Abstract
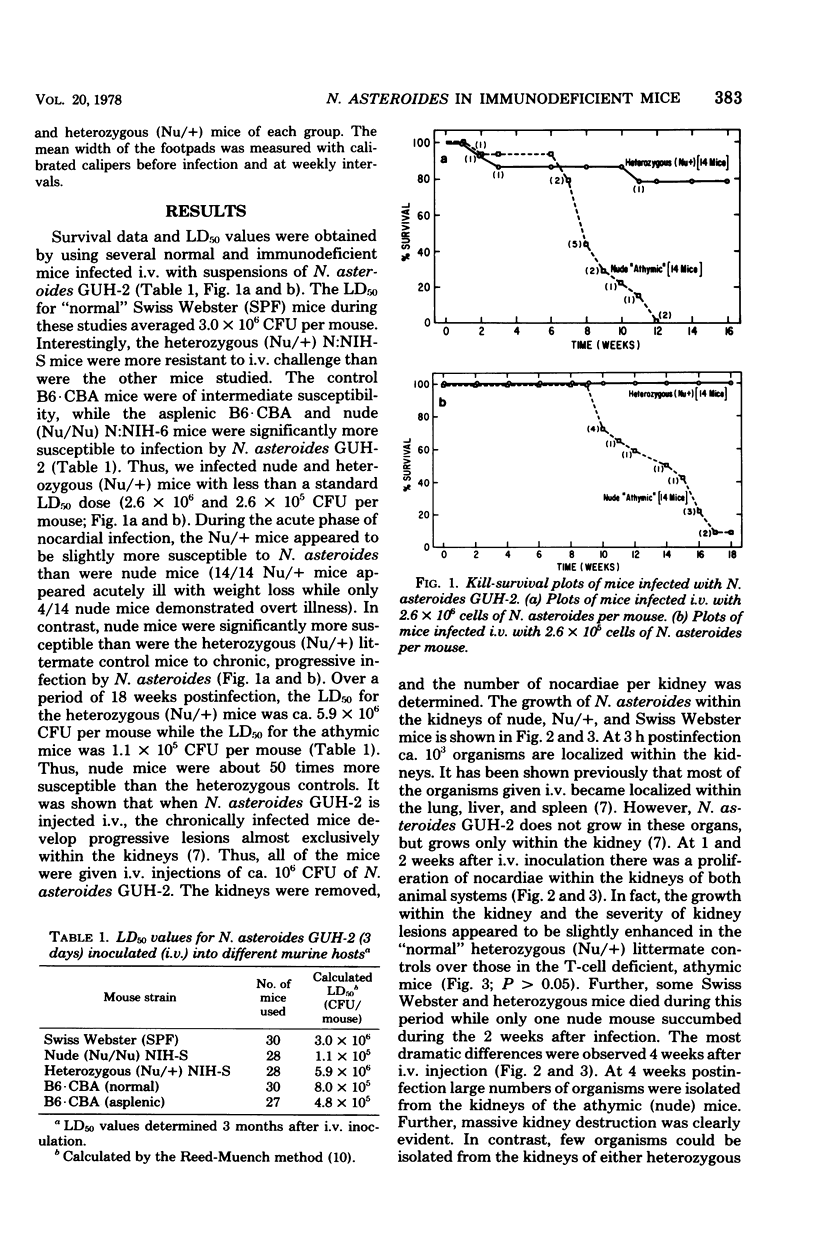
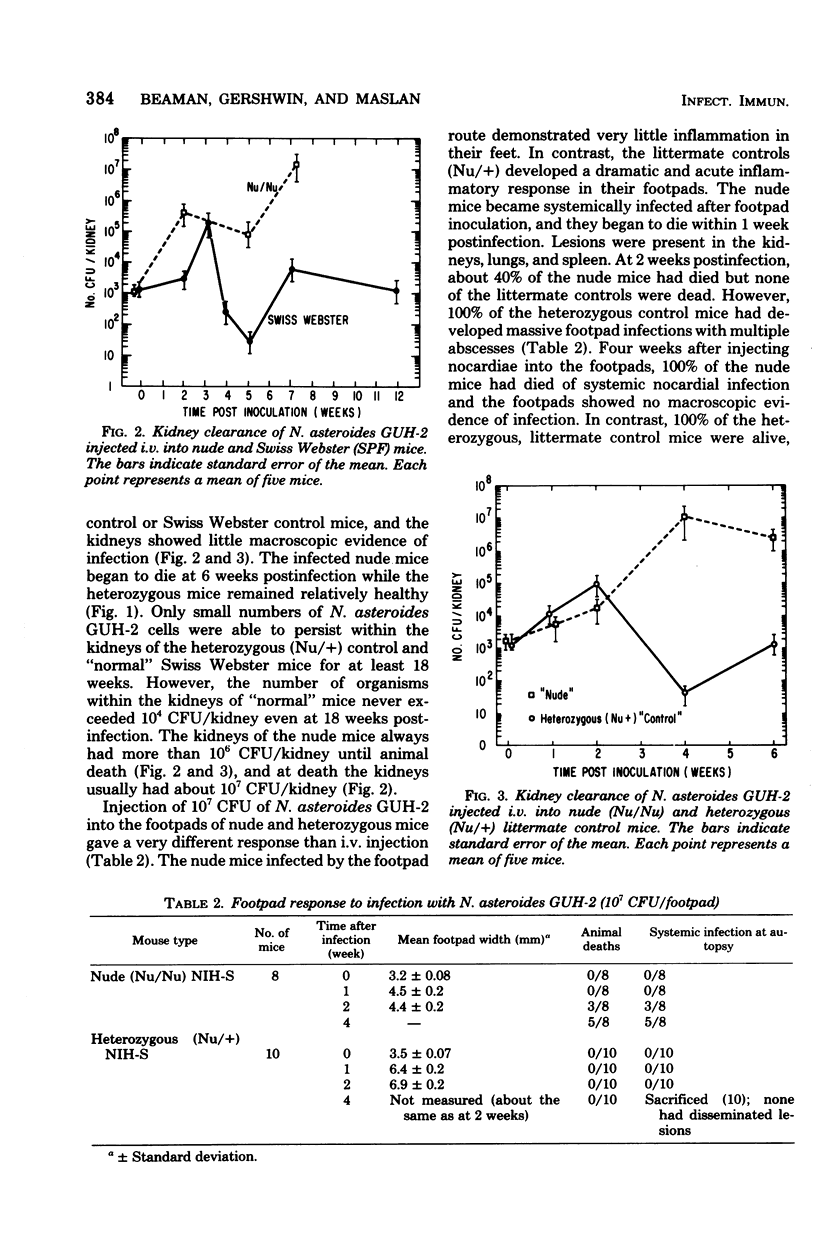
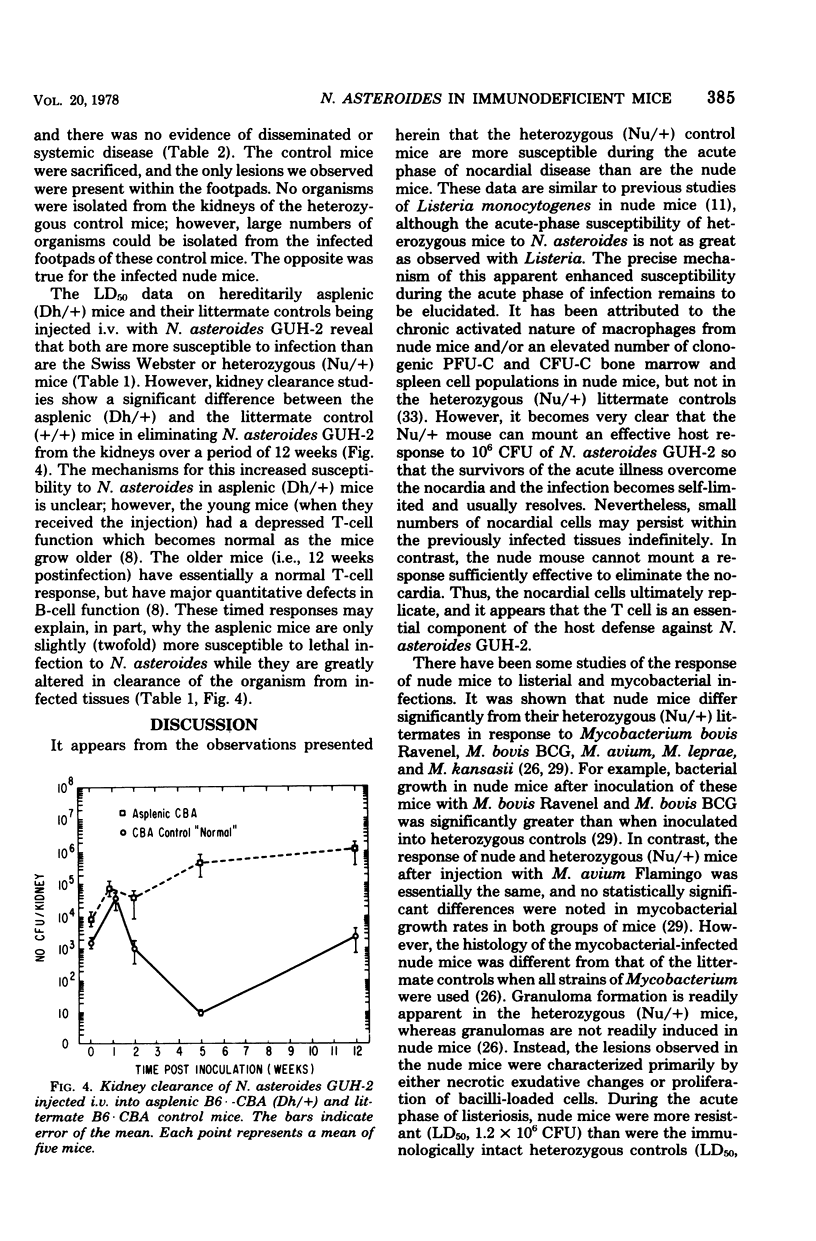
Congenitally athymic (Nu/Nu), hereditarily asplenic (Dh/+), and littermate control mice were given intravenous injections of homogeneous cell suspensions of the virulent Nocardia asteroides GUH-2. Kill curve, 50% lethal dose, and kidney clearance data were obtained over a period of 3 months postinfection. N. asteroides initiated both an acute infectious process and a chronic, progressive disease in these animals when given intravenously. The heterozygous (Nu/+) mice appeared to be slightly more susceptible to the acute phase of infection than their nude littermates. In contrast, nude mice were at least 50 times more susceptible to chronic nocardial infection than were the heterozygous (Nu/+) controls. Swiss Webster specific pathogen-free mice were similar to heterozygous (Nu/+) mice in their susceptibility to N. asteroides. The hereditarily asplenic (Dh/+) mice were not as susceptible to lethal infection as were nude mice. However, asplenic mice demonstrated an inability to eliminate nocardia from infected kidneys, whereas their littermate control (+/+) mice were able to mount an effective response and destroy most of the organisms within the kidneys. Similar observations were noted when nude and heterozygous (Nu/+) littermate mice were infected in the footpad. The nude mice developed a systemic infection and died within 4 weeks with little inflammation of the footpad and no macroscopic lesions. In contrast, heterozygous (Nu/+) mice developed extensive local abscesses in the foot that persisted for at least 4 weeks. There was no animal death and no evidence of dissemination. The data presented herein indicate that T cells are essential for adequate host response against infection with a virulent strain of N. asteroides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Beaman B. L. An ultrastructural analysis of Nocardia during experimental infections in mice. Infect Immun. 1973 Nov;8(5):828–840. doi: 10.1128/iai.8.5.828-840.