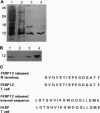
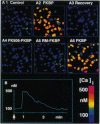
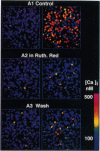
Abstract
The immunophilins of the FK506-binding protein (FKBP) family are intracellular proteins that bind the immunosuppresants FK506 and rapamycin. In this study we show that HMC-1 mast cells sensitized with IgE release FKBP12 upon stimulation with anti-IgE. The release is rapid and not affected by actinomycin D or cycloheximide, suggesting that it is due to exocytosis from a storage compartment. FKBP12 from HMC-1 mast cells exhibits biological activity. When applied extracellularly to human neutrophils, it induces transient changes in the intracellular Ca2+ concentration ([Ca2+]i) due to Ca2+ release from intracellular stores. Inhibition of [Ca2+]i changes by ruthenium red and ryanodine indicates that ryanodine receptor/Ca2+ release channels are involved in FKBP12-induced Ca2+ signaling. Neutrophil activation by mast cell-derived FKBP12 is prevented by complexing FKBP12 with FK506 or rapamycin. These results demonstrate that extracellular FKBP12 functions as a cytokine in cell-to-cell communication. They further suggest a pathophysiological role for FKBP12 as a mediator in immediate or type I hypersensitivity and may have implications for novel therapeutic strategies in the treatment of allergic disorders with FK506 and rapamycin.
Full text
PDF
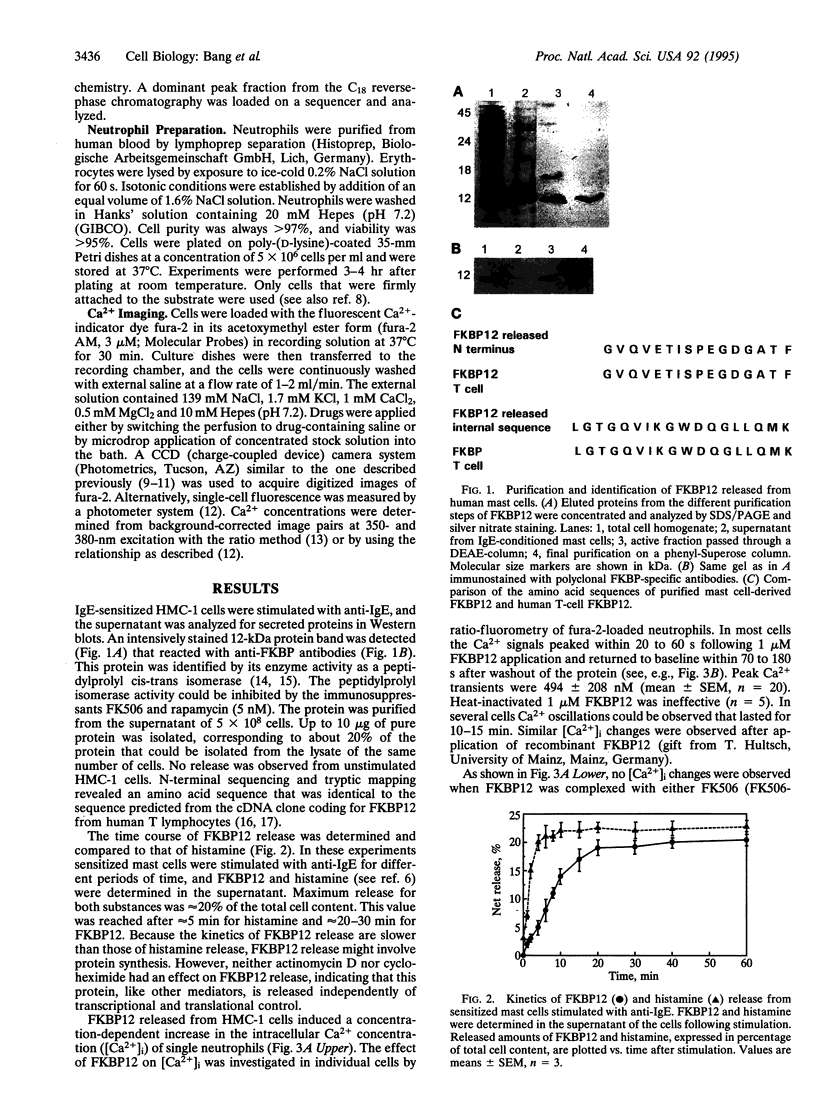

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benyon R. C., Lowman M. A., Church M. K. Human skin mast cells: their dispersion, purification, and secretory characterization. J Immunol. 1987 Feb 1;138(3):861–867. [PubMed] [Google Scholar]
- Bierer B. E., Mattila P. S., Standaert R. F., Herzenberg L. A., Burakoff S. J., Crabtree G., Schreiber S. L. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. T., Hendrickson B. A., Nigam S. K. Induction of the FK506-binding protein, FKBP13, under conditions which misfold proteins in the endoplasmic reticulum. Biochem J. 1994 Nov 1;303(Pt 3):705–708. doi: 10.1042/bj3030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Digital imaging of free calcium changes and of spatial gradients in growing processes in single, mammalian central nervous system cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6179–6183. doi: 10.1073/pnas.83.16.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich B. E., Kaftan E., Bezprozvannaya S., Bezprozvanny I. The pharmacology of intracellular Ca(2+)-release channels. Trends Pharmacol Sci. 1994 May;15(5):145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Fischer G., Bang H., Mech C. Nachweis einer Enzymkatalyse für die cis-trans-Isomerisierung der Peptidbindung in prolinhaltigen Peptiden. Biomed Biochim Acta. 1984;43(10):1101–1111. [PubMed] [Google Scholar]
- Franke E. K., Yuan H. E., Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994 Nov 24;372(6504):359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hultsch T., Albers M. W., Schreiber S. L., Hohman R. J. Immunophilin ligands demonstrate common features of signal transduction leading to exocytosis or transcription. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6229–6233. doi: 10.1073/pnas.88.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaconi M. E., Theler J. M., Schlegel W., Appel R. D., Wright S. D., Lew P. D. Multiple elevations of cytosolic-free Ca2+ in human neutrophils: initiation by adherence receptors of the integrin family. J Cell Biol. 1991 Mar;112(6):1249–1257. doi: 10.1083/jcb.112.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. J., Albers M. W., Lane W. S., Bierer B. E., Schreiber S. L., Burakoff S. J. Molecular cloning of a membrane-associated human FK506- and rapamycin-binding protein, FKBP-13. