Kidney stones affect up to 5% of the population, with a lifetime risk of passing a kidney stone of about 8-10%.1 Increased incidence of kidney stones in the industrialised world is associated with improved standards of living and is strongly associated with race or ethnicity and region of residence.2 A seasonal variation is also seen, with high urinary calcium oxalate saturation in men during summer and in women during early winter.3 Stones form twice as often in men as women. The peak age in men is 30 years; women have a bimodal age distribution, with peaks at 35 and 55 years. Once a kidney stone forms, the probability that a second stone will form within five to seven years is approximately 50%.1
Sources and search criteria
I searched Medline to identify recent articles (1990-2003) related to the evaluation and management of kidney stones. Key words used included kidney stones, urinary calculi, urolithiasis, urinary tract stones, and nephrolithiasis.
Classification and pathophysiology
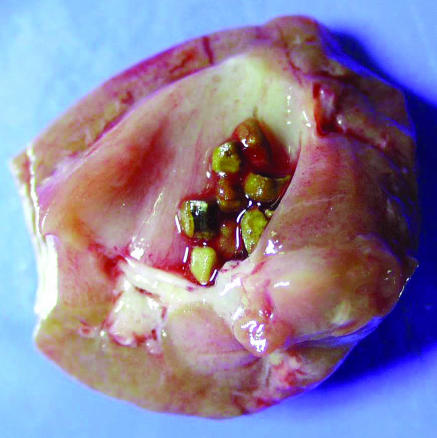
Kidney stones are broadly categorised into calcareous (calcium containing) stones, which are radio-opaque, and non-calcareous stones. On the basis of their composition, stones are classified as shown in the table. The figure shows multiple calcium oxalate stones.
Table 1.
Classification of kidney stones
| Composition | Causative factors | Frequency (%) |
|---|---|---|
| Calcium oxalate, phosphate, or both | Underlying metabolic abnormality
|
60-80 |
| Idiopathic (25%) | ||
| Struvite (triple phosphate) | Infection | 10-15 |
| Uric acid* | Hyperuricaemia and hyperuricosuria
|
5-10 |
| Idiopathic (50%) | ||
| Cystine | Renal tubular defect | 1 |
| Other (xanthine, indigo, triamterene, indinavir*, etc) | 1 |
Pure uric acid and indinavir stones are radiolucent. Cystine stones are radio-opaque because of the sulphur content.
Figure 1.
Multiple calcium oxalate stones (0.5 x 0.5 cm) in the collecting system of a kidney (reproduced courtesy of C F Verkoelen, Josephine Nefkens Institute, Netherlands)
Recent evidence indicates that formation of kidney stones is a result of a nanobacterial disease akin to Helicobacter pylori infection and peptic ulcer disease.4 Nanobacteria are small intracellular bacteria that form a calcium phosphate shell (an apatite nucleus) and are present in the central nidus of most (97%) kidney stones and in mineral plaques (Randall's plaques) in the renal papilla. Further crystallisation and growth of stone are influenced by endogenous and dietary factors. Urine volume, solute concentration, and the ratio of stone inhibitors (citrate, pyrophosphate, and urinary glycoproteins) to promoters are the important factors that influence crystal formation. Crystallisation occurs when the concentration of two ions exceeds their saturation point in the solution.
Risk factors for kidney stones
A precise causative factor is not identified in most cases. A family history of kidney stones (increases risk by three times), insulin resistant states, a history of hypertension, primary hyperparathyroidism, a history of gout, chronic metabolic acidosis, and surgical menopause are all associated with increased risk of kidney stones.5-11 In postmenopausal women, the occurrence of kidney stones is associated with a history of hypertension and a low dietary intake of magnesium and calcium.12 Incidence of stones is higher in patients with an anatomical abnormality of the urinary tract that may result in urinary stasis (box 1). Most patients (up to 80%) with calcium stones have one or more of the metabolic risk factors shown in box 2, and about 25% of stones are idiopathic in origin. Box 3 shows the various drugs that increase the risk of stone disease.