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. In vitro response of rabbit alveolar macrophages to infection with Nocardia asteroides. Infect Immun. 1977 Mar;15(3):925–937. doi: 10.1128/iai.15.3.925-937.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Effect of cyclophosphamide on experimental Nocardia asteroides infection in mice. Infect Immun. 1977 Jun;16(3):995–1004. doi: 10.1128/iai.16.3.995-1004.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Smathers M. Interaction of Nocardia asteroides with cultured rabbit alveolar macrophages. Infect Immun. 1976 Apr;13(4):1126–1131. doi: 10.1128/iai.13.4.1126-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. Structural and biochemical alterations of Nocardia asteroides cell walls during its growth cycle. J Bacteriol. 1975 Sep;123(3):1235–1253. doi: 10.1128/jb.123.3.1235-1253.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucsi R. A., Borek F., Battisto J. R. Splenic replenishment of synergistic ability to bone marrow and thymic cells of neonatally splenectomized CBA mice. J Exp Med. 1972 Oct 1;136(4):761–768. doi: 10.1084/jem.136.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Congdon C. C., Morrison N. E. Growth of mycobacterium bovis (BCG) in T lymphocyte-depleted mice. Infect Immun. 1975 Jan;11(1):57–64. doi: 10.1128/iai.11.1.57-64.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Hof H. Cell-mediated resistance to infection with Listeria monocytogenes in nude mice. Infect Immun. 1977 Feb;15(2):382–385. doi: 10.1128/iai.15.2.382-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan S. P. 'Nude', a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966 Dec;8(3):295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- Folb P. I., Timme A., Horowitz A. Nocardia infections in congenitally athymic (nude) mice and in other inbred mouse strains. Infect Immun. 1977 Nov;18(2):459–466. doi: 10.1128/iai.18.2.459-466.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin M. E., Ikeda R. M., Kawakami T. G., Owens R. B. Immunobiology of heterotransplanted human tumors in nude mice. J Natl Cancer Inst. 1977 May;58(5):1455–1461. doi: 10.1093/jnci/58.5.1455. