36; 30), ketorolac 60 mg intravenously | Pain score*, rescue analgesia, adverse events | Group 3 (n=35) receiving combined non-steroidal anti-inflammatory drug and opioid not included |
| Curry and Kelly 19954 | New Zealand | Group 1 (17), tenoxiam 40 mg intravenously; group 2 (24), pethidine 75 mg intravenously. Overall, 75% men | Pain score*, rescue analgesia, adverse events | |
| Hetherington and Philip 198619 | United Kingdom | Group 1 (28), pethidine 100 mg intramuscularly; group 2 (30), diclofenac 75 mg intramuscularly. Overall, 48 men | Rescue analgesia, adverse events | |
| Indudhara et al 199020 | India | Group 1 (33), diclofenac 150 mg orally; group 2 (31), pethidine 50 mg intramuscularly. Overall, 73% men | Pain relief measured but not defined, adverse events | Group 3 (n=30) not included because of drug type (“baralgin”) |
| Jonsson et al 198721 | Sweden | Group 1 (26; 24), oxyconchloride 5 mg and papaverine 50 mg intravenously; group 2 (35; 30), indomethacin 50 mg intravenously | Pain score*, adverse events | Crossover trial, data from postcrossover period not included |
| Larkin et al 199922 | United States | Group 1 (33; 26), ketorolac 60 mg intramuscularly; group 2 (37; 27), meperidine 100-150 mg intramuscularly | Pain score*, rescue analgesia, adverse events | |
| Lehtonen et al 198323 | Finland | Group 1 (93; 69), indomethacin 50 mg intravenously; group 2 (31; 26), pethidine 50 mg intravenously | Complete relief at 30 minutes, rescue analgesia, adverse events | Group 3 not included because of drug type (dipyrone) |
| Lundstam et al 198224 | Sweden | Group 1 (34; 25), diclofenac 50 mg intramuscularly; group 2 (32; 25), “spasmofen” (combination of multiple narcotics) 1 ml intramuscularly | Adverse events | Pain relief measured but not well defined and therefore not analysed |
| Marthak et al 199125 | India | Group 1 (25; 17), diclofenac 75 mg intramuscularly; group 2 (25; 20), pethidine 75 mg intramuscularly | Pain score*, complete relief at 30 minutes, adverse events | Second study comparing diclofenac with dipyrone not included |
| Oosterlinck et al 199026 | United Kingdom and Belgium | Group 1 (45; 32), ketorolac 10 mg intramuscularly; group 2 (37, 29), ketorolac 90 mg intramuscularly; group 3 (39; 29), pethidine 100 mg intramuscularly | Pain score*, complete relief at 60 minutes, adverse events | Patients from group 1 not included in visual analogue scale analysis owing to inability to combine data |
| Persson et al 1985,27 | Sweden | Group 1 (48; 35), indoprofen 400 mg intravenously; group 2 (46; 35), oxicone 10 mg and papaverine 20 mg intramuscularly | Pain score*, complete relief at 30 minutes, adverse events | |
| Quilez et al 198428 | Spain | Group 1 (24; 14), diclofenac 75 mg intramuscularly; group 2 (14; 8), pentazocine 30 mg intramuscularly | Complete relief at 30 minutes, adverse events | Group 3 (n=23) not included because of drug type (hyoscine) |
| Sandhu et al 199429 | United Kingdom | Group 1 (76; 59), ketorolac 30 mg intramuscularly; group 2 (78; 58), pethidine 100 mg intramuscularly | Adverse events | Data for pain scores and rescue analgesia not used in analysis due to format of information |
| Sommer et al 198930 | Denmark | Group 1 (27; 17), “ketogan” 3 ml intramuscularly; group 2 (29; 22), diclofenac 75 mg intramuscularly | Complete relief at 30 minutes, adverse events | Pain scores measured but not reported |
| Thompson et al 198931 | United Kingdom | Group 1 (29), pethidine 100 mg “injection”; group 2 (29), diclofenac 100 mg rectally† | Complete relief at 30 minutes, rescue analgesia, adverse events | Change in pain score but not absolute scores reported |
| Nicolas Torralba et al 199932 | Spain | Group 1 (24), ketorolac 30 mg intramuscularly; group 2 (24), tramadol 1 mg/kg subcutaneously† | Rescue analgesia, adverse events | Pain scores but no variance given |
| Uden et al 198333 | Sweden | Group 1 (25; 20), indomethacin 50 mg intravenously; group 2 (25; 22), hydromorphine chloride atropine 2 mg subcutaneously | Pain score*, complete relief at 30 minutes, rescue analgesia, adverse events |
Measured by visual analogue scale.
