Abstract
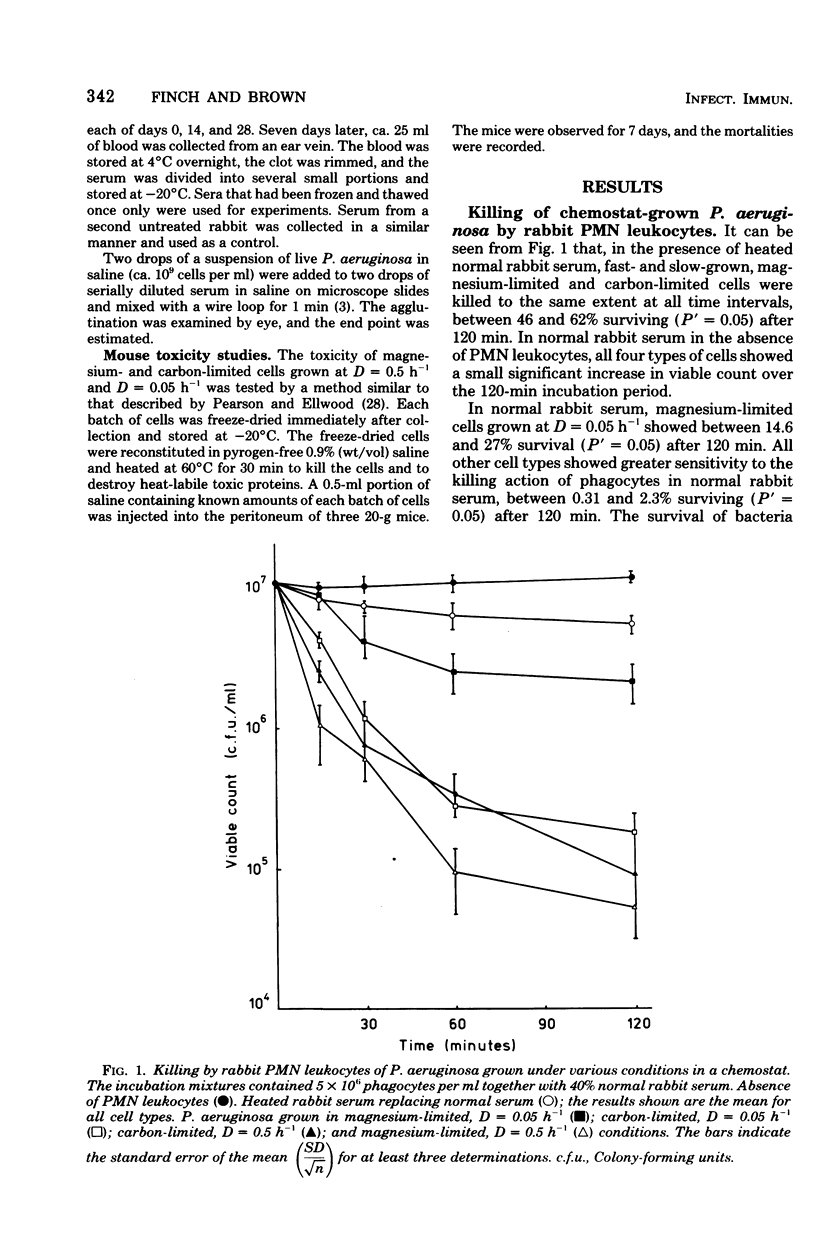
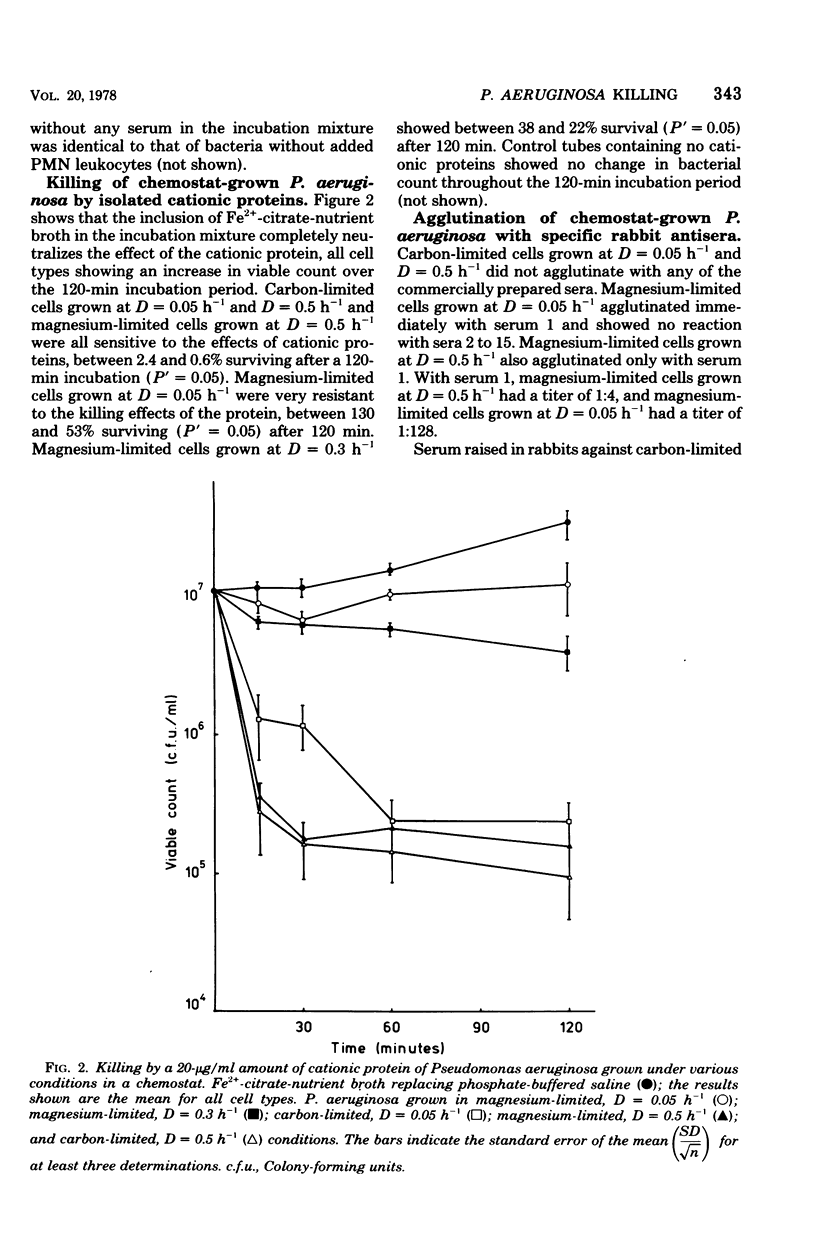
Pseudomonas aeruginosa grown in a chemostat under carbon- and magnesium-limited conditions showed varying resistance to killing by rabbit peritoneal exudate polymorphonuclear leukocytes. Slow-growing (D = 0.05 h-1), magnesium-limited cells were significantly more resistant to the lethal effects of the phagocytes than were fast-growing magnesium-limited cells and carbon-limited cells (D = 0.05 h-1 and D = 0.5 h-1, respectively). The resistance of magnesium-limited cells to killing by cationic proteins isolated from the leukocytes was shown to be growth-rate dependent, the slowest-growing (D = 0.05 h-1) cells being the most resistant. Carbon-limited cells were sensitive to killing by the cationic proteins at all growth rates tested. Antisera raised in rabbits to all types of cells and commercial anti-Pseudomonas serum rapidly agglutinated magnesium-limited cells but failed to agglutinate carbon-limited cells. There was some indication that slow-growing (D = 0.05 h-1), magnesium-limited cells agglutinated most readily with both types of antisera. No difference was detected in the mouse toxicity of heat-killed cells grown under the various conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUN W., POMALES-LEBRON A., STINEBRING W. R. Interactions between mononuclear phagocytes and Brucella abortus strains of different virulence. Proc Soc Exp Biol Med. 1958 Feb;97(2):393–397. doi: 10.3181/00379727-97-23752. [DOI] [PubMed] [Google Scholar]
- Bader J., Teuber M. Action of polymyxin B on bacterial membranes. 1. Binding to the O-antigenic lipopolysaccharide of Salmonella typhimurium. Z Naturforsch C. 1973 Jul-Aug;28(7):422–430. [PubMed] [Google Scholar]
- Beckerdite S., Mooney C., Weiss J., Franson R., Elsbach P. Early and discrete changes in permeability of Escherichia coli and certain other gram-negative bacteria during killing by granulocytes. J Exp Med. 1974 Aug 1;140(2):396–409. doi: 10.1084/jem.140.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Factors in human serum promoting phagocytosis of Pseudomonas aeruginosa. I. Interaction of opsonins with the bacterium. J Infect Dis. 1974 Nov;130 (Suppl)(0):S119–S126. doi: 10.1093/infdis/130.supplement.s119. [DOI] [PubMed] [Google Scholar]