Summary points
Calcium oxalate (alone or in combination) is the most common type of urinary stone
Low urine volume is the most common abnormality and the single most important factor to correct so as to avoid recurrences
Risk of a recurrent stone is about 50% within five to seven years
Diets low in salt (< 50 mmol/day) and animal proteins (< 52 g/day) are helpful in decreasing the frequency of recurrent calcium oxalate stones
Low calcium diets are not recommended to prevent recurrent stones, as they increase urinary oxalate excretion and may result in negative calcium balance
Most ureteral stones under 5 mm pass spontaneously
Hypercalciuria
Hypercalciuria is defined as excretion of urinary calcium exceeding 200 mg in a 24 hour collection or an excess of 4 mg calcium/kg/24 h. Hypercalciuria is the most common metabolic abnormality in patients with calcareous stones and results from various mechanisms.
Absorptive hypercalciuria—Increased absorption of calcium from the gut results in increased circulating calcium, resulting in increased renal filtered load. The exact mechanism is unknown but seems to be inherited in an autosomal dominant fashion, and the jejunal mucosa is hyper-responsive to vitamin D. Absorptive hypercalciuria is very common, but most patients remain asymptomatic and do not experience stone formation.
Box 1: Anatomical abnormalities that increase the risk of stone disease
Obstruction of the pelviureteral junction
Hydronephrotic renal pelvis or calices
Calyceal diverticulum
Horseshoe kidney
Ureterocele
Vesicoureteral reflux
Ureteral stricture
Tubular ectasia (medullary sponge kidney)
Renal hypercalciuria—Increased excretion of calcium in urine results from impaired renal tubular absorption of calcium. This occurs in about 2% of patients with recurrent stone formation.
Resorptive hypercalciuria—Increased resorption of bone occurs as a result of primary hyperparathyroidism. This occurs in about 5% of patients with recurrent stone formation. The risk of renal stones is increased in primary hyperparathyroidism and returns to baseline about 10 years after parathyroidectomy. Patients who had stones before undergoing parathyroidectomy have a 27 times greater risk of stone formation after parathyroidectomy than do patients without hyperparathyroidism.8
Hyperuricosuria
Uric acid is the end product of purine metabolism and is either derived from exogenous (dietary) sources or produced endogenously during cell turnover. Chronic metabolic acidosis can result in protein metabolism and thus increased excretion of urate and formation of kidney stones.10 Pure uric acid stones are rare but recur frequently. Low urinary pH (pH < 5.5) is the most common and important factor in uric acid nephrolithiasis; in normouricosuric stone disease the primary defect seems to be in the renal excretion of ammonia and is linked to an insulin resistant state.6 Hyperuricosuria occurs in 10% of patients with calcium stones, where uric acid crystals form the nidus for deposition of calcium and oxalate. A history of gout doubles the risk of kidney stones in men.9
Hyperoxaluria
Hyperoxaluria is defined as urinary excretion of oxalate in excess of 45 mg/day. On the basis of the mechanism, it is classified as follows.
Box 2: Metabolic risk factors for calcareous stones
Hypercalciuria (40-60%)
Hyperuricosuria (25%)
Hyperoxaluria
Hypocitriuria
Other (vitamin A deficiency, hot climates, immobilisation, urinary tract anomalies)
Enteric hyperoxaluria—This results from increased intestinal absorption due to ileal disease (Crohn's disease, ileal bypass) or short bowel syndrome, low calcium intake, or gastrointestinal decolonisation of Oxalobacter formigenes. Oxalobacter is an intestinal bacterium that degrades dietary oxalate, and decolonisation of the gut results in increased absorption of oxalate. Oral administration of Oxalobacter has been shown to decrease urinary oxalate concentration in animals and humans.13,14
Increased ingestion (oxalate gluttons)—Dietary oxalate contributes to about half of the urinary oxalate and is inversely proportional to calcium intake in healthy people without gastrointestinal disease.15 Spinach, rhubarb, beets, chocolate, nuts, tea, wheat bran, strawberries, and soya foods are known to increase urinary oxalate concentrations.16 Vitamin C supplementation may increase urinary oxalate excretion and the risk of calcium oxalate crystallisation in patients who form calcium stones.17 Ingestion of grapefruit juice increases excretion of both oxalate and citrate in urine with no net change in its lithogenicity.18
Primary hyperoxaluria—This is an inborn error of metabolism (glycolic aciduria).