Number of men not reported.
Many of the included trials did not report variance data or outcomes in a form suitable for meta-analysis, and we were unable to gain any further information from the authors. Six studies had treatment arms in addition to NSAIDs and opioids12,17,20,23,25,28; we analysed only data for the opioid and NSAID groups for these trials. Two studies used a crossover design, when the comparator drug was given if inadequate analgesia was achieved with the first drug. We included only data from the precrossover phase of these trials.18,21 One study included a third treatment arm with combined opioids and NSAIDs.11 Data from this treatment arm were not included.
No trial reported time to pain relief, although several reported the proportion of patients with complete pain relief within a fixed time. We therefore used this proportion as an alternative outcome measure. No trials reported rates of pain recurrence or specifically reported serious adverse events such as renal dysfunction or gastrointestinal bleeding.
Overall, no single study met all the quality criteria (table 2). For most studies, quality criteria were not met owing to lack of information rather than explicit reporting of methods that did not conform to the quality criteria.
Table 2.
Assessment of quality criteria for trial of efficacy of non-steroidal anti-inflammatory drugs and opioids for pain relief in acute renal colic
| Study | Allocation concealment | Intention to treat analysis | Completeness of follow up | Investigators blinded | Participants blinded | Outcome assessors blinded |
|---|---|---|---|---|---|---|
| al-Sahlawi and Tawfik 199612 | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Yes |
| Arnau et al 199117 | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Yes |
| Cordell et al 199418 | Adequate | Only patients with diagnosis confirmed on subsequent investigations included in analysis | Insufficient information | Yes | Yes | Yes |
| Cordell et al 199611 | Adequate | Yes; all patients with clinical diagnosis | Insufficient information | Yes | Yes | Yes |
| Curry and Kelly 19954 | Adequate | Only patients with diagnosis confirmed on subsequent investigations included in analysis | Reported | Yes | Yes | Yes |
| Hetherington and Philip 198619 | Insufficient information | Only patients with diagnosis confirmed on subsequent investigations included in analysis | Insufficient information | Insufficient information | Yes | Yes |
| Indudhara et al 199020 | Insufficient information | Insufficient information | Insufficient information | Insufficient information | NO | Insufficient information |
| Jonsson et al 198721 | Adequate | Only patients with diagnosis confirmed on subsequent investigations included in analysis | Insufficient information | Yes | Yes | Yes |
| Larkin et al 199922 | Adequate | Only patients with diagnosis confirmed on subsequent investigations included in analysis | Reported | Yes | Yes | Yes |
| Lehtonen et al 198323 | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Yes |
| Lundstam et al 198224 | Insufficient information | Only patients with diagnosis confirmed on subsequent investigations included in analysis | Insufficient information | Insufficient information | Insufficient information | Insufficient information |
| Marthak et al 199125 | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Insufficient information |
| Oosterlinck et al 199026 | Insufficient information | Only patients with diagnosis confirmed on subsequent investigations included in analysis | Reported | Insufficient information | Insufficient information | Yes |
| Persson et al 198526 | Insufficient information | Yes; all patients with clinical diagnosis | Insufficient information | Insufficient information | Insufficient information | Insufficient information |
| Quilez et al 198428 | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Insufficient information |
| Sandhu et al 199429 | Insufficient information | Yes; all patients with clinical diagnosis | Insufficient information | Insufficient information | Yes | Insufficient information |
| Sommer et al 198930 | Insufficient information | Only patients with diagnosis confirmed on subsequent investigations included in analysis | Reported | Insufficient information | Yes | Insufficient information |
| Thompson et al 198931 | Insufficient information | Only patients with diagnosis confirmed on subsequent investigations included in analysis | Insufficient information | Insufficient information | No | Insufficient information |
| Nicolas Torralba et al 199932 | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Insufficient information | Insufficient information |
| Uden et al 198333 | Insufficient information | Insufficient information | Insufficient information | Insufficient information | No | Yes |
As the results from random and fixed effects models did not differ, we report only results from the random effects model.