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Merchant B., Steinberg A. D. The effects of synthetic polymeric agents on immune responses of nude mice. Immunology. 1977 Mar;32(3):327–336. [PMC free article] [PubMed] [Google Scholar]
- Hsu C. K., Hsu S. H., Whitney R. A., Jr, Hansen C. T. Immunopathology of schistosomiasis in athymic mice. Nature. 1976 Jul 29;262(5567):397–399. doi: 10.1038/262397a0. [DOI] [PubMed] [Google Scholar]
- Isaak D. D., Jacobson R. H., Reed N. D. Thymus dependence of tapeworm (Hymenolepis diminuta) elimination from mice. Infect Immun. 1975 Dec;12(6):1478–1479. doi: 10.1128/iai.12.6.1478-1479.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krick J. A., Remington J. S. Resistance to infection with Nocardia asteroides. J Infect Dis. 1975 Jun;131(6):665–672. doi: 10.1093/infdis/131.6.665. [DOI] [PubMed] [Google Scholar]
- Mauel J., Behin R. Cell-mediated and humoral immunity to protozoan infections. Transplant Rev. 1974;19(0):121–146. doi: 10.1111/j.1600-065x.1974.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Gershwin M. E. T cell differentiation and the congenitally athymic (nude) mouse. Dev Comp Immunol. 1977 Oct;1(4):289–298. doi: 10.1016/s0145-305x(77)80012-8. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Hogarth-Scott R. S., Edwards R. D., Lewers H. M., Cousins G., Moore T. Studies on immune responses to parasite antigens in mice. I. Ascaris suum larvae numbers and antiphosphorylcholine responses in infected mice of various strains and in hypothymic nu/nu mice. Int Arch Allergy Appl Immunol. 1976;52(1-4):64–78. doi: 10.1159/000231669. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Hogarth-Scott R. S., Edwards R. D., Moore T. Studies on immune responses to parasite antigens in mice. III. Nippostrongylus brasiliensis infections in hypothymic nu/nu mice. Int Arch Allergy Appl Immunol. 1976;52(1-4):95–104. doi: 10.1159/000231671. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. Studies on immune responses to parasite antigens in mice. II. Aspects of the T cell dependence of circulating reagin production to Ascaris suum antigens. Int Arch Allergy Appl Immunol. 1976;52(1-4):79–94. doi: 10.1159/000231670. [DOI] [PubMed] [Google Scholar]