In experimental animals, testosterone promotes stone formation by suppressing osteopontin expression in the kidney and increasing urinary oxalate excretion. Oestrogen seems to inhibit stone formation by increasing osteopontin expression in the kidney and decreasing urinary oxalate excretion.19
Box 3: Drugs that may increase the risk of stone disease*
Decongestants: ephedrine, guaifenesinw1-2
Diuretics: triamterene
Protease inhibitors: indinavirw3
Anticonvulsants: felbamate,w4 topiramate, and zonisamidew5
*The non-dissolving carrier of osmotically controlled release oral (OROS) drugs may be misdiagnosed as kidney stones on x rayw6
Hypocitriuria
Hypocitriuria is defined as urinary citrate excretion of < 250 mg in 24 hours. Urinary citrate forms a soluble complex with calcium that inhibits the formation and propagation of crystals. It is a common correctable cause of recurrent pure calcium phosphate or brushite stones. Women excrete more citrate and have lower incidence of stone formation than men. Urinary citrate is mainly derived endogenously through the tricarboxylic acid cycle and is excreted by renal tubular cells. Intracellular acidosis, acidic diets (diets rich in animal proteins), and hypokalaemia decrease urinary citrate excretion. Fruits such as oranges and grapefruits are the main exogenous sources of urinary citrate. Hormonal replacement therapy in postmenopausal women results in higher urinary calcium excretion, but it also increases urinary excretion of citrate and leads to net inhibition of crystal precipitation, thereby decreasing the risk of calcium stones.20
Struvite (triple phosphate) and cystine stones
Various anatomical abnormalities (box 1) promote urine stasis and increase the risk of stone formation by promoting precipitation of crystals. Urinary infection with urea splitting organisms (Proteus, Klebsiella, Serratia, and Mycoplasma) creates alkaline urine that promotes the formation of struvite stones. Urinary saturation with struvite occurs only when supranormal excretion of ammonia and alkaline urine occur together. Alkalaemia suppresses renal ammoniagenesis, but the hydrolysis of urea by bacteria liberates ammonia that alkalises urine.
Cystinuria (cystine stones) is an autosomal recessive trait, with an inborn error in the transport of dicarboxylic acids—cystine, ornithine, lysine, and arginine, commonly known as “COLA.” The low solubility of cystine results in its precipitation and stone formation.
Urinary glycoproteins
Various urinary glycoproteins (Tamm-Horsfall proteins, bikunin, nephrocalcin, urinary prothrombin fragment I) are inhibitors of stone formation. Their deficiency may promote stone formation.