Patient rated pain scores
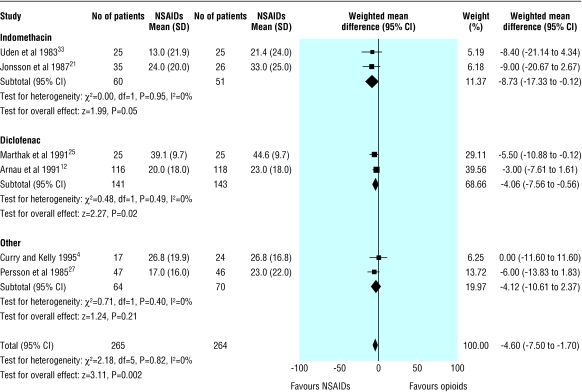
Fifteen trials measured pain scores at enrolment and at a fixed time after the study drug had been given. In two trials this outcome was measured but not reported.19,20 Four trials reported data that were not suitable for pooled analysis.29-32 All but one of these four trials showed a greater reduction in pain scores in the NSAID group than in the opioid group.30 Nine trials reported pain on a 100 mm visual analogue scale; six recorded scores at 30 minutes,4,11,17,25,27,33 two at 20 minutes,21,22 and one at 60 minutes.26 Seven of the nine trials favoured treatment with NSAIDs,11,17,21,25-27,33 one showed no difference,4 and one showed lower pain scores in patients treated with opioids.22 Subgroup analysis by type of NSAID showed heterogeneity for studies using ketorolac but homogeneity among all other trials using any other type of NSAID. Combined analysis of the six trials not using ketorolac showed the visual analogue scale was on average 4.6 mm (95% confidence interval 1.7 mm to 7.5 mm) lower in patients receiving NSAIDs than in those receiving opioids (fig 2). Subgroup analysis by type and route of opioid did not explain heterogeneity. Addition of the three trials using ketorolac to the pooled analysis showed a similar effect. We could find no obvious biological or clinical explanation for this heterogeneity.
Fig 2.
Patient rated scores on visual analogue scale for pain due to renal colic according to type of non-steroidal anti-inflammatory drug, excluding trials using ketorolac
Of the 13 trials with reported results, 10 found lower pain scores in patients treated with NSAIDs, two showed no difference, and only one found lower pain scores in patients treated with opioids.
Failure to achieve complete pain relief
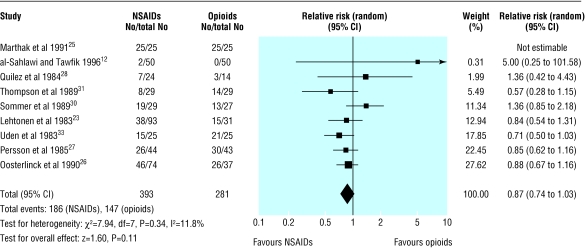
Nine trials (647 participants) reported the proportion of patients who failed to achieve complete pain relief at 30 or 60 minutes after receiving the study drug.12,23,25-31,33 No study found a significant difference in the proportion of patients with complete pain relief, and there was no significant heterogeneity between studies. Combined analysis of these studies showed a trend towards a higher rate of complete pain relief in patients treated with NSAIDs, but this finding was not significant (relative risk 0.87, 0.74 to 1.03; fig 3). Subgroup analysis by NSAID or opioid type did not show significant benefit for any one drug.
Fig 3.
