Abstract
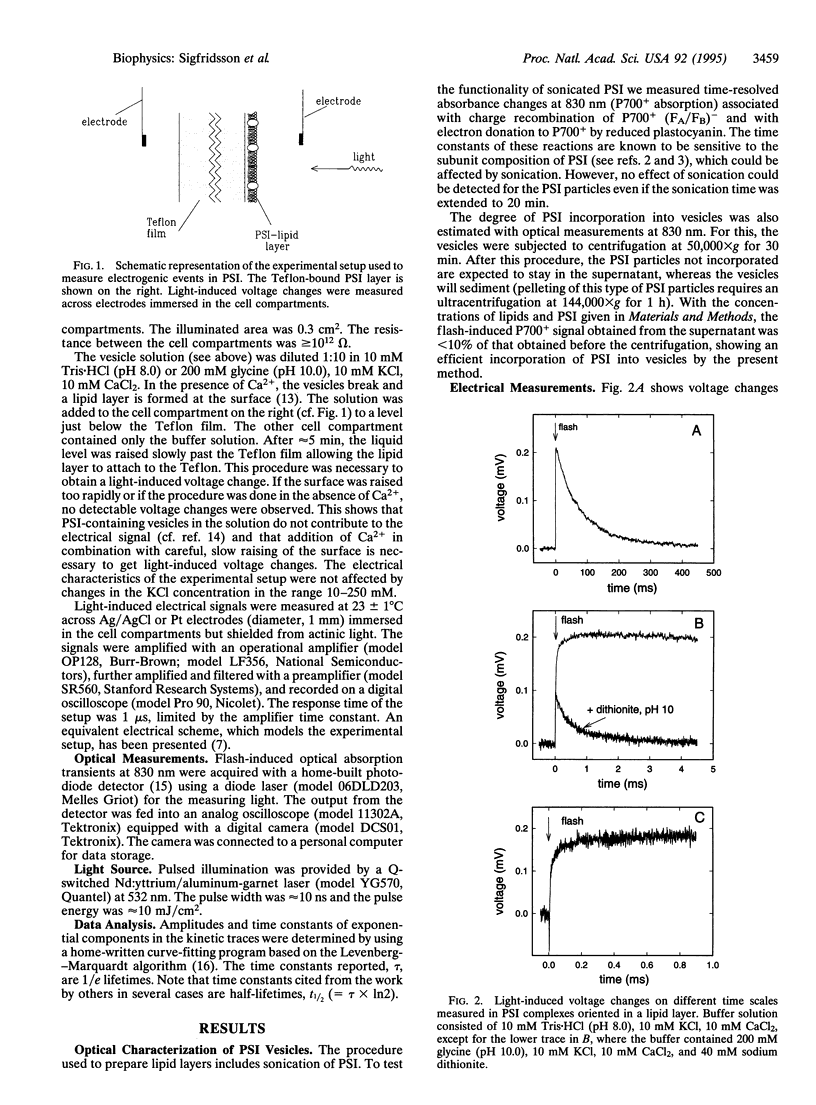
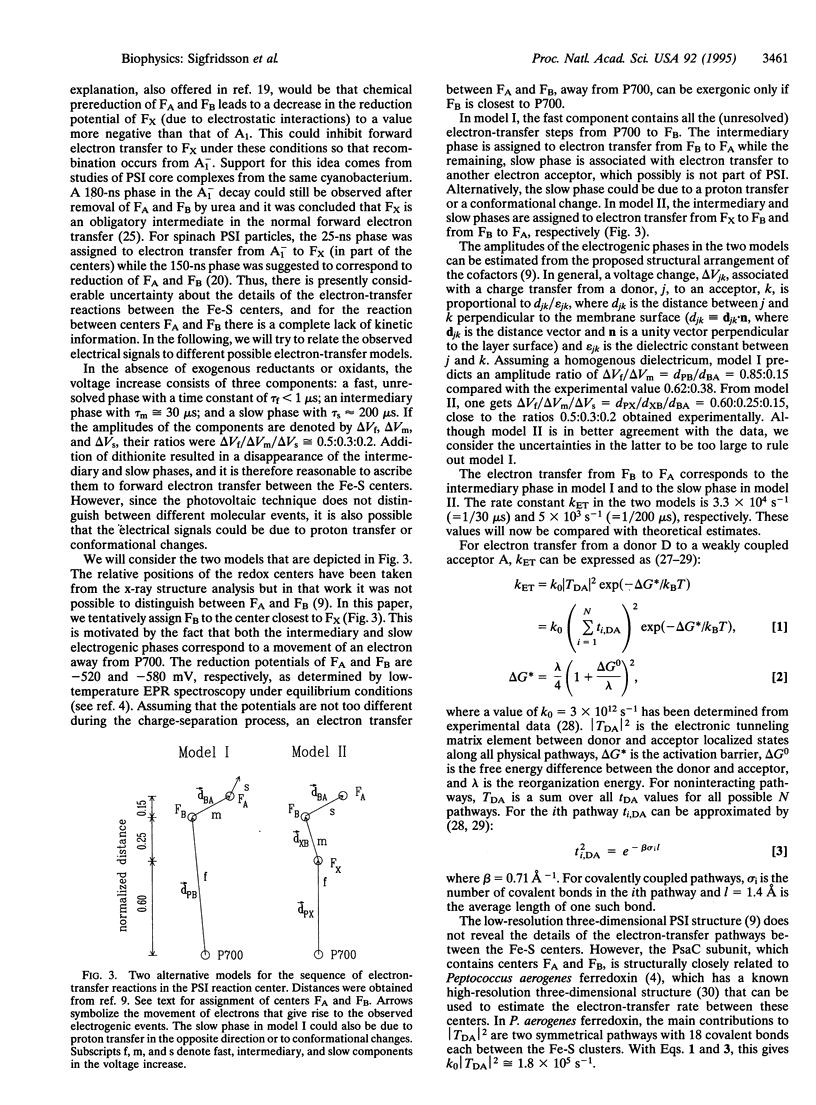
Flash-induced voltage changes (electrogenic events) in photosystem I particles from spinach, oriented in a phospholipid layer, have been studied at room temperature on a time scale ranging from 1 micros to several seconds. A phospholipid layer containing photosystem I particles was adsorbed to a Teflon film separating two aqueous compartments. Voltage changes were measured across electrodes immersed in the compartments. In the absence of added electron donors and acceptors, a multiphasic voltage increase, associated with charge separation, was followed by a decrease, associated with charge recombination. Several kinetic phases were resolved: a rapid (<1 micros) increase, ascribed to electron transfer from the primary electron donor P700 to the iron-sulfur electron acceptor FB, was followed by a slower, biphasic increase with time constants of 30 and 200 micros. The 30-micros phase is assigned to electron transfer from FB to the iron-sulfur center FA. The voltage decrease had a time constant of 90 ms, ascribed to charge recombination from FA to P700. Upon chemical prereduction of FA and FB the 30- and 200-micros phases disappeared and the decay time constant was accelerated to 330 micros, assigned to charge recombination from the phylloquinone electron acceptor (A1) or the iron-sulfur center FX to P700.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Siefker L. C., Jensen L. H. Structure of Peptococcus aerogenes ferredoxin. Refinement at 2 A resolution. J Biol Chem. 1976 Jun 25;251(12):3801–3806. doi: 10.2210/pdb1fdx/pdb. [DOI] [PubMed] [Google Scholar]
- De Vos H., Vauquelin G., De Keyser J., De Backer J. P., Van Liefde I. Regional distribution of alpha 2A- and alpha 2B-adrenoceptor subtypes in postmortem human brain. J Neurochem. 1992 Apr;58(4):1555–1560. doi: 10.1111/j.1471-4159.1992.tb11378.x. [DOI] [PubMed] [Google Scholar]
- Golbeck J. H. Structure, function and organization of the Photosystem I reaction center complex. Biochim Biophys Acta. 1987;895(3):167–204. doi: 10.1016/s0304-4173(87)80002-2. [DOI] [PubMed] [Google Scholar]
- Hallén S., Brzezinski P. Light-induced structural changes in cytochrome c oxidase: implication for the mechanism of electron and proton gating. Biochim Biophys Acta. 1994 Mar 8;1184(2-3):207–218. doi: 10.1016/0005-2728(94)90225-9. [DOI] [PubMed] [Google Scholar]