- Nelson D. S. Immunity to infection, allograft immunity and tumour immunity: parallels and contrasts. Transplant Rev. 1974;19(0):226–254. doi: 10.1111/j.1600-065x.1974.tb00134.x. [DOI] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Greenberg L. E., Merchant E. B., Vickers J. H. Response of congenitally athymic (nude) mice to infection with Mycobacterium bovis (strain BCG). J Natl Cancer Inst. 1975 Jun;54(6):1419–1426. doi: 10.1093/jnci/54.6.1419. [DOI] [PubMed] [Google Scholar]
- Sundararaj T., Agarwal S. C. Cell-mediated immunity in experimental Nocardia asteroides infection. Infect Immun. 1977 Feb;15(2):370–375. doi: 10.1128/iai.15.2.370-375.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Mori R., Nomoto K., Nakayama H. Experimental mycobacterial infections in neonatally thymectomized mice. Am Rev Respir Dis. 1967 Sep;96(3):469–477. doi: 10.1164/arrd.1967.96.3.469. [DOI] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Experimental mycobacterial infection in congenitally athymic "nude" mice. J Reticuloendothel Soc. 1976 Feb;19(2):77–90. [PubMed] [Google Scholar]
- Walzer P. D., Schnelle V., Armstrong D., Rosen P. P. Nude mouse: a new experimental model for Pneumocystis carinii infection. Science. 1977 Jul 8;197(4299):177–179. doi: 10.1126/science.301657. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Krick J. A., Remington J. S. Pulmonary infection in the compromised host: part I. Am Rev Respir Dis. 1976 Aug;114(2):359–394. doi: 10.1164/arrd.1976.114.2.359. [DOI] [PubMed] [Google Scholar]
- Wilson F. D., Gershwin M. E., Shifrine M., Graham R. Increased clonogenic (CFU-C, PFU-C) populations from bone marrow and spleen of nude mice. Dev Comp Immunol. 1977 Oct;1(4):373–384. doi: 10.1016/s0145-305x(77)80020-7. [DOI] [PubMed] [Google Scholar]