Number of patients failing to achieve complete pain relief from renal colic after receiving anti-inflammatory drugs (NSAIDs) or opioids
Need for rescue analgesia
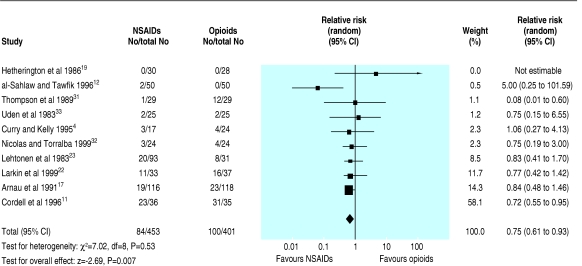
Ten trials (854 participants) reported the need for rescue analgesia within four hours of giving the study drug.4,11,12,17,19,22,23,31-33 The decision to use rescue analgesia was generally determined by clinician's preference in all trials, and the decision to give further analgesia had no objective criteria in eight of the studies. In four trials, pethidine was given if further analgesia was needed 30 minutes after the study drug had been given.4,11,12,17 In the remaining trials the drug used for rescue analgesia was either not specified, was a second dose of the study drug, or was the alternate study drug. The pooled analysis showed no statistical heterogeneity, and patients receiving NSAIDs were significantly less likely to require rescue analgesia than those receiving narcotics (0.75, 0.61 to 0.93; fig 4). Subgroup analysis of only those trials with blinding of investigators and participants continued to show in favour of NSAIDs. All but one of the pooled trials used pethidine as the opiate (dose range 50-150 mg).33 Based on this analysis, approximately 16 patients would require treatment with a NSAID rather than with an opioid for one additional patient to avoid the need for rescue analgesia.
Fig 4.
Number of patients requiring rescue analgesia after treatment with non-steroidal anti-inflammatory drugs (NSAIDs) or opioids for acute renal colic
Adverse events
The definition of adverse effects varied between trials, and many trials included any complaint recorded on general questioning after the study drug had been given. No trial specifically defined or reported serious adverse events such as gastrointestinal bleeding or renal impairment. Most trials had a short period of follow up (maximum 24 hours).29 All studies included reporting of adverse events, and all but four trials11,17,28,31 reported the total number of patients mentioning any adverse event, rather than total number of adverse events. Most of these 16 trials showed a higher incidence of adverse events in patients who were treated with opioids, but there was significant heterogeneity between studies. Subgroup analysis by type of opioid, route of opioid administration, and type of NSAID did not explain this heterogeneity. This heterogeneity may be explained by the ad hoc nature of reporting adverse events in most trials.
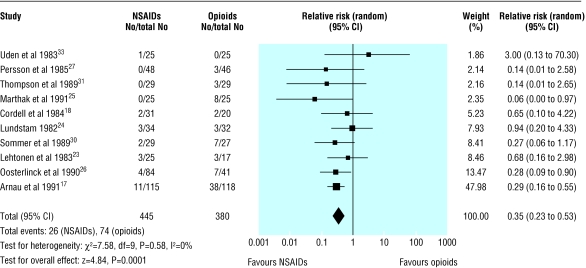
Vomiting was reported as a specific adverse event in 10 trials (826 participants), with no evidence of heterogeneity. The pooled analysis showed significantly less vomiting in patients treated with NSAIDs than in those treated with opioids (0.35, 0.23 to 0.53; fig 5): overall rate 5.8% in patients treated with NSAIDs and 19.5% in patients receiving opioids. Thus for every seven patients treated with NSAIDs rather than with opioids, one less patient will experience vomiting. Subgroup analysis by type of narcotic showed that the risk of vomiting was particularly dominant in patients receiving pethidine (0.30, 0.18 to 0.49). Adverse event rates did not vary according to dosage of opioid.
Fig 5.