- Brown M. R. Nutrient depletion and antibiotic susceptibility. J Antimicrob Chemother. 1977 May;3(3):198–201. doi: 10.1093/jac/3.3.198. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M., Pitt T. L. The relationship between the O-antigenic lipopolysaccharides and serological specificity in strains of Pseudomonas aeruginosa of different O-serotypes. J Gen Microbiol. 1973 Oct;78(2):305–318. doi: 10.1099/00221287-78-2-305. [DOI] [PubMed] [Google Scholar]
- Eudy W. W., Burrous S. E. Generation times of Proteus mirabilis and Escherichia coli in experimental infections. Chemotherapy. 1973;19(3):161–170. doi: 10.1159/000221451. [DOI] [PubMed] [Google Scholar]
- Finch J. E., Brown M. R. The influence of nutrient limitation in a chemostat on the sensitivity of Pseudomonas aeruginosa to polymyxin and to EDTA. J Antimicrob Chemother. 1975 Dec;1(4):379–386. doi: 10.1093/jac/1.4.379. [DOI] [PubMed] [Google Scholar]
- Friedberg D., Shilo M. Role of cell wall structure of salmonella in the interaction with phagocytes. Infect Immun. 1970 Sep;2(3):279–285. doi: 10.1128/iai.2.3.279-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone G. P., Walton E. Effect of iron on the bactericidal proteins from rabbit polymorphonuclear leukocytes. Nature. 1970 Aug 22;227(5260):849–851. doi: 10.1038/227849a0. [DOI] [PubMed] [Google Scholar]
- HABS I. Untersuchungen über die O-Antigene von Pseudomonas aeruginosa. Z Hyg Infektionskr. 1957;144(3):218–228. [PubMed] [Google Scholar]
- HIRSCH J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956 May 1;103(5):589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Quie P. G., Windhorst D. B., Pollara B., Good R. A. Protection of phagocytized bacteria from the killing action of antibiotics. Nature. 1966 Jun 11;210(5041):1131–1132. doi: 10.1038/2101131a0. [DOI] [PubMed] [Google Scholar]
- JANOFF A., ZWEIFACH B. W. ADHESION AND EMIGRATION OF LEUKOCYTES PRODUCED BY CATIONIC PROTEINS OF LYSOSOMES. Science. 1964 Jun 19;144(3625):1456–1458. doi: 10.1126/science.144.3625.1456. [DOI] [PubMed] [Google Scholar]