Clinical features
Kidney stones may present in different ways (box 4), but the classic presentation is with acute loin to groin colicky pain associated with nausea and vomiting. This, combined with renal angle tenderness and microscopic haematuria, is highly predictive of urinary tract stone disease with a sensitivity of 84% and a specificity of 99%.21 One third of incidental stones may become symptomatic.22
Box 4: Clinical features of urinary tract stones
Urinary tract symptoms
Pain—classic colicky loin to groin pain or renal pain
Haematuria, gross or microscopic (occurs in 90%)
Dysuria and strangury
Systemic symptoms
Restless patient, often writhing in distress
Nausea, vomiting, or both (shared innervation of renal capsule and intestines)
Fever and chills (if associated infection)
Asymptomatic
• Incidental stones (one third may become symptomatic)
Investigations
Some experts recommend detailed evaluation even after passage of a single stone, because of the high rate of recurrence. However, medical evaluation and prophylaxis may not be cost effective in patients who form stones less than once every three years.23 In addition to detailed history, including family history of stone disease and past history of stone passage, the basic investigations in a patient who has passed a stone are:
Box 5: General measures to prevent recurrent stone formation
-
Increase fluid intake to maintain urine output of 2-3 l/day:
Higher fluid intake is modestly effective in practice, and this effect is often offset by an increase in urine sodium as a result of increased sodium intake31
higher fluid intake alone will not prevent recurrent stones in patients with hypercalciuria
-
Decrease intake of animal protein (≤ 52 g/day)32: Reduces production of metabolic acids, resulting in a lower level of acid induced calcium excretion; increases excretion of citrate that forms a soluble complex with calcium; and reduces supersaturation with respect to calcium oxalate and limits the excretion of uric acid
- Restrict salt intake (≤ 50 mmol/day of sodium chloride)32: Dietary and urinary sodium is directly correlated with urinary calcium excretion, and lower urinary excretion of sodium reduces urinary calcium excretion
- Normal calcium intake (≥ 30 mmol/day)32: Low calcium diets increase urinary oxalate excretion, which may result in more stone formation and possibly a negative calcium balance
- Decrease dietary oxalate16: Reduce the intake of foods rich in oxalate—spinach, rhubarb, chocolate, and nuts
- Cranberry juice: Decreases oxalate and phosphate excretion and increases citrate excretion33
Urinalysis, including urine pH and urine culture
Serum electrolytes: calcium, phosphate, bicarbonate, uric acid
Blood urea nitrogen, serum creatinine (renal function)
Parathyroid hormone, if elevated serum calcium
Stone analysis, if possible
Radiological investigations:
unenhanced helical computed tomography is the best imaging method to confirm (99% diagnostic accuracy) the diagnosis of a urinary stone in a patient with acute flank pain; it also helps with the measurement of stone density and may guide treatment—stones with density > 1000 Housnfield units respond less well to lithotripsy
plain abdominal film (kidney-ureter-bladder view) is important to assess the radio-opacity of stone, to monitor stone progression, and to guide shock wave lithotripsy.
In a patient with recurrent stones, in addition to the baseline investigations, a 24 hour urine assessment should be done for urine volume and calcium, oxalate, uric acid, citrate, urine sodium, and creatinine excretion. Urine creatinine is measured to determine the accuracy of urine collection.
Management
Management of a kidney stone depends on its size, location, and composition and the presence of anatomical malformation (box 1) and complications. The presence of a complication (complicated stone)—infection or obstruction—may necessitate immediate intervention, whereas uncomplicated stones can be managed conservatively with adequate fluid intake and analgesia. If a stone does not pass spontaneously then definitive treatment is needed to remove it. The goals of treatment are to control symptoms, render the patient stone free, and prevent recurrence.
Management of acute renal colic
The best resolution of renal (ureteric) colic is by either spontaneous passage or removal of the stone. However, until then, the patient needs pain control and agents that may help the stone to pass. In a recent study, addition to conventional treatment of a calcium channel blocker (nifedipine) to relax ureteral muscles, short term prednisone (for five days) to reduce local oedema through its anti-inflammatory action, antibiotics to prevent and treat urinary tract infection, and paracetamol to raise the pain threshold and reduce the need for narcotics boosted the rate of passage of stones and led to fewer lost work days, emergency room visits, and surgical interventions, with a similar side effect profile.24 An intranasal spray of antidiuretic desmopressin with or without diclofenac sodium has also been shown to be effective in relieving the pain of renal colic, although experience is limited.25 Local active warming of the abdomen and lower back (42°C) has recently been shown to be an effective treatment of pain and nausea associated with renal colic.26
Box 6: Specific treatments to prevent recurrent stones
Calcareous stones
Normocalciuria
• Oral administration of potassium citrate—increases urine pH and citrate excretion in the urine
Hypercalciuria
Thiazide diuretics—decrease urinary calcium excretion by augmenting tubular reabsorption of calcium, but do not decrease intestinal absorption in absorptive hypercalciuria; the effect may be attenuated or lost after two or more years of treatment