Incidence of vomiting as adverse event in patients receiving non-steroidal anti-inflammatory drugs (NSAIDs) or opioids for acute renal colic
Other subgroup analysis and publication bias
Data were insufficient for subgroup analysis by participants' age and sex, size and site of stone, or drug dose for all outcomes. As all opioids and all but three NSAIDs were given parenterally it was not possible to analyse the effect of different routes of administration other than intravenous and intramuscular. Insufficient trials were available to perform funnel plot analysis.
Discussion
Our systematic review shows that non-steroidal anti-inflammatory drugs (NSAIDs) have better efficacy than opioids for relieving the pain of acute renal colic. Results favoured NSAIDs for the three outcomes of pain scores at a specified time after the study drug had been given, proportion of patients who achieved complete pain relief within a fixed time, and the need for rescue analgesia, although the differences reached significance for only two of the three outcomes.
Both opioids and NSAIDs showed a clinically important analgesic effect in patients with acute renal colic, with a noticeable reduction in pain scores over time. Significant heterogeneity between studies did not allow pooled analysis of pain scores for all studies, but qualitatively most studies showed lower pain scores for patients receiving NSAIDs rather than opioids, although the differences were small. In the subgroup of patients receiving NSAIDs other than ketorolac, there was a statistically significant reduction in pain scores of 4.6 mm. This difference is unlikely to be clinically important, however, as previous studies have shown the minimum clinically important difference in visual analogue scales to be around 9-13 mm.34,35
No significant difference was found between NSAIDs and opioids in the proportion of patients who achieved complete pain relief in the short term. Our findings are consistent with the review by Labrecque et al, which also found a non-significant increase in the proportion of patients achieving complete pain relief when treated with NSAIDs rather than with other analgesics.8 In our review the results varied widely between studies, with some showing almost all patients and others showing less than half of the patients achieving complete pain relief. This may reflect the wide range of agents, doses, and routes of administration for the study drugs.
Although both NSAIDs and opioids led to clinically important analgesia, a greater number of patients who received opioids required rescue analgesia within an hour of receiving the study drug. As nine of 10 trials pooled for this analysis used pethidine, this finding may not be generalisable to all opioids. The lack of clear objective guidelines for giving a rescue drug may also limit interpretation of this finding.
Adverse events were generally more common in patients receiving opioids than NSAIDs, but the ad hoc nature of reporting these events makes interpretation of this finding difficult. The specific adverse event of vomiting showed a clear association with opioids, particularly pethidine. Although no studies reported serious adverse events, the short follow up period and failure to specifically record renal dysfunction and gastrointestinal bleeding necessitates cautious interpretation of these results.
The comparative efficacy NSAIDs and opioids has been examined in several clinical settings. Several studies have shown that NSAIDs and opioids provide at least equivalent levels of postoperative analgesia, with higher rates of nausea, vomiting, and dizziness in patients treated with opioids.36-40 Similar results have been found in patients with acute biliary colic and isolated limb injuries and after lithotripsy.41-44 Our findings that NSAIDs provided slightly better analgesia with fewer side effects than opioids are in keeping with these studies, although the finding of improved analgesia in patients with renal colic may relate to the local synthesis and release of prostaglandins specific to this condition.
Limitations
We aimed to assess the effect of treatment in patients with a clinical diagnosis of renal colic because in practice most patients will be treated initially on the basis of a presumptive diagnosis. The applicability of our findings may be limited because most of the studies reviewed only included patients who had renal calculi confirmed on subsequent testing.
Pain scores were reported in all studies as means with variance, although it is well recognised that data from visual analogue scales are often skewed and therefore may be more accurately analysed as medians. We were unable to access individual patient data to assess whether comparison of medians rather than means may have altered our findings. In general, however, analysis of means rather than medians is unlikely to introduce bias unless the distribution of scores is severely skewed.45
All the included trials used fixed doses of opioids, rather than titration of opioids to an appropriate level of pain relief. The standard practice in most emergency departments is to titrate opioids to effect rather than to give single large boluses, and this limits the applicability of our findings to everyday practice.9 The wide variety of drug types and doses used in the studies make it difficult to identify appropriate dosing regimens for clinical practice.