- JENKIN C., BENACERRAF B. In vitro studies on the interaction between mouse peritoneal macrophages and strains of Salmonella and Escherichia coli. J Exp Med. 1960 Aug 1;112:403–417. doi: 10.1084/jem.112.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACEY B. W. Variation of the antigenic structure of certain bacteria with temperature of incubation and ionic composition of the medium. Biochem J. 1953 Nov 14;56(323RD):xiv–xiv. [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S94–S99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson A. D., Ellwood D. C. Growth environment and bacterial toxicity. J Med Microbiol. 1974 Aug;7(3):391–393. doi: 10.1099/00222615-7-3-391. [DOI] [PubMed] [Google Scholar]
- Roberts R. B. The interaction in vitro between group B meningococci and rabbit polymorphonuclear leukocytes. Demonstration of type specific opsonins and bactericidins. J Exp Med. 1967 Nov 1;126(5):795–818. doi: 10.1084/jem.126.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Edebo L. Phagocytosis of mutants of Salmonella typhimurium by rabbit polymorphonuclear cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):481–488. doi: 10.1111/j.1699-0463.1972.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Tagesson C., Edebo L. Influence of hyperimmune immunoglobulin G on the physicochemical properties of the surface of Salmonella typhimurium 395 MS in relation to interaction with phagocytic cells. Infect Immun. 1974 Aug;10(2):316–319. doi: 10.1128/iai.10.2.316-319.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagesson C., Stendahl O. Influence of the cell surface lipopolysaccharide structure of Salmonella typhimurium on resistance to intracellular bactericidal systems. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Aug;81(4):473–480. doi: 10.1111/j.1699-0463.1973.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Ellwood D. C. The influence of growth conditions on the composition of some cell wall components of Aerobacter aerogenes. Biotechnol Bioeng. 1969 Sep;11(5):775–783. doi: 10.1002/bit.260110507. [DOI] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]
- Young L. S. Role of antibody in infections due to Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S111–S118. doi: 10.1093/infdis/130.supplement.s111. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