Addition of potassium citrate may help to control the diuretic induced hypokalaemia
If magnesium loss is a concern because of chronic diuretic use, consider potassium magnesium citrate
Potassium phosphate—may suppress calcitriol synthesis and thereby decrease calcium absorption
Hyperuricaemia or hyperuricosuria
Allopurinol—to inhibit uric acid synthesis and decrease urinary uric acid excretion
Potassium citrate should be given in addition to increase urine pH, as uric acid precipitates in acidic urine
Hyperoxaluria
No specific drugs are available to reduce oxalate excretion in the urine
Pyridoxine, a cofactor in the alanine-glycoxylate pathway, may reduce production of oxalate by inducing enzyme activity; in an observational study, high intake of vitamin B6 (> 40 mg/day) was inversely associated with risk of oxalate stone formation in women
Calcium supplementation (250-1000 mg four times a day) to control enteric hyperoxaluria; urinary oxalate may decrease, but a concurrent rise in calcium may negate the beneficial effect
Cholestyramine reduces intestinal absorption of oxalate, but no trials have shown its efficacy in preventing recurrent stones
Probiotic treatment with Oxalobacter formigenes has recently been shown to significantly reduce oxalate excretion in both animals and humans13,14; however, trials are pending to show its role in clinical practice
Hypocitriuria
• Potassium citrate—to increase citrate excretion
Struvite stones
Treatment of infection is mandatory and may be needed in the long term
Acetohydroxamic acid, a urease inhibitor, has been shown to reduce the urinary saturation of struvite but is associated with high frequency of side effects (deep vein thrombosis, haemolytic anaemia), which limits its use
Cystine stones
Treatment must include increasing urine output to about 3 l/day and adequate alkalinisation (urine pH > 7.0) with potassium citrate
In addition, specific agents such as α mercaptopropionylglycine or d-penicillamine that form soluble complexes with cystine are used
About 90% of ureteric stones smaller than 5 mm pass spontaneously, compared with about 50% of stones between 5 mm and 10 mm, so conservative management is preferred for ureteric stones.27 Depending on the size of the stone, the average time to pass the stone ranges between one week and three weeks, and the passage of the stone is most accurately assessed by a plain film (kidney-ureter-bladder view) every one to two weeks to monitor progression. An observation period of three to four weeks is reasonable unless urgent intervention is indicated for intractable symptoms, infection, or obstruction.
Additional educational resources
Delvecchio FC, Preminger GM. Medical management of stone disease. Curr Opin Urol 2003;13:229-33
Tiselius H-G. Medical evaluation of nephrolithiasis. Endocrinol Metab Clin N Am 2002;31:1031-50
Borghi L, Meschi T, Schianchi T, Allegri F, Guerra A, Maggiore U, et al. Medical treatment of nephrolithiasis. Endocrinol Metab Clin N Am 2002;31:1051-64
Auge BK, Preminger GM. Surgical treatment of urolithiasis. Endocrinol Metab Clin N Am 2002;31:1065-82
Information for patients
National Institute of Diabetes and Digestive and Kidney Diseases (www.kidney.niddk.nih.gov/kudiseases/topics/stones.asp)—presents simple and concise information about kidney stones
Urology Channel (www.urologychannel.com/kidneystones/index.shtml)—provides a general overview of kidney stones, including the role of naturopathic treatments in prevention of stones
Patient UK (www.patient.co.uk/showdoc.asp?doc=23068865)—provides simple information on kidney stones
Your Medical Source (yourmedicalsource.com/library/kidneystones/KS_whatis.html)—provides information about diagnosis, management, and prevention of kidney stones
Surgical treatment
Surgical management has recently been reviewed elsewhere.28 About 10-20% of all kidney stones need radiological or surgical intervention to remove the stone. For proximal ureteric stones, shock wave lithotripsy is useful if the stone is less than 1 cm in size, and ureteroscopy is more successful for stones larger than 1 cm. The preferred approach for distal ureteric stones is controversial. Shock wave lithotripsy and ureteroscopy have shown similar stone-free rates in distal ureteric stones of less than 7 mm. Ureteroscopy is less expensive than shock wave lithotripsy but is more time consuming and technically demanding. Shock wave lithotripsy is less efficacious if the stone is dense (attenuation value of more than 1000 Housnfield units) on helical computed tomography and might adversely affect ovarian function when used for distal ureteric stones in women. Ureteroscopy using the holmium:yttrium-aluminum-garnet (YAG) laser (photothermal lithotripsy) is effective for stones of all compositions and sizes, with a success rate of 97-100%.29
Medical treatment to prevent recurrent stones
Medical management to prevent recurrence after a first stone episode is not cost effective.23 All patients should be advised to follow general treatment recommendations (box 5) for prevention of stone recurrence, and specific treatment (box 6) should be advised to patients with specific problems or with frequent recurrences (a stone at least every three years). Medical prophylaxis is effective in up to 80% of patients with recurrent calcium stones.30
Supplementary Material
 Extra references are on bmj.com
Extra references are on bmj.com
I thank C F Verkoelen, Department of Urology, Josephine Nefkens Institute, Erasmus MC, Rotterdam, Netherlands, for providing the photograph.