Conclusion
Single bolus doses of NSAIDs and opioids provide pain relief for patients with acute renal colic. Patients receiving NSAIDs, however, achieve greater reduction in pain scores and are less likely to require further analgesia in the short term. Opioids, particularly pethidine, are associated with a higher rate of vomiting than NSAIDs. We therefore recommend a NSAID rather than an opioid. If opioids are to be used either because of contraindications to NSAIDs or ease of titratability, we recommend that pethidine be avoided.
What is already known on this topic
Both non-steroidal anti-inflammatory drugs (NSAIDs) and opioids provide analgesia in acute renal colic
NSAIDs have well recognised side effects
What this study adds
NSAIDs achieve slightly greater reductions in pain scores than opioids in patients with renal colic
Patients with renal colic are less likely to need rescue analgesia if treated with NSAIDs
Opioids, particularly pethidine, are associated with a higher rate of vomiting and other adverse effects
This review was conducted with substantial support and advice from the Cochrane Renal Group, Sydney, Australia.
Contributors: AH and TP were involved in all stages of study design, data collection, data analysis, and manuscript preparation. AH will act as guarantor for the paper.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Stewart C. Nephrolithiasis. Emerg Med Clin North Am 1988;6: 617-30. [PubMed] [Google Scholar]
- 2.Drach GW. Urinary lithiasis: etiology, diagnosis, and medical management. In: Walsh PC, Refik AB, Stamey TA, Vaughan ED, eds. Campbell's urology. 6th ed. Philadelphia, WB Saunders, 1992: 2085-156.
- 3.Holdgate A, Hardcastle J. Renal colic: a diagnostic and therapeutic review. Emerg Med 1999;11: 9-16. [Google Scholar]
- 4.Curry C, Kelly AM. Intravenous tenoxicam for the treatment of renal colic. NZ Med J 1995;108: 229-30. [PubMed] [Google Scholar]
- 5.Smally AJ. Analgesia in renal colic. Ann Emerg Med 1997;29: 296. [PubMed] [Google Scholar]
- 6.Reich JD, Hanno PM. Factitious renal colic. Urology 1997;50: 858-62. [DOI] [PubMed] [Google Scholar]
- 7.Tramer MR, Williams JE, Carroll D, Wiffen PG, Moore RA, McQuay HJ. Comparing analgesic efficacy of non-steroidal anti-inflammatory drugs given by different routes in acute and chronic pain: a qualitative systematic review. Acta Anaesthesiol Scand 1998;42: 71-9. [DOI] [PubMed] [Google Scholar]
- 8.Labrecque M, Dostaler L-P, Rouselle R, Nguyen T, Poirier S. Efficacy of non-steroidal anti-inflammatory drugs in the treatment of acute renal colic. Arch Intern Med 1994;154: 1381-7. [PubMed] [Google Scholar]
- 9.Nicholson F. Renal colic. In: Cameron P, Jelinek G, Kelly AM, Murray L, Heyworth L, eds, Textbook of adult emergency medicine. Edinburgh: Churchill Livingstone, 2000: 372-4.
- 10.Moll J, Peacock WF. Urologic stone disease. In: Tintinalli JE, Kelen GD, Stapczynski JS, eds. Emergency medicine: a comprehensive study guide. 5th ed. New York: McGraw-Hill, 1999: 640-5.
- 11.Cordell WH, Wright SW, Wolfson AB, Timerding BL, Maneatis TJ, Lewis RH, et al. Comparison of intravenous ketorolac, meperidine, and both (balanced analgesia) for renal colic. Ann Emerg Med 1996;28: 151-8. [DOI] [PubMed] [Google Scholar]
- 12.al-Sahlawi KS, Tawfik OM. Comparative study of the efficacy of lysine acetylsalicylate, indomethacin and pethidine in acute renal colic. Eur J Emerg Med 1996;3: 183-6. [DOI] [PubMed] [Google Scholar]
- 13.Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs versus opioids for acute renal colic. Cochrane Database Syst Rev 2004;(1): CD004137. [DOI] [PubMed]
- 14.Edwards JE, Meseguer F, Faura C, Moore RA, McQuay HJ. Single dose dipyrone for acute renal colic. Cochrane Database Syst Rev 2003;(4): CD003867. [DOI] [PMC free article] [PubMed]
- 15.Willis NS, Mitchell R, Craig JC. Renal Group. In: Cochrane library, Issue 4. Chichester: Wiley, 2003.