- Hecks B., Wulf K., Breton J., Leibl W., Trissl H. W. Primary charge separation in photosystem I: a two-step electrogenic charge separation connected with P700+A0- and P700+A1- formation. Biochemistry. 1994 Jul 26;33(29):8619–8624. doi: 10.1021/bi00195a001. [DOI] [PubMed] [Google Scholar]
- Hök F., Brzezinski P. Light-induced voltage changes associated with electron and proton transfer in photosystem II core complexes reconstituted in phospholipid monolayers. Biophys J. 1994 Jun;66(6):2066–2072. doi: 10.1016/S0006-3495(94)81001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüneberg J., Fromme P., Jekow P., Schlodder E. Spectroscopic characterization of PS I core complexes from thermophilic Synechococcus sp. Identical reoxidation kinetics of A1- before and after removal of the iron-sulfur-clusters FA and FB. FEBS Lett. 1994 Jan 31;338(2):197–202. doi: 10.1016/0014-5793(94)80364-1. [DOI] [PubMed] [Google Scholar]
- Nordling M., Sigfridsson K., Young S., Lundberg L. G., Hansson O. Flash-photolysis studies of the electron transfer from genetically modified spinach plastocyanin to photosystem I. FEBS Lett. 1991 Oct 21;291(2):327–330. doi: 10.1016/0014-5793(91)81313-w. [DOI] [PubMed] [Google Scholar]
- Schindler H. Formation of planar bilayers from artificial or native membrane vesicles. FEBS Lett. 1980 Dec 15;122(1):77–79. doi: 10.1016/0014-5793(80)80405-4. [DOI] [PubMed] [Google Scholar]
- Sétif P. Q., Bottin H. Laser flash absorption spectroscopy study of ferredoxin reduction by photosystem I in Synechocystis sp. PCC 6803: evidence for submicrosecond and microsecond kinetics. Biochemistry. 1994 Jul 19;33(28):8495–8504. doi: 10.1021/bi00194a014. [DOI] [PubMed] [Google Scholar]
- Sétif P., Brettel K. Forward electron transfer from phylloquinone A1 to iron-sulfur centers in spinach photosystem I. Biochemistry. 1993 Aug 10;32(31):7846–7854. doi: 10.1021/bi00082a002. [DOI] [PubMed] [Google Scholar]
- Trissl H. W., Darszon A., Montal M. Rhodopsin in model membranes: charge displacements in interfacial layers. Proc Natl Acad Sci U S A. 1977 Jan;74(1):207–210. doi: 10.1073/pnas.74.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke D. S., Bjerrum M. J., Winkler J. R., Gray H. B. Electron-tunneling pathways in cytochrome C. Science. 1992 May 15;256(5059):1007–1009. doi: 10.1126/science.256.5059.1007. [DOI] [PubMed] [Google Scholar]