Funding: None.
Competing interests: None declared.
References
- 1.Asplin JR, Favus MJ, Coe FL. Nephrolithiasis. In: Brenner BM, ed. Brenner and Rector's the kidney. 5th ed. Philadelphia: Saunders, 1996: 1893-935.
- 2.Stamatelou KK, Francis ME, Jones CA, Nyberg LM Jr, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 2003;63: 1817-23. [DOI] [PubMed] [Google Scholar]
- 3.Parks JH, Barsky R, Coe FL. Gender differences in seasonal variation of urine stone risk factors. J Urol 2003;170: 384-8. [DOI] [PubMed] [Google Scholar]
- 4.Ciftcioglu N, Bjorklund M, Kuorikoski K, Bergstrom K, Kajander EO. Nanobacteria: an infectious cause for kidney stone formation. Kidney Int 1999;56: 1893-8. [DOI] [PubMed] [Google Scholar]
- 5.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. Family history and risk of kidney stones. J Am Soc Nephrol 1997;8: 1568-73. [DOI] [PubMed] [Google Scholar]
- 6.Sakhaee L, Adams-Huet B, Moe OW, Pak CYC. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int 2002;62: 971-9. [DOI] [PubMed] [Google Scholar]
- 7.Cappuccio FP, Siani A, Barba G, Mellone MC, Russo L, Farinaro E, et al. A prospective study of hypertension and the incidence of kidney stones in men. J Hypertens 1999;17: 1017-22. [DOI] [PubMed] [Google Scholar]
- 8.Mollerup CL, Vestergaard P, Frokjaer VG, Mosekilde L, Christiansen P, Blichert-Toft M. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. BMJ 2002;325: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer HJ, Choi HK, Atkinson K, Stampfer M, Curhan GC. The association between gout and nephrolithiasis in men: the health professionals' follow-up study. Kidney Int 2003;64: 1022-6. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin DN, Spencer JL, Jeffries-Stokes CA. Carbohydrate intolerance and kidney stones in children in the Goldfields. J Paediatr Child Health 2003;39: 381-5. [DOI] [PubMed] [Google Scholar]
- 11.Mattix Kramer HJ, Grodstein F, Stampfer MJ, Curhan GC. Menopause and postmenopausal hormone use and risk of incident kidney stones. J Am Soc Nephrol 2003;14: 1272-7. [DOI] [PubMed] [Google Scholar]
- 12.Hall WD, Pettinger M, Oberman A, Watts NB, Johnson KC, Paskett ED, et al. Risk factors for kidney stones in older women in Southern United States. Am J Med Sci 2001;322: 12-8. [DOI] [PubMed] [Google Scholar]
- 13.Sidhu H, Allison MJ, Chow JM, Clark A, Peck AB. Rapid reversal of hyperoxaluria in a rat model after probiotic administration of Oxalobacter formigenes. J Urol 2001;166: 1487-91. [PubMed] [Google Scholar]
- 14.Duncan SH, Richardson AJ, Kaul P, Holmes RP, Allison MJ, Stewart CS. Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol 2002;68: 3841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int 2001;59: 270-6. [DOI] [PubMed] [Google Scholar]
- 16.Grentz L, Massey LK. Contribution of dietary oxalate to urinary oxalate in health and disease. Topics in Clinical Nutrition 2002;17: 60-70. [Google Scholar]