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6677–6681. doi: 10.1073/pnas.88.15.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye R. E., Fruman D. A., Bierer B. E., Albers M. W., Zydowsky L. D., Ho S. I., Jin Y. J., Castells M. C., Schreiber S. L., Walsh C. T. Effects of cyclosporin A and FK506 on Fc epsilon receptor type I-initiated increases in cytokine mRNA in mouse bone marrow-derived progenitor mast cells: resistance to FK506 is associated with a deficiency in FK506-binding protein FKBP12. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8542–8546. doi: 10.1073/pnas.89.18.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiva M. C., Lyttle C. R. Leukocyte chemotactic activity of FKBP and inhibition by FK506. Biochem Biophys Res Commun. 1992 Jul 31;186(2):1178–1183. doi: 10.1016/0006-291x(92)90871-h. [DOI] [PubMed] [Google Scholar]
- Maki N., Sekiguchi F., Nishimaki J., Miwa K., Hayano T., Takahashi N., Suzuki M. Complementary DNA encoding the human T-cell FK506-binding protein, a peptidylprolyl cis-trans isomerase distinct from cyclophilin. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5440–5443. doi: 10.1073/pnas.87.14.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Connor J. A. Cholinergic input uncouples Ca2+ changes from K+ conductance activation and amplifies intradendritic Ca2+ changes in hippocampal neurons. Neuron. 1991 Jun;6(6):901–905. doi: 10.1016/0896-6273(91)90230-w. [DOI] [PubMed] [Google Scholar]
- Müller W., Connor J. A. Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature. 1991 Nov 7;354(6348):73–76. doi: 10.1038/354073a0. [DOI] [PubMed] [Google Scholar]
- Rosales C., Brown E. J. Signal transduction by neutrophil immunoglobulin G Fc receptors. Dissociation of intracytoplasmic calcium concentration rise from inositol 1,4,5-trisphosphate. J Biol Chem. 1992 Mar 15;267(8):5265–5271. [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Siekierka J. J., Hung S. H., Poe M., Lin C. S., Sigal N. H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989 Oct 26;341(6244):755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- Sigal N. H., Dumont F. J. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. M., Wandless T. J., Schreiber S. L., Crabtree G. R. Controlling signal transduction with synthetic ligands. Science. 1993 Nov 12;262(5136):1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- Thali M., Bukovsky A., Kondo E., Rosenwirth B., Walsh C. T., Sodroski J., Göttlinger H. G. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994 Nov 24;372(6504):363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- Thelen M., Dewald B., Baggiolini M. Neutrophil signal transduction and activation of the respiratory burst. Physiol Rev. 1993 Oct;73(4):797–821. doi: 10.1152/physrev.1993.73.4.797. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Van Duyne G. D., Standaert R. F., Karplus P. A., Schreiber S. L., Clardy J. Atomic structure of FKBP-FK506, an immunophilin-immunosuppressant complex. Science. 1991 May 10;252(5007):839–842. doi: 10.1126/science.1709302. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. Naming a targeting signal. Cell. 1991 Jan 11;64(1):13–15. doi: 10.1016/0092-8674(91)90202-a. [DOI] [PubMed] [Google Scholar]
- Wang T., Donahoe P. K., Zervos A. S. Specific interaction of type I receptors of the TGF-beta family with the immunophilin FKBP-12. Science. 1994 Jul 29;265(5172):674–676. doi: 10.1126/science.7518616. [DOI] [PubMed] [Google Scholar]
- Ye R. D., Quehenberger O., Thomas K. M., Navarro J., Cavanagh S. L., Prossnitz E. R., Cochrane C. G. The rabbit neutrophil N-formyl peptide receptor. cDNA cloning, expression, and structure/function implications. J Immunol. 1993 Feb 15;150(4):1383–1394. [PubMed] [Google Scholar]