- 16.Egger M, Davey-Smith G, Scneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ 1997;315: 629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnau JM, Cami J, Garcia-Alonso F, Laporte JR, Palop R. Comparative study of the efficacy of dipyrone, diclofenac sodium and pethidine in acute renal colic. Eur J Clin Pharmacol 1991;40: 543-6. [DOI] [PubMed] [Google Scholar]
- 18.Cordell WH, Larson TA, Lingeman JE, Nelson DR, Woods JR, Burns LB, et al. Indomethacin suppositories versus intravenously titrated morphine for the treatment of ureteral colic. Ann Emerg Med 1994;23: 262-9. [DOI] [PubMed] [Google Scholar]
- 19.Hetherington JW, Philp NH. Diclofenac sodium versus pethidine in acute renal colic. BMJ 1986;292: 237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Indudhara R, Vaidyanathan S, Sankaranarayanan A. Oral diclofenac sodium in the treatment of acute renal colic. A prospective randomized study. Clin Trials J 1990;27: 295-300. [Google Scholar]
- 21.Jonsson PE, Olsson AM, Petersson BA, Johansson K. Intravenous indomethacin and oxycone-papaverine in the treatment of acute renal colic. A double-blind study. BJU Int 1987;59: 396-400. [DOI] [PubMed] [Google Scholar]
- 22.Larkin GL, Peacock WF, Pearl SM, Blair GA, D'Amico F. Efficacy of ketorolac tromethamine versus meperidine in the ED treatment of acute renal colic. Am J Emerg Med 1999;17: 6-10. [DOI] [PubMed] [Google Scholar]
- 23.Lehtonen T, Kellokumpu I, Permi J, Sarsila O. Intravenous indomethacin in the treatment of ureteric colic. A clinical multicentre study with pethidine and metamizol as the control preparations. Ann Clin Res 1983;15: 197-9. [PubMed] [Google Scholar]
- 24.Lundstam SOA, Leissner K-H, Wahlander LA, Kral JG. Prostaglandin-synthetase inhibition with diclofenac sodium in treatment of renal colic: comparison with use of a narcotic analgesic. Lancet 1982;i: 1096-7. [DOI] [PubMed] [Google Scholar]
- 25.Marthak KV, Gokarn AM, Rao AV, Sane SP, Mahanata RK, Sheth RD, et al. A multi-centre comparative study of diclofenac sodium and a dipyrone/spasmolytic combination, and a single-centre comparative study of diclofenac sodium and pethidine in renal colic patients in India. Curr Med Res Opin 1991;12: 366-73. [DOI] [PubMed] [Google Scholar]
- 26.Oosterlinck W, Philp NH, Charig C, Gillies G, Hetherington JW, Lloyd J. A double-blind single dose comparison of intramuscular ketorolac tromethamine and pethidine in the treatment of renal colic. J Clin Pharm 1990;30: 336-41. [DOI] [PubMed] [Google Scholar]
- 27.Persson NH, Bergqvist D, Melander A, Zederfelt B. Comparison of a narcotic (oxicone) and a non-narcotic anti-inflammatory analgesic (indoprofen) in the treatment of renal colic. Acta Chir Scand 1985;151: 105-8. [PubMed] [Google Scholar]
- 28.Quilez C, Perez-Mateo M, Hernandez P, Rubio I. Usefulness of a non-steroid anti-inflammatory, sodium diclofenac, in the treatment of renal colic. Comparative study with a spasmolytic and an opiate analgesic. Med Clin (Barc) 1984;82: 754-5. [In Spanish.] [PubMed] [Google Scholar]
- 29.Sandhu DP, Lacovou JW, Fletcher MS, Kaisary AV, Philip NH, Arkell DG. A comparison of intramuscular ketorolac and pethidine in the alleviation of renal colic. BJU Int 1994;74: 690-3. [DOI] [PubMed] [Google Scholar]