- 17.Baxmann AC, Mendonca CD, Heilberg IP. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int 2003;63: 1066-71. [DOI] [PubMed] [Google Scholar]
- 18.Goldfarb DS, Asplin JR. Effect of grapefruit juice on urinary lithogenicity. J Urol 2001;166: 263-7. [PubMed] [Google Scholar]
- 19.Yagisawa T, Ito F, Osaka Y, Amano H, Kobayshi C, Toma H. The influence of sex hormones on renal osteopontin expression and urinary constituents in experimental urolithiasis. J Urol 2001;166: 1078-82. [PubMed] [Google Scholar]
- 20.Dey J, Creighton A, Lindberg JS, Fuselier HA, Kok DJ, Cole FE, et al. Estrogen replacement increased the citrate and calcium excretion in rates in postmenopausal women with recurrent urolithiasis. J Urol 2002;167: 169-71. [PubMed] [Google Scholar]
- 21.Eskelinen M, Ikonen J, Lipponen P. Usefulness of history-taking, physical examination and diagnostic scoring in acute renal colic. Eur Urol 1998;34: 467-73. [DOI] [PubMed] [Google Scholar]
- 22.Glowacki LS, Beecroft ML, Cook RJ, Pahl D, Churchill DN. The natural history of urolithiasis. J Urol 1992;147: 319-21. [DOI] [PubMed] [Google Scholar]
- 23.Chandhoke PS. When is medical prophylaxis cost effective for recurrent calcium stones? J Urol 2002;168: 937-40. [DOI] [PubMed] [Google Scholar]
- 24.Cooper JT, Stack GM, Cooper TP. Intensive management of ureteral calculi. Urology 2000;56: 575-8. [DOI] [PubMed] [Google Scholar]
- 25.Lopes T, Dias JS, Marcelino J, Varela J, Ribeiro S, Dias J. An assessment of the clinical efficacy of intranasal desmopressin spray in the treatment of renal colic. BJU Int 2001;87: 322-5. [DOI] [PubMed] [Google Scholar]
- 26.Kober A, Dobrovits M, Djavan B, Marberger M, Barker R, Bertalanffy P, et al. Local active warming: an effective treatment for pain, anxiety and nausea caused by renal colic. J Urol 2003;170: 741-4. [DOI] [PubMed] [Google Scholar]
- 27.Segura JW, Preminger GM, Assimos DG, Dretler SP, Kahn RL, Lingeman JE, et al. Ureteral stones clinical guidelines panel summary report on the management of ureteral calculi. J Urol 1997;158: 1915-21. [DOI] [PubMed] [Google Scholar]
- 28.Auge BK, Preminger GM. Surgical management of urolithiasis. Endocrinol Metab Clin North Am 2002;31: 1065-82. [DOI] [PubMed] [Google Scholar]
- 29.Sofer M, Watterson JD, Wollin TA, Nott L, Razvi H, Denstedt JD. Holmium:YAG laser lithotripsy for upper urinary tract calculi in 598 patients. J Urol 2002;167: 31-4. [DOI] [PubMed] [Google Scholar]
- 30.Delvecchio FC, Preminger GM. Medical management of stone disease. Curr Opin Urol 2003;13: 229-33. [DOI] [PubMed] [Google Scholar]
- 31.Parks JH, Goldfischer ER, Coe FL. Changes in urine volume accomplished by physicians treating nephrolithiasis. J Urol 2003;169: 863-6. [DOI] [PubMed] [Google Scholar]
- 32.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002;346: 77-84. [DOI] [PubMed] [Google Scholar]
- 33.McHarg T, Rodgers A, Charlton K. Influence of cranberry juice on the urinary risk factors for calcium oxalate stone formation. BJU Int 2003;92: 765-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.