- 30.Sommer P, Kromann-Andersen B, Lendorf A, Lyngdorf P, Moller P. Analgesic effect and tolerance of Voltaren and Ketogan in acute renal or ureteric colic. BJU Int 1989;63: 4-6. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JF, Pike JM, Chumas PD, Rundle JS. Rectal diclofenac compared with pethidine injection in acute renal colic. BMJ 1989;299: 1140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolas Torralba JA, Rigabert Montiel M, Banon Perez V, Valdelvira Nadal P, Perez Albacete M. Ketorolaco intramuscular frente a Tramadol subcutaneo en el tratemiento inicial de urgencia del colico renal. Arch Esp Urol 1999;52: 435-7. [PubMed] [Google Scholar]
- 33.Uden P, Rentzhog L, Berger T. A comparative study of the analgesic effects of indomethacin and hydromorphine-chloride-atropine in acute, ureteral-stone pain. Acta Chir Scand 1983;149: 497-9. [PubMed] [Google Scholar]
- 34.Todd KH, Funk KG, Funk JP. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996;27: 485-9. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med 2001;38: 633-8. [DOI] [PubMed] [Google Scholar]
- 36.Smith LA, Carroll D, Edwards JE, Moore RA, McQuay HJ. Single-dose ketorolac and pethidine in acute postoperative pain: systematic review with meta-analysis. Br J Anaesth 2000;84: 48-58. [DOI] [PubMed] [Google Scholar]
- 37.McEvoy A, Livingstone JI, Cahill CJ. Comparison of diclofenac sodium and morphine sulphate for postoperative analgesia after day case inguinal hernia surgery. Ann R Coll Surg Engl 1996;78: 363-6. [PMC free article] [PubMed] [Google Scholar]
- 38.Zackova M, Taddei S, Calo P, Bellochchio A, Zanello M. Ketorolac vs tramadol in the treatment of postoperative pain during maxillofacial surgery. Minerva Anestesiol 2001;67: 641-6. [PubMed] [Google Scholar]
- 39.DeAndrade JR, Maslanka M, Reines HD, Howe, D, Rasmussen GL, Cardea J, et al. Ketorolac versus meperidine for pain relief after orthopaedic surgery. Clin Orthop 1996;1: 302-12. [PubMed] [Google Scholar]
- 40.Shende D, Das K. Comparative effects of intravenous ketorolac and pethidine on peri-operative analgesia and postoperative nausea and vomiting (PONV) for paediatric strabismus surgery. Acta Anaesthesiol Scand 1999;43: 265-9. [DOI] [PubMed] [Google Scholar]
- 41.Dula DJ, Anderson R, Wood GC. A prospective study comparing i.m. ketorolac with i.m. meperidine in the treatment of acute biliary colic. J Emerg Med 2001;20: 121-4. [DOI] [PubMed] [Google Scholar]
- 42.Henderson SO, Swadron S, Newton E. Comparison of intravenous ketorolac and meperidine in the treatment of biliary colic. J Emerg Med 2002;23: 237-41. [DOI] [PubMed] [Google Scholar]
- 43.Rainer TH, Jacobs P, Ng YC, Cheung NK, Tam M, Lam PK, et al. Cost effectiveness analysis of intravenous ketorolac and morphine for treating pain after limb injury: double blind randomised controlled trial. BMJ 2000;321: 1247-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chia YY, Liu K. Prospective randomized trial of intravenous tenoxicam versus fentanyl and tramadol for analgesia in outpatient extracorporeal lithotripsy. Acta Anaesthesiol Sin 1998;36: 17-22. [PubMed] [Google Scholar]
- 45.Streiner DL, Norman GR. Health measurement scales—a practical guide to their development and use, 2nd ed. Oxford: Oxford University Press, 1995.